Abstract
A brief (<4 seconds) period of neural activation evokes a stereotypical sequence of vascular and metabolic events to create the hemodynamic response function (HRF) measured using functional magnetic resonance imaging (fMRI). Linear analysis of fMRI data requires that the HRF be treated as an impulse response, so the character and temporal stability of the HRF are critical issues. Here, a simple audiovisual stimulus combined with a fast-paced task was used to evoke a strong HRF across a majority, ∼77%, of cortex during a single scanning session. High spatiotemporal resolution (2-mm voxels, 1.25-s acquisition time) was used to focus HRF measurements specifically on the gray matter for whole brain. The majority of activated cortex responds with positive HRFs, while ∼27% responds with negative (inverted) HRFs. Spatial patterns of the HRF response amplitudes were found to be similar across subjects. Timing of the initial positive lobe of the HRF was relatively stable across the cortical surface with a mean of 6.1±0.6 seconds across subjects, yet small but significant timing variations were also evident in specific regions of cortex. The results provide guidance for linear analysis of fMRI data. More importantly, this method provides a means to quantify neurovascular function across most of the brain, with potential clinical utility for the diagnosis of brain pathologies such as traumatic brain injury.
Keywords: Cerebral hemodynamics, fMRI, Neurovascular coupling, Cerebral pathology, Multisensory stimulation
1. Introduction
In functional magnetic resonance imaging (fMRI) experiments, use is made of the hemodynamic response function (HRF), the vascular response evoked by brief (<4 s) neural activation (Boynton et al., 1996). The HRF is predominantly formed by changes in oxygen uptake and blood flow, and typically exhibits a 3-phase response: initial delay/dip, hyperoxic peak, and undershoot with frequent ringing (Buxton, 2012; Kim et al., 2013; Thompson et al., 2003).
To enable linear analysis, the HRF is often assumed to be sufficiently stereotypical that a standard form can be used as a neurovascular impulse response. Principal component analysis (PCA) has demonstrated a moderately unimodal character to the HRF (Aguirre et al., 1998; Friman et al., 2003; Steffener et al., 2010; Woolrich et al., 2001). To match the temporal profile of the HRF, other works have suggested a heuristic, double gamma model for the HRF (Friston et al., 1998; Glover, 1999; Handwerker et al., 2004) that has been commonly used for fMRI analysis. This model fits empirical HRFs better than the single gamma model by including an undershoot, but it does not capture all temporal variability (particularly undershoot ringing effects) evident in many cortical regions.
Some experiments have shown noticeable variation of the HRF across subjects and sessions (Aguirre et al., 1998; Fransson et al., 1999; Handwerker et al., 2004; Miezin et al., 2000). They found significant variation in HRF magnitude and shape across specific regions of interest and even greater variation between subjects. However, previous characterization experiments have used stimuli that activated only limited portions of cortex, so the generality of these results across cortex was not clear.
Because structural MRI often does not reveal the underlying causes of brain pathologies (which present diverse collections of symptoms), use of the HRF to evaluate neurovascular function is attractive. It has often been noted that neurovascular coupling is affected by brain pathologies (Bonakdarpour et al., 2007; Carusone et al., 2002; Marshall, 2004; Roc et al., 2006; Rombouts et al., 2005; Siegel et al., 2015). Impaired and/or inverted neurovascular coupling have been described during cortical spreading depressions after severe traumatic brain injury and subarachnoid hemorrhage (Hinzman et al., 2014; Koide et al., 2013). Hemodynamic time lag was found to be affected by stroke (Siegel et al., 2015). Changes in neurovascular coupling have been observed after traumatic brain injury (TBI) and strongly related to functional recovery (Werner and Engelhard, 2007). Changes in activation patterns were observed in mild Alzheimer patients (Sperling et al., 2003); and slow resting state HRFs were observed in early Alzheimer’s disease (Rombouts et al., 2005). Changes to neurovascular coupling could thus provide a much stronger metric to indicate pathology in specific brain regions, and thereby track their recovery, either naturally or by medical intervention.
There are two main challenges to such clinical applications. First, we need a method to evoke the HRF broadly across the brain, and measure the response specifically in parenchymal gray matter. The task should be very simple, but effective for majority of patient population. Second, clinical use of the HRF will require a better understanding of its variability across brain regions and subjects to provide a database of normal response properties against which pathology can be detected.
Our previous modeling work (Kim et al., 2013; Kim and Ress, 2016), based upon upstream arterial dilation by brief stimulation (Drew et al., 2011), asserted that the BOLD HRF predominantly corresponds to competition between oxygen supply (blood flow response) and demand (metabolic response). When applied to detailed measurements of tissue oxygen levels in cerebral cortex (Thompson et al., 2003), the model predicted that the blood flow response was well modeled with a simple underdamped sinusoidal kernel with a stable oscillation frequency. For brief stimuli, the results suggested that the physiology of the vascular coupling is largely independent of the detailed sequence of neural processes evoked by the stimulus: the aggregate activation evoked a temporally stereotypical flow response. The modeling further indicated that differences in the metabolic demand created relatively small changes observed in HRFs; the dynamics of the HRF were dominated by this stereotypical flow response. Accordingly, we hypothesize that HRFs corresponding to strong neural activation will be largely stereotypical, but with small variations corresponding to variable metabolic demands.
Here, we present a simple event-related paradigm to effectively evoke the HRF across cerebral cortex, and use high spatiotemporal resolution (2-mm voxels, 1.25-s volume acquisition time) fMRI to extract the HRF predominantly in gray-matter at 3T. A strong HRF was observed across a majority, ∼77%, of the cortical surface after a 42-minute fMRI scan session. Of this strongly activated cortical surface, ∼73% responded with a positive HRF, while ∼27% responded with a negative HRF. The positive HRF was remarkably unimodal based on PCA, but the negative HRF had a more variable character. After moderate blurring across the gray matter manifold (Gaussian disc blur filter with 8-mm FWHM) onto a curvature-based surface reference (Dale et al., 1999; Mazziota et al., 2001), the spatial patterns of positive and negative HRF response amplitudes were found to be similar across subjects. An undershoot was observed with an amplitude that was strongly correlated with the peak amplitude. As expected, early temporal dynamics were stereotypical: time-to-peak (TTP) and full-width at half-maximum (FWHM) were stable across the cortical surface and across subjects. Time-to-undershoot (TTU) was also stable, showing reliable late-time behavior of the HRF. Small but significant timing differences were evident within all subjects, and these differences had a consistent spatial pattern across subjects.
The results reveal similar spatial HRF dynamics within cortex across healthy individuals and may provide guidance for linear analysis of fMRI data throughout the brain. More importantly, this method provides an efficient means to quantify neurovascular function across the majority of the brain with potential clinical utility to diagnose and monitor the treatment of vascular brain pathologies.
2. Methods
Imaging experiments were performed on a 3T Siemens Trio scanner. Twenty subjects, ages 20—60 years, participated in the experiments. We balanced the number of subjects between younger (21—39) and late middle age (50—64). All gave informed consent under procedures reviewed and authorized by the Baylor College of Medicine Institutional Review Board.
