Abstract
Our understanding of the typical human brain has benefitted greatly from studying different kinds of brains and their associated behavioral repertoires, including animal models and neuropsychological patients. This same comparative perspective can be applied to early development — the environment, behavior, and brains of infants provide a model system for understanding how the mature brain works. This approach requires non-invasive methods for measuring brain function in awake, behaving infants. fMRI is becoming increasingly viable for this purpose, with the unique ability to precisely measure the entire brain, including both cortical and subcortical structures. Here we discuss potential lessons from infant fMRI for several domains of adult cognition and consider the challenges of conducting such research and how they might be mitigated.
Keywords: cognitive development, memory systems, attention, perception, neuroimaging
Infant fMRI
The main objective of cognitive neuroscience has been to understand how the propensities of the mind are implemented in the brain. One of the most prolific and insightful tools in this endeavor has been functional magnetic resonance imaging (fMRI, see Glossary) — a technique now used in thousands of studies. However, a vanishingly small number of these studies involved infant participants, and in only a handful were the infants awake and having their behavior monitored (Box 1).
Box 1. Infant fMRI literature.
Infants seemingly do not make good fMRI participants: they are easily bored, cannot follow instructions or provide verbal responses, and are hard to keep still. Hence, most fMRI with infants has been done with them sleeping [74, 75] or sedated [76, 77]. These studies have often been conducted in a medical setting [78], although not always [79]. They have provided resting-state data that can be directly compared with adults [80].
Only a handful of fMRI studies have tested awake infants. The first prominent example involved collecting data while sleeping infants listened to speech [81]. Serendipitously, some participants woke up during the scan, which allowed researchers to study the awake brain’s responses to language. Scant other research of this kind has been done, until two recent studies:
The first examined motion perception [62]. Optic flow motion was shown to infants through VR goggles and contrasted with random motion. The infant data were compared to adult data, revealing that some properties of the adult motion processing system are apparent as early as 7 weeks of age.
The second examined category selectivity [22]. A custom bed and headcoil were used to study neural responses to faces, objects, and scenes. The spatial organization of face and scene responses in infants 4–6 months old was adult-like, though objects differed, as did overall selectivity, suggesting variation across categories in the role of experience in tuning perception.
What might be gained by studying the infant brain with fMRI? Perhaps the most apparent benefits are for developmental science, as discussed elsewhere [1]. In brief, this field has long been constrained by the limited range of dependent measures that can be obtained from infants, resulting in considerable disputes about the interpretation of findings, such as from looking time [2]. In this context, fMRI provides a rich, incidental measure that can be used to study the infant brain itself and also to unpack and understand widely used behavioral measures.
Rather than focus on these benefits for developmental science per se, here we consider potential benefits of infant fMRI for adult cognitive neuroscience. We explore how investigating the infant brain with fMRI could improve our understanding of mature brain function.
Theoretical framework
Infants and adults differ in a number of ways, including behavioral outputs (e.g., Figure 1A/B), brain structure (e.g., Figure 1C), and environmental inputs (e.g., Figure 1D/E). This results in fortuitous quasi-experiments in which two periods of development differ in an important and specific way, akin to comparative research with animal models or neuropsychological patients. Such comparisons can lack control due to confounding or unknown differences between groups. Moreover, because adults were once infants, their comparison is inherently different and closer than the comparison of humans to other species, which contrasts both phylogeny and ontogeny, and the comparison of typical to atypical brains, which contrasts abnormal experience, disease, and/or genetics. Indeed, it might seem more intuitive to just study adults directly.
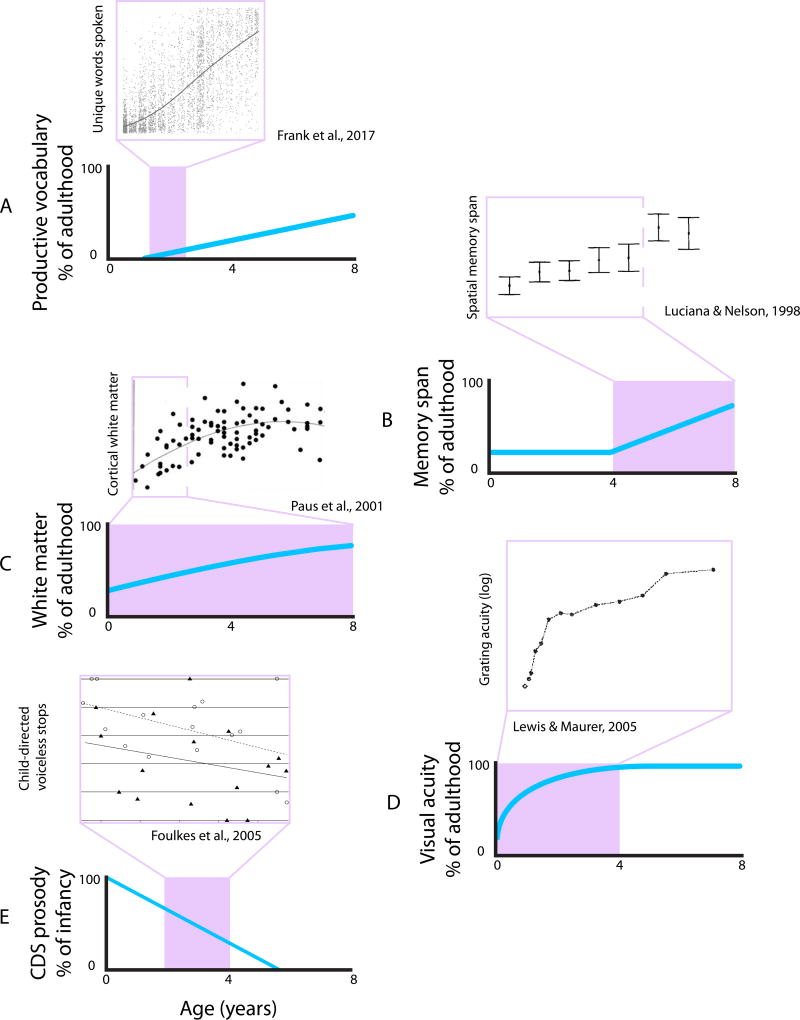
Figure 1. Approximate Developmental Trajectories for Certain Neural, Cognitive, and Behavioral Systems from 0 to 8 Years of Age.
(A) Language acquisition reflected in the size of spoken vocabulary [105]. (B) The development of memory span up to adult capacity [106]. (C) The amount of myelination across development relative to adults [107, 108]. (D) The visual acuity of children relative to adults [8]. (E) Changes in speech prosody from child- to adult-directed speech [11, 12]. Abbreviation: CDS, child-directed speech. Subpanels were adapted from figures in cited sources.
What then might be gained by comparing adults to infants? In their most basic form, some comparisons can be considered “knock-outs”: when a system is immature or unavailable in infants, their behavior and brain function can provide insight into the necessity of that system for the corresponding behavior and brain function in adults. However, the potential gains may run deeper. Specifically, whereas the aforementioned approach views infants as a deficient or simpler version of adults (analogous to an adult with a brain lesion), infants can also be viewed, at least in some circumstances, as adapted to the unique challenges they face in development. Through this lens, infant behavior and brain function could help explain adult behavior and brain function by revealing general principles about what computations are possible under different biological and environmental constraints.
Language acquisition provides a clear example of such adaptation [3, 4]. Infants are unique, among humans, in needing to acquire a first language (Figure 1), and may in turn be more plastic to linguistic input than adults [5]. For example, they must learn the sounds in their native language, but because they cannot know in advance which language this will be, they are born with broad sensitivity to phonemes and then during development lose contrasts that are not meaningful in their language [6, 7]. Infants must also learn the names of objects, but this is complicated by the fact that these labels are used sporadically and in environments with multiple objects. In practice, this complexity can be mitigated by the lower acuity of early vision (Figure 1D)[8, 9], by infants bringing individual objects close to their face and obscuring competing objects [10], and by caregivers speaking with exaggerated prosody to help infants segment words (Figure 1E)[11, 12]. Infants are adapted for more advanced aspects of language acquisition as well, including the benefit of their smaller working memory capacity for bootstrapping knowledge of grammatical rules (Figure 1B)[3, 13].
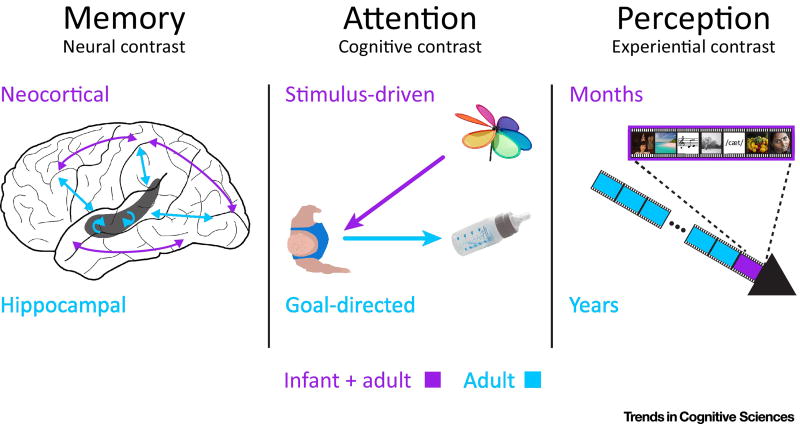
In sum, we argue that infants provide a rich and useful model system for adults. To illustrate the value of this approach, below we present possible quasi-experiments between infants and adults in different cognitive domains. These examples were chosen to highlight three types of quasi-experiments (Figure 2): (a) neural contrasts, in which a brain system in one group does not have the same organization, function, or connectivity in the other group; (b) cognitive contrasts, in which a mental process in one group is inoperative, differently constrained, or less efficient in the other group; and (c) experiential contrasts, in which the current and past environments of one group leads to different types and amounts of experience than the other group. All three types could be examined in one cognitive domain (e.g., neural, cognitive, and experiential contrasts in perception), and one type could be examined across multiple domains (e.g., neural contrasts in memory, attention, and perception), but below we consider each type in a different domain to provide more varied examples (i.e., neural contrast in memory, cognitive contrast in attention, and experiential contrast in perception).
Figure 2.
Different types of contrasts across three cognitive domains for which quasi-experiments with infant fMRI may be informative about adult cognitive neuroscience. Neural contrast in memory: infants may not need a dedicated memory system for rapid learning (the hippocampus) because there is less risk of catastrophic interference to stable prior knowledge (in neocortex). Cognitive contrast in attention: infant deficits in goal-directed attention may reflect impoverished goal representations and help isolate behaviors and brain networks associated with stimulus-driven attention. Experiential contrast in perception: infants have much less sensory experience and thus every new experience may lead to more substantial perceptual changes than adults and a greater ability to tease apart proposed theoretical explanations of such learning.
Neuroscientific methods can be useful for all types of quasi-experiments, especially in comparisons with pre-verbal infants who afford few dependent measures. This usefulness is clearest for neural contrasts, but also applies to cognitive contrasts (i.e., for indexing cognitive processes) and experiential contrasts (i.e., for assessing the impact of experience). Because adult cognitive neuroscience depends so heavily on fMRI, we focus in each case on what might be learned from infant fMRI. Other neuroimaging techniques have valuable and complementary strengths, of course, including being more amenable to data collection from developing populations (Box 2). Moreover, although we focus on theories and findings from adult cognitive neuroscience, we believe that this approach could have implications for our understanding of development itself (Box 3).
Box 2. Infant neuroimaging methods.
Although we have focused on fMRI, a number of other valuable techniques are available for examining infant brain function:
Electroencephalography (EEG): records electrical changes on the scalp with high temporal resolution to measure rapid cognitive processes, at the cost of precise spatial localization of these functions. As an example, EEG has been used with infants to identify processing stages in the visual hierarchy that distinguish predicted from unpredicted events [82].
Magnetoencephalography (MEG): captures changes in the magnetic field generated by electrical activity in the brain. MEG has similar temporal resolution to EEG but potentially better spatial resolution. It may be particularly amenable to infant studies because of reduced acoustic noise and relative tolerance for motion [83, 84]. For instance, MEG has been used to study changes in the attention system of pre-term infants and to predict delays in visual development [85].
Functional near-infrared spectroscopy (fNIRS): uses infrared light on the scalp to measure blood flow in the brain. Robust to a variety of testing conditions (including movement), fNIRS can measure cortical activity with potentially similar spatial precision to fMRI in some parts of the brain near to the cortical surface [86]. As one example, fNIRS has been used in infants to study multisensory prediction in visual cortex [87].
Box 3. Impact for developmental research.
Different periods throughout development present unique challenges and a child’s behavior, cognition, and brain may be adapted to overcome these challenges [10]. We have emphasized the comparative implications of this perspective for adult cognitive neuroscience, but it also has implications for development itself.
For example, this perspective can help identify which aspects of the environment infants represent. Visual perception is severely impoverished at birth and improves over the first year of life [9]. As in congenital blindness [88], these visual deficits may increase reliance on, and sensitivity to, other modalities for infants. Indeed, in auditory perception, thresholds for detecting high-frequency sounds are adult-like by 6 months of age [89]. Furthermore, neonatal olfaction may in some cases be superior to adult olfaction [90]. These enhanced perceptual abilities in non-visual modalities may underlie functions that become visual in adulthood, such as identifying individuals [91].
This perspective can also inform how individuals will be affected by atypical challenges and constraints. Premature infants are often placed in neonatal intensive care units with vastly different environmental statistics than the womb, including more exposure to high-frequency sounds [92], full-spectrum light, and painful stimuli, as well as reduced exposure to language, touch, and physical constraints on movement. Insofar as a fetus develops in response to their maternal environment, the sensory and motor systems of premature infants may not unfurl in the same way during this same biological age range. In turn, this may impair learning of subsequent information for which the womb provides optimal preparation, relative to full-term infants [92]. At the same time, these differential experiences may improve some discrimination abilities in premature infants, though with still-greater risks overall for developmental delay and disorders.
Finally, this perspective should apply across the lifespan, with aspects of adolescence and senescence reflecting adaptation to environmental challenges. Consider adolescents for example: Although commonly thought to be deficient because of impairments in self-control, accompanying increases in novelty-seeking and risk-taking, especially in social contexts, may facilitate familial independence [93]. This transition to independence may also be associated with an increased need for flexible thinking, and correspondingly, adolescents are more likely to update their beliefs based on new information than adults or younger children [94].
This perspective does not explain all or likely even most of development, but the examples above illustrate how it may nevertheless prove informative and productive for developmental science.
Example cognitive domains
In what follows, we explore potential gains from conducting quasi-experiments with infant and adult populations for current topics in the cognitive neuroscience of adult memory, attention, and perception (Figure 2). These fields are used as examples to show how neural, cognitive, and experiential contrasts between infants and adults, using fMRI as a method, can inform cognitive neuroscience. Of special interest are questions for which research on adults alone would be difficult or tell only a partial story.
Memory: How do we avoid interference while learning?
Neural contrasts can be made in the domain of memory by comparing the brain systems that support different forms of learning/memory. In particular, because infants and adults are able to achieve at least somewhat analogous memory behaviors despite having different memory systems, the way in which these behaviors are generated may differ, providing a window into the computational requirements of brain systems for producing specific behaviors. Here we discuss the potential implications of this approach for a prominent theory of memory, complementary learning systems (CLS) [14].
Adults are able to quickly learn individual episodes (e.g., where I parked my car today) without confusing them with related prior experiences (e.g., where I parked my car yesterday), while also being able to learn regularities across these episodes abstracted away from the details of any individual episode (e.g., where I tend to park my car). CLS and the neural networks that instantiate its variations reveal that these two goals — fast learning and stable retention — trade off against one another and cannot be optimized simultaneously within the same system [14, 15]. Specifically, model simulations indicate that if learning happens quickly, then previously stored information may be overwritten (i.e., catastrophic interference).
In CLS, fast learning and stable retention are achieved in the adult brain via a division of labor between two memory systems — the hippocampus and neocortex. The hippocampus is necessary for rapidly encoding information from even a single experience into episodic memory. Representations of recently encoded information are reflected in the patterns of hippocampal activity [16] and the hippocampus reinstates these representations when memories are retrieved [17]. The neocortex is responsible for storing information more stably from multiple experiences. This includes semantic knowledge about concepts [18], visual knowledge about objects and their parts [19], and spatial knowledge about the environment [20]. When hippocampal and neocortical systems work in concert, the brain can achieve both fast and robust storage of information: individual episodes encoded rapidly in the hippocampus are consolidated slowly into neocortex, resulting in the storage of long-term memories for these episodes and their regularities without retroactive interference to pre-existing knowledge.
Although CLS as described above does a good job of accounting for neural and behavioral data from adults, the computational requirements of infant memory may suggest a different solution. For example, the risk of catastrophic interference in infants is lower simply because they have less knowledge than adults. Moreover, one reason the hippocampus is necessary for episodic memory in adults is that it can bind inputs from disparate sensory regions [21]; however, these regions in infants are more broadly tuned [22] and multisensory [23]. This greater cortical overlap could in turn allow for local Hebbian learning, eliminating the need for hippocampal binding. Finally, the hippocampus has a protracted maturation, with the trisynaptic pathway that supports episodic memory coming online only in adulthood in non-human primates (Box 4). For these reasons, it seems plausible that rapid learning in infants circumvents the hippocampus and happens directly in neocortex, consistent with evidence of greater cortical plasticity early in life [24]. Fast cortical learning would aid the infant in adapting to the new and constantly changing challenges it faces in the world. Because of this speed, such cortical learning may even be of a different nature (i.e., more episodic) than cortical learning in adulthood.
Box 4. What does the hippocampus do in early development?
