Abstract
The cochlea has an immune environment dominated by macrophages under resting conditions. When stressed, circulating monocytes enter the cochlea. These immune mediators, along with cochlear resident cells, organize a complex defense response against pathological challenges. Since the cochlea has minimal exposure to pathogens, most inflammatory conditions in the cochlea are sterile. Although the immune response is initiated for the protection of the cochlea, off-target effects can cause collateral damage to cochlear cells. A better understanding of cochlear immune capacity and regulation would therefore lead to development of new therapeutic treatments. Over the past decade, there have been many advances in our understanding of cochlear immune capacity. In this review, we provide an update and overview of the cellular components of cochlear immune capacity with a focus on macrophages in mammalian cochleae. We describe the composition and distribution of immune cells in the cochlea and suggest that phenotypic and functional characteristics of macrophages have site-specific diversity. We also highlight the response of immune cells to acute and chronic stresses and comment on the potential function of immune cells in cochlear homeostasis and disease development. Finally, we briefly review potential roles for cochlear resident cells in immune activities of the cochlea.
Keywords: Cochlea, Immunity, Macrophage, Inflammation
The cochlea is the sensory organ responsible for hearing perception. Sensory cells in the cochlea are susceptible to various acute and chronic stresses such as acoustic overstimulation, ototoxicity and age-related degeneration. In response to these stresses, the cochlea launches an inflammatory response and this response is an integral component of the defense mechanism. Indeed, immune activities and inflammatory responses have been implicated in all major causes of acquired hearing loss (Fujioka et al., 2014; Goodall et al., 2015; Iwai et al., 2008; Iwai et al., 2003; Malik et al., 2012; Oh et al., 2011; Tan et al., 2013; Toubi et al., 2004). Moreover, therapeutic intervention targeting inflammation has been shown to be effective for reducing the level of damage in many inner ear diseases that concur with inflammatory activities (Arpornchayanon et al., 2013; Arslan et al., 2011; Canlon et al., 2007; Rauch, 2004; Wakabayashi et al., 2010).
Immune cells are the primary players in immune responses. A scarcity of leukocyte activity in the cochlea was suggested by early investigations and this was attributed to the blood-labyrinth barrier that isolates the cochlea from the systemic immune system. However, recent studies provide evidence to the contrary. The cochlea in fact contains not only immune cells but also resident cells with immune capabilities. These cells reside within all of the major cochlear partitions important for cochlear function, and participate in the maintenance of cochlear homeostasis through immune surveillance of the cochlear microenvironment. Immune cells also regulate cochlear responses to disease formation following pathological insults. Their activities are believed to contribute to the progression and final outcome of cochlear pathogenesis. Here, we review properties of immunocompetent cells in the cochlea with a focus on macrophages that are the predominant immune cell in the cochlea. Over the past few years, several review articles have been published on cochlear immunity and immune cell responses to disease development (Fujioka et al., 2014; Goodall et al., 2015; Hirose et al., 2017; Kalinec et al., 2017; Wood et al., 2017). Interested readers may refer to these references for additional information.
1. Leukocyte composition of the cochlea
Leukocytes are the first line of defense against invasive pathogens and endogenous damage-associated molecules. There are three types of leukocytes: lymphocytes, granulocytes and monocytes, each consisting of subgroups. Lymphocytes comprise T cells, B cells and natural killer cells. Granulocytes include neutrophils, eosinophils and basophils. Monocytes, once extravasated from the bloodstream into tissues, differentiate into macrophages and dendritic cells. The abundance of each type of leukocyte is tissue-specific: neutrophils are the most common leukocyte in the bloodstream and constitute roughly 50–70% of all leukocytes in humans (Bainton et al., 1971; Mayadas et al., 2014); the brain and eyes, which are protected by a tissue-blood barrier, are rich in monocytes and macrophages (Korin et al., 2017; Liyanage et al., 2016); tissues under constant exposure to microbial-derived molecules and intrinsic cytotoxic molecules host a more diverse population of resident immune cells including mast cells, natural killer cells and T cells (Mowat et al., 2014; Racanelli et al., 2006; Tay et al., 2014). This diversity reflects the difference in tissue immune microenvironments and is a biological basis for tissue-specific immune activity.
Leukocyte composition in the cochlea is not completely clear; several studies have shown that the macrophage is the most abundant immune cell population in the cochlea (Hirose et al., 2005; Okano et al., 2008), accounting for more than 80% of bone-marrow derived cells in the cochlea (Okano et al., 2008). Cochlear macrophages are identified by their expression of macrophage specific markers, including F4/80 (Frye et al., 2017; Okano et al., 2008; Tornabene et al., 2006; Yang et al., 2015), Iba1 (Hirose et al., 2005; Okano et al., 2008), CD68 (Hirose et al., 2005; Okano et al., 2008), and CD11b (Shi, 2010). This macrophage-dominated immune capacity in the cochlea differs from T cell-dominated immune capacity reported in the endolymphatic sac (Takahashi et al., 1988b).
The cochlea has a small population of lymphocytes that express T cell marker proteins under resting conditions and their number increases after immune challenge (Takahashi and Harris, 1988a). The cochlea also contains immune cells that bear features of dendritic cells, including expression of major histocompatibility complex II (MHC-II), an antigen presenting protein (Yang et al., 2015). However, under steady-state conditions, these cells do not express CD11c (Yang et al., 2015), an integrin that is enriched in classical dendritic cells. Thus, the identity of these cells requires further clarification.
Systematic analysis of immune cell populations in the cochlea is scarce. In a recent study, Matern and co-workers used fluorescence-activated cell sorting to characterize the composition of CD45-positive immune cells isolated from the cochleae of postnatal GfiCre mice (Matern et al., 2017). This study not only confirmed macrophages, which account for 81.3% of CD45-positive cells, as the most abundant immune cell population but also identified several groups of minority immune cells, including granulocytes (3.1%), T cells (0.8%), B cells (0.4%), and natural killer cells (3.4%). At present, it is not clear whether this composition in postnatal cochleae is applicable to mature cochleae. Because the murine cochlea undergoes active postnatal remodeling, its immune cell composition is likely to change during maturation. Therefore, the immune cell makeup in mature cochleae requires additional studies.
2. Distribution of macrophages in the cochlea
Immune cells are present in the cochlea starting from the early stages of development (Brown et al., 2017; Hinton et al., 2017; Hirose et al., 2017; Hu et al., 2017; Kaur et al., 2017; Kim et al., 2011), where they remain for life (Frye et al., 2017). While multiple leukocyte populations have been identified in the cochlea, only the distribution of macrophages has been studied in detail. Under steady-state conditions, macrophages are found in multiple cochlear regions and are particularly abundant in the spiral ligament and neural tissues (Hirose et al., 2005; Okano et al., 2008). Okano and co-workers reported that approximately 62% of cochlear macrophages reside in the neural tissue and about 36% are in the spiral ligament and spiral limbus (Okano et al., 2008). In general, macrophages are uniformly distributed along the apex-to-base gradient of the cochlea, and aggregation or gaps in macrophage distribution are rare. This spatial configuration ensures that each cell has its own territory for immune inspection and that all cochlear regions are under immune surveillance.
2.1. Lateral wall macrophages
Macrophages reside in both the spiral ligament and the stria vascularis. In the spiral ligament, macrophages exhibit irregular shapes with branches and processes (Fig. 1). They are more abundant in the inferior site of the spiral ligament. This region houses type II and IV fibrocytes, both of which are susceptible to pathological insults (Hirose and Liberman, 2003; Wang et al., 2002b). The inferior site is adjacent to the scala tympani, a major site of leukocyte accumulation after acute damage (Du et al., 2011; Miyao et al., 2008; Sautter et al., 2006). Morphological analysis has revealed micropores on the surface of the spiral ligament facing the scala tympani (Lim, 1970). These fenestrae allow the perilymph to access the spiral ligament that could serve as a communication interface between spiral ligament leukocytes and the perilymph. This region also expresses intercellular adhesion molecule 1 (ICAM-1) in vascular endothelial cells and fibrocytes, and acoustic overstimulation promotes the expression of this molecule (Miyao et al., 2008; Tornabene et al., 2006). ICAM-1 is an intercellular adhesion molecule that could play a role in mediating adhesion of leukocytes to vascular endothelial cells for extravasation into the cochlea.
Figure 1. Macrophages in the spiral ligament.
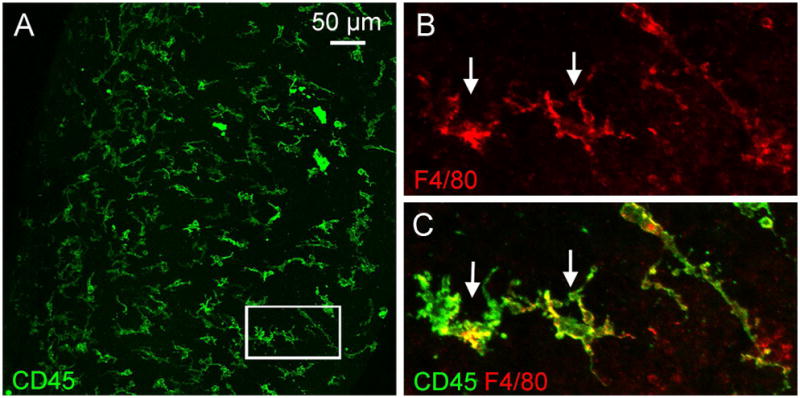
The images show macrophages in a whole-mount preparation of the spiral ligament collected from a young C57BL/6J mouse. The tissue was doubly stained with antibodies against CD45, a pan-leukocyte marker, and F4/80, a macrophage marker, using a method that has been previously described (Yang et al., 2015). A. Typical macrophages in the spiral ligament exhibit irregular shapes with large branches and short processes. B and C. Enlarged view of the macrophages in the area marked by the rectangle in A. B shows F4/80 immunoreactivity and C is the overlap of F4/80 and CD45 immunostaining of the same region shown in B.
Macrophages are also found in the stria vascularis, where they serve as perivascular cells around endothelial cells and participate in the formation of the blood-labyrinth barrier (Shi, 2010; Zhang et al., 2012).
2.2. Macrophages in neural tissues
The neural tissue of the cochlea comprises spiral ganglion cell bodies and their peripheral and central processes. Leukocytes are found around ganglion neurons as well as the nerve fibers inside the osseous spiral lamina and the modiolus (Fig. 2). Inside the bony tunnel of the osseous spiral lamina, macrophages lie in roughly two rows: one close to the edge of the osseous spiral lamina and the other close to ganglion neurons. Many of these macrophages are oriented parallel to the tunnel radial fibers of ganglion neurons, while some at the lateral edge of the osseous spiral lamina lie in a perpendicular direction. Some macrophages at the edge of the osseous spiral lamina extend their processes through the habenula perforata to the region of inner hair cells; however, these processes do not contact inner hair cells. While the function of these processes is not completely clear, their proximity to cochlear nerve synapses suggests a role in synapse homeostasis, a function that has been recognized in microglial cells, which are the resident macrophage in the brain (Wake et al., 2009). Moreover, these processes have been implicated in clearance of degenerated sensory cells in the organ of Corti after damage (Hirose et al., 2017).
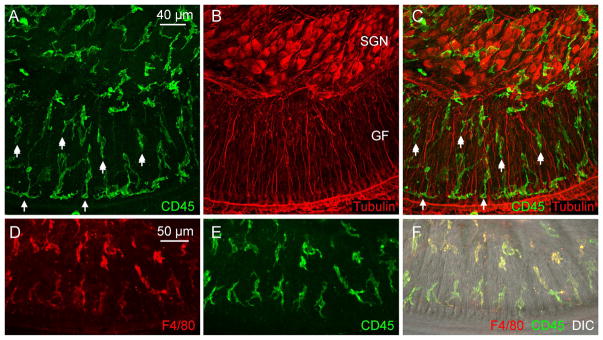
Figure 2. Identification of macrophages in the neural region inside the osseous spiral lamina and among ganglion cell bodies.
The images show macrophages in whole-mount preparations of cochleae in young C57BL/6J mice. The images were derived by projecting serial optical sections of confocal images covering only the depth of neural tissues in the osseous spiral lamina and ganglion cell regions. A. The image shows CD45 immunoreactivity in cochlear immune cells. B. Tubulin immunoreactivity is used to illustrate spiral ganglion neurons (marked by SGN) and their peripheral fibers (marked by GF, which stands for ganglion fiber). C. Merged view of A and B. Notice that macrophages are present in the regions of both ganglion cell bodies and their fibers. In the osseous spiral lamina, macrophages are arranged in two rows: one close to the edge of the osseous spiral lamina (shown by single-arrows) and the other close to ganglion neurons (marked by double-arrows). Many of these macrophages are oriented in parallel to the tunnel radial fibers of ganglion neurons. D, E, and F. Identity of macrophages is confirmed by the presence of F4/80 immunoreactivity. D shows the F4/80 immunoreactivity of immune cells. E. The same cells also display CD45 immunoreactivity. F. A merged view of A and B, as well as a differential interference contrast view (DIC) of the same region that illustrates the tissue orientation.
2.3. Basilar membrane macrophages
Under steady-state conditions, the organ of Corti is devoid of immune cells (Du et al., 2011; Hirose et al., 2005; Okano et al., 2008; Yang et al., 2015). However, its surrounding tissues are rich in macrophages. Basilar membrane macrophages that reside on the scala tympani side of the basilar membrane are in close proximity to the organ of Corti. These cells are distributed along the entire length of the basilar membrane and are present under steady-state conditions. Basilar membrane macrophages face two distinct extracellular milieus: their basal surface contacts mesothelial cells and the basement membrane, while their luminal surface is immersed in perilymph. This unique location enables them to survey the immune environments of both the organ of Corti and perilymph. The acellular environment of the scala tympani imposes a less physical constraint on macrophages, facilitating their shape change and migration. Our recent studies used these macrophages as an immune sensor for detecting changes in the microenvironment of the organ of Corti (Frye et al., 2017; Yang et al., 2015; Zhang et al., 2017). These studies reveal that basilar membrane macrophages are able to respond to sensory cell pathogenesis by changing their phenotypical characteristics and that these changes are correlated with the progression of sensory cell lesions.
Immune cells are also found in the spiral limbus, a cochlear site that attracts infiltrating monocytes under stress conditions. Macrophages identified in this region have a dendritic morphology with fine processes (Fig. 3). Moreover, immune cells are found within the blood vessels of the cochlea (Hirose et al., 2005). These cells are circulating leukocytes and some can egress into the cochlea in the event of cochlear inflammation (Harris et al., 1990; Stearns et al., 1993).
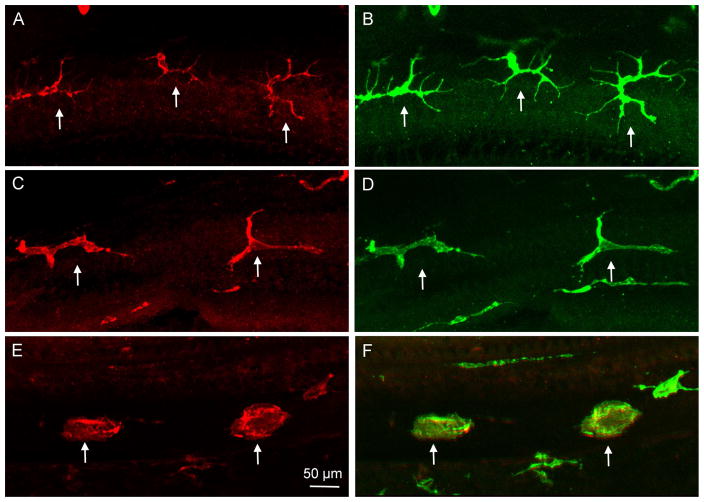
Figure 3. Identification of macrophages in the spiral limbus.
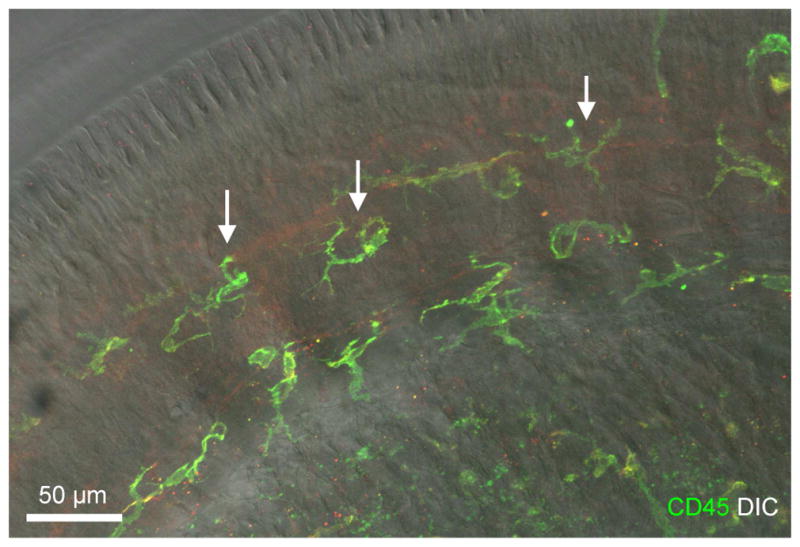
The image shows spiral limbus macrophages in a whole-mount preparation of a cochlea collected from a young C57BL/6J mouse. The image was derived by projecting serial optical sections of confocal image covering only the depth of the spiral limbus. The image is a superimposed image of CD45 immunostaining and DIC view of the same region. F4/80 immunoreactivity is weak and thus is not shown in the image. Macrophages identified in the spiral limbus region have a dendritic morphology with fine processes (marked by arrows).
It has been observed that the organ of Corti lacks immune cells (Du et al., 2011; Hirose et al., 2005; Okano et al., 2008; Yang et al., 2015) despite the fact that sensory cells in this region are susceptible to various pathological insults. The lack of immune cells in the organ of Corti indicates its immune privileged status, even though the traditional notion that the cochlea is an immune privileged organ has been challenged by the finding of immune cell activity under both steady-state and pathological conditions. Lack of immune cells could be beneficial because this organ has an elegant architecture formed by well-organized sensory cells and supporting cells. Any immune cell intrusion could alter its mechanical property and microenvironment, and consequently compromise its functional integrity.
Due to the limited availability of human cochlear tissues, the distribution of immune cells in human cochleae has not been studied extensively. In a recent study with human temporal bone tissues, O’Malley and co-workers observed macrophages in the spiral ligament, the stria vascularis and the neural region of the cochlea (O’Malley et al., 2016); this macrophage distribution pattern is consistent with those reported in rodent cochleae. However, the study also uncovered macrophages in multiple regions inside the organ of Corti, including the regions around the tunnel of Corti, Hensen cells and inner hair cells. These findings are inconsistent with the results from previous studies in rodent cochleae. Part of the discrepancy might be explained by differences in age among individuals from whom the tissues were collected (young animals for rodent studies vs. 52–88-year-old individuals for the human temporal bone observation). Moreover, we have found that, after developmental death, macrophage corpses are well preserved in the organ of Corti of the mouse cochlea (Hu et al., 2017). These residual bodies could be misidentified as immune cells in cochlear sectioning preparation, the method used in the human temporal bone study.
3. Macrophage properties and phenotypic diversity
Macrophages are a diverse group of immune cells with distinct phenotypic features and functional roles. They adopt their phenotypes and functions based on signals from the microenvironment around them. In the cochlea, macrophages reside in multiple anatomic areas, each with unique microenvironment and physiological function. Therefore, macrophages at different cochlear partitions are expected to have disparate phenotypic properties and functional states.
In addition to the inter-partition variation, cochlear macrophages display an intra-partition diversity; that is, macrophages within a tissue partition where the cell composition and function are presumably the same can display phenotypic differences. This diversity is evident in basilar membrane macrophages located on the scala tympani side of the basilar membrane. These macrophages display a progressive change in their morphology along the apex-to-base gradient (Frye et al., 2017; Yang et al., 2015). In the apical portion, macrophages exhibit a highly ramified morphology, characterized by a slim cell body with multiple branching processes and long extensions (Fig. 4A). By contrast, macrophages in the middle portion of the cochlea exhibit a less branched shape with short processes (Fig. 4B). In the basal end of the cochlea, the cells are more flattened and rounded with very short processes or without any processes (Fig. 4C). In addition to these morphological distinctions, basilar membrane macrophages display site-specific differences in the expression of immune proteins. In a recent study (Yang et al., 2015), we examined the constitutive expression of two immune proteins (CD11c and CD14) that have roles in inflammation (Anas et al., 2010), and found that these proteins were expressed primarily in basal macrophages. Because macrophage phenotypes are affected by signals from their environments, this site-specific difference could suggest a difference in the immune environment between the apical and basal portions of the cochlea. This speculation is supported by the observation of monocyte differentiation that occurs after settling in the cochlea. One example is the differentiation of infiltrated monocytes after acoustic trauma. The monocytes that populate the apex of the cochlea adopt a dendritic morphology, whereas the cells that arrive in the basal region acquire a breached or rounded shape (Yang et al., 2015). This result indicates that the apical-basal difference in macrophage phenotypes is not intrinsically programmed, but is acquired during differentiation.
Figure 4. Distinct morphologies of basilar membrane macrophages along the apical-to-basal gradient of the cochlea.
The images show macrophages in a whole-mount preparation of the cochlea collected from a young C57BL/6J mouse. The tissues was stained for F4/80 and CD45. A. and B. Morphology of apical macrophages (approximately 0–30% distance from the apex). These cells display a dendritic shape with long thin projections (arrows). C and D. Morphology of macrophages in the middle region of the basilar membrane (approximately 30–70% distance from the apex). The long projections are shortened and the dendritic processes are reduced in number (arrows). E and F. Morphology of macrophages in the basal region of the basilar membrane (approximately 70–100% distance from the apex). Macrophages in this region display an amoeboid morphology without long projections or processes (arrows).
4. Immune cell responses to acute cochlear pathogenesis
Acute damage to the cochlea occurs after a variety of pathological insults such as acoustic injury, ototoxicity, immune challenge and mechanical trauma due to cochlear implantation, all of which provoke inflammatory responses in the cochlea (Fujioka et al., 2006; Nakamoto et al., 2012; Tan et al., 2016; Verschuur et al., 2015; Wakabayashi et al., 2010 1869; Warchol et al., 2012). Cochlear inflammation caused by acute damage is characterized by a massive influx of inflammatory cells. In fact, our current knowledge of immune cell responses to cochlear pathogenesis has been derived primarily from studying these acute immune and inflammatory responses. Pathologically, acute damage is characterized by a rapid onset of tissue pathogenesis and in particular sensory cell pathogenesis. This disease process evolves rapidly, but is usually temporary. Once inciting events are removed, the pathogenesis as well as the inflammatory responses are resolved.
The magnitude of immune cell infiltration has been found to be massive after acute damage, although the actual level is dependent on the nature and severity of the triggering event. Hirose and co-workers revealed a six-fold increase in CD45-positive cells, which represent all leukocytes, in the basal end of the cochlea after exposure to a broadband noise at 112 or 120 dB SPL for 2 h (Hirose et al., 2005). An investigation by Tornabene and co-workers (Tornabene et al., 2006) revealed a larger increase (<1 cell/section for controls and 88 cells/section for traumatized cochleae) after exposure to a similar level of noise (118 dB SPL for 2 hours). Even on the surface of the basilar membrane where immune cell infiltration is relatively less active, the number of inflammatory cells is doubled after exposure to a noise at 120 dB SPL (Yang et al., 2015). Massive infiltration of inflammatory cells is also observed in other forms of cochlear damage including ototoxicity, surgical trauma, selective hair cell ablation, and immune challenge (Hirose et al., 2014; Kaur et al., 2015; Okano et al., 2008; Sato et al., 2010; Satoh et al., 2002). Notably, the level of influx can be further augmented if the subjects have a prior history of systemic immune challenge (Hirose et al., 2014; Oh et al., 2011), possibly due to the activation of adaptive immune activity (Hashimoto et al., 2005). These infiltrating cells are the major players in inflammatory responses to acute damage in the cochlea.
Infiltration is selective after acute damage. Studies have shown that monocytes are the predominant cell population that enter the cochlea after acute damage (Hirose et al., 2005; Takahashi et al., 1988a; Yang et al., 2015) despite the fact that neutrophils constitute approximately 40–60% of the white blood cell population in circulation. This monocyte-dominated infiltration differs from the acute inflammatory response commonly seen in other organs and tissues, which features two waves of immune cell infiltration (Fleming et al., 2006; Kochanek et al., 1992). The first wave involves neutrophils. These cells isolate, engulf, and kill pathogens using oxidative and non-oxidative mechanisms (Hickey et al., 2009; Kawamoto et al., 2004). The second wave of infiltration involves monocytes. These cells act as sentinels that ingest and clear apoptotic neutrophils, a key event of inflammation resolution (Godson et al., 2000). Infiltration of neutrophils in large numbers has been found to increase tissue damage in many pathological conditions including ischemic or toxic damage to the heart (Romson et al., 1983), lung (Kishi et al., 1999), liver (Liu et al., 2006), brain (Abulafia et al., 2009), and kidney (Kelly et al., 1996). In many of these disease conditions, depletion of neutrophils reduces the level of tissue damage. Although the precise mechanism for selective recruitment of immune cells to the cochlea is unclear, the lack of neutrophil influx in the cochlea could suggest a protective mechanism against cytotoxic inflammatory reaction.
Infiltrating cells migrate to multiple cochlear partitions, including the spiral ligament, spiral limbus, neural tissues and scala tympani. All these sites are important for cochlear homeostasis and function. Notably, the organ of Corti, the site of sensory cell pathogenesis, is devoid of immune cell infiltration (Du et al., 2011; Hirose et al., 2005; Okano et al., 2008), unless substantial damage to sensory cells and supporting cells takes place (Fredelius et al., 1990). The lack of inflammatory cell activity could serve as a defense mechanism against collateral damage. Despite the lack of inflammatory cell influx, the extension of macrophage processes toward the inner hair cell region has been reported and these processes are thought to play a role in phagocytosis of hair cell debris (Hirose et al., 2017). However, such extension has not been reported in the outer hair cell region, the major site of sensory cell pathogenesis.
Infiltration of inflammatory cells is a dynamic process during acute damage. Quantification of the number of inflammatory cells in acutely damaged cochleae reveals the kinetics of infiltration that starts at 12 hours to 1 day after exposure to noise, peaks at 3–7 days, and then subsides gradually (Fredelius et al., 1990; Hirose et al., 2005; Tornabene et al., 2006; Wakabayashi et al., 2010). The time at which monocyte infiltration is at its maximum appears not to coincide with the early production of inflammatory molecules that occurs within 1 day after acoustic injury (Cai et al., 2014; Fujioka et al., 2006; Patel et al., 2013; Vethanayagam et al., 2016). Clearly, infiltrating cells are not the early responders to cochlear inflammation. Instead, they are attracted to the cochlea by inflammatory mediators produced during the early inflammatory reaction.
The duration of the acute episode of inflammatory cell infiltration is dependent on the nature and persistence of inflammatory triggers. For acute acoustic trauma that causes sensory cell death, infiltration persists for approximately 2 weeks (Hirose et al., 2005; Tornabene et al., 2006; Wakabayashi et al., 2010; Yang et al., 2015). For severe ototoxic damage, infiltration can last for 10 weeks due to slow progression of supporting cell pathogenesis that occurs after sensory cell death (Ladrech et al., 2007). At the resolution phase of inflammation, the number of inflammatory cells reduces or returns to the pre-damage level, but at present, it is unclear how they are cleared from the cochlea. This is an important question because the failure to clear inflammatory cells could delay the recovery process, causing chronic inflammation that has been associated with a number of inflammatory pathologies such as rheumatoid arthritis and cardiovascular diseases (McInnes and Schett, 2007; Nahrendorf et al., 2010).
5. Macrophage responses to chronic cochlear pathogenesis
Similar to acute damage, chronic diseases can compromise cochlear physiology. Typical examples of such disease conditions include age-related and genetic defect-associated cochlear degeneration. Pathologically, age-related cochlear degeneration is characterized by a slow progression of sensory cell lesions, commonly starting from the basal end of the cochlea. Unlike acute damage, chronic damage develops progressively rather than episodically, and therefore the trigger event for the immune response persists. Chronic cochlear pathogenesis provokes immune cell responses in a way that is similar to, but not exactly the same as, the response incited by acute damage.
Our recent study examined macrophage response to age-related cochlear degeneration in C57BL/6J mice (Frye et al., 2017), a murine model of age-related hearing loss. This study focused on macrophages on the surface of the scala tympani side of the basilar membrane because of their close proximity to sensory cells in the organ of Corti. In these cochleae, massive infiltration of inflammatory cells is absent. Instead, the number of macrophages decreases as the age of mice increases (Frye et al., 2017; Neng et al., 2015). The primary immune cell reaction to chronic pathogenesis is the inflammatory activation of tissue-matured macrophages as evidenced by changes in macrophage morphology, including size enlargement and amoeboid transformation (Frye et al., 2017; Zhang et al., 2017). These changes coincide with the progression of sensory cell lesions. Specifically, morphological transition from a branched appearance to a more rounded or amoeboid shape occurs in the region where active sensory cell pathogenesis takes place. Once sensory cell degeneration is completed, the number of macrophages in that region decreases. Clearly, the major immune cell response to chronic sensory cell pathogenesis is the inflammatory activation of tissue macrophages that are pre-stationed in the cochlea although cochleae affected by chronic lesions can display an increased number of tissue macrophages (O’Malley et al., 2017; Zhang et al., 2017).
6. Potential functional roles of macrophages in the cochlea
Macrophages are an essential executor of inflammatory response in the innate immune system and are known to participate in all phases of inflammation including initiation, maintenance and resolution, as well as in tissue repair. Macrophages also have roles in immune surveillance under physiological conditions. Despite increased awareness of the presence of macrophages in the cochlea, data demonstrating their functional roles in cochlear homeostasis and disease development remain limited.
6.1. Phagocytosis
Phagocytosis is an essential function of macrophages. Like macrophages in other organs and tissues, cochlear macrophages are phagocytic. They constantly survey their surrounding environment and are ready to engulf and clear cell debris. As shown in Figure 5A, a macrophage is wrapping up a red blood cell for phagocytosis on the surface of the scala tympani in a normal mouse cochlea. Such macrophage phagocytosis is more evident in cochleae that have sustained pathological insults (Fig. 5B), and this process is rapid as demonstrated in an ex vivo model of hair cell damage induced by kanamycin cytotoxicity (Hirose et al., 2017).
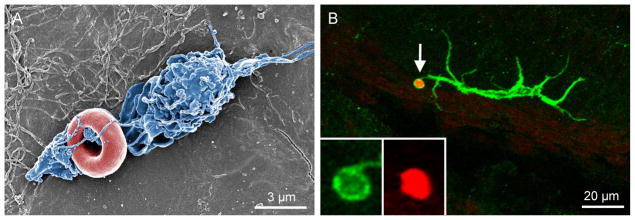
Figure 5. Macrophage phagocytosis in the cochlea.
A.A scanning electron microscope image of a macrophage on the lumen surface of the scala tympani collected from a normal young C57BL/6J mouse. The image was captured using a Hitachi scanning electron microscope (Hitachi SU-70). Notice that the processes of the macrophage (marked in blue) are entangling a red blood cell (marked in red). B. Image shows macrophage phagocytosis of a cell debris. The tissue was stained with CD45 and propidium iodide, a fluorescent dye that binds to nuclear DNA. Notice that the tip of a dendritic process of the macrophage (white arrow) has enclosed a condensed nuclear fragment for engulfment. Insets show the enlarged view of the tip of the macrophage process.
Sensory cells in the organ of Corti are susceptible to pathological insults, and severe damage causes these cells to die by either apoptosis, necrosis or a mixed mode of death (Bohne et al., 2007 ; Hu et al., 2000; Nicotera et al., 2001; Niu et al., 2003; Shibuya et al., 2003; Wang et al., 2002a; Ylikoski et al., 2002). Because the organ of Corti is devoid of macrophages under steady-state conditions, phagocytes have to migrate into the organ of Corti or extend their processes to the organ of Corti in order to clear sensory cell debris. Migration of macrophages into the organ of Corti occurs only in severely damaged cochleae in which the gross structure of the organ of Corti is compromised (Fredelius et al., 1990). In situations where the damage is not drastic, the overall structure of the organ of Corti maintains as supporting cells survive. In this circumstance, Deiters’ cells assume the role of phagocytes in engulfing sensory cell debris (Abrashkin et al., 2006). In addition, macrophages outside the organ of Corti can extend their processes to the inner hair cell region for clearance of cellular debris (Hirose et al., 2017). As discussed in a recent review article by Hirose and colleagues (Hirose et al., 2017), macrophages and supporting cells are jointly responsible for removing cellular debris from the organ of Corti.
The molecular mechanism that mediates phagocytosis for removing cellular debris after cochlear damage is not yet identified. It is known, however, that macrophages express multiple pattern recognition receptors for detecting damaged tissues and molecules. Toll-like receptors (TLRs) are a family of membrane receptors responsible for identifying both invasive pathogens and internal molecules from damaged tissues. These receptors are expressed in cochlear tissues and cochlear macrophages (Cai et al., 2014; Oh et al., 2011; Vethanayagam et al., 2016). At present, it is not known whether TLRs have a role in macrophage removal of cellular debris in the cochlea. Furthermore, it is unclear which other pattern recognition receptors are expressed in cochlear macrophages. It would be of importance to define the detailed molecular mechanisms responsible for macrophage detection of cellular debris, because removal of cellular debris is a prerequisite for the restoration of tissue homeostasis after injury.
6.2. Developmental roles
Macrophages are known to have prominent roles in tissue development (Pollard, 2009; Stefater et al., 2011). While cochlear macrophages have been identified in embryonic and postnatal cochleae (Brown et al., 2017; Hinton et al., 2017; Hirose et al., 2017; Hu et al., 2017; Kaur et al., 2017; Kim et al., 2011), their contribution to cochlear development remains to be determined. Bank and co-workers recently reported a neurotrophic role of macrophage migration inhibitory factor (MIF), an immune molecule that is involved in regulating immune cell behavior, in the development of the inner ear (Bank et al., 2012). This study revealed that MIF knockout leads to aberrant innervation to the organ of Corti. While the authors attribute this observation to the neurotrophic effect of the molecule, it is probable that this molecule, given its role in immune cell regulation, could also affect cochlear development by modulating the function of cochlear macrophages. This notion is supported by a recent study that demonstrated macrophage engulfment of nerve fibers for neural refinement during postnatal development of the cochlea (Brown et al., 2017). This study also demonstrated that diminishing macrophage function caused the elevation of auditory brainstem potential thresholds, suggesting a developmental role for cochlear macrophages in neural circuit refinement.
In rodent ears, structural development of the cochlea continues after birth. This structural maturation includes the removal of excessive cells from the sensory epithelium, formation of Corti’s lymph-filled spaces, and vascular pruning (Kraus et al., 1981; Pujol et al., 1998; Souter et al., 1997). This gross structural refinement requires elimination of supernumerary cells from the cochlea. Our recent study revealed the presence of developmental macrophages within the organ of Corti during the early stages of postnatal maturation of the cochlea in mice (Hu et al., 2017). As the organ of Corti matures, these macrophages undergo developmental death. Notably, this degradation starts from the basal region and advances to the apical region of the cochlea, coinciding with the maturation pattern of the sensory epithelium that also progresses from the base to the apex of the cochlea. Although there is still much to learn about the role of macrophages in the maturation of the organ of Corti, the presence of developmental macrophages within the organ of Corti suggests their involvement in the postnatal development of the cochlea.
6.3. Production of immune and inflammatory molecules
While the cochlea is not an immunological organ, it expresses a variety of immune molecules. Several research groups have investigated cochlear inflammatory activity by examining expression profiles of immune molecules under normal and pathogenic conditions including noise-induced cochlear damage (Cai et al., 2014; Cho et al., 2004; Fujioka et al., 2006; Gratton et al., 2011; Kirkegaard et al., 2006; Oh et al., 2011; Patel et al., 2013; Tornabene et al., 2006; Yang et al., 2016). Using an RNA-sequencing technique, we have identified constitutive expression of a large group of immune-related molecules that are involved in multiple immune signaling pathways (Patel et al., 2013; Yang et al., 2016). The expression alteration of myriad immune-related genes have also been reported in the cochlea following pathological insult (Fujioka et al., 2006; Gratton et al., 2011; Nakamoto et al., 2012; Tan et al., 2016; Vethanayagam et al., 2016; Wakabayashi et al., 2010). These include, but are not limited to Tnf, Il1b, Il6, Ccl2, Ccl4, Cxcl10, Socs3, Ifrd1, Ifi202b, Igh-6 and Tcl1b1. Our transcriptome analyses using an RNA-sequencing technique have revealed a comprehensive change in the expression profile of cochlear genes after acoustic injury (Cai et al., 2014; Patel et al., 2013; Yang et al., 2016). A large number of differentially expressed genes have immune/inflammation function. In addition to the upregulation and downregulation of particular immune-related genes, researchers have investigated myriad molecular pathways associated with differentially expressed genes. Cytokine-cytokine receptor interaction, complement and coagulation cascades, chemokine signaling, NOD-like receptor signaling and TLR signaling, amongst others, have all been reported (Vethanayagam et al., 2016; Yang et al., 2016). While immune cells are known to be a major source of immune-related molecules, the precise contribution of cochlear immune cells to immune molecule production is poorly understood. Lack of such important knowledge is in part due to technical challenges for collecting immune cells from the cochlea for a cell-type specific analysis.
6.4. Antigen presenting function
Enhanced immune responses have been observed in the cochlea after repeated challenges. For example, activation of innate cochlear immunity has been shown to enhance the adaptive immune response to subsequent immune challenge in mouse cochleae (Hashimoto et al., 2005). Similarly, a prior history of acoustic trauma augments cochlear immune response to subsequent antigen challenge (Miyao et al., 2008). These observations suggest that repeated stresses boost immune responses by activating adaptive immune activity in the cochlea (Hashimoto et al., 2005).
Antigen presentation is an important event for activating adaptive immune activity. Antigen presentation involves phagocytosis of foreign pathogens or internal molecules from damaged tissues by antigen presenting cells. Antigen presenting cells are able to process engulfed molecules and present them onto their membrane surface, which in turn activate T cells and provoke the adaptive immune response (Steinman, 1991; Unanue, 1984). The primary antigen-presenting cells are dendritic cells. These cells have a dendritic shape and express antigen-presenting proteins, including MHC-II, CIITA and CD11c. Macrophages and monocytes also bear the function of antigen presentation.
Under steady-state conditions, immune cells that have a dendritic shape and express MHC-II are present in the cochlea (Yang et al., 2015). After acute acoustic trauma, the number of macrophages that express MHC-II and CIITA is increased. These macrophages reside in the cochlear regions that display maximal sensory cell damage. In parallel, the number of T cells increases as well. The presence of T cells is important because of their roles in receiving antigens and in activating the adaptive immune response. These observations suggest that acoustic stress is able to activate the antigen presenting function in cochlear macrophages. Currently, the molecular details linking the innate and adaptive immunity in the cochlea remain unclear. Such knowledge is important for our understanding of how the adaptive immune system affects cochlear responses to stresses.
7. Cochlear resident cells have immune capacity
In addition to immune cells, resident cells of the cochlea have immune capacity. These cells include supporting cells in the organ of Corti, fibrocytes in the lateral wall, and mesothelial cells on the scala tympani side of the basilar membrane.
A strong piece of evidence for immune capacity of resident cells in the organ of Corti originates from the observation of phagocytic activity of supporting cells. Using prestin as a marker for outer hair cells, Abrashkin and colleagues reported the accumulation of prestin-positive debris in Deiters cells in the areas of sensory cell lesions after acoustic overstimulation (Abrashkin et al., 2006). This observation suggests a phagocytic role for Deiters cells. At present, it is not clear how supporting cells detect sensory cell debris. It has been known that phagocytes express a genus of receptors for sensing damage-associated molecular pattern molecules. TLRs are a group of membrane receptors that recognizes both exogenous molecules from pathogens and endogenous molecules from damaged tissues (Miyake, 2007; Ohashi et al., 2000; Termeer et al., 2002; Vabulas et al., 2001). TLR4 expression has been found in the outer sulcus cells that form the boundary between the organ of Corti and the spiral ligament (Hashimoto et al., 2005). Our recent study showed an increase in protein expression of TLR4 in Deiters cells adjacent to damaged sensory cells after acoustic injury (Cai et al., 2014), suggesting that TLR4-mediated signaling might be involved in supporting cell detection of damaged sensory cells. In addition to TLRs, other sensor molecules have been found in the cochlea. For example, two members of the retinoic acid inducible gene-I (RIG-I) receptor family, RIG-I and melanoma differentiation-associated gene 5 (MDA5), have been localized to Hensen and Claudius cells and their expression increases when the organ of Corti tissue is infected by virus (Hayashi et al., 2013). These receptors are responsible for sensing double-stranded RNA (Takeuchi and Akira, 2010; Yoneyama et al., 2004) and play an important role in recognizing the genomic RNA of viruses. In addition, these receptors act as sensors of cellular damage and have been implicated in the inflammatory response to tissue pathogenesis, such as spinal cord injury and Alzheimer’s disease (de Rivero Vaccari et al., 2014; de Rivero Vaccari et al., 2012). Furthermore, our expression profiling of immune-related genes in the cochlear sensory epithelium has identified RIG-I-like receptor signaling as a potential molecular pathway in cochlear response to acoustic injury (Yang et al., 2016). These observations provide clues for future investigation into the molecular mechanism responsible for Deiters cell phagocytosis.
In addition to phagocytic activity, resident cells in the organ of Corti express a plethora of immune-related molecules. In a recent study, we isolated the cells of the organ of Corti for transcriptional analysis of 148 immune and inflammation-related genes (Cai et al., 2014). Over 60% of examined genes are constitutively expressed in the organ of Corti. These expressed genes are functionally related to multiple immune pathways. Further analysis of immune proteins revealed that supporting cells are the dominant site of immune molecule expression. These observations underscore the importance of resident cells, particularly supporting cells, in local immune activity of the organ of Corti.
Fibrocytes reside in large numbers in the spiral ligament. These cells are functionally differentiated and specialized for ion transport, which is essential for proper maintenance of the cochlear microenvironment. These cells also produce immune mediators, including proinflammatory molecules following treatment with proinflammatory cytokines, lysates from otitis media pathogens, or acoustic overstimulation (Fujioka et al., 2006; Hashimoto et al., 2005; Moon et al., 2006; Satoh et al., 2002; Yoshida et al., 1999). While the molecular details for the production of inflammatory mediators are not clear, expression of nuclear factor kappa B (NF-κB), a transcriptional factor that mediates the expression of numerous proinflammatory genes, has been observed in fibrocytes after acoustic trauma and systemic immune challenge (Adams et al., 2009). As inflammatory molecules produced by immune cells, the immune mediators generated by fibrocytes could participate in cochlear immune activities, such as recruiting inflammatory cells, as a drastic increase in immune cell population has been found around fibrocytes in the spiral ligament after stresses (Hirose et al., 2005; Sautter et al., 2006 1873; Tornabene et al., 2006). These inflammatory cells could subsequently contribute to the loss of fibrocytes in the spiral ligament, as discussed in a publication by Hirose and colleagues (Hirose et al., 2005).
Mesothelial cells are spindle-shaped cells that line the scala tympani side of the basilar membrane, forming a protective layer. These cells have a direct cell-cell contact with basilar membrane macrophages. Evidence suggests a phagocytic role for mesothelial cells in the cochlea. Angelborg used thorotrast tracers to detect the phagocytic activity of mesothelial cells and demonstrated an accumulation of thorotrast particles, in these cells after the tracers were surgically administered into the scala tympani (Angelborg, 1974). This finding suggests the phagocytic capacity of cochlear mesothelial cells, a function that has been demonstrated in mesothelial cells of other organs, such as the lung pleura (Kuwahara et al., 1995). As cochlear mesothelial cells are co-localized with basilar membrane macrophages, it is likely that these cells can work in conjunction with macrophages for clearing cellular debris that is released into the scala tympani after damage.
8. Concluding remarks
In this review, we have discussed the immune and inflammatory activities of the cochlea. Under physiological conditions, the cochlea contains tissue immune cells that reside in multiple cochlear compartments, many of which are known to be important for cochlear function. Tissue macrophages have diverse morphologies and gene expression patterns, suggesting the presence of different functional states. In addition to resident immune cells, the cochlea is able to recruit circulating immune cells in the event of stress. These immune responders, together with cochlear resident cells, orchestrate a complex inflammatory response. At present, despite an increased awareness of the presence of immune cells in the cochlea, data demonstrating their functional roles in cochlear homeostasis and disease formation are still lacking. Moreover, the current observations focus on the induction of inflammation; little is known about the resolution of inflammation. Since the proper resolution of inflammation is an essential component of tissue recovery after stress, a better understanding of its biological process and regulation will lead to new therapeutic targets for improving the outcome of cochlear inflammation.
HIGHLIGHTS.
Tissue macrophages are present in multiple cochlear sites except for the organ of Corti.
Immune cells migrate to the cochlea in response to stress
Cochlear macrophages have site-specific diversity in their phenotypes and functional states.
Macrophages have functional roles in cochlear development and responses to stress.
Resident cells of the cochlea present with immune functions.
Acknowledgments
Research reported in this publication was supported by National Institutes on Deafness and Other Communication Disorders of the National Institutes of Health under award number: R01DC010154 (BHH). The authors thank Dr. Henry Adler for his help in preparing the manuscript.
Abbreviations
- TLR
Toll-like receptor
- RIG-I
Retinoic acid inducible gene-I
- MDA5
Melanoma differentiation-associated gene 5
- TGF-β
Transforming growth factor beta
- MHC-II
major histocompatibility complex II
- ICAM-1
Intercellular Adhesion Molecule 1
- DIC
Differential interference contrast view
- MIF
macrophage migration inhibitory factor
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006;218:20–9. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. Journal of Cerebral Blood Flow & Metabolism. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- Adams JC, Seed B, Lu N, Landry A, Xavier RJ. Selective activation of nuclear factor kappa B in the cochlea by sensory and inflammatory stress. Neuroscience. 2009;160:530–9. doi: 10.1016/j.neuroscience.2009.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anas A, van der Poll T, de Vos AF. Role of CD14 in lung inflammation and infection. Crit Care. 2010;14:209. doi: 10.1186/cc8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelborg C. Distribution of macromolecular tracer particles (Thorotrast-r) in the cochlea. An electron microscopic study in guinea pig. Part I. The organ of Corti, the basilar membrane and the tympanic covering layer. Acta Otolaryngol Suppl. 1974;319:19–41. [PubMed] [Google Scholar]
- Arpornchayanon W, Canis M, Ihler F, Settevendemie C, Strieth S. TNF-alpha inhibition using etanercept prevents noise-induced hearing loss by improvement of cochlear blood flow in vivo. Int J Audiol. 2013;52:545–52. doi: 10.3109/14992027.2013.790564. [DOI] [PubMed] [Google Scholar]
- Arslan N, Oguz H, Demirci M, Safak MA, Islam A, Kaytez SK, Samim E. Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2011;32:393–7. doi: 10.1097/MAO.0b013e318206fdfa. [DOI] [PubMed] [Google Scholar]
- Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. Journal of Experimental Medicine. 1971;134:907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank LM, Bianchi LM, Ebisu F, Lerman-Sinkoff D, Smiley EC, Shen Y-c, Ramamurthy P, Thompson DL, Roth TM, Beck CR. Macrophage migration inhibitory factor acts as a neurotrophin in the developing inner ear. Development. 2012;139:4666–4674. doi: 10.1242/dev.066647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223:61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Brown L, Xing Y, Barth J, Panganiban C, Smythe N, Bridges M, Lang H. Macrophages contribute to auditory nerve refinement by eliminating excessive glial cells in the postnatal mouse cochlea. 40th Annual MidWinter Meeting of ARO; Baltimore, MD. 2017. p. 338. [Google Scholar]
- Cai Q, Vethanayagam RR, Yang S, Bard J, Jamison J, Cartwright D, Dong Y, Hu BH. Molecular profile of cochlear immunity in the resident cells of the organ of Corti. Journal of Neuroinflammation. 2014;11:173. doi: 10.1186/s12974-014-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlon B, Meltser I, Johansson P, Tahera Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear Res. 2007;226:61–9. doi: 10.1016/j.heares.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Cho Y, Gong TW, Kanicki A, Altschuler RA, Lomax MI. Noise overstimulation induces immediate early genes in the rat cochlea. Brain Res Mol Brain Res. 2004;130:134–48. doi: 10.1016/j.molbrainres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Brand FJ, 3rd, Sedaghat C, Mash DC, Dietrich WD, Keane RW. RIG-1 receptor expression in the pathology of Alzheimer’s disease. J Neuroinflammation. 2014;11:67. doi: 10.1186/1742-2094-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Minkiewicz J, Wang X, De Rivero Vaccari JC, German R, Marcillo AE, Dietrich WD, Keane RW. Astrogliosis involves activation of retinoic acid-inducible gene-like signaling in the innate immune response after spinal cord injury. Glia. 2012;60:414–21. doi: 10.1002/glia.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Choi CH, Chen K, Cheng W, Floyd RA, Kopke RD. Reduced formation of oxidative stress biomarkers and migration of mononuclear phagocytes in the cochleae of chinchilla after antioxidant treatment in acute acoustic trauma. International journal of otolaryngology. 2011;2011:612690. doi: 10.1155/2011/612690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–69. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Fredelius L, Rask-Andersen H. The role of macrophages in the disposal of degeneration products within the organ of corti after acoustic overstimulation. Acta Otolaryngol. 1990;109:76–82. doi: 10.3109/00016489009107417. [DOI] [PubMed] [Google Scholar]
- Frye MD, Yang W, Zhang C, Xiong B, Hu BH. Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hear Res. 2017;344:125–134. doi: 10.1016/j.heares.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Okano H, Ogawa K. Inflammatory and immune responses in the cochlea: potential therapeutic targets for sensorineural hearing loss. Front Pharmacol. 2014;5:287. doi: 10.3389/fphar.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575–83. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–7. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Goodall AF, Siddiq MA. Current understanding of the pathogenesis of autoimmune inner ear disease: a review. Clin Otolaryngol. 2015;40:412–9. doi: 10.1111/coa.12432. [DOI] [PubMed] [Google Scholar]
- Gratton MA, Eleftheriadou A, Garcia J, Verduzco E, Martin GK, Lonsbury-Martin BL, Vazquez AE. Noise-induced changes in gene expression in the cochleae of mice differing in their susceptibility to noise damage. Hear Res. 2011;277:211–26. doi: 10.1016/j.heares.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JP, Fukuda S, Keithley EM. Spiral modiolar vein: its importance in inner ear inflammation. Acta Otolaryngol. 1990;110:357–65. doi: 10.3109/00016489009107455. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Billings P, Harris JP, Firestein GS, Keithley EM. Innate immunity contributes to cochlear adaptive immune responses. Audiol Neurootol. 2005;10:35–43. doi: 10.1159/000082306. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Onomoto K, Narita R, Yoneyama M, Kato H, Nakagawa T, Ito J, Taura A, Fujita T. Virus-induced expression of retinoic acid inducible gene-I and melanoma differentiation-associated gene 5 in the cochlear sensory epithelium. Microbes and infection / Institut Pasteur. 2013;15:592–8. doi: 10.1016/j.micinf.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nature reviews Immunology. 2009;9:364–75. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- Hinton A, Kaur T, Warchol M. Time course of macrophage numbers and morphology in the developing mouse cochlea. 40th Annual MidWinter Meeting of ARO; Baltimore, MD. 2017. p. 512. [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–52. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Rutherford MA, Warchol ME. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear Res. 2017 doi: 10.1016/j.heares.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005;489:180–94. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Hirose K, Li SZ, Ohlemiller KK, Ransohoff RM. Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide. J Assoc Res Otolaryngol. 2014;15:555–70. doi: 10.1007/s10162-014-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BH, Yang W, Dong Y. Site-specific transformation and re-organization of tissue macrophages in the sensory epithelium during postnatal development. 40th Annual MidWinter Meeting of ARO; Baltimore, MD. 2017. p. 513. [Google Scholar]
- Hu BH, Guo W, Wang PY, Henderson D, Jiang SC. Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Otolaryngol. 2000;120:19–24. [PubMed] [Google Scholar]
- Iwai H, Baba S, Omae M, Lee S, Yamashita T, Ikehara S. Maintenance of systemic immune functions prevents accelerated presbycusis. Brain Res. 2008;1208:8–16. doi: 10.1016/j.brainres.2008.02.069. [DOI] [PubMed] [Google Scholar]
- Iwai H, Lee S, Inaba M, Sugiura K, Baba S, Tomoda K, Yamashita T, Ikehara S. Correlation between accelerated presbycusis and decreased immune functions. Exp Gerontol. 2003;38:319–25. doi: 10.1016/s0531-5565(02)00177-8. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Lomberk G, Urrutia RA, Kalinec F. Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss. Front Cell Neurosci. 2017;11:192. doi: 10.3389/fncel.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Ohlemiller KK, Schrader A, Warchol M. Macrophages influence noise-induced cochlear synaptopathy and neuropathy via fractalkine signaling. 40th Annual MidWinter Meeting of ARO; Baltimore, MD. 2017. p. 470. [Google Scholar]
- Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, Warchol ME. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. Journal of Neuroscience. 2015;35:15050–15061. doi: 10.1523/JNEUROSCI.2325-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto H, Minato N. Myeloid cells. The international journal of biochemistry & cell biology. 2004;36:1374–1379. doi: 10.1016/j.biocel.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Kelly K, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutiérrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. Journal of Clinical Investigation. 1996;97:1056. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Rodriguez-Vazquez JF, Verdugo-Lopez S, Cho KH, Murakami G, Cho BH. Early fetal development of the human cochlea. Anat Rec (Hoboken) 2011;294:996–1002. doi: 10.1002/ar.21387. [DOI] [PubMed] [Google Scholar]
- Kirkegaard M, Murai N, Risling M, Suneson A, Jarlebark L, Ulfendahl M. Differential gene expression in the rat cochlea after exposure to impulse noise. Neuroscience. 2006;142:425–35. doi: 10.1016/j.neuroscience.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Kishi M, Richard LF, Webster RO, Dahms TE. Role of neutrophils in xanthine/xanthine oxidase-induced oxidant injury in isolated rabbit lungs. Journal of Applied Physiology. 1999;87:2319–2325. doi: 10.1152/jappl.1999.87.6.2319. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23:1367–79. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, Koren T, Rolls A. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci. 2017 doi: 10.1038/nn.4610. [DOI] [PubMed] [Google Scholar]
- Kraus HJ, Aulbach-Kraus K. Morphological changes in the cochlea of the mouse after the onset of hearing. Hearing research. 1981;4:89–102. doi: 10.1016/0378-5955(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Kagan E. The mesothelial cell and its role in asbestos-induced pleural injury. Int J Exp Pathol. 1995;76:163–70. [PMC free article] [PubMed] [Google Scholar]
- Ladrech S, Wang J, Simonneau L, Puel JL, Lenoir M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J Neurosci Res. 2007;85:1970–9. doi: 10.1002/jnr.21335. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Surface ultrastructure of the cochlear perilymphatic space. J Laryngol Otol. 1970;84:413–28. doi: 10.1017/s0022215100072029. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Liyanage SE, Gardner PJ, Ribeiro J, Cristante E, Sampson RD, Luhmann UF, Ali RR, Bainbridge JW. Flow cytometric analysis of inflammatory and resident myeloid populations in mouse ocular inflammatory models. Exp Eye Res. 2016;151:160–70. doi: 10.1016/j.exer.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MU, Pandian V, Masood H, Diaz DA, Varela V, Davalos-Balderas AJ, Parra-Cardenas M, Seo P, Francis HW. Spectrum of immune-mediated inner ear disease and cochlear implant results. Laryngoscope. 2012;122:2557–62. doi: 10.1002/lary.23604. [DOI] [PubMed] [Google Scholar]
- Matern M, Vijayakumar S, Margulies Z, Milon B, Song Y, Elkon R, Zhang X, Jones SM, Hertzano R. Gfi1Cre mice have early onset progressive hearing loss and induce recombination in numerous inner ear non-hair cells. Scientific reports. 2017;7:42079. doi: 10.1038/srep42079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nature reviews Immunology. 2007;7:429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Seminars in immunology. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Miyao M, Firestein GS, Keithley EM. Acoustic trauma augments the cochlear immune response to antigen. Laryngoscope. 2008;118:1801–8. doi: 10.1097/MLG.0b013e31817e2c27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SK, Park R, Lee HY, Nam GJ, Cha K, Andalibi A, Lim DJ. Spiral ligament fibrocytes release chemokines in response to otitis media pathogens. Acta Otolaryngol. 2006;126:564–9. doi: 10.1080/00016480500452525. [DOI] [PubMed] [Google Scholar]
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nature Reviews Immunology. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–45. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T, Mikuriya T, Sugahara K, Hirose Y, Hashimoto T, Shimogori H, Takii R, Nakai A, Yamashita H. Geranylgeranylacetone suppresses noise-induced expression of proinflammatory cytokines in the cochlea. Auris, nasus, larynx. 2012;39:270–4. doi: 10.1016/j.anl.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Neng L, Zhang J, Yang J, Zhang F, Lopez IA, Dong M, Shi X. Structural changes in thestrial blood–labyrinth barrier of aged C57BL/6 mice. Cell and tissue research. 2015;361:685–696. doi: 10.1007/s00441-015-2147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera T, Henderson D, Hu BH, Zheng XY. Noise exposure and mechanisms of hair cell death. In: Henderson D, Prasher D, Kopke R, Salvi R, Hamernik R, editors. Noise Induced Hearing Loss: Basic Mechanisms, Prevention and Control. Noise Research Network Publications; London: 2001. pp. 99–117. [Google Scholar]
- Niu X, Shao R, Canlon B. Suppression of apoptosis occurs in the cochlea by sound conditioning. Neuroreport. 2003;14:1025–9. doi: 10.1097/01.wnr.0000070830.57864.32. [DOI] [PubMed] [Google Scholar]
- O’Malley J, Burgess B, Santos F, MaKenna MJ. Sensorineural hearing loss in otosclerosis: The role of cochlear macrophages, a potential therapeutic target Newsletter of the NIDCD National Temporal Bone, Hearing and balance. Pathology Resource Registry. 2017;24:4–6. [Google Scholar]
- O’Malley JT, Nadol JB, Jr, McKenna MJ. Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol Neurotol. 2016;37:99–108. doi: 10.1097/MAO.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Choi JH, Shen A, Kim CH, Kim SJ, Shin SR, Hong SH, Kim Y, Park C, Lee SJ, Akira S, Park R, So HS. Activation of lipopolysaccharide-TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. J Immunol. 2011;186:1140–50. doi: 10.4049/jimmunol.1002183. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008;86:1758–67. doi: 10.1002/jnr.21625. [DOI] [PubMed] [Google Scholar]
- Patel M, Hu Z, Bard J, Jamison J, Cai Q, Hu BH. Transcriptome characterization by RNA-Seq reveals the involvement of the complement components in noise-traumatized rat cochleae. Neuroscience. 2013;248C:1–16. doi: 10.1016/j.neuroscience.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nature reviews Immunology. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Lavigne-Rebillard M, Lenoir M. Development of sensory and neural structures in the mammalian cochlea, Development of the auditory system. Springer; 1998. pp. 146–192. [Google Scholar]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006:43. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061–74. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Sato E, Shick HE, Ransohoff RM, Hirose K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol. 2010;11:223–34. doi: 10.1007/s10162-009-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope. 2002;112:1627–34. doi: 10.1097/00005537-200209000-00019. [DOI] [PubMed] [Google Scholar]
- Sautter NB, Shick EH, Ransohoff RM, Charo IF, Hirose K. CC chemokine receptor 2 is protective against noise-induced hair cell death: studies in CX3CR1(+/GFP) mice. J Assoc Res Otolaryngol. 2006;7:361–72. doi: 10.1007/s10162-006-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell and tissue research. 2010;342:21–30. doi: 10.1007/s00441-010-1040-2. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Kato Y, Saito M, Isobe T, Tsuboi R, Koga M, Toyota H, Mizuguchi J. Induction of apoptosis and/or necrosis following exposure to antitumour agents in a melanoma cell line, probably through modulation of Bcl-2 family proteins. Melanoma Res. 2003;13:457–64. doi: 10.1097/00008390-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Souter M, Nevill G, Forge A. Postnatal maturation of the organ of Corti in gerbils: morphology and physiological responses. Journal of Comparative Neurology. 1997;386:635–651. [PubMed] [Google Scholar]
- Stearns GS, Keithley EM, Harris JP. Development of high endothelial venule-like characteristics in the spiral modiolar vein induced by viral labyrinthitis. Laryngoscope. 1993;103:890–8. doi: 10.1288/00005537-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: macrophages in development, homeostasis and regeneration. Trends in molecular medicine. 2011;17:743–52. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annual review of immunology. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Harris JP. Analysis of immunocompetent cells following inner ear immunostimulation. Laryngoscope. 1988a;98:1133–8. doi: 10.1288/00005537-198810000-00018. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Harris JP. Anatomic distribution and localization of immunocompetent cells in normal mouse endolymphatic sac. Acta Otolaryngol. 1988b;106:409–16. doi: 10.3109/00016488809122264. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tan WJ, Thorne PR, Vlajkovic SM. Noise-induced cochlear inflammation. World J Otorhinolaryngol. 2013;3:89–99. [Google Scholar]
- Tan WJ, Thorne PR, Vlajkovic SM. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem Cell Biol. 2016;146:219–30. doi: 10.1007/s00418-016-1436-5. [DOI] [PubMed] [Google Scholar]
- Tay SS, Roediger B, Tong PL, Tikoo S, Weninger W. The skin-resident immune network. Current dermatology reports. 2014;3:13. doi: 10.1007/s13671-013-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene SV, Sato K, Pham L, Billings P, Keithley EM. Immune cell recruitment following acoustic trauma. Hear Res. 2006;222:115–24. doi: 10.1016/j.heares.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Toubi E, Ben-David J, Kessel A, Halas K, Sabo E, Luntz M. Immune-mediated disorders associated with idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2004;113:445–9. doi: 10.1177/000348940411300605. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Antigen-presenting function of the macrophage. Annual review of immunology. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Verschuur C, Causon A, Green K, Bruce I, Agyemang-Prempeh A, Newman T. The role of the immune system in hearing preservation after cochlear implantation. Cochlear implants international. 2015;16:S40–S42. doi: 10.1179/1467010014Z.000000000233. [DOI] [PubMed] [Google Scholar]
- Vethanayagam RR, Yang W, Dong Y, Hu BH. Toll-like receptor 4 modulates the cochlear immune response to acoustic injury. Cell Death Dis. 2016;7:e2245. doi: 10.1038/cddis.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Fujioka M, Kanzaki S, Okano HJ, Shibata S, Yamashita D, Masuda M, Mihara M, Ohsugi Y, Ogawa K, Okano H. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neuroscience research. 2010;66:345–52. doi: 10.1016/j.neures.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dib M, Lenoir M, Vago P, Eybalin M, Hameg A, Pujol R, Puel J. Riluzole rescues cochlear sensory cells from acoustic trauma in the guinea-pig. Neuroscience. 2002a;111:635–648. doi: 10.1016/s0306-4522(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002b;3:248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Schwendener RA, Hirose K. Depletion of Resident Macrophages Does Not Alter Sensory Regeneration in the Avian Cochlea. PloS one. 2012;7:e51574. doi: 10.1371/journal.pone.0051574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MB, Zuo J. The Contribution of Immune Infiltrates to Ototoxicity and Cochlear Hair Cell Loss. Front Cell Neurosci. 2017;11:106. doi: 10.3389/fncel.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SZ, Cai QF, Vethanayagam RR, Wang JM, Yang WP, Hu BH. Immune defense is the primary function associated with the differentially expressed genes in the cochlea following acoustic trauma. Hearing Research. 2016;333:283–294. doi: 10.1016/j.heares.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience. 2015;303:1–15. doi: 10.1016/j.neuroscience.2015.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;166:33–43. doi: 10.1016/s0378-5955(01)00388-4. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5:730. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Ichimiya I, Suzuki M, Mogi G. Effect of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hear Res. 1999;137:155–9. doi: 10.1016/s0378-5955(99)00134-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Sun W, Li J, Xiong B, Frye MD, Ding D, Salvi R, Kim M-J, Someya S, Hu BH. Loss of Sestrin 2 potentiates the early onset of age-related sensory cell degeneration in the cochlea. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109:10388–93. doi: 10.1073/pnas.1205210109. [DOI] [PMC free article] [PubMed] [Google Scholar]