2.1 Stimulus and task
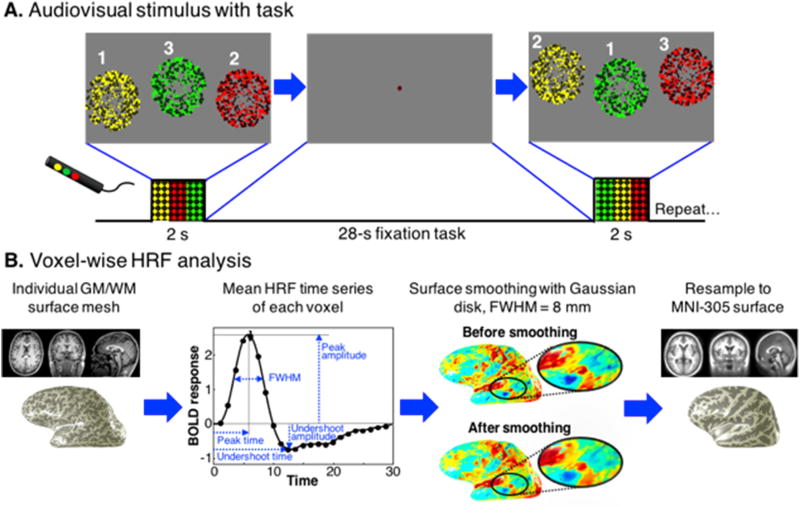
We used a simple audiovisual stimulus with fast-paced task to evoke brief periods of brain activity broadly across cerebral cortex. We specifically chose a simple, low-level and abstract audiovisual stimulus to limit variability due to higher-level cognitive responses across subjects. Subjects attended a display while wearing MR-compatible earphones. Every 30 seconds, a central fixation dot changed color to cue the subject 0.5 s before a 2-s duration stimulation period. Visual stimulation consisted of three circular regions (5° radius) of flickering colored dots (half bright, half dark). A region of dots appeared at a random selection of one of the three screen locations for 667 ms, followed by a second differently colored (red, yellow, green) region of dots at a different location for the next 667 ms, then a third (Fig. 1A). An auditory stimulus of bandpass-filtered white noise accompanied each regional dot display. The center frequency of the noise filter was also coded to the dot color: low pitch for red, medium for yellow, and high pitch for green. Subjects were instructed to follow the dot presentations with their eyes, and press a button that matched the color and position of the dot display. 17 audiovisual impulses were presented per run, and 5 runs were collected in each 60-minute session to create a total of 85 measurements of the HRF. Between impulses, subjects were instructed to maintain fixation on a central colored dot and perform a slow-paced, non-challenging color-detection task. The fixation dot changed color every 0.6 s, and subjects were instructed to push a button each time the dot color matched a target color, which occurred on average every 8 s (exponential distribution).
Fig. 1.
A) Subjects attended visual stimuli consisting of three circular patches of flickering colored dots, half bright and half dark, each presented in random sequence for 667 ms. Bandpass filtered sounds were presented simultaneously: medium pitch with the yellow circular patch; low pitch for red; and high pitch for green. Order of presentation was randomized across all 30-s-duration stimulus trials. Subjects were instructed to press a button corresponding to the color of the stimulus patch during the presentation. After each presentation, a fixation point appeared and subjects were instructed to perform an easy, fixation-point color-detection task until the next task sequence. B) High-resolution T1-weighted image volumes obtained for each subject were classified into gray and white matter using the FreeSurfer software package. The HRF time series for each voxel was then obtained by averaging across trials. The HRF time series were analyzed to obtain peak amplitude, peak time, and other HRF parameters. These data were depth-averaged onto the gray-white surface. Next, these maps of each parameter were smoothed along the surface using a Gaussian disk filter with 8-mm FWHM. Finally, the smoothed surface data were resampled onto the MNI-152 surface provided by FreeSurfer for across-subject comparisons.
2.2 Magnetic resonance imaging
Imaging was performed using the Siemens product 32-channel head coil. FMRI data were collected using an echo-planar imaging sequence with 3× blipped-CAIPI simultaneous multi-slice (Breuer et al., 2005; Setsompop et al., 2012) acceleration (shift-factor 4) together with 2× GRAPPA acceleration to obtain 2-mm cubic voxels on 57 slices using FOV = 200 mm, TR = 1.25 s, TE = 30 ms, and Ernst flip angle for gray matter. Fat saturation was used to reduce chemical-shift artifacts. High-order shimming was performed every two functional runs to accommodate subject head motion and thereby minimize phase-encode distortion.
At the end of each functional imaging session, a set of T1-weighted structural images centered on the same prescription as the functional images were obtained using a 3D FLASH sequence (minimum TE and TR, 15° flip angle, 1-mm inplane pixel size). These images were used to align functional data to a segmented reference volume anatomy, collected for each subject using a MP-RAGE sequence (TR = 2300 ms; TI = 900 ms; flip angle = 9°; 1-mm voxels). This volume was analyzed using the FreeSurfer software suite to segment the gray and white matter (Dale et al., 1999).
2.3 Data analysis
Subject motion in fMRI data was compensated using a robust intensity-based scheme (Nestares and Heeger, 2000). Data were corrected for spatial variations due to receiver-coil inhomogeneity by dividing by spatially smoothed version of the temporal mean of the fMRI time series with an additive correction to make the correction robust (Ress et al., 2007). Next, data were high-pass filtered to reduce the effects of slow image-intensity drifts. The anatomical images collected in each session were then used to align and transform the functional data to a structural 3D reference volume using the same intensity-based scheme.
We used depth mapping to enable additional analysis. We calculated a normalized distance map (w = 0 at white/gray matter interface, w = 1 at the pial surface) using a morphing approach (Khan et al., 2011; Kim and Ress, 2017). We then track between the two surfaces to measure physical gray-matter thickness.
For each subject, 85 HRFs were averaged together and analyzed on a voxel-by-voxel basis. To reduce partial volume effects, functional data were constrained to a normalized depth range of 0.2 < w < 0.8. Voxels were averaged across this depth range and mapped onto the gray-white surface (Fig. 1B). The mean HRF time series of each surface vertex was then characterized by its peak amplitude, TTP, FWHM, undershoot amplitude, TTU, and contrast-to-noise ratio (CNR). A zero baseline was estimated as the average of the first and last points of the each HRF time series. Voxel HRFs were separated into positive and negative based on the sign of its amplitude.
CNR of each voxel was defined as the ratio of the peak amplitude (absolute value) to its standard-error-of-the-mean across the 85 HRFs. We chose a particular CNR threshold for analysis. Voxels with CNR > 2 have significant activations with p < 0.05, but we chose the more conservative CNR > 3 to improve our ability to distinguish HRF shape and discern temporal parameters.
Principal component analysis of the HRF time series was performed for each subject. First, voxels with positive and negative HRFs were segregated. Then the top 75% of voxels by CNR of each group were decomposed into principal components by singular value decomposition in Matlab (Mathworks Inc., Natick, MA). Each principal component was a time series that explained a fraction of variance in the voxel time series.
Surface maps of each HRF parameter were created and then smoothed using a Gaussian disk kernel of FWHM = 8 mm. Filtering was performed in the surface space by performing convolutions within 16-mm diameter disk-shaped regions, making use of manifold-distance coordinates. For across-subject comparisons, the smoothed surface data were resampled onto the FreeSurfer MNI-305 surface (provided in FreeSurfer), then averaged across subjects (Collins et al., 1994; Fischl et al., 1999). This smoothing is necessary for the resampling onto the standardized surface, which down-samples the data to a similar spatial scale.
For across-subject comparison of HRF peak amplitudes, we normalized the peak amplitudes by their spatial mean for across above-threshold (CNR >3) voxels in each subject. Then, after averaging this normalized amplitude data across subjects, the average was returned to experimentally meaningful units by multiplying by the mean peak amplitude across subjects. We obtained the mean and standard deviation of the normalized peak amplitude across subjects for each voxel on the MNI template. Similarly, we extracted parameters for the undershoot amplitude of each positive HRF.
Many voxels also exhibited negative HRFs (Fig. 2). These tended to be somewhat weaker and were more temporally complex than the positive HRFs. We therefore chose to defer detailed parametric evaluation of the negative HRFs for later work.
Fig. 2.
A) Audiovisual stimulus with fast-paced task evokes strong HRFs (CNR > 3) over ≥84% of cortex in subjects 1 and 2 as shown by the CNR overlay. The average and standard deviation (STD) of CNR across subjects are depicted on the standard MNI brain surfaces. B) Left bar graph shows fraction of cortex exhibiting HRFs with CNR > 3 for each subject; black bar on right shows average and STD across subjects. Right bar graph shows fraction of activated voxels that have negative peak amplitude for each subject. C) Sample HRFs averaged from 3-mm-diameter disks of gray matter correspond to color-coded dots in the inset brain depicting patterns of positive (yellow overlay) and negative (blue overlay) HRFs across cortex. D) Peak amplitudes across the surface of S1, S2, and the average brain show a stereotypical spatial pattern.
For each temporal parameter on each subject, the mean value across cortex was calculated for positive HRFs. Then, this mean was subtracted from all of the values across cortex, giving us delta parameters, specifically ΔTTP, ΔFWHM, and ΔTTU. These delta values were calculated per the individual mean value in the MNI space, and then compared across subjects, with significance values obtained under the assumption of normal statistical distribution.
Because individual trial timing parameters can exhibit non-normal distributions, bootstrapping was used to establish statistical significance of the timing differences in a few selected individuals. The 85 HRFs were resampled with replication 2000 times on a HRF-by-HRF basis for strongly activated voxels (CNR >3), then averaged. The resulting HRF was parameterized in our standard fashion, thus generating estimates of the statistical distribution of the timing-difference parameter, e.g., ΔTTP; these distributions provide estimates of their significance (p value).
We performed correlations between all HRF parameters and cortical thickness to test for potential anatomic dependence. The significance of the correlation included a 4:1 correction for multiple comparisons to account for correlation of the noise between neighboring voxels. Along with the correlation and significance, slopes were calculated from linear fits of each parameter data with thickness to visualize the correlation trends.
3. Results
3.1 Behavioral performance
Subjects performed the main multisensory task well, but not perfectly. Because the task required quick responses, subjects occasionally failed to produce a valid button press within the allocated 667-ms time interval. However, valid response rates were very high, varying from 85—100%, with a mean valid fraction of 91% and reaction time of 497±88 ms. Of the valid responses, accuracy was quite high, varying from 69—96%, with a mean accuracy of 83%. Thus, the task fulfilled its design to be moderately challenging.
3.2 Global activation
The audiovisual stimulus with its fast-paced task was effective in evoking strong HRFs across the majority of cerebral cortex. Raw amplitude data, projected onto partially inflated cortical surfaces (Fig 2A), show that more than 84% of the cortical surface was strongly activated, CNR > 3, in two representative subjects. The CNR surface overlays were calculated for all subjects (N = 20) and then resampled onto the MNI surface. Overlays of the average and standard deviation (STD) across subjects show that visual, auditory, motor/motor-planning, and dorsolateral prefrontal cortices were strongly activated by the audiovisual stimulus and task. Activity was weaker or absent over portions of prefrontal cortex and ventral temporal and occipital lobes.
Prior to resampling onto MNI surface, the percentage of strongly activated cortex (CNR > 3) was calculated for each subject. The mean fraction of activated cortical surface is 77±13% (Fig. 2B, left). Of this activated cortex, a subset produced negative HRFs: mean of 27±9% across subjects (Fig. 2B, right). The median CNR in the active positive regions was a 5.08±1.38 across subjects, while active negative regions had median CNR of 3.64±0.40.
Figure 2C shows samples of BOLD signals selected from the cortex of an individual subject. Positive (yellow overlay) and negative (blue overlay) HRFs found across cortex display the large variations in HRF amplitude within a single subject. Two individuals, as well as the average across subjects, show HRF amplitudes ranging from −2 to 2% change in BOLD signal (Fig. 2D). The mean peak amplitude across subjects showed distinct regions of positive and negative HRFs. HRF parameter overlays for all individual subjects (Supplemental Information, Fig. S1) confirmed similar patterns of activation across subjects. Most strong activation was positive. However, regions of strong negative HRFs were also evident across the cortical surface, and were strongest and most heavily clustered on lateral temporal lobe, much of prefrontal cortex, and medial parietal lobe.
We evaluated correlations between all parameters and gray-matter thickness to test any dependence of the hemodynamics on anatomic structure. Pearson correlations, R, are shown for each HRF parameter for each subject (S1—S20) and across all subjects (Supplemental Information, Table S1). Most of the HRF correlations to thickness were significant (p < 0.01) in the individuals, and all correlations were significant across the collection of subjects; however, the correlations were generally weak. Strongest correlations across subjects were observed in CNR (R = −0.068, slope = −0.371 mm−1), FWHM (R = −0.058, slope = −0.136 s/mm), and peak amplitude (R = −0.049, slope = − 0.032 %/mm). Plots of these correlation data are shown for these parameters in representative subjects S2 and S3, and for all subjects combined (Supplemental Information, Fig. S2). The correlations (and slopes) were negative for these parameters: peak amplitude, FWHM, and CNR all decreased as cortex thickens. We found little variation of the widths (e-folding values) of the distributions as a function of thickness, a feature that is not obvious from the scatter plots in Fig. S2.
3.2 Principal component analysis
Principal component analysis was performed on positive and negative HRF time series separately for each subject. The first principal components of positive HRFs were similar across subjects, shown in Figure 3A (left) as time series with each subject a different color, and confirms the expected temporal pattern of HRF dynamics with initial latency, hyperoxic peak at 5.7±0.9 s, followed by a prolonged undershoot with minimum at 10.9±2.7 s. Note that the early period of all principal components was particularly consistent up to the hyperoxic peak, with much greater subsequent variability across subjects. Meanwhile, the first principal components of negative HRFs were highly variable across subjects (Fig. 3A, right).
