Abstract
The matrix (MA) domain of the HIV-1 precursor Gag protein (PrGag) has been shown interact with the HIV-1 envelope (Env) protein, and to direct PrGag proteins to plasma membrane (PM) assembly sites by virtue of its affinity to phosphatidylinositol-4,5-bisphosphate (PI[4,5]P2). Unexpectedly, HIV-1 viruses with large MA deletions (ΔMA) have been shown to be conditionally infectious as long as they are matched with Env truncation mutant proteins or alternative viral glycoproteins. To characterize the interactions of wild type (WT) and ΔMA Gag proteins with PI(4,5)P2 and other acidic phospholipids, we have employed a set of lipid biosensors as probes. Our investigations showed marked differences in WT and ΔMA Gag colocalization with biosensors, effects on biosensor release, and association of biosensors with virus-like particles. These results demonstrate an alternative approach to the analysis of viral protein-lipid associations, and provide new data as to the lipid compositions of HIV-1 assembly sites.
Keywords: HIV-1, matrix, Gag, lipid biosensors, phospholipids
1. INTRODUCTION
The matrix (MA) domain of the HIV-1 Gag protein serves at least two major functions (Alfadhli and Barklis, 2014; Freed, 2015). One of these is to interact with the HIV-1 envelope (Env) protein in such a way as to facilitate assembly of the wild type (WT) Env protein into virus particles (Checkley et al., 2011; Tedbury et al., 2013; Freed, 2015). The second major function is to direct precursor Gag (PrGag) proteins to assembly sites at the plasma membranes (PMs) of cells (Ono and Freed, 2001; Alfadhli and Barklis, 2014; Freed, 2015). In part, the N-terminal myristoylation of MA (Bryant and Ratner, 1990) is responsible for the membrane-binding of PrGag. However, MA also binds preferentially to phosphatidylinositol-4,5-bisphosphate (PI[4,5]P2), which is enriched at the PMs of cells (Varnai and Balla, 1998; Ono et al., 2004; Saad et al., 2006; Chukapalli et al., 2008; Lemmon, 2008; Alfadhli et al., 2009; Alfadhli and Barklis, 2014; Freed, 2015; Platre and Jaillais, 2016). Despite the high degree of MA conservation among all HIV-1 isolates, we and others have reported that HIV-1 viruses deleted for up to 80% of the MA coding region (retaining myristoylation and viral protease cleavage sites) are conditionally infectious (Wang et al., 1993; Reil et al., 1998). Specifically, these ΔMA viruses are not infectious in the context of full-length HIV-1 Env proteins, but can produce infectious virions if matched with cytoplasmic domain-truncated HIV-1 Env, or alternative virus envelope glycoprotein pseudotypes (Wang et al., 1993; Reil et al., 1998). Interestingly, in virus producing cells, ΔMA virus particles appear to accumulate within intracellular compartments, and it is not clear whether extracellular virus particles derive from a minority of virions that assemble at the PM, or via vesicular trafficking of particles that have assembled intracellularly (Facke et al, 1993).
For wild type (WT) HIV-1, evidence indicates that HIV-1 budding occurs at PI(4,5)P2-rich, lipid raft-like PM domains, and viral lipidomic analyses have shown that HIV-1 particles are enriched in PI(4,5)P2 (Aloia et al., 1993; Ono and Freed, 2001; Ono et al, 2004; Brugger et al, 2006; Chan et al., 2008; Chukkapalli et al., 2008; Lorizate et al., 2013; Alfadhli and Barklis, 2014; Freed, 2015). Lipidomic analyses also have shown that, relative to total cell lipids, HIV-1 particles are enriched for phosphatidylserine (PS), cholesterol, and sphingomyelin (SM) (Aloia et al., 1993; Brugger et al., 2006; Chan et al., 2008; Lorizate et al., 2013). However, some variability has been observed depending on the virus producer cell line and, relative to the PM content of cells, there may be only a slight enrichment in virus particles for cholesterol (Lorizate et al., 2013). Despite the progress in the characterization of the HIV-1 lipidome, difficulties in the chemistry of lipid analysis have made it so that the levels of a number of different lipids, particularly acidic phospholipids, have not been examined (Aloia et al., 1993; Brugger et al., 2006; Chan et al., 2008; Lorizate et al., 2013).