Although we do not yet understand the function of the hippocampus in infancy, there is some progress in understanding its anatomy [95]. At around 6 months, the hippocampus has adult-like glucose use and synaptic density [96]. By 12 months, the dentate gyrus (DG) has adult-like morphology but an overabundance of synapses that are pruned until about age 5 [97]. Based on non-human primates, the monosynaptic pathway (MSP) connecting entorhinal cortex with CA1 develops relatively earlier than the trisynaptic pathway (TSP) connecting entorhinal cortex, through DG, then CA3, to CA1 [98]. However, the timing of these transitions in humans is unknown, though some have speculated that the MSP is functional before the age of 2 whereas the TSP pathway develops between 2 and 5 years of age [95, 98].
This trajectory of hippocampal development leads to an interesting hypothesis about what kinds of learning and memory are possible early in life. A recent computational model showed that the MSP can integrate across experiences to support statistical learning, even without a functioning TSP [99], which is consistent with the ability of even very young infants to do statistical learning [100–102]. Episodic memory behavior, in contrast, should emerge only when the TSP (and the pattern separation it enables) comes online, consistent with the ongoing development of episodic memory into childhood [103, 104]. An alternative hypothesis is that the hippocampus can perform adult-like computations from infancy, but that the immaturity of cortex prevents it from being able to support episodic memory [14]. A third possibility, described in the main text, is that infant hippocampus plays a reduced role in memory and instead statistical learning and memory encoding are supported directly by cortex. We hope that infant fMRI will provide valuable data to help evaluate these and other accounts.
There is not yet enough data to constrain these ideas and build developmental models. In moving forward, fMRI is well-suited to assessing the relative contributions of the hippocampus and neocortex to infant memory, as it can resolve activity and representations from deep brain structures with high spatial resolution, including from hippocampal subfields and circuits [25].
Attention: How do we prioritize sensory information?
Cognitive contrasts can be made in the domain of attention by comparing the component processes that underlie different ways of prioritizing sensory information. The standard definition of attention is broad, spanning functions like maintenance, orienting, facilitation, and inhibition [26]. These functions interact to support two competing needs: pursuing a goal while minimizing distraction (e.g., reading this article) while monitoring the environment for information that might require changing tasks (e.g., someone calling your name). These needs are reflected in a common dichotomy in the attention literature between top-down, goal-directed attention (i.e., volitional orienting to goal-relevant stimuli) and bottom-up, stimulus-driven attention (i.e., involuntary capture by salient stimuli) [27, 28]. These processes are recruited differently by various attention tasks [27] and require different brain systems [28].
Despite these distinctions, it is often difficult to isolate goal-directed and stimulus-driven attention in humans [29]. For instance, a typical task for assessing stimulus-driven attention involves responding as fast as possible based on the appearance of a stimulus. Yet, in order to execute this behavior, a participant must follow task instructions, including remembering stimulus-response mappings and volitionally trying to respond quickly and accurately; in essence, the stimulus becomes goal-relevant. These otherwise intertwined processes can be isolated by examining how disruption in one system affects behavior. This has been a productive approach in animal models [30], neuropsychological patients [31], and healthy participants receiving stimulation [32].
Infants can similarly be viewed as having an impairment in one system — they seem to lack adult-like goal-directed attention. Although infants are capable of selective attention [33, 34] and sustained attention [35], stimulus salience is the primary driver of orienting early in childhood [36, 37]. This orienting is automatic and manifests without instructions or explicit rewards. A deficit in goal-directed attention is also evident in the developing brain (albeit in studies of older children to date) with less differentiation and usage of the attention networks that will become responsible for goal-directed orienting in adults [38–40].
These apparent deficits in goal-directed attention may actually better serve the current needs of infants [4]. Without adult-like knowledge and expertise, infants may learn more when guided by the environment than by their own intentions. Relatedly, infants may be limited to stimulus-driven attention, having had less opportunity to learn reward contingencies and lacking the locomotive abilities needed to attain goals.
In addition to such adaptive value, and a better normative understanding of the purpose of stimulus-driven attention, early deficits in goal-directed attention allow infants to be used as a model system. The mechanisms supporting stimulus-driven attention can be investigated more cleanly in the infant brain, by disentangling them from goal-directed attention. Infant fMRI, combined with child-friendly orienting tasks [33, 34], could thus help strengthen the link between brain regions/networks and exogenous behavior, as well as help elucidate the interdependence and causal relationships of stimulus-driven and goal-directed attention.
Perception: How do we adapt to our environment?
Experiential contrasts can be made in the domain of perception by comparing the recognition of different types of stimuli that have (or have not) been encountered. By adulthood, extensive experience has increased sensitivity for identifying or discriminating categories of objects that are frequent in the observer’s environment. For instance, Caucasian adults are faster at distinguishing male and female Caucasian faces compared to Japanese faces, whereas Japanese adults show the opposite pattern of behavior [41]. Such perceptual learning extends beyond faces to a wide variety of inputs, including letters, phonemes, and perceived actions [6].
There are several proposed psychological mechanisms of how adults gain these proficiencies; however, the circumstances under which they apply remains unclear [42]. At a neural level, perceptual learning could reflect attention, inference, and/or decision-making, enabling enhanced read-out of sensory information without changing the sensory code itself [43–47]. Alternatively, perceptual learning may result from refined tuning of even early sensory areas, enhancing representations of learned stimuli [48], or some combination of these mechanisms [42, 49, 50].
Adjudicating these accounts may be complicated by the strong focus in this field on studying perceptual learning in adults. Participants in these studies have undergone extensive learning throughout their lifetime, which has fine-tuned their perceptual system pre-experimentally. New learning must occur on top of this prior knowledge, entrenched associations, and expertise, biasing what adults can learn and how quickly. Moreover, many categories of objects like faces and letters, as well as basic features like orientation and motion, are so familiar and ingrained that experimental manipulations of these stimuli may conflict with natural statistics and prior learning, further diminishing effects. The challenge of inducing perceptual learning in adults is evident in the extensive training employed in such studies (often over weeks) and in the hyperspecificity of the resulting improvements to trained features and locations [51].
Infants provide an opportunity to examine the mechanisms of perceptual learning in a nubile, more plastic brain. Although infants begin with some tuning, such as for face-like stimuli [52, 53], experience adapts this tuning to the statistics of environmental input [7, 54]. Even by 4 months of age infants exhibit greater discrimination of faces from their own ethnicity relative to faces from ethnicities that they do not see regularly [55], a learning process often referred to as perceptual narrowing [6, 56]. Importantly, these changes in infants are more drastic than in adults, in terms of the scope of stimuli impacted, persistently generalizing to and affecting entire classes of frequently occurring objects.
By allowing for greater and more impactful perceptual learning, infants provide a useful model system for studying how humans gain such expertise and for evaluating different potential mechanisms. For example, specific regions of the adult brain respond selectively to faces [41, 57, 58] and the infant brain seems to have a similar organization [22]. At the same time, however, the selectivity of such responses is lower in infants, suggesting that experience serves to differentiate object representations [59]. Such findings from infant fMRI may help uncover general principles of human perceptual systems and how they adapt to the sensory environment.
Challenges of infant fMRI
Despite the potential value of infant fMRI in different domains, there are substantial practical obstacles that have prevented widespread adoption of this technique [1, 60, 61]. Some of these challenges apply more generally to developmental work (e.g., task design, behavioral measures, fussiness, etc.), and so leaning on developmental science when adapting adult fMRI protocols to infancy will be helpful. However, other challenges are more specific to fMRI studies and so require novel technical solutions. Below we outline three challenges that we and others have been encountering in conducting infant fMRI studies, and consider how insights from developmental studies and other new innovations are helping to overcome them.
Experimental design
The way behavioral experiments are conducted in adult and infant populations is very different: infants cannot be given instructions, their responses are usually indirect and hard to interpret (e.g., heart rate, looking time [2]), and testing sessions are very short and often take place on a caregiver’s lap. Perhaps surprisingly, many of these design elements can be preserved in infant fMRI. For example, caregivers can still participate in the experiment by entering [22, 62] or standing next to the scanner bore. It can be very helpful to also have an experimenter in the scanner room to help direct when to start and stop scans based on fussiness, motion, fatigue, etc. Other experimenters in the control room can then manage a menu of tasks, start movies during downtimes, and track the stream of fMRI and behavioral data. Individual scanning runs can be made quite short to maintain attention (e.g., 30–60 s), even just the duration of one block, if repeated and if the conditions are counterbalanced. Tasks can be made more engaging by employing salient visual and auditory stimuli, such as faces, looming motion, colors, and videos. Presenting these stimuli may require a new approach: mirror or goggle systems typically used in adult fMRI involve placing something on or over an infant’s face, which can be distracting and reduce visibility of the environment and caregiver. We have circumvented such issues by projecting panoramically on the ceiling of the scanner bore over the infant’s face. Visibility and comfort are further enhanced in our ongoing work by using only the bottom half of the headcoil, which we have found retains adequate SNR, likely because of the smaller head size of infants. Given this open-face apparatus, looking time can be measured easily with a small camera mounted in the bore. With these procedures, it is possible to conduct infant versions of cognitive neuroscience experiments.
Motion
Head movement presents a substantial challenge to fMRI research in general and may be especially problematic in infants, who tend to squirm and cannot be instructed to stay still. However, this motion can be mitigated in various ways. For example, a mock scanner session or other orientation may be helpful for acclimating infants (and parents) and reducing movement [63]. Moreover, we have observed that, at least for short periods of time in the scanner, infants move considerably less when presented with engaging stimuli. Training may also play a role in reducing movement, though in infants this would require instrumental conditioning or other implicit methods, rather than the incentives used with older children [64]. A final prevention technique is the use of foam padding and/or vacuum pillows, which reduce incidental motion without constricting the infant [65, 66]. Beyond prevention, motion can also be corrected during or after data acquisition. For example, prospective motion correction can adjust the field of view in real time to keep images aligned. Algorithms that depend on shifts in the brain images themselves work well in adults (e.g., PACE sequences) but fail under excessive motion, whereas camera-based approaches with fiducial markers can be more robust. After acquisition, extensive quality assurance and preprocessing help scrub motion artifacts out of the data.
Alignment
Registering brain data across infants — in either a cross-sectional or longitudinal study — is extremely difficult, let alone comparisons between infant and adult brains. In adults, differences in brain shape and size are often assumed to preserve the relative organization of functions, and thus data can be aligned by stretching or squeezing brains into the space of a “standard” brain. Fortunately, atlases and standard brains are now becoming available for infants [67–69], allowing for age-specific alignment and group analyses. Even so, such techniques make assumptions about the mapping of function onto anatomy that are unproven in development. For example, although brain function may be relatively stable at maturity in adults, the development of function (and anatomy) over time can follow a different trajectory across infants, increasing the variability at any given age. Similarly, comparisons to adults based on anatomical alignment require a questionable assumption that the brain’s functional organization is preserved across development. One approach for improving alignment is to incorporate multiple modalities of data, including structural, diffusion, and resting [70]. Task data can also be useful for this purpose, potentially allowing infant data to be aligned functionally. Such approaches — known as hyperalignment [71] or shared response modeling [72] — seek to map voxels from one infant onto other infants, or from an infant at one age onto the same infant at different ages, based on the similarity of temporal response profiles to a common stimulus. Even if such response profiles change over development, limiting the success of such methods, these techniques could help quantify the amount and nature of developmental changes in a brain-wide manner. Partly mitigating the alignment problem, modern cognitive neuroscience approaches such as multivariate pattern analysis focus on extracting the contents of mind rather than on localizing responses anatomically [73].
Concluding remarks
Cognitive science has often advanced by taking on new perspectives and adopting new tools. fMRI has been a valuable technique in adults and older children, and there is great promise in using it to elucidate the infant mind and brain. The opportunity to assess cognition incidentally in infants could have a significant impact on developmental science by providing a new kind of dependent measure. Here we emphasized the additional influence this tool could have on cognitive neuroscience more generally (see ‘Outstanding Questions’). We argued that viewing the infant brain as a model for how intelligent systems organize themselves under different biological and environmental constraints may ultimately help explain why memory, attention, perception, and cognition more generally work the way they do in adults.
Outstanding questions.