Fig. 3.
A) The first principal components of each subject (colored lines) show stereotypical HRF behavior in voxels with positive peak amplitude (left). Atypical, highly variable HRF behavior is found in voxels with negative peak amplitude (right). B) Mean variance explained of the first 15 principal components across subjects for positive (left) and negative (right) HRFs.
Eigenvalue plots (Fig. 3B) show that most of the variance was captured in the first components with a mean of 53±11% for positive HRFs. The substantial decline of the second and third components (accounting for 14±4% and 6±2% of variance, respectively) demonstrates the largely unimodal character of the positive HRF. Negative HRF eigenvalues drop off more slowly suggesting more complex underlying mechanisms, but could also reflect their lower CNR values. Characterization of the highly variable and non-canonical negative HRFs is reserved to future studies. Because positive HRFs were stronger, more prevalent, and more stable, we will restrict subsequent presentation of the results to the positive HRFs, and mask out regions of negative BOLD signal.
3.3 Parametric analysis of positive HRFs
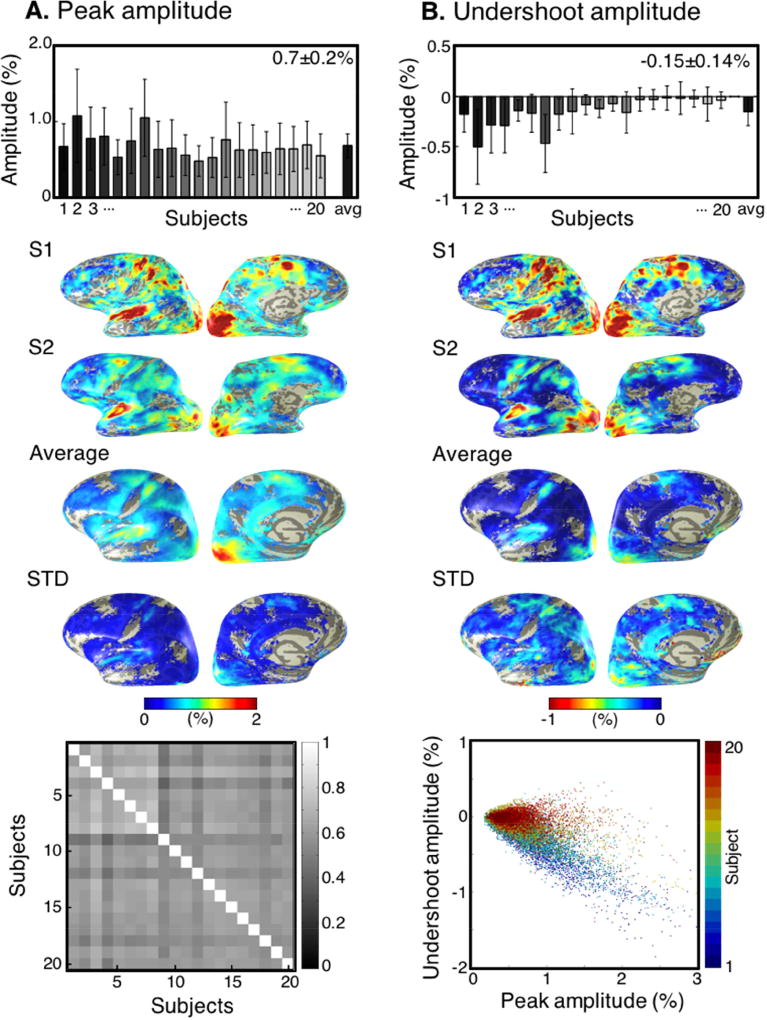
Peak amplitudes of the positive HRFs varied substantially across the cortical surface with spatial standard deviations ranging from 0.2—0.6%. Strong subject-to-subject amplitude variations were also evident with a mean across subjects of 0.7±0.2% (Fig. 4A, bar plot). However, spatial patterns of positive peak amplitude were similar across subjects. Patterns appear qualitatively similar in amplitude overlays on two individual subjects, and upon the MNI average brain (Figure 4A, brain overlays). Amplitude overlays for each subject (N = 20) qualitatively confirm their similarity (Fig. S1). Amplitude standard deviation was generally low, reflecting the high quality of the data. After resampling the overlay of each subject to the MNI surface, a correlation plot shows the consistency of the spatial pattern (Fig. 4A, bottom-left). Correlations varied from 0.42—0.77 across subjects, with <R> = 0.60±0.06. Similar to CNR patterns (above), regions of strongest positive peak amplitude were found in the primary sensory areas and motor strip. Patches of negative BOLD signal were masked out in the overlays; however, surrounding these empty patches were rings of low positive peak amplitude, consistent with a hemodynamic transition between positive and negative regions.
Fig. 4.
A) Mean peak amplitude across voxels is highly variable within and across subjects. However, normalized positive HRF peak amplitude overlays on subjects, S1 and S2, with the average and STD across subjects illustrate similar spatial patterns of activation on the healthy brain. Subject-to-subject correlations are large (<R>=0.60), quantifying the consistency of spatial patterns across subjects. Undershoot amplitudes of the positive HRFs are smaller in magnitude than peak amplitudes and highly variable across cortex and subjects. However, undershoot amplitude overlays on subjects, S1 and S2, with the average and STD across subjects illustrate similar spatial patterns as peak amplitude. Undershoot and peak amplitudes are strongly correlated across voxels (illustrated by a randomly chosen sample of 800 colored dots for each subject), with <R>=−0.67 across subjects.
Like peak amplitude, undershoot amplitude was highly variable within and across subjects, mean −0.15±0.14% (Fig. 4B, bar plot). Overlays of undershoot amplitude on individuals and the average surface and STD across subjects show its higher degree of spatial variability than for peak amplitude. The stronger variability is confirmed by the STD overlay. Undershoot amplitude was strongly correlated (<R> = 0.67) with the hyperoxic peak amplitude, but with a much smaller magnitude (Fig. 4B, bottom).
We found the “initial dip” to be weak or absent. In individual subjects, only a few voxel HRFs exhibited a significantly negative initial dip. Averaged across subjects, none of the initial dip amplitudes were significant. Thus, a meaningful initial dip was not evident to our analysis procedure for this stimulus protocol.
Temporal parameters of the positive HRF across the cortical surface and across subjects were far more spatially consistent than the amplitude parameters (Fig. 5). TTP varied little across cortex (Fig. 5A gives overview; details in Fig. S3); the global mean across cortex in all subjects was 6.1±0.6 s. Likewise, the other temporal parameters showed only weak spatial variability. FWHM of the hyperoxic lobe had a mean of 4.1±0.5 s across cortex for all subjects (Fig. 5B), and the TTU had a mean of 10.8±1.1 s (Fig. 5C). For clinical applications, it is useful to characterize the sensitivity of each of these temporal metrics (Fig. S4). The sensitivity of the temporal parameters is quite high, typically in the range of 6—15, so our method can reliably characterize timing shifts in the range of 7—17%.
