Abstract
Background
Hypertension is a highly prevalent cardiovascular risk factor. It is possible that air pollution, also an established cardiovascular risk factor, may contribute to cardiovascular disease through increasing blood pressure. Previous studies evaluating associations between air pollution and blood pressure have had mixed results.
Methods
We examined the association between long-term (one-year moving average) air pollutant exposures, prevalent hypertension and blood pressure in 4,121 older Americans (57+ years) enrolled in the National Social Life, Health, and Aging Project. We estimated exposures to PM2.5 using spatio-temporal models and used logistic regression accounting for repeated measures to evaluate the association between long-term average PM2.5 and prevalence odds of hypertension. We additionally used linear regression to evaluate the associations between air pollutants and systolic, diastolic, mean arterial, and pulse pressures. Health effect models were adjusted for a number of demographic, health and socioeconomic covariates.
Results
An inter-quartile range (3.91 µg/m3) increase in the one-year moving average of PM2.5 was associated with increased: Odds of prevalent hypertension (POR 1.24, 95% CI: 1.11, 1.38), systolic blood pressure (0.93 mm Hg, 95% CI: 0.05, 1.80) and pulse pressure (0.89 mm Hg, 95% CI: 0.21, 1.58). Dose-response relationships were also observed.
Conclusions
PM2.5 was associated with increased odds of prevalent hypertension, and increased systolic pressure and pulse pressure in a cohort of older Americans. These findings add to the growing evidence that air pollution may be an important risk factor for hypertension and perturbations in blood pressure.
Keywords: Air pollution, Elderly, Hypertension, Blood pressure, Pulse pressure
1. INTRODUCTION
Approximately 75 million people, or 29% of all adults in the United States (US), have hypertension, and an additional third of Americans have pre-hypertension.[1] In 2013, 360,000 Americans died from hypertension-related diseases such as myocardial infarctions, cerebrovascular accidents, congestive heart failure and renal insufficiency.[1 2] It is estimated that treatment of hypertension and its sequelae in the U.S. costs over 46 billion dollars per year.[2] Since hypertension is both easily diagnosed and treatable, with successful treatment correlated with decreased subsequent morbidity and mortality,[3] control of hypertension has emerged as a cornerstone of preventative cardiovascular care.[4] While current clinical recommendations focus on individual, modifiable exposures to hypertension risk factors such as physical inactivity, poor diet, alcohol and tobacco use,[5] it is possible that environmental risk factors, such as air pollution, may also be important determinants of hypertension risk.
Air pollution, like hypertension, is a known, key risk factor for adverse cardiovascular health outcomes.[6 7] Evidence from animal [8 9] and epidemiologic [10] studies suggests that air pollution may impair cardiovascular function by inducing a chronic, systemic inflammatory response, increasing oxidative stress and plasma viscosity and altering autonomic nervous input to the heart and vasculature.[11] These pathophysiologic events can in turn lead to endothelial dysfunction, altered arterial diameter [12] or vascular tone and changes in heart rate, all of which ultimately can result in increased blood pressure and hypertension.[12 13]
While studies have provided key evidence linking air pollution exposures to adverse cardiovascular outcomes, epidemiological findings for hypertension are less consistent. Several studies have identified positive associations between air pollution and incident [14–17] and prevalent [18–20] hypertension; however, others have reported no association.[21–23] This inconsistency may reflect heterogeneity in a number of factors between studies, including differences in the studied populations, the exposure assessment methods, and the methods used to assess hypertension. Few studies, for example, have investigated the air pollution-hypertension association in large US populations, and none in nationally representative samples, significantly limiting inference.
A number of studies have examined associations between air pollution and blood pressure as a continuous measure, and those that have report heterogeneous results.[21 23 25–27] As cardiovascular risk increases at pressures lower than those used to diagnose hypertension (such as in pre-hypertension),[28 29] studies that evaluate hypertension as a dichotomous outcome might fail to adequately quantify the effects of air pollution on blood pressure-related cardiovascular outcomes. Furthermore, blood pressure has a number of component measurements, each a quantification of a different aspect of cardiovascular function. The systolic pressure (the maximum arterial pressure during the cardiac cycle) and the diastolic pressure (the minimum arterial pressure during a cardiac cycle) are perhaps the best studied, and increases in either lead to a diagnosis of hypertension due to their established, adverse long-term cardiovascular effects. In addition to maximum and minimum measurements, blood pressure is further composed of steady components (quantified by mean arterial pressure) and pulsatile components (quantified by pulse pressure) which describe the cardiovascular function between blood pressure peaks and troughs.[30] Physiologically, mean arterial pressure is a proxy measure for tissue and organ perfusion pressure, while pulse pressure is proportional to cardiac stroke volume and a measure of conduit artery stiffness. Importantly, increases in all of these aspects of blood pressure have been previously associated with elevated cardiovascular risk,[30 31] while a number of recent studies have shown pulse pressure to have the greatest predictive ability for cardiovascular disease in older (>60 years) populations.[32] To date, few studies have investigated the effects of air pollution on mean arterial pressure and pulse pressure, but those that have observed significant, positive associations.[25 27] While elevations in these measures have both been associated with cardiovascular outcomes, they are not incorporated into the diagnosis of hypertension. So studies examining hypertension as an outcome may fail to completely capture the cardiovascular risk associated with changes in these blood pressure parameters.
To address these gaps in the current literature, we investigate whether fine particulate air pollution (PM2.5) is associated with odds of prevalent hypertension in a nationally representative cohort of older Americans utilizing high quality exposure estimates of PM2.5. We additionally investigate the association between particulate air pollutants and a number of continuous blood pressure measures, including systolic blood pressure, diastolic blood pressure, mean arterial pressure and pulse pressure.
2. METHODS
2.1 Population
We used demographic, health, and other data from a nationally representative probability sample of Americans participating in the National Social Life, Health, and Aging Project (NSHAP). NSHAP is a national area probability sample of 4,121 community residing, older (57+ year) adults, selected from households identified in the Health and Retirement Study (a national, multi-stage area probability sample with a target population of all U.S. adults) in 2004. [33 34] Participants included 4,121 older Americans. Wave 1 recruited 3,005 participants examined in 2005–2006 and Wave 2 included 3,377 participants in 2011–2012, with 2,261 individuals participating in both waves; 744 Wave 1 participants were either too sick to participate in Wave 2 or deceased. Additional Wave 2 participants were selected from eligible respondent (n=907) and non-respondent (n=209) households originally identified from the HRS probability sample. The survey over-sampled African-Americans, Latinos, men and individuals between 75–84 years.[33] Response rates for each wave were high, with 75% and 74% of individuals selected for Wave 1 and Wave 2 opting to participate, respectively. For each data collection wave, participants underwent interviews to obtain demographic, social (social networks, social support, marital history and intimate partnerships and sexuality), and health (self-reported health, physical function and morbidity) data, including medical history and a comprehensive list of current medications. At the time of the interviews, biomeasure data on anthropometrics (height, weight, waist circumference) and cardiovascular health (blood pressure, pulse) were also collected.[33 34]
2.2 Outcome Assessment
During each data collection wave, two blood pressure measurements were collected for each participant from the left arm using a Lifesource digital blood pressure monitor (Model: UA-767PVL) according to manufacturer specifications.[35] If measured systolic blood pressure differed by >20 mm Hg or diastolic blood pressure differed by >14 mm Hg across the two measurements, then a third measurement was taken. Systolic blood pressure and diastolic blood pressure were calculated for inclusion in statistical models as the arithmetic mean of all measurements taken for an individual. Mean arterial pressure was calculated as [systolic blood pressure + (2*diastolic blood pressure)]/3; pulse pressure was calculated as the difference between systolic blood pressure and diastolic blood pressure.[36] In addition, hypertensive status was assessed using 1) Self-report of history of physician-diagnosed hypertension and 2) Self-report of current anti-hypertensive medication consumption. From these data, we defined prevalent hypertension as having a self-report of either hypertension history or medication use or having a measured systolic blood pressure or diastolic blood pressure greater than or equal to 140 mm Hg or 90 mm Hg, respectively.[37]
2.3 Covariates
We controlled for potential confounding using wave-specific covariates previously associated with hypertension or air pollution,[19 21 23 27] including body mass index (BMI), race/ethnicity, socio-economic status (SES), current smoking status, physical activity, alcohol consumption and residence location. Race/ethnicity was categorized as White, Black, Hispanic or other. Socioeconomic status (SES) was assessed on an individual level using self-reported educational attainment and on a neighborhood level using median household income and percent of households below the federal poverty line as reported in the 2000 US Census. Behavioral factors included smoking status (current, historical or none), self-reported frequency of physical activity, alcohol use (ever versus none), and estimated number of alcoholic drinks consumed per week. We additionally control for self-reported use of anti-hypertensive medications in any of the following classes: Diuretics, calcium channel blockers, beta blockers, alpha blockers, angiotensin converting enzyme (ACE) inhibitors, vasodilators and angiotensin receptor blockers (ARBs). Each class of anti-hypertensive was modeled with an individual indicator variable to reduce potential for residual confounding in those on more than one anti-hypertensive. Geographic covariates included region of residence (North Atlantic, South, Great Lakes region, Plains States, Pacific) as well as six categories of urbanicity (Categorized as: [1) 12 largest Standard Metropolitan Statistical Areas (SMSAs) 2) 13–100 largest SMSAs, 3) 12 largest suburbs, 4) 13–100 largest suburbs, 5) Other urban, 6) Other rural].
2.4 Exposure assessment
For each person, we estimated exposures to PM2.5 using spatio-temporal models as described in Yanosky et al..[38] Briefly, PM2.5 data were obtained from the US Environmental Protection Agency (EPA) Air Quality System database and Interagency Monitoring of Protected Visual Environments (IMPROVE) network.[39 40] These data, along with meteorological (wind speed, temperature, total precipitation) and geospatial (county population density, line-source traffic density, point-source PM2.5 emissions, elevation) covariates were used to fit a series of generalized additive mixed models. The daily spatio-temporal estimates derived from these models were used to calculate long-term moving average exposure estimates for each point on a 6 km grid of the conterminous US. NSHAP participants were assigned exposure estimates based on the grid point closest to their permanent addresses (mean distance, 2.23 km). The spatio-temporal estimates have been previously validated using cross-validation techniques, demonstrating high accuracy and low bias (cross-validation R2 of 0.76).[38]
2.5 Statistical Analysis
Logistic regression models with a generalized estimating equation (GEE) approach to account for repeated measures were employed to investigate the associations between an interquartile range (IQR) increase in one-year PM2.5 moving average and prevalence odds ratios (POR) of hypertension as a dichotomous outcome. Linear regression models using GEEs to account for repeated measures were used to examine associations between air pollution and various measures of blood pressure. All fully adjusted models included all participants (4,121 total participants, of which 2,261 had repeated measures) and controlled for BMI, age, sex, education, race/ethnicity, history of diabetes, behavioral variables (self-reported tobacco and alcohol use, physical activity) area-level socioeconomic indicators (including census-track data on median household income and percent of households living below the poverty line) and geographic covariates (region and urbanicity). Linear models of blood pressure additionally controlled for hypertensive medication use, while logistic models included did not, as hypertension medication use was a component of the outcome definition. All models were weighted to account for non-response and oversampling of certain populations..
Effect modification was investigated by education, physical activity, smoking status, age, sex, diabetes status, race and BMI. Last, dose-response relationships were modeled utilizing indicator variables for quartiles of exposure and non-parametric dose-response curves.
Data completeness for the blood pressure measurements was high, with only 84 (2.8%) Wave 1 and 120 (3.6%) Wave 2 participants missing blood pressure measurements. Data completeness for covariates was similarly high, with most covariates having less than 5% missing data, while BMI had a slightly higher (6.3%) missingness. Multiple imputation with chained equations was used to handle all covariate and outcome variable missingness by creating ten datasets with complete data and pooling estimates across datasets.[41]
A number of sensitivity analyses were conducted. First, mean arterial pressure was alternatively calculated as mean arterial pressure = diastolic blood pressure + 0.412 (systolic blood pressure – diastolic blood pressure) as a prior study identified this as producing a better approximation of actual mean arterial pressure.[42] Second, to minimize outcome misclassification for hypertension, we examined a more conservative definition of hypertension defined as: 1) Self report of current anti-hypertensive medication consumption, or 2) Systolic blood pressure ≥ 140 mm Hg, or diastolic blood pressure ≥ 90 mm Hg. Third, variables (i.e., BMI, history of diabetes) that are potentially on the causal pathway between air pollution and hypertension were excluded from models. Last, complete case analyses were undertaken to examine the potential effects of the imputation methods. Analyses were completed using SAS Version 9.4 (SAS INC, Cary, NC).
3. RESULTS
3.1 Participant characteristics
Table 1 shows participant demographic, socioeconomic, health and average exposure characteristics. The average age of participants was approximately 70 years (SD 8.1) and 53.7% were female. Hypertension was highly prevalent, with 80.1% percent in Wave 1 hypertensive and 83.2% in Wave 2 meeting the study definition of hypertension and 58.6% of participants across both waves reporting anti-hypertension medication use.
Table 1.