Infantile amnesia refers to the fact that memories encoded in the first few years of life are inaccessible in adulthood. Theories have been proposed to explain infantile amnesia but have not been tested with functional imaging of memory systems in the developing human brain. What can be learned about infantile amnesia from an improved understanding of hippocampal development?
Our understanding of mental health disorders has been improved by fMRI and its ability to characterize normal and dysfunctional brain activity. How will the diagnosis and treatment of developmental disorders be informed by infant fMRI?
It may be more difficult to learn a new language in adulthood relative to infancy because perceptual systems are tuned by early experience and become inflexible. How can such perceptual narrowing be overcome in adults to unleash new learning?
Standards have emerged over the past two decades for the acquisition, preprocessing, and analysis of adult fMRI data. However, many of these conventions are suboptimal in infants because of their different brain size, structure, and function, and different comportment in the scanner. How should such methods be adjusted for infant fMRI data?
Multivariate analysis methods, emphasizing distributed patterns and networks, have made it possible to extract mental representations from fMRI data. This may be valuable in developmental studies, in which choice of dependent measures is limited. What can be learned about development from decoding and other advanced approaches?
Looking time is perhaps the most pervasive and useful dependent measure in infant cognition, but its interpretation is controversial [2]. Part of the difficulty might be that it is a relatively simple behavior over-determined by multiple complex mechanisms in the brain (i.e., attention networks, oculomotor control systems, neuromodulatory circuits). How would an improved understanding of brain mechanisms help dissect and interpret looking behavior?
Highlights.
Brain imaging methods provide a useful dependent measure for developmental psychology
Advances in acquisition and analysis make fMRI feasible for awake and behaving infants
Infant research provides an important constraint on theories from adult cognitive neuroscience
Theories about learning and memory are starting to account for this period of dramatic change
Acknowledgments
We thank J. Junge, J. Fan, and N. Malek for comments on an early version of this paper. N.B.T-B. was supported by NIH grants R01 EY021755 and R01 MH069456.
Glossary
- Complementary learning systems
A rapid, high-fidelity memory system, thought to be instantiated in the hippocampus, supports the gradual consolidation of memories into stable, often generalized representations in another memory system, thought to be instantiated in the neocortex.
- Episodic memory
The ability to encode an event in the context of the space and time in which it occurs after experiencing it once, and then to retrieve such details based on partial cues in the future.
- fMRI
Brain imaging tool to acquire spatially precise measurements of activity across the whole brain every 1–2 s. fMRI measures the blood oxygenation level dependent (BOLD) response, a proxy for the metabolic activity of neurons in a volumetric cube of brain tissue.
- Goal-directed attention
The volitional selection and facilitation of internal or external stimuli to achieve behavioral goals.
- Hebbian learning
Neural learning rule that reweights the strength of synapses based on the correlations of activity between pre-synaptic and post-synaptic neurons.
- Perceptual learning
Improvement in discrimination abilities as a result of extensive experience, often but not necessarily due to training, feedback, and/or reward.
- Perceptual narrowing
A transition from broad sensitivity across diverse stimuli to more selective sensitivity for stimuli that are frequent in an observer’s environment.
- Quasi-experiment
Between group comparison where group assignment is not performed by the experimenter. Quasi-experiments can be considered well controlled when all factors other than the intended “manipulation” are balanced between the groups.
- Statistical learning
The ability to represent regularities that are extracted from continuous sensory input across multiple episodes and often without effort or awareness.
- Stimulus-driven attention
The automatic orienting and selection of stimuli based on their inherent salience and distinctiveness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morita T, et al. Contribution of Neuroimaging Studies to Understanding Development of Human Cognitive Brain Functions. Frontiers in Human Neuroscience. 2016;10:464. doi: 10.3389/fnhum.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslin RN. What's in a look? Developmental Science. 2007;10:48–53. doi: 10.1111/J.1467-7687.2007.00563.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newport EL. Maturational constraints on language learning. Cognitive Science. 1990;14:11–28. [Google Scholar]
- 4.Thompson-Schill SL, et al. Cognition without control: When a little frontal lobe goes a long way. Current Directions in Psychological Science. 2009;18:259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singleton JL, Newport EL. When learners surpass their models: The acquisition of American Sign Language from inconsistent input. Cognitive Psychology. 2004;49:370–407. doi: 10.1016/j.cogpsych.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Scott LS, et al. A domain-general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16:197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant behavior and development. 1984;7:49–63. [Google Scholar]
- 8.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Developmental Psychobiology. 2005;46:163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 9.Mayer DL, Dobson V. Visual acuity development in infants and young children, as assessed by operant preferential looking. Vision research. 1982;22:1141–1151. doi: 10.1016/0042-6989(82)90079-7. [DOI] [PubMed] [Google Scholar]
- 10.Smith LB, et al. Not your mother’s view: The dynamics of toddler visual experience. Developmental Science. 2011;14:9–17. doi: 10.1111/j.1467-7687.2009.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H-M, et al. Age-related changes in acoustic modifications of Mandarin maternal speech to preverbal infants and five-year-old children: a longitudinal study. Journal of child language. 2009;36:909–922. doi: 10.1017/S030500090800929X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulkes P, et al. Phonological variation in child-directed speech. Language. 2005;81:177–206. [Google Scholar]
- 13.Elman JL. Learning and development in neural networks: The importance of starting small. Cognition. 1993;48:71–99. doi: 10.1016/0010-0277(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 14.McClelland JL, et al. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 15.Kumaran D, et al. What learning systems do intelligent agents need? complementary learning systems theory updated. Trends in Cognitive Sciences. 2016;20:512–534. doi: 10.1016/j.tics.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka KZ, et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84:347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Rogers TT, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychological Review. 2004;111:205. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- 19.Murray EA, et al. Visual Perception and Memory: A New View of Medial Temporal Lobe Function in Primates and Rodents. Annual Review of Neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- 20.Brodt S, et al. Rapid and independent memory formation in the parietal cortex. Proceedings of the National Academy of Sciences. 2016;113:13251–13256. doi: 10.1073/pnas.1605719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayes A, et al. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Deen B, et al. Organization of high-level visual cortex in human infants. Nature Communications. 2017;8:13995. doi: 10.1038/ncomms13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner K, Dobkins KR. Synaesthetic associations decrease during infancy. Psychological Science. 2011;22:1067–1072. doi: 10.1177/0956797611416250. [DOI] [PubMed] [Google Scholar]