Fig. 5.
Temporal parameters (A, TTP, B, FWHM, and C, TTU) of positive HRFs show moderate stability across cortex and across subjects. Timing overlays on S1, S2, the average, and STD reveal weak-to-moderate consistency in spatial patterns across subjects. D) TTU (left) strongly correlated with TTP (<R> = 0.74) and FWHM (<R> = 0.70). Hyperoxic peak parameters, FWHM and TTP, are more weakly correlated, <R> = 0.34. The scatter plots include 800 randomly selected points from the positive HRF overlay of each subject.
Strong correlations were observed between undershoot peak time (TTU) and both the hyperoxic peak time (TTP) and its FWHM (Fig. 5D, left). Mean correlation across subjects between TTU and TTP was <R> = 0.74 (blue points). Correlation between TTU and FWHM was slightly smaller, <R> = 0.70 (red points). However, the timing parameters of the hyperoxic peak, FWHM and TTP, were more weakly correlated, <R> = 0.34 (Fig. 5D, right).
Although temporal parameters were relatively stable, detailed analysis showed regions where timing differed significantly from the mean across the cortical surface. Timing differences were calculated for TTP, FWHM, and TTU for each subject. HRFs that peaked more slowly than the mean yielded a positive ΔTTP, and similarly for ΔTTU. Regions of significant (p < 0.05) ΔTTP were observed using a bootstrapping procedure on subjects, S1 and S2 (Fig. 6, left overlays). Mean fraction of the cortical surface with significant ΔTTP was 22%. Similar areas of significant timing shifts were seen on S3 and S4. The spatial patterns show a fair degree of similarity. In fact, when the timing parameters were averaged across subjects onto the MNI brain (Fig. S5), we observed regular patterns of statistically significant (p < 0.05) Δ times across subjects for TTP, FWHM, and TTU (Fig. 6). Most of the significantly fast TTP appeared in regions of auditory, visual, and motor cortices. The ΔFWHM values reveal particularly narrow HRFs in auditory and ventral visual cortex. Timing differences in undershoot time resemble those of both TTP and FWHM. A slow and broad region of evoked HRFs exists in portions of the angular and supramarginal gyri, as well as intraparietal and Jensen sulci.
Fig. 6.
Timing differences within each subject are found in similar regions across subjects; S1 and S2 overlays show comparable regions of fast (blue) and slow (red) ΔTTP. The average overlays (ΔTTP, ΔFWHM, and ΔTTU) show the regions of significantly fast or slow (p < 0.05) timing differences across subjects.
4. Discussion
Activation of a large portion of cortex is not trivial. Our speeded audiovisual stimulus paradigm evoked strong activation across the majority of cortex (∼77%) with both positive and negative responses. The auditory and visual stimulus components are relatively simple, but the task sufficiently challenging to make subjects focus continuously upon the stimulus. The fast nature of our task, its multisensory character, and the more complex motor response that it requires may also contribute to its efficacy. With a brief stimulus (2 s), we characterized the temporal shape of the HRF and examined its parameters, thus providing more details to enable the understanding of neurovascular and neurometabolic coupling. We showed not only positive and negative BOLD responses related to the task, but also provided temporal characteristics (e.g. TTP and TTU) induced by the competition between cerebral blood flow (CBF) and the cerebral metabolic rate of oxygen (CMRO2) responses related to the task.
It has been shown that a simple visual stimulus can generate a meaningful BOLD signal in the majority of the brain (Gonzalez-Castillo et al., 2012). The interregional shape differences of the BOLD signal were interpreted to indicate variability in the evoked neural response. Their follow-up study showed task dependence and spatial distribution of BOLD response in a majority of cerebral cortex with block-designed stimulation (Gonzalez-Castillo et al., 2015). The addition of an attention demanding task activated a greater extent of cortex, and produced both significant positive and negative BOLD responses. However, all of these experiments utilized a blocked design, which did not permit measurement of the HRF.
Longer stimuli are less desirable for three main reasons. First, the time course of neural responses to longer stimuli will exhibit temporal variability because of cognitive issues such as adaptation and variable attention. Second, longer stimuli will temporally blur the dynamical details of the HRF. Third, additional hemodynamic processes may be evoked on longer time-scales, and these mechanisms are less relevant to the typical time scales used in fMRI experiments. Accordingly, this work focused on the HRF evoked by brief neural activation. However, we chose a 2-second stimulus duration to avoid the non-linear regime observed for very short stimuli. These non-linearities may be partly the consequence of the inefficiency of matching metabolic demands by increasing blood flow to enhance diffusive transmural oxygen transport ((Buxton and Frank, 1997; Kim and Ress, 2016; Uludag et al., 2004)); task switching effects may also play a role (Pfeuffer et al., 2003). The strong, extensive, and fairly stereotypical HRF that we observed in these experiments suggests that the 2-second stimulus provides an optimal time scale for measurement of the hemodynamic response across cortex: short enough to avoid long-term cognitive and neural changes, but long enough to avoid non-linearity in the neurovascular coupling.
Principal component analysis shows that positive HRFs have a substantially stereotypical character across subjects. The first principal components of positive HRFs shows reliable response characteristics of the initial positive lobe of the HRF, peak amplitude, TTP and FWHM, among subjects, Fig. 3A (left, colored lines). However, there are noticeable variations in the late-time dynamics of the HRFs. This characteristic of the HRF have been also observed in previous findings (Dilharreguy et al., 2003; Drew et al., 2011; Kim et al., 2013). This could imply strong and stereotypical competition between CBF and CMRO2 for the first few seconds due to the transient neural activation; then more complicated dynamics drive the response after the hyperoxic peak, such as CBF ringing (Ress et al., 2009), slow/fast decay of CMRO2 (Vazquez et al., 2008) or spatial propagation of the BOLD response (Drysdale et al., 2010). Late-time variability could also reflect neural variability during the long inter-stimulus intervals (ISI) between the brief impulse stimuli. Combinations of some (or all) of these dynamics could result in heterogeneous late-time responses across subjects.
We observed that hyperoxic peak amplitudes varied substantially across cortical regions; however, similar spatial patterns of activation were observed across subjects. Strong and reliable positive peaks were evident in early visual area, auditory areas, primary sensory areas and motor areas, which is consistent with our audiovisual stimulus and button-press task. Thus, our simple stimulus and task evoked a stereotypical pattern of functional responses. This reliably similar spatial pattern across subjects for HRF peak amplitude could be used as a clinical metric for assessment of the integrity of neurovascular coupling. Significant deviations from this healthy pattern to that observed in a patient could be a metric for pathologies, either neural or vascular in character.
The undershoot amplitude was highly correlated with the hyperoxic peak amplitude across subjects, suggesting a strong link between the dynamics of the hyperoxic peak and the undershoot. This correlation has been observed previously (Davis et al., 1994; Hu et al., 1997). Here, we have expanded this correlation for HRFs across the majority of cortex. In our previous HRF modeling studies, CBF response evoked by brief neural activation was assumed to have the form of an underdamped response because of the inertia of upstream arterial flow (Kim et al., 2013; Kim and Ress, 2016; Ress et al., 2009). An underdamped flow response is consistent with the observed correlation.
We saw little evidence for an “initial dip” in our data. It has been proposed that the presence of such an initial decrease in BOLD signal can indicate an increase in local CMRO2 increase prior to increase in local CBF (Frostig et al., 1990; Hu and Yacoub, 2012; Menon et al., 1995; Siero et al., 2015). In our analysis, spatial averaging could mask this feature in the healthy brain.
Our measurements showed that temporal parameters of the HRF are fairly stable across both cortex and subjects. Our use of brief stimulation periods was critical to observe this stability. Furthermore, correlations of temporal parameters (TTP vs. TTU, and FWHM vs. TTU) are also consistent with a unimodal HRF profile. The rough temporal stability suggests that neurovascular coupling is a stereotypical process across the cortical surface, regardless of local variations in cytoarchitecture and functional specialization, and consistent with the vascular coupling dynamics asserted by the Arterial Impulse Model (Kim and Ress, 2016). This motivates the use of the HRF as a diagnostic for cortical health. Delayed time-to-peak of the BOLD HRF has been observed in stroke patients (Bonakdarpour et al., 2007; Roc et al., 2006), which was putatively explained by prolonged delivery time of CBF from upstream pial vasculature evoked by local neural activity. These results suggest that the temporal behavior of neurovascular coupling can be altered by pathology, and that our protocol could discern such temporal changes in a patient population.
Despite the rough stability of the temporal parameters, we did observe small but significant variations in timing across cortex (Fig. 6). This suggests the need for caution in the use of a generic form to describe the HRF (Friston et al., 1998; Glover, 1999), because such timing changes can affect linear analysis of fMRI experiments (Handwerker et al., 2004; Hernandez et al., 2002).
In fact, the spatial patterns of these timing shifts across cortex were quite similar across subjects. The fastest regions exist around the primary sensory and motor areas, consistent with immediate processing of the stimulus. Slow ΔTTP values appear in portions of Brodmann areas 7, 39, and 40. These later and broader HRFs may arise from the task-related aspects of the stimulus, such as sequence processing and coordination of finger movements. There are also interesting fine-grained variations across visual cortex. For example, pericalcarine visual cortex has a relatively broad FHWM and late TTU, suggesting particularly robust blood flow and subsequent oxygen metabolism during the brief stimulus and task. Similar timing is observed for portions of the intraparietal sulcus, which may reflect the attentional and integrative demands of our task (Corbetta and Shulman, 2002; Jovicich et al., 2001; Scolari et al., 2015; Silver et al., 2005). In contrast, ventral visual cortex exhibits faster and narrower HRFs, perhaps indicating reduced metabolic demand in these regions, which are likely less involved in the processing of our simple but dynamic stimuli.
Although we cannot precisely account for detailed changes in the neural response across these many brain regions, we clearly observe here that they introduce relatively small changes to the shape of the HRF. Strong HRFs will inevitably be dominated by strong flow responses, and thus not strongly affected by changes in neurometabolic coupling. Detailed modeling studies will be useful to evaluate these fine-grained changes in HRF dynamics in terms of blood flow and oxygen metabolism.
Negative HRFs have been observed in many previous studies, leading to a consensus that it is as an actual signal, rather than an artifact (Allison et al., 2000; Harel et al., 2002; Pasley et al., 2007; Puckett et al., 2014; Shmuel et al., 2002; Stefanovic et al., 2004; Tootell et al., 1998; Wade, 2002). PCA of our observed negative HRFs showed multiple modes with diverse temporal characteristics. However, their median CNR was lower than that of positive HRFs, which raises the possibility that their higher noise content may have distorted the PCA. Nevertheless, our data show negative HRFs in many cortical regions, further bolstering its existence as a true hemodynamic signal. We find many cortical regions exhibiting a strong negative HRF adjacent to voxels exhibiting strong positive HRFs. This, and the highly varied temporal dynamics in these regions suggest competition between positive and negative BOLD. Such observations are consistent with the “vascular-steal” hypothesis, with the negative HRFs induced by a vascular response to neural activity in nearby regions where strong positive HRFs occur (Harel et al., 2002; Kannurpatti and Biswal, 2004; Puckett et al., 2014; Shmuel et al., 2002; Stefanovic et al., 2004). However, we also observe a spatial distribution of strong negative BOLD reminiscent of the default-mode network (Broyd et al., 2009; Raichle et al., 2001; Raichle and Snyder, 2007). Because the default-mode network should be inhibited during our task, this observation is consistent with the neuronal suppression hypothesis for negative BOLD (Gonzalez-Castillo et al., 2015; Pasley et al., 2007; Shmuel et al., 2006; Shmuel et al., 2003; Shmuel et al., 2002; Stefanovic et al., 2004).
There are some limitations to our large-scale measurements of the HRF. First and foremost, subject behavior is only well-controlled during the 2-s duration task period. Although we utilized a simple task during the long ISI, it was intentionally not very demanding of cognitive resources, therefore permitting subjects to enter a “resting” state. This is consistent with the pattern of negative HRFs generated by our paradigm. Because such resting-state activity is intrinsically less controlled, the “baseline” for our HRFs is less certain. This limitation is consistent with the temporal variability of the HRFs, which exhibit far more stereotypical dynamics during their early periods (through the hyperoxic peak) than during later periods. Task switching may also cause longer timescale neural activity that distort our measurement. Subjects may take a variable amount of time to “cool down” after the demands of our speeded paradigm, and such switching is likely to vary by cognitive domain.
Second, our experimental paradigm sought to activate as much cortex as possible while evoking a uniform response across individuals, so we utilized a very simple audiovisual stimulus with the understanding that some regions would not be sufficiently activated. Despite this limitation, our observed HRF late-time dynamics are stable – the variability of TTU is similar to that of TTP – and we were still able to activate the majority of cortex.
Third, there are resolution limitations to our MRI acquisitions. The 2-mm voxels are not sufficient to resolve the thinnest gray matter located in the sulcal folds. HRFs in these regions, therefore, will be subject to contamination from white matter and superficial vascular responses. The latter are of some concern, as we have previously characterized their strong signals but lower reliability (Kim & Ress, 2017). Correlations between most HRF parameters and gray-matter thickness showed weak but significant downward trends. CNR values decreased most strongly with thickness, an effect that is partly explained by peak-amplitude values, which also decreased with thickness. Thus, our measurements from the thickest cortex likely represent the most veridical data, while those measurements in thinner cortex reflect partial-volume contamination from the superficial vasculature. Likewise, the trend of narrowing FWHM with thickness suggests reduced temporal blurring from partial volume effects in the thickest portions of cortex. Nevertheless, the weakness of these correlations suggest that partial volume issues had only weak effects on the HRF measurements. Beyond partial-volume effects, we also chose to blur our data to 8-mm FWHM spatial resolution along the gray-matter ribbon. This blurring was necessary to permit across-subject variations, but reduced our ability to discern fine-scale differences within the spatial distribution of the HRFs. This may have tended to exaggerate the degree of correlation between individual subjects, and in the relative stability of the HRF timing parameters across subjects.
Finally, we made no attempt to correct for EPI shear distortion along the phase-encode direction. Although, we did make use of high-order shimming, and repeated the shim after each run to account for subject head motion, some amount of distortion is likely close to strong susceptibility gradients, such as ventromedial frontal cortex. Such effects may have reduced CNR in these areas.
Hypercapnic challenge has often been used to assess neurovascular coupling and cerebrovascular reactivity (Lin et al., 1999; Rostrup et al., 1995; Turner et al., 1991; van der Zande et al., 2005). However, such methods rely on either carbogen inhalation or breath holding. The former is disagreeable and both are physically challenging, reducing their utility for clinical application. The current protocol requires no special MRI gas-handling equipment and should be accessible to a large range of clinically impaired individuals. Moreover, such methods generally focus on time-averaged metrics for assessment. With our procedure, the full temporal dynamics of the HRF become accessible. Nevertheless, it would be useful to compare the current approach to hypercapnic metrics in future experiments.
There are several other useful directions to pursue to extend this research. First, these methods can provide copious data against to test models for the BOLD HRF (Buxton et al., 2004; Drysdale et al., 2010; Friston et al., 2000; Griffeth and Buxton, 2011; Huppert et al., 2007; Kim et al., 2013; Kim and Ress, 2016; Ress et al., 2009; Zheng et al., 2002). Such analyses can give insight into the physiology of any observed changes in the HRF, such as distinguishing CBF from CMRO2 changes. Also, the strong evocation of the negative HRF by our protocol could provide a useful means for further experiments and theoretical analysis of this intriguing phenomena. For example, regions of negative BOLD may be examined in the context of intrinsic connectivity networks (Yeo et al., 2011). Finally, this simple task could likely be improved to further expand and strengthen the evoked activation by small modifications of the audiovisual stimulus and its associated task. Altogether, our results provide a broadly useful set of directions to further explore the phenomena of neurovascular and neurometabolic coupling for the study of the human brain in health and disease.
Conclusion
We demonstrated that our simple stimulus and task protocol evoked a strong HRF across the majority of cortex. The spatial pattern of the HRF amplitude evoked by our protocol was similar among subjects. Strong correlation was observed between the undershoot amplitude and the peak amplitude. Timing parameters of the HRFs were moderately stable, with strong correlations between undershoot and hyperoxic peak timing. However, there were small but significant timing differences evident in certain regions of cortex. Thus, the data bear out our hypothesis that strong HRFs should have stereotypical flow-dominated dynamics, while the small but significant variations in temporal dynamics reflect interesting variations in metabolic demands. Altogether, these finding suggest that our procedure could be adapted to diagnose and explore neuropathology, such as mild-to-moderate TBI and neurodegenerative conditions, that often exhibit little-or-no structural abnormality. Our findings could provide a means to localize such pathology by way of abnormal neurovascular coupling. Our characterization of the HRF for a healthy population provides a normative dataset of amplitude and timing parameters. Local pathology, neural or vascular, could be identified if a patient’s HRF parameter is significantly different than recorded for this control population.
Supplementary Material
Acknowledgments
We thank Michael Beauchamp, Elizabeth Halfen, and John Magnotti for providing thoughtful comments and advice. This work was supported by NIH R21HL108143, NIH R01NS095933, and NIH K25 HL131997.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre G, Zarahn E, D’esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright J. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–135. doi: 10.1212/wnl.54.1.135. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B, Parrish T, Thompson C. Hemodynamic response function in patients with stroke-induced aphasia: implications for fMRI data analysis. NeuroImage. 2007;36:322–331. doi: 10.1016/j.neuroimage.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53:684–691. doi: 10.1002/mrm.20401. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience & biobehavioral reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Dynamic models of BOLD contrast. NeuroImage. 2012;62:953–961. doi: 10.1016/j.neuroimage.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Carusone LM, Srinivasan J, Gitelman DR, Mesulam MM, Parrish TB. Hemodynamic response changes in cerebrovascular disease: implications for functional MR imaging. American Journal of Neuroradiology. 2002;23:1222–1228. [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis TL, Weisskoff RM, Kwong KK, Savoy R, Rosen BR. Society of Magnetic Resonance. San Francisco, CA, USA: 1994. Susceptibility contrast undershoot is not matched by inflow contrast undershoot; p. 435. [Google Scholar]
- Dilharreguy B, Jones RA, Moonen CT. Influence of fMRI data sampling on the temporal characterization of the hemodynamic response. NeuroImage. 2003;19:1820–1828. doi: 10.1016/s1053-8119(03)00289-1. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc Natl Acad Sci U S A. 2011;108:8473–8478. doi: 10.1073/pnas.1100428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale PM, Huber JP, Robinson PA, Aquino KM. Spatiotemporal BOLD dynamics from a poroelastic hemodynamic model. J Theor Biol. 2010;265:524–534. doi: 10.1016/j.jtbi.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fransson P, Krüger G, Merboldt K, Frahm J. Temporal and spatial MRI responses to subsecond visual activation. Magnetic resonance imaging. 1999;17:1–7. doi: 10.1016/s0730-725x(98)00163-5. [DOI] [PubMed] [Google Scholar]
- Friman O, Borga M, Lundberg P, Knutsson H. Adaptive analysis of fMRI data. NeuroImage. 2003;19:837–845. doi: 10.1016/s1053-8119(03)00077-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: The balloon model, volterra kernels, and other hemodynamics. NeuroImage. 2000;12:466–477. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proceedings of the National Academy of Sciences. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of Impulse Response in Event-Related BOLD fMRI. NeuroImage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Hoy CW, Handwerker DA, Roopchansingh V, Inati SJ, Saad ZS, Cox RW, Bandettini PA. Task Dependence, Tissue Specificity, and Spatial Distribution of Widespread Activations in Large Single-Subject Functional MRI Datasets at 7T. Cerebral Cortex. 2015;25:4667–4677. doi: 10.1093/cercor/bhu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Saad ZS, Handwerker DA, Inati SJ, Brenowitz N, Bandettini PA. Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc Natl Acad Sci U S A. 2012;109:5487–5492. doi: 10.1073/pnas.1121049109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffeth VE, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. NeuroImage. 2011;58:198–212. doi: 10.1016/j.neuroimage.2011.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee S-P, Nagaoka T, Kim D-S, Kim S-G. Origin of negative blood oxygenation level-dependent fMRI signals. Journal of Cerebral Blood Flow & Metabolism. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Badre D, Noll D, Jonides J. Temporal sensitivity of event-related fMRI. NeuroImage. 2002;17:1018–1026. [PubMed] [Google Scholar]
- Hinzman JM, Andaluz N, Shutter LA, Okonkwo DO, Pahl C, Strong AJ, Dreier JP, Hartings JA. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain. 2014;137:2960–2972. doi: 10.1093/brain/awu241. [DOI] [PubMed] [Google Scholar]