Characteristics of participants at study entry
| Covariates | Total Observations (N=4121) |
With HTN (N=3250) |
Without HTN (N=871) |
p-value for difference |
|---|---|---|---|---|
| Age (mean, SD) | 69.6 ± 8.1 | 70.2 ± 0.14 | 66.8 ± 8.1 | <0.001 |
| Male (n, %) | 1909 (46.3%) | 1568 (47.3%) | 341 (42.3%) | 0.010 |
|
| ||||
| HEALTH STATUS VARIABLES | ||||
| BMI kg/m2 (mean, SD) | 29.2 ± 6.2 | 29.8 ± 6.4 | 26.8 ± 5.0 | <0.001 |
| -Obese (n, %) | 1471 (35.7%) | 1272 (42.3%) | 199 (23.8%) | <0.001 |
| -Overweight (n, %) | 1430 (34.7%) | 1109 (34.5%) | 321 (35.3%) | 0.660 |
|
| ||||
| Systolic pressure (mean, SD) | 136.9 ± 20.4 | 140.5 ± 20.5 | 121.8 ± 10.6 | <0.001 |
| Diastolic pressure (mean, SD) | 80.8 ± 11.9 | 82.0 ± 12.3 | 75.5 ± 7.8 | <0.001 |
| Mean arterial pressure (mean, SD) | 99.5 ± 13.0 | 101.5 ± 13.2 | 90.9 ± 7.6 | <0.001 |
| Pulse pressure (mean, SD) | 56.2 ± 16.9 | 58.5 ± 17.4 | 46.3 ± 45.6 | <0.001 |
| History of diabetes (n, %) | 933 (22.6%) | 866 (26.1%) | 67 (8.3%) | <0.001 |
|
| ||||
| RACE/ETHNICITY | ||||
| -White (n, %) | 2920 (70.0%) | 2316 (70.1%) | 604 (75.0%) | <0.001 |
| -Black (n, %) | 651 (15.8%) | 574 (17.4%) | 77 (9.6%) | |
| -Hispanic (n, %) | 439 (10.7%) | 332 (10.1%) | 107 (13.3%) | |
| -Other (n, %) | 97 (2.4%) | 80 (2.4%) | 17 (2.1%) | |
|
| ||||
| BEHAVIORAL VARIABLES | ||||
| Current tobacco use | 0.115 | |||
| -Yes (n, %) | 714 (17.3%) | 559 (16.9%) | 155 (19.2%) | |
| -No (n, %) | 1719 (41.7%) | 2755 (83.1%) | 652 (80.8%) | |
| Physical Activity | <0.001 | |||
| - <3 times per week (n, %) | 1,172 (28.4%) | 1017 (31.3%) | 155 (17.8%) | |
| - ≥3 times per week (n, %) | 2,949 (71.6%) | 2292 (70.5%) | 651 (74.7%) | |
|
| ||||
| SOCIOECONOMIC VARIABLES | ||||
| Education level | <0.001 | |||
| - <High school (n, %) | 887 (21.5%) | 758 (22.9%) | 129 (16.0%) | |
| - High school equivalent (n, %) | 1059 (25.7%) | 879 (26.5%) | 180 (22.3%) | |
| - Some college (n, %) | 1239 (30.1%) | 990 (29.9%) | 249 (30.9%) | |
| - College degree or greater (n, %) | 936 (22.7%) | 687 (20.7%) | 249 (30.9%) | |
| Median household income (mean, SD) | 54,259 ± 26,039 | 53,152 ± 28,783 | 58,838 ± 28,783 | <0.001 |
| Percent census track below poverty line (mean, SD) | 14.8 ± 12.2 | 15.2 ± 12.3 | 13.2 ± 11.3 | <0.001 |
|
| ||||
| POLLUTANT (mean, SD) | ||||
| - PM2.5 (µg/m3) | 10.4 ± 3.0 | 10.5 ± 3.0 | 10.1 ± 3.0 | 0.001 |
Participants with hypertension tended to be older (P<0.001), male (47.3% versus 42.3%, p=0.010), have a higher BMI (29.8 kg/m2 versus 26.8 kg/m2, p<0.001), be more likely to have diabetes (26.1% versus 8.3%, p<0.001) and be less physically active than participants without hypertension. Hypertensive patients also had significantly higher one-year moving average PM2.5 exposures as compared to non-hypertensive participants (10.5 µg/m3 versus 10.1 µg/m3, p=0.001).
3.2 Associations between PM2.5 and hypertension
PM2.5 exposures were positively associated with prevalent hypertension (Table 2). In base models, an IQR (3.91 µg/m3) increase in PM2.5 was associated with an 18% increased odds of prevalent hypertension (POR 1.18, 95% CI: 1.08, 1.29). Upon adjusting for geographic, behavioral, and area-level socioeconomic variables, the PM2.5-associated POR increased 33% (POR 1.24, 95% CI: 1.11, 1.38). In models of quartiles of PM2.5 exposure and prevalent hypertension, the PORs associated with the third and fourth quartiles of exposure were significantly higher than those in the lowest quartile of exposure, suggesting a dose-response relationship (Table 2; p- trend<0.001).
Table 2.