As an alternative strategy to the examination of the lipid associations of HIV-1, we have taken advantage of the fact that a variety of fluorescently tagged lipid biosensors have been developed for the detection of PS and phosphaditylinositol phosphates (PIPs; Varnai and Balla, 1998; Kanai et al., 2001; Lemmon, 2008; Yeung et al., 2008; Yip et al., 2008; Gozani et al., 2003; Hammond et al., 2013; Ariotti et al., 2015; Platre and Jaillais, 2016). These include the following: the lactadherin synthase C2 domain (lact-C2) domain for PS, which is present throughout the endosomal system and the PM (Yeung et al., 2008); the p40 phox (p40PX) domain for phosphatidylinositol-3-phosphate (PI3P), enriched in early endosomes and occasionally the PM (Kanai et al., 2001); the P4M-SidM domain of the legionella pneumophila effector protein for phosphatidylinositol-4-phosphate (PI4P) that localizes to the golgi, late endosomes and PM (Hammond et al., 2014); the Ing2 plant homeodomain (ING2-pHD) for phosphatidylinositol-5-phosphate (PI5P), which mainly localizes to nuclei (Gozani et al., 2003); the TAPP1 pleckstrin homology (TAPP1-PH) domain for phosphatidylinositol-3,4-bisphosphate (PI[3,4]P2) that is reported to accumulate at the PM (Ariotti et al., 2015); a tandem dimer of the N-terminus of the mucolipin cation channel TRPML1 (ML1N*2) for phosphatidylinositol-3,5-bisphosphate (PI[3,5]P2) that appears to accumulate in late endosomes and lysosomes (Ariotti et al., 2015); the PH domain of PLCδ1 (PLCδ1-PH) for detection of PM-enriched PI(4,5)P2 (Varnai and Balla, 1998); and the Bruton’s tyrosine kinase PH (Btk-PH), a marker for phosphatidylinositol-3,4,5-triphosphate (PIP3), which also accumulates at the PM (Varnai and Balla, 1998).
We have employed these green fluorescence protein (GFP) tagged lipid biosensors as probes to examine the lipid interactions of WT and ΔMA HIV-1 Gag proteins. Using biosensors and Gag expression vectors, we have analyzed Gag-biosensor colocalization in cells, Gag-induced biosensor release from cells, and biosensor association with extracellular virus-like particles. Our results demonstrate a significant colocalization of PS, PI(4,5)P2 and PIP3 biosensors with WT Gag in cells, and an association of these biosensors with HIV-1 VLPs. Interestingly, ΔMA Gag co-localization was reduced with the PI(4,5)P2 and PIP3 biosensors and, relative to WT Gag, ΔMA Gag expression was associated with lower levels of PI(4,5)P2 biosensor release and VLP association. In contrast, we found that the PI3P biosensor colocalized with ΔMA Gag, and that ΔMA Gag expression increased levels of PI3P biosensor release and VLP association. These results demonstrate an alternative approach to the analysis of viral protein-lipid associations, and have provided new information as to the lipid composition of HIV-1 assembly sites.
2. RESULTS
2.1 Colocalization of lipid biosensors and Gag proteins
As an initial analysis of GFP-tagged lipid biosensor expression in cells (Figure 1), we transfected cells with expression vectors for enhanced GFP (EGFP) and the indicated biosensors, collected cell lysates, and subjected proteins to denaturing gel electrophoresis and immunoblot detection of GFP-tagged proteins, with an anti-GFP primary antibody (see Materials and Methods). Major bands for EGFP and the biosensors were observed to migrate at their predicted sizes, relative to size markers run in parallel (Figure 1). Moreover, only small levels of breakdown products were observed, supporting the notion that subsequent fluorescence analyses would pertain mostly to the full-length biosensors, rather than proteolytic fragments.
Figure 1. Expression of lipid biosensors.
Expression vectors for EGFP and the indicated EGFP-tagged lipid biosensors were transfected into cells. Three days post-transfection, proteins in cell lysate samples were separated electrophoretically, electroblotted onto nitrocellulose filters, and processed for detection of EGFP-tagged proteins using a primary antibody to GFP. The mobilities of 50 and 37 kDa marker proteins, run in parallel with the samples, are indicated on the left-hand side of each panel.
To verify the reported (Varnai and Balla, 1998; Kanai et al., 2001; Lemmon, 2008; Yeung et al., 2008; Yip et al., 2008; Gozani et al., 2003; Hammond et al., 2013; Ariotti et al., 2015; Platre and Jaillais, 2016) localization patterns for the lipid biosensors, they were expressed in cells that were processed for fluorescence detection. As shown in Figure 2, EGFP itself yielded the expected cytoplasmic staining pattern, while the PS biosensor gave a significant perinuclear signal, as well as a heterogeneous cytoplasmic staining pattern, as seen previously (Yeung et al., 2008). The PI(4,5)P2 and PIP3 reporters, which localized to cell PMs (Varnai and Balla, 1998) accumulated at the periphery of cells; while the PI(3,5)P2 biosensor showed a speckled pattern in accordance with expectations (Ariotti et al., 2015). Also in agreement with prediction (Gozani et al., 2003), the PI5P sensor appeared nuclear localized. Finally, the PI3P, PI4P and PI(3,4)P2 biosensors also showed a degree of nuclear staining, but additionally stained cytoplasmic and membrane structures, as seen previously (Kanai et al., 2001; Hammond et al., 2014; Ariotti et al., 2015).
Figure 2. Lipid biosensor localization patterns.
The indicated lipid biosensor vectors were transfected into cells and were processed at three days post-transfection for GFP detection. Image panels correspond to 40 × 40 microns each.
To compare localization patterns of lipid biosensors and WT Gag in cells, WT HIV-1 Gag and GagPol proteins were co-expressed in cells with either EGFP or the lipid biosensors, which were processed for dual fluorescence analysis of GFP, and Gag, using a primary anti-capsid (anti-CA) antibody, and a secondary AlexaFluor594-tagged secondary antibody. As expected (Scholz et al., 2008; Alfadhli and Barklis, 2014; Ritchie et al., 2015; Freed, 2015), HIV-1 Gag appeared to stain cells with a heterogeneous pattern, indicative of cell membrane localization (Figure 3, Gag panel). The EGFP and lipid biosensor probes also stained in their anticipated ways (Figure 3, lipid panel), and thier patterns in the presence of HIV-1 Gag/GagPol were similar to those seen in the absence of HIV-1 expression (Figure 2). Overlays of Gag and biosensor signals (Figure 3, right panel) yielded significant yellow patterns, indicative colocalization, especially with the PS, PI(4,5)P2, and PIP3 biosensors, and to a lesser extent with the PI4P biosensor. These colocalization signals were quantified as described below.
Figure 3. Immunofluorescent detection of lipid biosensors and wild type Gag proteins.

The indicated lipid biosensor vectors were co-transfected into cells with an expression vector for wild type HIV-1 Gag and GagPol proteins. Three days post-transfection, cells were processed for detection of EGFP fluorescence (lipid; green), and HIV-1 Gag, using a primary anti-CA antibody (Gag; red). The rightmost panels depict overlays of lipid and Gag signals, in which yellow is indicative of localization. Image panels correspond to 40 × 40 microns each.
In contrast to WT Gag staining, ΔMA Gag appeared to localize preferentially to perinuclear puncta (Figure 4, Gag panel). These results are consistent with previous electron microscopy (EM) analysis of ΔMA Gag proteins, where virus particle assembly predominantly occurred on intracellular membranes (Facke et al., 1993). Staining patterns for EGFP and biosensors in ΔMA Gag-expressing cells (Figure 4, center panels) were largely similar to the patterns in WT Gag-expressing cells, and ΔMA Gag proteins appeared to colocalize to some degree with all the GFP-tagged proteins, excepting EGFP and PI5P (Figure 4, right panel).
Figure 4. Immunofluorescent detection of lipid biosensors and ΔMA Gag proteins.

The indicated lipid biosensor vectors were co-transfected into cells with an expression vector for ΔMA HIV-1 Gag and GagPol proteins. Three days post-transfection, cells were processed for detection of EGFP fluorescence (lipid; green), and HIV-1 Gag, using a primary anti-CA antibody (Gag; red). The rightmost panels depict overlays of lipid and Gag signals, in which yellow is indicative of localization. Image panels correspond to 40 × 40 microns each.
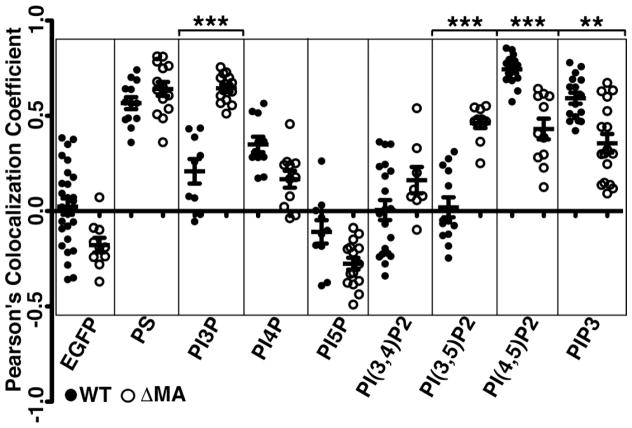
To quantify colocalization levels, we calculated Pearson’s Correlation Coefficients (PCCs; Adler and Parmryd, 2010), which vary from -1 (inversely correlated) to +1 (completely correlated) for multiple cells from each Gag plus lipid biosensor co-transfection combination (Figure 5). While EGFP and the PI5P biosensor distributions correlated poorly with both WT and ΔMA Gag, PCC values for both Gags were above 0.4 for the PS, PI(4,5)P2, and PIP3 biosensors, and slightly less for PI4P. Nevertheless, relative to WT Gag, colocalization levels of ΔMA Gag with the PM biosensors for PI(4,5)P2 and PIP3 were significantly reduced, and a similar, but lesser effect was seen with PI4P. Additionally, PCC values for the PI3P and PI(3,5)P2 biomarkers were notably higher with ΔMA Gag than with WT Gag, supporting the notion that ΔMA Gag accumulates at intracellular membranes.
Figure 5. Lipid biosensor correlation with Gag localization.
Pearson’s correlation coefficient values, which vary from -1 (inversely correlated) to +1 (perfectly correlated), were determined from at least twelve cells of every co-transfection combination of the indicated lipid biosensors plus either WT (black dots) or ΔMA (white dots) Gag expression vectors. Experiments were performed as in Figures 3–4. Correlation coefficients were calculated as detailed in the Materials and Methods. Averages and standard deviations are as shown. Pairwise WT versus ΔMA comparisons determined to be significant (** P < 0.01) or highly significant (** * P < 0.001) are indicated.
2.2 Lipid biosensor release from cells
Assuming that lipid biosensors localize to virus assembly sites, then it is possible that they will be incorporated into assembling VLPs. As one method of testing this, we monitored biosensor release from cells in the presence or absence of WT or ΔMA Gag. To do so, immunoblot analyses of lipid biosensor levels were performed as in Figure 1 for cell samples, and for pelleted media samples, which contain VLPs, microvesicles, exosomes, and PM-derived membrane blebs and fragments (Bess et al., 1997; Gluschankof et al., 1997; