Abstract
Background and Purpose
Little is known about how chemokine systems influence the behavioral effects of designer cathinones and psychostimulants. The chemokine CXCL12 and its principal receptor target, CXCR4, are of particular interest because CXCR4 activation enhances mesolimbic dopamine output that facilitates psychostimulant reward, reinforcement, and locomotor activation. Repeated cocaine enhances CXCL12 gene expression in the midbrain and produces conditioned place preference (CPP) that is inhibited by a CXCR4 antagonist. Yet, interactions between chemokines and synthetic cathinones remain elusive.
Methods
We tested the hypothesis that an FDA-approved CXCR4 antagonist (AMD3100) inhibits MDPV-induced reward, locomotor activation and positive affective state in rats using a triad of behavioral assays (CPP, open field, and 50-kHz ultrasonic vocalizations [USVs]).
Key Results
AMD3100 (1, 2.5, 5, 10 mg/kg, ip) significantly reduced MDPV (2 mg/kg, ip)-evoked hyper-locomotion in a dose-related manner. AMD3100 (1, 5, 10 mg/kg) administered during CPP conditioning caused a significant, dose-dependent reduction of MDPV (2 mg/kg × 4 days) place preference. MDPV injection elicited significantly greater 50-kHz USVs in vehicle-pretreated rats but not in AMD3100-pretreated rats.
Conclusion and Implication
A CXCR4 antagonist reduced the rewarding and locomotor-activating effects of MDPV. Our results identify the existence of chemokine/cathinone interactions and suggest the rewarding and stimulant effects of MDPV, similar to cocaine, require an active CXCL12/CXCR4 system.
Keywords: MDPV, Bath Salts, Conditioned Place Preference, Psychostimulant, Reward, Locomotor, Chemokine, Cytokine, USV
1. Introduction
CXCL12 (C-X-C motif chemokine 12), also known as stromal cell-derived factor 1 (SDF-1α), is one of the few chemokines found in the brain (Kim et al., 2017; Trojan et al., 2017). It activates two receptors, CXCR4 (C-X-C chemokine receptor type 4) and CXCR7 (C-X-C chemokine receptor type 7), with the former being its major brain receptor (Bleul et al., 1996; Trecki et al., 2010). CXCR4 is one of the few chemokine receptors that has a commercially-available antagonist (AMD3100) to investigate receptor mechanisms. AMD3100 (plerixafor, Mozobil) displays high selectivity for CXCR4 receptors (e.g., calcium flux assays revealed no interaction of AMD3100 with the chemokine receptors CXCR1 through CXCR3, or CCR1 through CCR9) and is also approved by the FDA as an immunostimulant to mobilize stem cells in cancer patients (Khan et al., 2007; Bilgin et al., 2016).
Notably, CXCL12 is the chemokine most linked to psychostimulant addiction. Acute cocaine increases plasma levels of CXCL12 in mice (Araos et al., 2015). In human cocaine abusers, CXCL12 is the only chemokine correlated to the history of pathological cocaine use and severity of dependence (Araos et al., 2015). Cocaine exposure also increases CXLC12 gene expression in the rat ventral tegmental area (VTA) and produces place preference that is inhibited by a CXCR4 antagonist (Kim et al., 2017). Other psychostimulant drugs, notably methamphetamine, interact with chemokine systems. Methamphetamine induces persistent immune dysregulation, and CCL2 (CC-chemokine ligand 2) facilitates conditioned place preference to methamphetamine through the activation of dopamine systems (Loftis et al., 2011; Wakida et al., 2014). Since enhanced dopamine transmission in the NAC contributes to reward, reinforcement and relapse, interactions of CXCL12/CXCR4 with dopamine systems may be potentially important for psychostimulant addiction. Effects of CXCL12 on dopamine transmission are supported by electrophysiological studies showing that CXCL12 acts directly as a neuromodulator of dopamine activity in the midbrain through activation of calcium currents (Guyon et al., 2008). CXCL12 injected by intracerebroventricularly or into the VTA enhances hyper-locomotion produced by acute cocaine through a mechanism that requires active CXCR4 receptors (Trecki and Unterwald, 2009). Injection of CXCL12 into the substantia nigra elevates extracellular dopamine in the dorsal striatum, an effect that is also dependent on CXCR4 activation (Skrzydelski et al., 2007).