- Hu X, Le TH, Uğurbil K. Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magnetic Resonance in Medicine. 1997;37:877–884. doi: 10.1002/mrm.1910370612. [DOI] [PubMed] [Google Scholar]
- Hu X, Yacoub E. The story of the initial dip in fMRI. NeuroImage. 2012;62:1103–1108. doi: 10.1016/j.neuroimage.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Allen MS, Benav H, Jones PB, Boas DA. A multicompartment vascular model for inferring baseline and functional changes in cerebral oxygen metabolism and arterial dilation. J Cereb Blood Flow Metab. 2007;27:1262–1279. doi: 10.1038/sj.jcbfm.9600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Peters RJ, Koch C, Braun J, Chang L, Ernst T. Brain areas specific for attentional load in a motion-tracking task. J Cogn Neurosci. 2001;13:1048–1058. doi: 10.1162/089892901753294347. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. Negative functional response to sensory stimulation and its origins. Journal of Cerebral Blood Flow & Metabolism. 2004;24:703–712. doi: 10.1097/01.WCB.0000121232.04853.46. [DOI] [PubMed] [Google Scholar]
- Khan R, Zhang Q, Darayan S, Dhandapani S, Katyal S, Greene C, Bajaj C, Ress D. Surface-based analysis methods for high-resolution functional magnetic resonance imaging. Graphical models. 2011;73:313–322. doi: 10.1016/j.gmod.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Khan R, Thompson JK, Ress D. Model of the transient neurovascular response based on prompt arterial dilation. Journal of Cerebral Blood Flow & Metabolism. 2013 doi: 10.1038/jcbfm.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Ress D. Arterial impulse model for the BOLD response to brief neural activation. NeuroImage. 2016;124:394–408. doi: 10.1016/j.neuroimage.2015.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Ress D. Reliability of the depth-dependent high-resolution BOLD hemodynamic response in human visual cortex and vicinity. Magnetic resonance imaging. 2017;39:53–63. doi: 10.1016/j.mri.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Sukhotinsky I, Ayata C, Wellman GC. Subarachnoid hemorrhage, spreading depolarizations and impaired neurovascular coupling. Stroke research and treatment 2013. 2013 doi: 10.1155/2013/819340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Celik A, Paczynski RP, Hsu CY, Powers WJ. Quantitative magnetic resonance imaging in experimental hypercapnia: improvement in the relation between changes in brain R2 and the oxygen saturation of venous blood after correction for changes in cerebral blood volume. J Cereb Blood Flow Metab. 1999;19:853–862. doi: 10.1097/00004647-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Marshall RS. The functional relevance of cerebral hemodynamics: why blood flow matters to the injured and recovering brain. Curr Opin Neurol. 2004;17:705–709. doi: 10.1097/00019052-200412000-00010. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Ugurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: Echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magnetic Resonance in Medicine. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger J, Petersen S, Buckner R. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Nestares O, Heeger DJ. Robust multiresolution alignment of MRI brain volumes. Magn Reson Med. 2000;43:705–715. doi: 10.1002/(sici)1522-2594(200005)43:5<705::aid-mrm13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. NeuroImage. 2007;36:269–276. doi: 10.1016/j.neuroimage.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, McCullough JC, Van de Moortele PF, Ugurbil K, Hu X. Spatial dependence of the nonlinear BOLD response at short stimulus duration. NeuroImage. 2003;18:990–1000. doi: 10.1016/s1053-8119(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Puckett AM, Mathis JR, DeYoe EA. An investigation of positive and inverted hemodynamic response functions across multiple visual areas. Human brain mapping. 2014;35:5550–5564. doi: 10.1002/hbm.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Ress D, Glover GH, Liu J, Wandell B. Laminar profiles of functional activity in the human brain. NeuroImage. 2007;34:74–84. doi: 10.1016/j.neuroimage.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Ress D, Thompson JK, Rokers B, Khan R, Huk AC. A model for transient oxygen delivery in cerebral cortex. Frontiers in neuroenergetics. 2009;1:3. doi: 10.3389/neuro.14.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roc AC, Wang J, Ances BM, Liebeskind DS, Kasner SE, Detre JA. Altered hemodynamics and regional cerebral blood flow in patients with hemodynamically significant stenoses. Stroke. 2006;37:382–387. doi: 10.1161/01.STR.0000198807.31299.43. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. NeuroImage. 2005;26:1078–1085. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Rostrup E, Larsson HB, Toft PB, Garde K, Henriksen O. Signal changes in gradient echo images of human brain induced by hypo-and hyperoxia. NMR Biomed. 1995;8:41–47. doi: 10.1002/nbm.1940080109. [DOI] [PubMed] [Google Scholar]
- Scolari M, Seidl-Rathkopf KN, Kastner S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr Opin Behav Sci. 2015;1:32–39. doi: 10.1016/j.cobeha.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67:1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature neuroscience. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Rounis E, Logothetis N, Smirnakis S. Negative BOLD response ipsi-lateral to the visual stimulus: Origin is not blood stealing. Ninth Annual Meeting of the Organization for Human Brain Mapping (OHBM 2003) 2003 [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele P-F, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Siegel JS, Snyder AZ, Ramsey L, Shulman GL, Corbetta M. The effects of hemodynamic lag on functional connectivity and behavior after stroke. Journal of Cerebral Blood Flow & Metabolism, 0271678×15614846. 2015 doi: 10.1177/0271678X15614846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siero JC, Hendrikse J, Hoogduin H, Petridou N, Luijten P, Donahue MJ. Cortical depth dependence of the BOLD initial dip and poststimulus undershoot in human visual cortex at 7 Tesla. Magnetic Resonance in Medicine. 2015;73:2283–2295. doi: 10.1002/mrm.25349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike GB. Hemodynamic and metabolic responses to neuronal inhibition. NeuroImage. 2004;22:771–778. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Steffener J, Tabert M, Reuben A, Stern Y. Investigating hemodynamic response variability at the group level using basis functions. NeuroImage. 2010;49:2113–2122. doi: 10.1016/j.neuroimage.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Turner R, Le Bihan D, Moonen CT, Despres D, Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991;22:159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. NeuroImage. 2004;23:148–155. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- van der Zande FH, Hofman PA, Backes WH. Mapping hypercapnia-induced cerebrovascular reactivity using BOLD MRI. Neuroradiology. 2005;47:114–120. doi: 10.1007/s00234-004-1274-3. [DOI] [PubMed] [Google Scholar]
- Vazquez AL, Masamoto K, Kim SG. Dynamics of oxygen delivery and consumption during evoked neural stimulation using a compartment model and CBF and tissue P(O2) measurements. NeuroImage. 2008;42:49–59. doi: 10.1016/j.neuroimage.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade AR. The negative BOLD signal unmasked. Neuron. 2002;36:993–995. doi: 10.1016/s0896-6273(02)01138-8. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British journal of anaesthesia. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Martindale J, Johnston D, Jones M, Berwick J, Mayhew J. A model of the hemodynamic response and oxygen delivery to brain. NeuroImage. 2002;16:617–637. doi: 10.1006/nimg.2002.1078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.