Association between odds of prevalent hypertension and quartiles of one-year moving average of PM2.5
| PM2.5 IQR incrementa |
POR (95% CI) | |
|---|---|---|
|
| ||
| Base modelb | Adjusted Modelc | |
|
| ||
| 1 year | 1.18 (1.08, 1.29) | 1.24 (1.11, 1.38) |
|
| ||
| Trend Analysis | ||
| Quartile1 | -- | -- |
| Quartile 2 | 1.12 (0.94, 1.34) | 1.15 (0.94, 1.39) |
| Quartile 3 | 1.39 (1.16, 1.68) | 1.48 (1.19, 1.84) |
| Quartile 4 | 1.35 (1.12, 1.62) | 1.46 (1.17, 1.81) |
| P trend | <0.001 | <0.001 |
IQR PM: 1 year: 3.91 µg/m3
Base model includes PM2.5, BMI, age, sex, education, diabetes status, race
Adjusted model additionally controls for tobacco and alcohol use, physical activity, region, urbanicity, census track data on median household income and percent of households living below the poverty line
3.3 Associations between PM2.5 and blood pressure measures
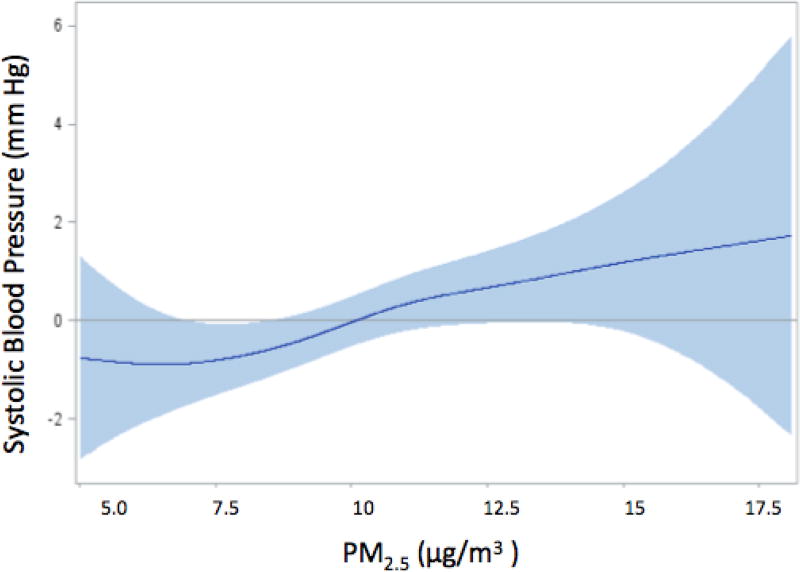
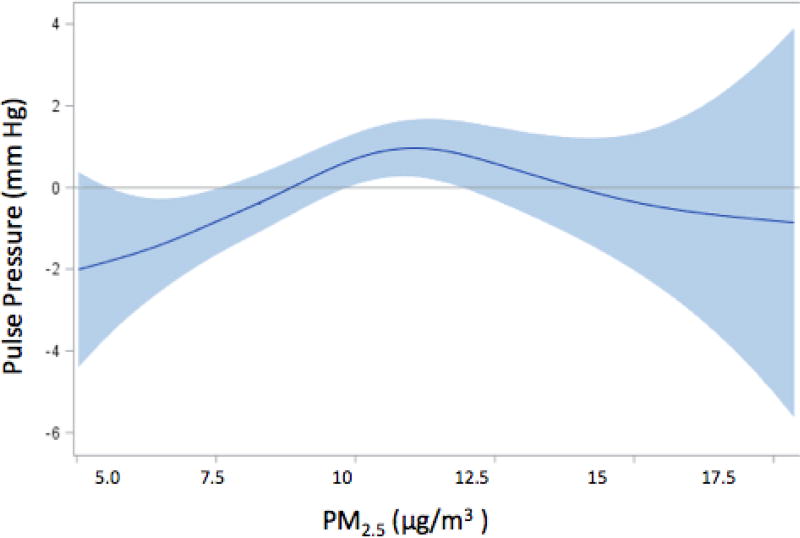
Table 3 summarizes the associations between PM2.5 and the blood pressure measures. In base models, an IQR increase in one-year moving average PM2.5 exposure was associated with an increase in systolic blood pressure of 0.73 mm Hg (95% CI: 0.00, 1.46); the effect estimate was 27.4% higher (0.93 mm Hg, 95% CI: 0.05, 1.80) in the fully adjusted models compared to that in base models. Similar magnitude and trend were also found for pulse pressure, with an IQR increase in PM2.5 associated with an 0.89 mm Hg (95% CI: 0.21, 1.58) increase in pulse pressure in fully adjusted models. On the other hand, one-year PM2.5 exposures were not associated with diastolic blood pressure or mean arterial pressure in either base or fully-adjusted models. Further analyses of systolic blood pressure and pulse pressure show the presence of dose-response relationships, with the third and fourth quartile effects higher than those in lower quartiles of exposure (Table 4, ptrend=0.056 for systolic blood pressure and ptrend=0.069 for pulse pressure). Interestingly, for both outcomes third quartile effect estimates were higher than those in the fourth quartile. This was particularly true for pulse pressure, suggesting a non-linear association. Consistent with these findings, in dose-response curves fit with natural cubic splines, the associations between air pollution and systolic blood pressure (Figure 1) were largely linear, while a more pronounced deviation from linearity was observed for pulse pressure (Figure 2), consistent with a threshold effect.
Table 3.
Association between blood pressure measures and one-year moving averages of PM2.5
| PM2.5a | β (95% CI) | |
|---|---|---|
|
| ||
| Base modelb | Adjusted Modelc | |
|
| ||
| Systolic pressure (mm Hg) | 0.73 (0.00, 1.46) | 0.93 (0.05, 1.80) |
| Diastolic pressure (mm Hg) | −0.01 (−0.41, 0.40) | 0.02 (−0.47, 0.50) |
| Mean arterial pressure (mm Hg) | 0.24 (−0.23, 0.70) | 0.32 (−0.24, 0.88) |
| Pulse pressure (mm Hg) | 0.73 (0.16, 1.30) | 0.89 (0.21, 1.58) |
IQR PM: 1 year: 3.91 µg/m3
Base model includes PM2.5, BMI, age, sex, , anti-hypertensive medication (loop diuretics, thiazide diuretics, potassium sparing diuretics, calcium channel blockers, beta blockers, alpha blockers, ACE inhibitors, vasodilators and ARBs), education, diabetes status, race,
Adjusted model additionally controls for tobacco and alcohol use, physical activity, region, urbanicity, census track data on median household income and percent of households living below the poverty line
Table 4.
Association between systolic and pulse pressures and quartiles of one-year moving averages of PM2.5
| PM2.5 IQR incrementa |
β (95% CI) | |
|---|---|---|
|
| ||
| Systolic pressure (mm Hg)b | Pulse pressure (mm Hg)b | |
|
| ||
| 1 year | 0.93 (0.05, 1.80) | 0.89 (0.21, 1.58) |
|
| ||
| Trend Analysis | ||
| Quartile1 | -- | -- |
| Quartile 2 | 0.47 (−1.13, 2.07) | 1.20 (−0.06, 2.46) |
| Quartile 3 | 1.76 (0.00, 3.52) | 2.51 (1.14, 3.87) |
| Quartile 4 | 1.47 (−0.30, 3.25) | 1.24 (−0.15, 2.64) |
| P trend | 0.056 | 0.069 |
IQR PM: 1 year: 3.91 µg/m3
Model includes PM2.5, BMI, age, sex, , anti-hypertensive medication (loop diuretics, thiazide diuretics, potassium sparing diuretics, calcium channel blockers, beta blockers, alpha blockers, ACE inhibitors, vasodilators and ARBs), education, diabetes status, race, tobacco and alcohol use, physical activity, region, urbanicity, census track data on median household income and percent of households living below the poverty line
Figure 1. Spline representation of the association between an IQR increment in PM2.5 and levels of systolic pressurea.
aThe shaded region indicates 95% confidence intervals
Figure 2. Spline representation of the association between an IQR increment in PM2.5 and levels of pulse pressurea.
aThe shaded region indicates 95% confidence intervals
3.4 Effect modification
No significant effect modifiers were identified for prevalent hypertension (data not shown). Participants with a history of type 2 diabetes mellitus, however, were found to have increased PM2.5-associated impacts on pulse pressure (1.85 mm Hg versus 0.64 mm Hg), although the interaction term was borderline statistically significant (pinteract=0.071; Table 5).
Table 5.
Association between systolic and pulse pressures and one-year moving averages of PM2.5a stratified by participant characteristics
| Characteristics | Systolic pressure (mm Hg)b β (95% CI) |
Interaction p value |
Pulse Pressure (mm Hg)b P (95% CI) |
Interaction p value |
|---|---|---|---|---|
| Sex | 0.999 | 0.648 | ||
| -Male | 0.93 (−0.24, 2.09) | 0.75 (−0.15, 1.66) | ||
| -Female | 0.93 (−0.16, 2.01) | 1.01 (0.16, 1.87) | ||
|
| ||||
| Age | 0.770 | |||
| -Below Median (70) | 1.04 (0.11, 1.96) | 0.466 | 0.93 (0.21, 1.65) | |
| -Above Median (70) | 0.81 (−0.13, 1.75) | 0.85 (0.12, 1.58) | ||
|
| ||||
| BMI | 0.453 | 0.078 | ||
| <30 | 0.83 (−0.67, 2.65) | 1.08 (0.37, 1.80) | ||
| ≥30 | 1.06 (0.11, 2.01) | 0.64 (−0.09, 1.38) | ||
|
| ||||
| Smoker | 0.930 | 0.081 | ||
| -Current tobacco | 0.99 (−0.82, 2.83) | 1.93 (0.61, 3.25) | ||
| -No current | 0.91 (−0.03, 1.86) | 0.68 (−0.06, 1.41) | ||
|
| ||||
| Physical Activity | 0.834 | 0.464 | ||
| >3 times/week | 0.99 (−0.07, 2.05) | 1.07 (0.24, 1.90) | ||
| ≤3 times/week | 0.85 (−0.30, 2.00) | 0.68 (−0.22, 1.57) | ||
|
| ||||
| Education | 0.166 | 0.218 | ||
| >High school | 0.49 (−0.55, 1.54) | 0.59 (−0.25, 1.42) | ||
| ≤High school | 1.53 (0.27, 2.78) | 1.33 (0.35, 2.31) | ||
|
| ||||
| History of Diabetes | 0.220 | 0.071 | ||
| -Yes | 1.75 (0.18, 3.31) | 1.85 (0.62, 3.09) | ||
| -No | 0.71 (−0.24, 1.66) | 0.64 (−0.10, 1.38) | ||
|
| ||||
| Race/Ethnicity | 0.571 | 0.485 | ||
| -White | 1.07 (0.11, 2.02) | 1.00 (0.26, 1.75) | ||
| -Non-White | 0.54 (−1.14, 2.22) | 0.49 (−0.83, 1.81) | ||
IQR PM: 1 year: 3.91 µg/m3
Model includes PM2.5, BMI, age, sex, , anti-hypertensive medication (loop diuretics, thiazide diuretics, potassium sparing diuretics, calcium channel blockers, beta blockers, alpha blockers, ACE inhibitors, vasodilators and ARBs), education, diabetes status, race, tobacco and alcohol use, physical activity, region, urbanicity, census track data on median household income and percent of households living below the poverty line
3.5 Sensitivity Analysis
Models using an alternative calculation of mean arterial pressure did not significantly affect the null findings for this outcome (data not shown), nor did logistic regression models investigating a more conservative definition of HTN (Supplement Table 1). Models excluding covariates potentially on the causal pathway (BMI, diabetes) between air pollution and systolic blood pressure (0.89 mm hg, 95% CI: 0.02, 1.77), pulse pressure (0.93 mm hg, 95% CI: 0.25, 1.62) and hypertension (OR 1.22, 95% CI: 1.10, 1.35) and analyses restricted to complete cases (Supplement Table 2) did not differ appreciably from our fully adjusted, imputed models.
4. DISCUSSION
We observed that PM2.5 was significantly associated with increased prevalence of hypertension and with increased measures of blood pressure, as assessed using systolic blood pressure and pulse pressure in a nationally representative cohort of older Americans. The impact of PM2.5 on hypertension prevalence and systolic blood pressure was greatest in the third and fourth as compared to lowest quartile of exposure, consistent with a dose-response relationship, while a non-linear dose response was identified for pulse pressure. Diabetic participants had larger associations between pulse pressure and PM2.5 exposures, although this was marginally significant (pinteract < 0.10).
Our finding of an association between PM2.5 and hypertension prevalence is consistent with some, but not all, previous literature. Babisch et al. (2014) reported positive associations (OR 1.15, 95% CI: 1.02, 1.30) between increases in PM2.5 (1 µg increment) and prevalent hypertension,[20] as did Pitchika et al. [43], who observed a 1 µg increment increase in PM2.5 to be associated with a 15% increase in hypertension prevalence (95% CI: 2.5%, 28.0%) in a German population. This is consistent with findings by Liu et al. [44] who observed a 41.7 µg/m3 increment in PM2.5 to be significantly associated with increased odds of prevalent hypertension (POR 1.11, 95% CI: 1.05, 1.17) in a Chinese population. Dong et al. (2013) similarly observed increased odds of prevalent hypertension for long-term PM10 exposures (OR 1.12, 95% CI: 1.08, 1.16),[19] in a Chinese population. In contrast, Fuks et al. [24] examined the association between prevalent hypertension and residential distance to nearest major roadway in a population-based German cohort and observed no association, as did Foraster et al. (2014) in a European cohort.[21] This heterogeneity in study findings may reflect design differences in terms of populations studied and differences in pollution constituents, as both studies which found null effects were in European populations.[21 22] Our study, in a nationally-representative cohort of older Americans using high-quality PM2.5 exposure estimates, adds significantly to the extant literature on PM2.5 and hypertension prevalence.
Most literature investigating the association of air pollution with blood pressure as a continuous measure has reported similar, positive associations to those we observe for PM2.5.[21 23–27 45] Auchincloss et al. (2008) reported positive associations between PM2.5, systolic blood pressure and pulse pressure in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort [25] while in a Taiwanese cohort Chuang et al. (2010) observed particulate matter exposure to be associated with systolic blood pressure, but not diastolic blood pressure.[45] Similarly, Fuks et al. (2011) observed long-term PM2.5 exposure to be positively associated with both systolic blood pressure and diastolic blood pressure in a German cohort,[46] while Chan et al. (2015) reported a 10 µg/m3 increase in PM2.5 to be associated with increases in systolic blood pressure (1.4 mm Hg, p<0.001), mean arterial pressure (0.8 mm Hg, p=0.01) and pulse pressure (1.0 mm Hg, p=0.01), but not diastolic blood pressure.[27] This study is similar to ours in that it used a national sample, high-quality exposure estimates and controlled for a number of likely confounders. In contrast, Pitchika et al. (2017) found 1 µg/m3 increase in long-term PM2.5 exposure to be associated with diastolic pressure (0.7 mmHg, 95% CI: 0.2; 1.2), but not systolic pressure in a German cohort, [43] while Zhang et al. (2018) observed a 10 µg/m3 2-year moving average increment in PM2.5 concentration was associated with increases in SBP (0.45 mmHg, 95% CI: 0.40, 0.50), DBP (0.07 mmHg, 95% CI: 0.04, 0.11), and PP (0.38 mmHg, 95% CI: 0.33, 0.42) in a Taiwanese population.[47] The observed heterogeneity in the literature is likely influenced by differences in populations and exposure constituents and magnitudes across studies.