Given some of the mechanistic similarities between cocaine and the designer cathinone MDPV (3,4-methylenedioxypyrovalerone; (Baumann et al., 2013), we hypothesized that a CXCR4 antagonist (AMD3100) would inhibit MDPV-induced hyper-locomotion, place conditioning and positive affective state. At the behavioral level, preclinical studies show that MDPV is self-administered, produces acute hyper-locomotion and locomotor sensitization following repeated exposure, and produces CPP that is partly dependent on glutamate uptake systems (Kohler et al., 2017; Watterson and Olive, 2017; Hicks et al., 2017; Berquist et al., 2016). MDPV, akin to cocaine, inhibits cellular monoamine reuptake but with different potencies at monoamine transporters (Simmler et al., 2013; Lehner and Baumann et al., 2013; i.e., 50-fold more potent at the dopamine transporter, 10-fold more potent at the norepinephrine transporter, and 10-fold less potent at the serotonin transporter. Unlike cocaine, MDPV has not been shown to interact with sigma receptors or block sodium channels. While most prior work with cathinones has been aimed at the monoamine system, we now provide the first evidence that the chemokine system facilitates rewarding and locomotor-stimulant effects of a cathinone.
2. Materials and Methods
2.1. Animals and Chemicals
Male Sprague-Dawley rats (275-300 g) were pair-housed on a 12-h light/dark cycle. Procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (the Guide, NRC 2011; McGrath et al., 2010). ((±)-MDPV; synthesized by Dr. Allen Reitz) and AMD3100 (AstaTech) were dissolved in sterile water and injected intraperitoneally (ip). Doses were based on AMD3100 (1-10 mg/kg) reducing cocaine's rewarding effects (Kim et al., 2017) and MDPV (2 mg/kg) causing CPP, locomotor activation, and 50-kHz USV calls (Gregg et al., 2016; Simmons et al., 2017). Each rat was used once for an experiment (either locomotor, CPP or USV) and then euthanized immediately following behavioral experimentation.
2.2. Locomotor experiments
Locomotor activity (ambulation + stereotypy) was assessed using a Digiscan DMicro system (Hicks et al., 2017). Following a 60-min habituation in activity chambers, rats were pretreated with AMD 3100 (5 mg/kg) or vehicle 30 min before MDPV (2 mg/kg) or vehicle and activity was measured for 90 min. Experiments were repeated with different AMD3100 doses (1, 2.5, and 10 mg/kg).
2.3. CPP experiments
CPP was measured with a manual system as described previously in detail (Hicks et al., 2017). The CPP apparatus consisted of two equal-sized environmentally distinguishable compartments separated by a removable door. The CPP test followed a 4-day biased design and was carried out during the light cycle. Prior to conditioning, a “pre-test” was conducted during which individual rats were allowed to explore both compartments for 30 min in a drug-free state. The time spent in each compartment was manually scored by a naïve experimenter. The compartment in which rats spent less time during the pre-test was designated their “less-preferred compartment.” The subsequent 4-day conditioning phase consisted of two 30-min sessions each day conducted 4 h apart. During conditioning, each animal was administered MDPV or vehicle immediately before being confined to their initially least preferred side for 30 min. Four hours later, animals were administered vehicle and confined to their preferred side. One day following conditioning (post-test), rats were placed back into the chambers, with free access to both sides for 30 min, and the time spent in each compartment was determined. Rats injected with MDPV were pretreated (30 min) with vehicle or AMD3100 (1, 5, 10 mg/kg).
2.4. Ultrasonic Vocalization (USV) 50-kHz Recordings
50-kHz USVs were used to assess positive affective state since increased 50-kHz calls during cocaine or MDPV exposure is thought to reflect a positive subjective response (Simmons et al., 2016, 2017). All recording sessions were 90 min long with 10 mg/kg AMD3100 or vehicle given at minute 0 and 2 mg/kg MDPV or vehicle given at minute 30. USV's recorded during minutes 0-30 were considered anticipatory calls and USV's emitted during minutes 30-90 were considered MDPV-evoked calls. Baseline USV's were measured on day 1 wherein rats were administered vehicle at minute 0 and minute 30. On days 2-5, rats were administered vehicle or AMD3100 at minute 0 and MDPV was administered at minute 30. USV's were recorded during the baseline session (day 1) and the last session (day 5). Putative 50-kHz USVs were detected using an automated scoring program (Barker et al., 2014) and manually confirmed using spectrograms by a trained experimenter.