- 24.Johnston MV, et al. Plasticity and injury in the developing brain. Brain and Development. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisse LE, et al. A harmonized segmentation protocol for hippocampal and parahippocampal subregions: Why do we need one and what are the key goals? Hippocampus. 2017;27:3–11. doi: 10.1002/hipo.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun MM, et al. A taxonomy of external and internal attention. Annual Review of Psychology. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- 27.Jonides J. Voluntary versus automatic control over the mind’s eye’s movement. Attention and performance IX. 1981;9:187–203. [Google Scholar]
- 28.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 29.Awh E, et al. Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeWeerd P, et al. Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nature Neuroscience. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- 31.Hu P, et al. Attention network impairments in patients with focal frontal or parietal lesions. Neuroscience Letters. 2013;534:177–181. doi: 10.1016/j.neulet.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 32.Kehrer S, et al. Timing of spatial priming within the fronto-parietal attention network: a TMS study. Neuropsychologia. 2015;74:30–36. doi: 10.1016/j.neuropsychologia.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Johnson MH, et al. Facilitation of saccades toward a covertly attended location in early infancy. Psychological Science. 1994;5:90–93. [Google Scholar]
- 34.Johnson MH, et al. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. Journal of Cognitive Neuroscience. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- 35.Xie W, Richards JE. Effects of interstimulus intervals on behavioral, heart rate, and event-related potential indices of infant engagement and sustained attention. Psychophysiology. 2016;53:1128–1142. doi: 10.1111/psyp.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslin RN, Salapatek P. Saccadic localization of visual targets by the very young human infant. Attention, Perception, & Psychophysics. 1975;17:293–302. [Google Scholar]
- 37.Jakobsen KV, et al. Look here! The development of attentional orienting to symbolic cues. Journal of Cognition and Development. 2013;14:229–249. [Google Scholar]
- 38.Fair DA, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey B, et al. Early development of subcortical regions involved in non-cued attention switching. Developmental Science. 2004;7:534–542. doi: 10.1111/j.1467-7687.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- 40.Bunge SA, et al. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Toole AJ, et al. A perceptual learning theory of the information in faces. In: Valentine T, editor. Cognitive and computational aspects of face recognition. Routledge; 1995. pp. 159–182. [Google Scholar]
- 42.Goldstone RL, Byrge LA. Perceptual Learning. In: Matthen M, editor. The Oxford Handbook of Philosophy of Perception. Oxford University Press; 2015. [Google Scholar]
- 43.Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends in Cognitive Sciences. 2004;8:457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Polley DB, et al. Perceptual learning directs auditory cortical map reorganization through top-down influences. Journal of Neuroscience. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahnt T, et al. Perceptual learning and decision-making in human medial frontal cortex. Neuron. 2011;70:549–559. doi: 10.1016/j.neuron.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 46.Bejjanki VR, et al. Perceptual learning as improved probabilistic inference in early sensory areas. Nature Neuroscience. 2011;14:642–648. doi: 10.1038/nn.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dosher BA, Lu Z-L. Mechanisms of perceptual learning. Vision research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 48.Schoups A, et al. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 49.Chen N, et al. Sharpened cortical tuning and enhanced cortico-cortical communication contribute to the long-term neural mechanisms of visual motion perceptual learning. Neuroimage. 2015;115:17–29. doi: 10.1016/j.neuroimage.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 50.Hung S-C, Seitz AR. Prolonged training at threshold promotes robust retinotopic specificity in perceptual learning. Journal of Neuroscience. 2014;34:8423–8431. doi: 10.1523/JNEUROSCI.0745-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fahle M. Perceptual learning: specificity versus generalization. Current Opinion in Neurobiology. 2005;15:154–160. doi: 10.1016/j.conb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Johnson MH, et al. Newborns' preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 53.Macchi CV, et al. Can a nonspecific bias toward top-heavy patterns explain newborns' face preference? Psychological Science. 2004;15:379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- 54.Pascalis O, et al. Is face processing species-specific during the first year of life? Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- 55.Heron-Delaney M, et al. An adult face bias in infants that is modulated by face race. International Journal of Behavioral Development. 2016 doi: 10.1177/0165025416651735. 0165025416651735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Needham A, et al. Learning Visual Units After Brief Experience in 10-Month-Old Infants. Cognitive Science. 2014;38:1507–1519. doi: 10.1111/cogs.12123. [DOI] [PubMed] [Google Scholar]
- 57.Kanwisher N, et al. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarthy G, et al. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- 59.Arcaro MJ, et al. Seeing faces is necessary for face-domain formation. Nature Neuroscience. 2017;20:1404–1412. doi: 10.1038/nn.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frith C, Frith U. What can we learn from structural and functional brain imaging. In: Rutter M, B D, Pine D, Scott S, Stevenson J, Taylor E, Thapar A, editors. Rutter’s child and adolescent psychiatry. Blackwell; 2008. pp. 134–144. [Google Scholar]
- 61.Grill-Spector K, et al. Developmental neuroimaging of the human ventral visual cortex. Trends in Cognitive Sciences. 2008;12:152–162. doi: 10.1016/j.tics.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biagi L, et al. BOLD response selective to flow-motion in very young infants. PLoS Biol. 2015;13:e1002260. doi: 10.1371/journal.pbio.1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnea-Goraly N, et al. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner–the Diabetes Research in Children Network (DirecNet) experience. Pediatric radiology. 2014;44:181–186. doi: 10.1007/s00247-013-2798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slifer KJ, et al. Operant-Contingency-based preparation of children for functional magnetic resonance imaging. Journal of applied behavior analysis. 2002;35:191–194. doi: 10.1901/jaba.2002.35-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doria V, et al. Emergence of resting state networks in the preterm human brain. Proceedings of the National Academy of Sciences. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merchant N, et al. A patient care system for early 3.0 Tesla magnetic resonance imaging of very low birth weight infants. Early human development. 2009;85:779–783. doi: 10.1016/j.earlhumdev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Fonov VS, et al. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- 68.Altaye M, et al. Infant brain probability templates for MRI segmentation and normalization. Neuroimage. 2008;43:721–730. doi: 10.1016/j.neuroimage.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi F, et al. Infant brain atlases from neonates to 1-and 2-year-olds. PloS one. 2011;6:e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu H, et al. 2012 IEEE Statistical Signal Processing Workshop (SSP) IEEE; 2012. Regularized hyperalignment of multi-set fMRI data; pp. 229–232. [Google Scholar]
- 72.Chen P-HC, et al. A reduced-dimension fMRI shared response model. Advances in Neural Information Processing Systems. 2015:460–468. [Google Scholar]
- 73.Cohen JD, et al. Computational approaches to fMRI analysis. Nature Neuroscience. 2017;20:304. doi: 10.1038/nn.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damaraju E, et al. Functional connectivity in the developing brain: a longitudinal study from 4 to 9 months of age. NeuroImage. 2014;84:169–180. doi: 10.1016/j.neuroimage.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dinstein I, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Konishi Y, et al. Functional brain imaging using fMRI and optical topography in infancy. Sleep medicine. 2002;3:S41–S43. doi: 10.1016/s1389-9457(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 77.Uematsu A, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS one. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seghier ML, et al. Combination of event-related fMRI and diffusion tensor imaging in an infant with perinatal stroke. Neuroimage. 2004;21:463–472. doi: 10.1016/j.neuroimage.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 79.Leroy F, et al. Early maturation of the linguistic dorsal pathway in human infants. Journal of Neuroscience. 2011;31:1500–1506. doi: 10.1523/JNEUROSCI.4141-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smyser CD, et al. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56:1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dehaene-Lambertz G, et al. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 82.Kouider S, et al. Neural dynamics of prediction and surprise in infants. Nature Communications. 2015;6:8537. doi: 10.1038/ncomms9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheour M, et al. Magnetoencephalography is feasible for infant assessment of auditory discrimination. Experimental Neurology. 2004;190:44–51. doi: 10.1016/j.expneurol.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 84.Kuhl PK, et al. Infants’ brain responses to speech suggest analysis by synthesis. Proceedings of the National Academy of Sciences. 2014;111:11238–11245. doi: 10.1073/pnas.1410963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doesburg SM, et al. Region-specific slowing of alpha oscillations is associated with visual-perceptual abilities in children born very preterm. Frontiers in Human Neuroscience. 2013;7:791. doi: 10.3389/fnhum.2013.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lloyd-Fox S, et al. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neuroscience & Biobehavioral Reviews. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Emberson LL, et al. Top-down modulation in the infant brain: Learning-induced expectations rapidly affect the sensory cortex at 6 months. Proceedings of the National Academy of Sciences. 2015;112:9585–9590. doi: 10.1073/pnas.1510343112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wan CY, et al. Early but not late-blindness leads to enhanced auditory perception. Neuropsychologia. 2010;48:344–348. doi: 10.1016/j.neuropsychologia.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 89.Schneider B, et al. High-frequency sensitivity in infants. Science. 1980;207:1003–1004. doi: 10.1126/science.7352294. [DOI] [PubMed] [Google Scholar]
- 90.Loos HM, et al. Responses of Human Neonates to Highly Diluted Odorants from Sweat. Journal of Chemical Ecology. 2017;43:106–117. doi: 10.1007/s10886-016-0804-x. [DOI] [PubMed] [Google Scholar]
- 91.Varendi H, Porter R. Breast odour as the only maternal stimulus elicits crawling towards the odour source. Acta Paediatrica. 2001;90:372–375. [PubMed] [Google Scholar]
- 92.Lahav A, Skoe E. An acoustic gap between the NICU and womb: a potential risk for compromised neuroplasticity of the auditory system in preterm infants. Frontiers in Neuroscience. 2014;8:381. doi: 10.3389/fnins.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casey B, Caudle K. The teenage brain: Self control. Current directions in psychological science. 2013;22:82–87. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gopnik A, et al. Changes in cognitive flexibility and hypothesis search across human life history from childhood to adolescence to adulthood. Proceedings of the National Academy of Sciences. 2017;114:7892–7899. doi: 10.1073/pnas.1700811114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gómez RL, Edgin JO. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Developmental Cognitive Neuroscience. 2016;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ábrahám H, et al. Myelination in the human hippocampal formation from midgestation to adulthood. International Journal of Developmental Neuroscience. 2010;28:401–410. doi: 10.1016/j.ijdevneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 97.Bauer PJ. Remembering the times of our lives: Memory in infancy and beyond. Psychology Press; 2014. [Google Scholar]
- 98.Lavenex P, Lavenex PB. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behavioural Brain Research. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 99.Schapiro AC, et al. Complementary learning systems within the hippocampus: a neural network modelling approach to reconciling episodic memory with statistical learning. Phil. Trans. R. Soc. B. 2017;372:20160049. doi: 10.1098/rstb.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saffran JR, et al. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 101.Kirkham NZ, et al. Visual statistical learning in infancy: Evidence for a domain general learning mechanism. Cognition. 2002;83:B35–B42. doi: 10.1016/s0010-0277(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 102.Bulf H, et al. Visual statistical learning in the newborn infant. Cognition. 2011;121:127–132. doi: 10.1016/j.cognition.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 103.Scarf D, et al. To have and to hold: Episodic memory in 3-and 4-year-old children. Developmental Psychobiology. 2013;55:125–132. doi: 10.1002/dev.21004. [DOI] [PubMed] [Google Scholar]
- 104.Lee JK, et al. Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 105.Frank MC, et al. Wordbank: An open repository for developmental vocabulary data. Journal of Child Language. 2017;44:677–694. doi: 10.1017/S0305000916000209. [DOI] [PubMed] [Google Scholar]
- 106.Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four-to eight-year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 107.Pujol J, et al. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- 108.Paus T, et al. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]