Importantly, our findings of significantly increased systolic blood pressure and pulse pressure are potentially of clinical relevance, as both have been associated with increased risk of cardiovascular events in older individuals.[30 32 48 49] In a 2001 study Franklin et al. found that the importance of each blood pressure fraction as a measure of risk differs by age. In individuals <50 years old, diastolic blood pressure was found to be the strongest predictor of coronary heart disease (CHD) development (HR per 10 mm Hg increment, 1.34; 95% CI, 1.18 to 1.51) while systolic blood pressure (HR 1.14, 95% CI: 1.06, 1.24) and pulse pressure (HR 1.02, 95% CI: 0.89, 1.17) were less strongly associated. In individuals 50–59 years, risks for all three measures were comparable in terms of CHD risk, while in individuals >60 years (the age group with by far the largest representation in our study) pulse pressure was the strongest predictor of CHD risk (HR 1.24, 95% CI: 1.16, 1.33) while diastolic blood pressure became a non-significant predictor (HR 1.12, 95% CI: 0.99, 1.27).[32] Similar results were reported in a 2003 analysis of the Framingham Heart Study in which systolic blood pressure (HR 1.56, 95% CI: 1.37, 1.77) and pulse pressure (HR 1.55, 95% CI: 1.37, 1.75) elevations conferred the greatest risk for incident congestive heart failure among adults 50–79 years.[50] As we observed air pollution exposures to be significantly associated with elevated systolic blood pressure and pulse pressure but not diastolic blood pressure in a population >57 years, these previous studies suggest that air pollution may confer a blood pressure phenotype which is at particularly high risk for detrimental cardiovascular outcomes in this age group.
Interestingly, the observed pattern of results suggests physiologic mechanisms through which PM2.5 increases blood pressure in older individuals. Blood pressure is a function of cardiac output (stroke volume and heart rate) as well as peripheral resistance.[51] Peripheral resistance (R) is described by:
in which resistance is directly proportional to vessel length (L) and blood viscosity (η), but inversely proportional to the fourth power of vessel radius (r), making vessel radius the most important determinant of peripheral vascular resistance.[52] Current biological hypotheses involve air pollution affecting blood viscosity, autonomic nervous activity and endothelial function via direct irritation of pulmonary receptors, oxidative stress, and systemic inflammation.[12 13] Plasma viscosity increases due to pulmonary macrophages increasing cytokine production, leading to increases in hepatic soluble acute phase protein production (i.e. fibrinogen, CRP, complement, haptoglobin).[9] The resultant increase in plasma viscosity is directly proportional to increased blood pressure.[53] Increasing sympathetic autonomic activity (or conversely, withdrawing parasympathetic activity) increases heart rate and myocardial contractility (and thus cardiac output) as well as peripheral resistance due to constriction of vascular smooth muscle. Endothelial dysfunction, characterized by decreased production of vasodilators (i.e. nitric oxide) and increased production of vasoconstrictors (i.e. endothelin), also leads to net arterial vasoconstriction.[11] As both of these latter mechanisms serve to decrease vessel radius, the single most important determinant of peripheral resistance, even small changes in sympathetic tone and endothelial function can have dramatic effects on all blood pressure measures we investigate.
These mechanisms, however, do not completely explain the pattern of our findings, as we observed significant increases in pulse pressure and systolic pressure associated with increased air pollution, but not diastolic pressure or mean arterial pressure. Pulse pressure and systolic pressure are impacted by heart rate, left ventricular ejection volume, large artery elasticity and the magnitude and speed of the backward pressure wave arising in the peripheral circulation.[30] Elastin, a ubiquitous protein in the large conduit arteries which contributes to arterial wall elasticity, breaks down with increased age, leading to increased aortic stiffness, impedance and pulse wave velocity.[54 55] Pulse pressures and systolic pressures increase with increased pulse wave velocity, as the systolic pressure wave is reflected back into the large conduit arteries earlier than in normal physiology (in systole rather than diastole). These processes, taken together, serve to increase systolic blood pressure and pulse pressure, but not diastolic blood pressure or mean arterial pressure.[55] Thus, while all of the aforementioned mechanisms likely contribute to the observed increased hypertension risk, increased large arterial stiffness may explain the pattern of significant and non-significant associations seen in our study. Concordant with this, systemic inflammation, a major biological pathway by which air pollution is hypothesized to exert cardiovascular health effects, has also been related to increased aortic arterial stiffness.[56]
We observed borderline significant effect modification by diabetes status. Interestingly, diabetes has also been shown to accelerate age-associated increases in conduit artery stiffness which may help mechanistically explain the observed effect modification.[57] Importantly, concomitant hypertension has been estimated to account for up to 75% of cardiovascular disease among diabetics.[58] As diabetics are already at increased risk of cardiovascular events as compared to non-diabetics,[59] the observed increased risk of blood pressure elevations among diabetics may thus be of significant clinical import. Specifically, it may demonstrate that diabetics are a particularly susceptible population to the increased cardiovascular risk consistently associated with air pollution.
Our study has a number of limitations. First, the observational nature of the study design limits our ability to draw causal inferences. Second, exposure error for PM2.5 is probable. While our PM2.5 estimates have been previously validated, the estimates are for outdoor pollution concentrations and do not estimate personal exposures per se. However, previous studies in elderly populations report high correlations between outdoor PM2.5 concentrations and personal exposures.[60 61] Last, outcome misclassification is possible, as use of any medication in a pharmacologic class traditionally used to treat hypertension was incorporated into the dichotomous definition of the outcome. It is likely that at least some individuals were taking antihypertensive medications for treatment of non-hypertensive conditions.
Despite these limitations, our study is among very few to examine these associations in a nationally representative cohort of older Americans. We employed PM2.5 estimates that have been shown to have high accuracy and precision in previous validation studies [38] and were able to control for an extensive list of likely confounders. Our reliance on three distinct measures of hypertension and consistency in results using both conservative and more liberal hypertension definitions limits the likelihood that outcome misclassification significantly affected our results.