2.5. Statistical Analysis
Locomotor data were analyzed by two-way or one-way ANOVA followed by a Bonferroni test. CPP data were analyzed by one-way ANOVA followed by a Dunnett's test. Ultrasonic vocalization (USV) data were normalized using change-scores [(B-A) / (A+B)]. A “USV Change-Score” metric ranged from -1 to +1 and was used to determine the change in 50-kHz USVs from treatment against the within-subjects baseline. T-tests compared USV Change-Score for each group during “Anticipation” and “Post-MDPV” time-points against 0 (the point of no-change from baseline).
3. Results
3.1. CXCR4 Antagonist Reduces MDPV-Evoked Locomotor Activation
For time-course data, two-way ANOVA showed effects of MDPV treatment [F(3,684)=423.84, p<0.0001] and time [F(18,684)=13.25, p<0.0001] and a significant interaction [F(54,684)=5.35, p<0.0001] (Fig. 1A). For MDPV-treated rats, AMD3100 (5 mg/kg; AMD-MDPV) pretreatment reduced hyper-locomotion compared to vehicle pretreatment (VEH-MDPV). For cumulative data (Fig. 1A inset), two-way ANOVA showed an effect of MDPV treatment [F(1,36)=59.94, p<0.0001] but not an effect of pretreatment [F(1,36)=3.18, p>0.05] or significant interaction [F(1,36)=2.82, p>0.05]. Rats treated with MDPV (VEH-MDPV) displayed greater hyper-locomotion than MDPV-naïve rats (VEH-VEH; p<0.001). However, for rats treated with MDPV, pretreatment with AMD3100 (AMD-MDPV) significantly reduced locomotor activity compared to pretreatment with saline (VEH-MDPV) (p<0.01). For dose-effect results with AMD3100 (Fig. 1B), one-way ANOVA indicated a main effect [F(4,47)=11.55, p<0.0001]. Locomotor activation produced by MDPV was reduced by each dose of AMD3100 (1 mg/kg, p<0.01; 2.5 mg/kg, p<0.001; 5 mg/kg, p<0.05; 10 mg/kg, p<0.001).
Figure 1.
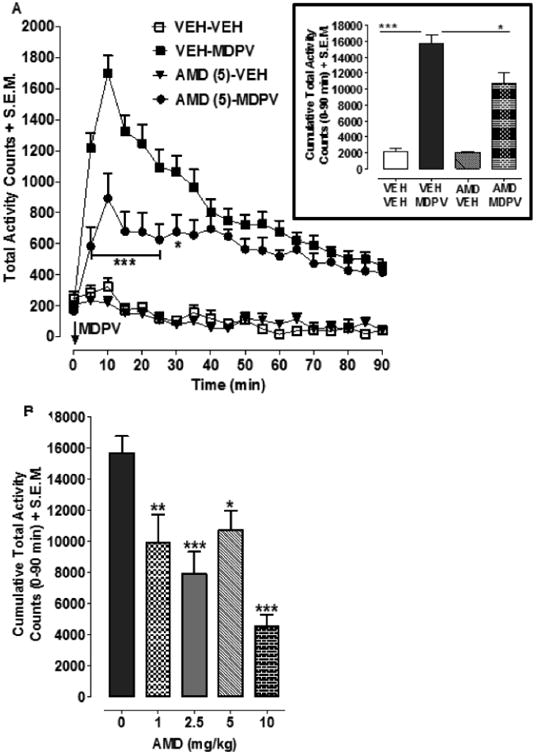
AMD3100 reduced MDPV-induced hyper-locomotion. (1A) Time-course: Rats pretreated with AMD3100 (5 mg/kg) or vehicle were injected 30 min later with MDPV (2 mg/kg) or vehicle. N=8-16 rats/group. ***p<0.001 or *p<0.05 compared to VEH-MDPV. Inset: Data from time-course are expressed as cumulative locomotor counts (0-90 min following MDPV injection). (1B) AMD3100 dose-effect: Rats pretreated with AMD3100 (0, 1, 2.5, 5, 10 mg/kg) were injected 30 min later with MDPV (2 mg/kg) and data were expressed as cumulative locomotor counts for the 90 min following MDPV (2 mg/kg) injection. N=8-16 rats/group. ***p<0.001, **p<0.001, or *p<0.05 compared to AMD (0 mg/kg) (i.e., MDPV alone).
3.2. CXCR4 Antagonist Reduces MDPV-Induced CPP
One-way ANOVA conducted on the differences scores obtained from CPP experiments indicated a main effect [F(5,47)=3.490, p=0.01] (Fig. 2A). Rats treated with MDPV (VEH-MDPV) produced a greater difference score than did drug-naive controls (VEH-VEH; p<0.01). In rats treated with MDPV, pretreatment with 5 mg/kg AMD3100 [(5) AMD-MDPV] or 10 mg/kg AMD3100 [(10) AMD-MDPV] reduced the difference score relative to MDPV-treated rats naïve to AMD3100 (VEH-MDPV; p<0.01).
Figure 2.

AMD3100 reduced MDPV place preference and modulated USV calls. (2A) CPP: Data are presented as a difference score (difference in time spent on MDPV (2 mg/kg)-paired side between post-test and pre-test). N=8 rats/group. **p<0.01 or *p<0.05 compared to VEH-MDPV. (2B) USVs: There was a non-significant trend toward AMD3100 reducing 50-kHz USVs during the “Anticipation” time-point (p=0.057). After MDPV injection (Post-MDPV), vehicle-pretreated rats emitted significantly greater 50kHz USVs (p<0.05) relative to baseline rates (compared to ‘0’) whereas no significant elevation in 50-kHz USVs was found in AMD3100-pretreated rats. N=8 rats/group. *p<0.05 compared to “0”.
3.3. CXCR4 Antagonist Modestly Suppresses 50-kHz Usvs Associated with MDPV Injection
When examining within-subjects USV Change-Score data (Fig. 2B), AMD3100 modestly suppressed 50-kHz USVs during “Anticipation” to MDPV on the fourth day of injection relative to baseline USVs [t(7)=1.81, p=0.057; H1: μ<0]. Consistent with the hypothesis that MDPV enhances 50-kHz USVs above baseline rate, MDPV injection elicited significantly greater 50-kHz USVs in vehicle-pretreated rats [t(7)=2.33, p=0.026; H1: μ>0]. In contrast, MDPV injection did not produce a significant elevation in 50-kHz USVs in AMD3100-pretreated rats [t(7)=0.75, n.s.]. No significant between-subjects differences emerged during “Anticipation” [t(14)=0.84, n.s.] or “Post-MDPV” [t(14)=0.93, n.s.] time-points.
4. Discussion
A CXCR4 antagonist reduced hyper-locomotion and place preference evoked by MDPV, suggesting tonically active CXCR4 receptors enable stimulant and rewarding effects of MDPV. Our findings concur with evidence that AMD3100 inhibits cocaine-induced hyper-locomotion and CPP (Kim et al., 2017). In the cocaine study (Kim et al., 2017), AMD3100 efficacy was evident at the same dose (5 mg/kg) that was effective here against MDPV. Moreover, in the Kim et al. (2017) study, AMD3100, when administered up to a dose of 10 mg/kg, did not significantly affect basal locomotor activity, making it unlikely that AMD3100 efficacy against MDPV (or cocaine) was due to generalized behavioral suppression. AMD3100 also modestly suppressed MDPV-elicited 50-kHz USVs relative to within-subject baseline rates, and AMD-pretreated rats elicited fewer USVs prior to the fourth MDPV injection (during “anticipation”). We previously showed that MDPV elicits 50-kHz USVs following injection or self-administration, and that an MDPV-paired context evokes 50-kHz USVs (Simmons et al. 2016, 2017).
A potential mechanism is that AMD3100 antagonizes CXCR4 receptors in the VTA, thereby reducing mesolimbic dopamine output during MDPV exposure that underlies locomotor activation and reward. MDPV enhances extracellular dopamine in the nucleus accumbens of conscious rats (Schindler et al., 2015). CXCR4 receptor activation in the VTA also appears apt to enhance mesolimbic dopamine because CXCL12 administered intracerebroventricularly or into the VTA augments cocaine hyper-locomotion (Trecki and Unterwald, 2009). While the cellular location of CXCR4 within the VTA is unknown, expression of CXCR4 by dopaminergic neurons is predictable based on evidence from the substantia nigra, where activation of CXCR4 receptors located on dopamine neurons enhances extracellular dopamine in the dorsal striatum (Skrzydelski et al., 2007). Future studies will examine effects of MDPV on endogenous CXCL12 levels in the mesolimbic circuit, as well as related brain reward substrates. Interestingly, a cocaine-conditioning paradigm identical to that used here for MDPV increases CXCL12 gene expression in the VTA and produces place preference that is antagonized by AMD3100 (Kim et al., 2017). MDPV may act similarly to increase CXCL12 levels in the VTA, perhaps through a TNF-α-related mechanism (Lewitus et al., 2016; Blaževski et al., 2015), causing CXCR4 receptor activation that enhances mesolimbic dopamine output.
In summary, the synthetic cathinone MDPV produced rewarding and locomotor-stimulant effects in rats that were CXCR4-dependent. Since AMD3100 is approved by the FDA to treat cancer (Khan et al., 2007; Bilgin et al., 2016), repurposing to psychostimulant addiction is possible, although translational potential will likely be limited by teratogenic effects and a parenteral administration route. It will be important in future studies, using self-administration models, to examine a role for CXCL12/CXCR4 in psychostimulant reinforcement, motivation and relapse in both male and female rats to uncover possible sex-specific effects of CXCR4 on the psychostimulant action. Since chemokines, including CXCL12, are pathological biomarkers for CNS and psychiatric disorders that are often comorbid with stimulant abuse (Trojan et al., 2017), it may prove fruitful also to investigate a chemokine-based approach for treating stimulant abusers who have schizophrenia, mood disorders, or traumatic brain injury.
Highlights.
A role for CXCL12/CXCR4 in designer cathinone (MDPV) pharmacology was investigated.
AMD3100 reduced MDPV-induced locomotor activation.
AMD3100 reduced MDPV-induced conditioned place preference.
AMD3100 modulated MDPV-induced increase in 50 k-Hz USV calls.
MDPV rewarding and stimulant efficacy requires active CXCR4 receptors.
Acknowledgments
The authors would like to thank the National Institute of Health and National Institute on Drug Abuse for funding through grants R01 DA039139, P30 DA013429-16, and T32 DA007237.
Role of Funding Source: The present work was supported by National Institutes of Health and National Institute on Drug Abuse through grants R01 DA039139, P30 DA013429-16, and T32 DA007237.
Footnotes
Author Disclosures: Contributors: Chicora Oliver conducted behavioral testing (locomotor experiments and place preference experiments) and was assisted by Sunil Nayak. Steve Simmons conducted the USV studies, analysis and interpretation. Allen Reitz synthesized MDPV. Chicora Oliver, Steve Simmons, and Scott Rawls contributed to data analysis, interpretation, and manuscript preparation. All authors have contributed to and approved the final manuscript.
Conflict of Interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, Campos-Cloute R, Ruiz JJ, Romero P, Suárez J, Baixeras E, de la Torre R, Montesinos J, Guerri C, Rodríguez-Arias M, Miñarro J, Martínez-Riera R, Torrens M, Chowen JA, Argente J, Mason BJ, Pavón FJ, Rodríguez de Fonseca F. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict Biol. 2015;20:756–772. doi: 10.1111/adb.12156. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostène W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003;18:1593–1606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Herrera C, West MO. Automated detection of 50-kHz ultrasonic vocalizations using template matching in XBAT. J Neurosci Methods. 2014;236:68–75. doi: 10.1016/j.jneumeth.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, Baker LE. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depend. 2016;164:128–134. doi: 10.1016/j.drugalcdep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin YM, de Greef GE. Plerixafor for stem cell mobilization: the current status. Curr Opin Hematol. 2016;23:67–71. doi: 10.1097/MOH.0000000000000200. [DOI] [PubMed] [Google Scholar]