5. CONCLUSIONS
Particulate matter was significantly associated with elevated odds of prevalent hypertension as well as increases in systolic and pulse pressures in a nationally representative cohort of older Americans. These findings add further to the growing body of evidence that air pollution may be an important risk factor for hypertension and perturbations in blood pressure.
Supplementary Material
Highlights.
Studies of air pollution and hypertension have reported mixed results.
Few studies have examined systolic, diastolic, pulse pressure, and mean arterial blood pressure components.
Increased long-term particulate matter exposure was found to be associated with increased odds of prevalent hypertension.
Significant associations between air pollution and systolic and pulse pressures were also identified.
Acknowledgments
FUNDING: This work was supported by NIEHS grant 1R01ES022657-01A1, with health and other covariate data obtained through NIH R01-AG021487, R37-AG030481, R01-AG033903, and R01-ES019168.
We thank Dr. Jeffrey Yanosky from the Pennsylvania State University for providing daily PM2.5 grid data.
Abbreviations
- PM2.5
Particulate matter with an aerodynamic diameter of ≤2.5µm
- POR
Prevalence odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DUALITY OF INTEREST: The authors declare that there is no duality of interest associated with this manuscript.
Ethics approval and consent to participate: This study was approved by the institutional review board (IRB) of Northeastern University and all participants provided written informed consent: IRB# 13-07-18
CONTRIBUTION STATEMENT: TH, VP, JM and HS were involved in the data analysis, interpretation and drafting the manuscript. All authors reviewed/edited the manuscript and approved the final version. TH is the guarantor of this work.
References
- 1.Nwankwo T, Yoon S, Burt V, Gu Q. NCHS data brief, no. 133. National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD, US Dept of Health and Human Services; Hypertension among adults in the US: National Health and Nutrition Examination Survey, 2011–2012. Ref Type: Report 2013. [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2015 Update A Report From the American Heart Association. Circulation. 2015;131(4):E29–E322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. The Lancet. 373(9667):929–40. doi: 10.1016/S0140-6736(09)60330-5. doi: http://dx.doi.org/10.1016/S0140-6736(09)60330-5%5Bpublished Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services, National Institutes of Health (NIH), National Heart, Lung, and Blood Institute. Your guide to lowering your blood pressure with DASH. DASH eating plan. 2006 [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. doi: 10.1161/CIR.0b013e3181dbece1. doi: CIR.0b013e3181dbece1 [pii] [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Gehring U, Heinrich J, Kramer U, et al. Long-term exposure to ambient air pollution and cardiopulmonary mortality in women. Epidemiology. 2006;17(5):545–51. doi: 10.1097/01.ede.0000224541.38258.87. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–46. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Eeden SF, Tan WC, Suwa T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) American journal of respiratory and critical care medicine. 2001;164(5):826–30. doi: 10.1164/ajrccm.164.5.2010160. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Kramer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118(9):1273–9. doi: 10.1289/ehp.0901689. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Q, Hong X, Wold LE. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. 2010;121(25):2755–65. doi: 10.1161/CIRCULATIONAHA.109.893461. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urch B, Brook JR, Wasserstein D, et al. Relative Contributions of PM2.5 Chemical Constituents to Acute Arterial Vasoconstriction in Humans. Inhalation toxicology. 2004;16(6–7):345–52. doi: 10.1080/08958370490439489. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Mills NL, Törnqvist H, Robinson SD, et al. Diesel Exhaust Inhalation Causes Vascular Dysfunction and Impaired Endogenous Fibrinolysis. Circulation. 2005;112(25):3930–36. doi: 10.1161/circulationaha.105.588962. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Burnett RT, Kwong JC, et al. Spatial Association between Ambient Fine Particulate Matter and Incident Hypertension. Circulation. 2013 doi: 10.1161/circulationaha.113.003532. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 15.Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in los angeles. Circulation. 2012;125(6):767–72. doi: 10.1161/circulationaha.111.052753. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Laden F, Forman JP, Hart JE. Long-Term Exposure to Particulate Matter and Self-Reported Hypertension: A Prospective Analysis in the Nurses’ Health Study. Environmental health perspectives. 2016 doi: 10.1289/EHP163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda T, Eliot MN, Eaton CB, et al. Long-term exposure to residential ambient fine and coarse particulate matter and incident hypertension in postmenopausal women. Environment International. 2017;105:79–85. doi: 10.1016/j.envint.2017.05.009. doi: https://doi.org/10.1016/j.envint.2017.05.009%5Bpublished Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirwa K, Eliot MN, Wang Y, et al. Residential proximity to major roadways and prevalent hypertension among postmenopausal women: results from the Women's Health Initiative San Diego Cohort. Journal of the American Heart Association. 2014;3(5):e000727. doi: 10.1161/JAHA.113.000727. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong G-H, Qian ZM, Xaverius PK, et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61(3):578–84. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 20.Babisch W, Wolf K, Petz M, Heinrich J, Cyrys J, Peters A. Associations between traffic noise, particulate air pollution, hypertension, and isolated systolic hypertension in adults: the KORA study. Environmental Health Perspectives (Online) 2014;122(5):492. doi: 10.1289/ehp.1306981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foraster M, Basagana X, Aguilera I, et al. Association of long-term exposure to traffic-related air pollution with blood pressure and hypertension in an adult population-based cohort in Spain (the REGICOR study) Environ Health Perspect. 2014;122(4):404–11. doi: 10.1289/ehp.1306497. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuks K, Moebus S, Hertel S, et al. Long-Term Urban Particulate Air Pollution, Traffic Noise and Arterial Blood Pressure. Environ Health Perspect. 2011 doi: 10.1289/ehp.1103564. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen M, Hoffmann B, Hvidberg M, et al. Long-term exposure to traffic-related air pollution associated with blood pressure and self-reported hypertension in a danish cohort. Environ Health Perspect. 2012;120(3):418–24. doi: 10.1289/ehp.1103631. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuks KB, Weinmayr G, Foraster M, et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE) Environ Health Perspect. 2014;122(9):896–905. doi: 10.1289/ehp.1307725. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auchincloss AH, Roux AVD, Dvonch JT, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-Ethnic Study of Atherosclerosis (MESA) Environmental Health Perspectives. 2008;116(4):486. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viehmann A, Hertel S, Fuks K, et al. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occupational and environmental medicine. 2015;72(9):656–63. doi: 10.1136/oemed-2014-102800. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Chan SH, Van Hee VC, Bergen S, et al. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environmental health perspectives. 2015;123(10):951–58. doi: 10.1289/ehp.1408125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi AI, Suri MFK, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke; a journal of cerebral circulation. 2005;36(9):1859–63. doi: 10.1161/01.STR.0000177495.45580.f1. [DOI] [PubMed] [Google Scholar]