- Blaževski J, Petković F, Momčilović M, Jevtić B, Mostarica Stojković M, Miljković D. Tumor necrosis factor stimulates expression of CXCL12 in astrocytes. Immunobiology. 2015;220:845–850. doi: 10.1016/j.imbio.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, Rovère C, Apartis E, Rostène W, Kitabgi P, Mélik Parsadaniantz S, Nahon JL. Stromal-cell-derived factor 1alpha /CXCL12 modulates high-threshold calcium currents in rat substantia nigra. Eur J Neurosci. 2008;28:862–870. doi: 10.1111/j.1460-9568.2008.06367.x. [DOI] [PubMed] [Google Scholar]
- Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, Rawls SM. Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology. 2016;108:111–119. doi: 10.1016/j.neuropharm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C, Gregg RA, Nayak SU, Cannella LA, Schena GJ, Tallarida CS, Reitz AB, Smith GR, Rawls SM. Glutamate carboxypeptidase II (GCPII) inhibitor 2-PMPA reduces rewarding effects of the synthetic cathinone MDPV in rats: A role for N-acetylaspartylglutamate (NAAG) Psychopharmacology (Berl) 2017;234:1671–1681. doi: 10.1007/s00213-017-4568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Greenman J, Archibald SJ. Small molecule CXCR4 chemokine receptor antagonists: developing drug candidates. Curr Med Chem. 2007;14:2257–2277. doi: 10.2174/092986707781696618. [DOI] [PubMed] [Google Scholar]
- Kim J, Connelly KL, Unterwald EM, Rawls SM. Chemokines and cocaine: CXCR4 receptor antagonist AMD3100 attenuates cocaine place preference and locomotor stimulation in rats. Brain Behav Immun. 2017;62:30–34. doi: 10.1016/j.bbi.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler RJ, Perrine SA, Baker LE. Concurrent repeated exposure to 3,4-Methylenedioxypyrovalerone and cocaine produce locomotor sensitization with minimal effects on brain monoamines. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.10.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner KR, Baumann MH. Psychoactive ‘bath salts’: Compounds, mechanisms, and toxicities. Neuropsychopharmacology. 2013;38:243–244. doi: 10.1038/npp.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D. Microglial TNF-α suppresses cocaine-induced plasticity and behavioral sensitization. Neuron. 2016;90:483–491. doi: 10.1016/j.neuron.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, Baumann MH. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 2016;233:1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SJ, Gregg RA, Tran FH, Mo L, von Weltin E, Barker DJ, Gentile TA, Watterson LR, Rawls SM, Muschamp JW. Comparing rewarding and reinforcing properties between ‘bath salt’ 3,4-methylenedioxypyrovalerone (MDPV) and cocaine using ultrasonic vocalizations in rats. Addict Biol. 2016;23:102–110. doi: 10.1111/adb.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SJ, Martorana R, Philogene-Khalid H, Tran FH, Gentile TA, Xu X, Su S, Rawls SM, Muschamp JW. Role of hypocretin/orexin receptor blockade on drug-taking and ultrasonic vocalizations (USVs) associated with low-effort self-administrationof cathinone-derived 3,4-methylenedioxypyrovalerone (MDPV) in rats. Psychopharmacology (Berl) 2017;234:3207–3215. doi: 10.1007/s00213-017-4709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Daugé V, Rovère C, Apartis E, Kitabgi P, Nahon JL, Rostène W, Parsadaniantz SM. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem. 2007;102:1175–1183. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- Trecki J, Brailoiu GC, Unterwald EM. Localization of CXCR4 in the forebrain of the adult rat. Brain Res. 2010;1315:53–62. doi: 10.1016/j.brainres.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Unterwald EM. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience. 2009;161:13–22. doi: 10.1016/j.neuroscience.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan E, ślusarczyk J, Chamera K, Kotarska K, Głombik K, Kubera M, Basta-Kaim A. The modulatory properties of chronic antidepressant drugs treatment on the brain chemokine-chemokine receptor network: A Molecular Study in an Animal Model of Depression. Front Pharmacol. 2017;8:779. doi: 10.3389/fphar.2017.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakida N, Kiguchi N, Saika F, Nishiue H, Kobayashi Y, Kishioka S. CC-chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. J Pharmacol Sci. 2014;125:68–73. doi: 10.1254/jphs.14032fp. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Olive MF. Reinforcing effects of cathinone NPS in the intravenous drug self-administration paradigm. Curr Top Behav Neurosci. 2017;32:133–143. doi: 10.1007/7854_2016_33. [DOI] [PubMed] [Google Scholar]