- 29.Hsia J, Margolis KL, Eaton CB, et al. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115(7):855–60. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 30.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13(4):392–400. doi: 10.1161/01.hyp.13.4.392. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 31.Madhavan S, Ooi WL, Cohen H, Alderman MH. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23(3):395–401. doi: 10.1161/01.hyp.23.3.395. [DOI] [PubMed] [Google Scholar]
- 32.Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103(9):1245–9. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 33.Waite LJ, Laumann EO, Levinson W, Lindau ST, O'Muircheartaigh CA. National Social Life, Health, and Aging Project (NSHAP): Wave 1. ICPSR20541-v6. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2014. pp. 04–30. [Google Scholar]
- 34.Waite LJCK, Dale W, Huang E, Laumann EO, McClintock M, et al. National Social Life, Health, and Aging Project (NSHAP): Wave 2 and Partner Data Collection. ICPSR34921-v1. Inter-university Consortium for Political and Social Research; Ann Arbor, MI: 2014. [Google Scholar]
- 35.Williams SR, Pham-Kanter G, Leitsch SA. Measures of chronic conditions and diseases associated with aging in the national social life, health, and aging project. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64(suppl 1):i67–i75. doi: 10.1093/geronb/gbn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cywinski J, Tardieu B, Heiss H. Essentials in pressure monitoring blood and other fluids. Wiley Online Library. 1982 [Google Scholar]
- 37.Wassertheil-Smoller S, Anderson G, Psaty BM, et al. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension. 2000;36(5):780–9. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 38.Yanosky JD, Paciorek CJ, Laden F, et al. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environmental health : a global access science source. 2014;13:63. doi: 10.1186/1476-069x-13-63. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EPA US. U.S. Environmental Protection Agency Air Quality System. 2009. Secondary U.S. Environmental Protection Agency Air Quality System. 2009 2009. http://www.epa.gov/ttn/airs/airsaqs/
- 40.IMPROVE. Interagency Monitoring of Protected Visual Environments Homepage. Secondary Interagency Monitoring of Protected Visual Environments Homepage. 2015 Jan 8; 2013. http://vista.cira.colostate.edu/improve/
- 41.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Statistics in medicine. 2011;30(4):377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 42.Meaney E, Alva F, Moguel R, Meaney A, Alva J, Webel R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart. 2000;84(1):64–64. doi: 10.1136/heart.84.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitchika A, Hampel R, Wolf K, et al. Long-term associations of modeled and self-reported measures of exposure to air pollution and noise at residence on prevalent hypertension and blood pressure. Science of The Total Environment. 2017;593–594:337–46. doi: 10.1016/j.scitotenv.2017.03.156. doi: https://doi.org/10.1016/j.scitotenv.2017.03.156%5Bpublished Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Chen R, Zhao Y, et al. Associations between ambient fine particulate air pollution and hypertension: A nationwide cross-sectional study in China. Science of The Total Environment. 2017;584–585:869–74. doi: 10.1016/j.scitotenv.2017.01.133. doi: https://doi.org/10.1016/j.scitotenv.2017.01.133%5Bpublished Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuang K-J, Yan Y-H, Cheng T-J. Effect of Air Pollution on Blood Pressure, Blood Lipids, and Blood Sugar: A Population-Based Approach. Journal of Occupational and Environmental Medicine. 2010;52(3):258–62. doi: 10.1097/JOM.0b013e3181ceff7a. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 46.Fuks K, Moebus S, Hertel S, et al. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environmental Health Perspectives. 2011;119(12):1706. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Guo C, Lau AKH, et al. Long-Term Exposure to Fine Particulate Matter, Blood Pressure, and Incident Hypertension in Taiwanese Adults. Environ Health Perspect. 2018;126(1):017008. doi: 10.1289/ehp2466. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kannel WB, Wolf PA, McGee DL, Dawber TR, McNamara P, Castelli WP. Systolic blood pressure, arterial rigidity, and risk of stroke: The framingham study. JAMA. 1981;245(12):1225–29. doi: 10.1001/jama.1981.03310370017013. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 49.Benetos A, Safar M, Rudnichi A, et al. Pulse Pressure: A Predictor of Long-term Cardiovascular Mortality in a French Male Population. Hypertension. 1997;30(6):1410–15. doi: 10.1161/01.hyp.30.6.1410. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 50.Haider AW, Larson MG, Franklin SS, Levy D. Systolic Blood Pressure, Diastolic Blood Pressure, and Pulse Pressure as Predictors of Risk for Congestive Heart Failure in the Framingham Heart Study. Annals of Internal Medicine. 2003;138(1):10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 51.Lund-Johansen P. Haemodynamics in essential hypertension. Clinical Science. 1980;59(s6):343s–54s. doi: 10.1042/cs059343s. [DOI] [PubMed] [Google Scholar]
- 52.Klabunde R. Cardiovascular physiology concepts. Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 53.Letcher RL, Chien S, Pickering TG, Sealey JE, Laragh JH. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects: role of fibrinogen and concentration. The American journal of medicine. 1981;70(6):1195–202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- 54.Nichols W, O'Rourke M, Vlachopoulos C. McDonald's blood flow in arteries: theoretical, experimental and clinical principles. CRC Press; 2011. [Google Scholar]
- 55.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated Systolic Hypertension: Prognostic Information Provided by Pulse Pressure. Hypertension. 1999;34(3):375–80. doi: 10.1161/01.hyp.34.3.375. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 56.Mahmud A, Feely J. Arterial Stiffness Is Related to Systemic Inflammation in Essential Hypertension. Hypertension. 2005;46(5):1118–22. doi: 10.1161/01.HYP.0000185463.27209.b0. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 57.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non–Insulin-Dependent Diabetes Mellitus and Fasting Glucose and Insulin Concentrations Are Associated With Arterial Stiffness Indexes. The ARIC Study. 1995;91(5):1432–43. doi: 10.1161/01.cir.91.5.1432. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 58.Sowers JR, Epstein M, Frohlich ED. Diabetes, Hypertension, and Cardiovascular Disease: An Update. Hypertension. 2001;37(4):1053–59. doi: 10.1161/01.hyp.37.4.1053. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention (CDC) National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Vol. 2014 Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 60.Williams R, Suggs J, Creason J, et al. The 1998 Baltimore Particulate Matter Epidemiology-Exposure Study: part 2. Personal exposure assessment associated with an elderly study population. J Expo Anal Environ Epidemiol. 2000;10(6 Pt 1):533–43. doi: 10.1038/sj.jea.7500108. [DOI] [PubMed] [Google Scholar]
- 61.Janssen NA, de Hartog JJ, Hoek G, et al. Personal exposure to fine particulate matter in elderly subjects: relation between personal, indoor, and outdoor concentrations. Journal of the Air & Waste Management Association. 2000;50(7):1133–43. doi: 10.1080/10473289.2000.10464159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.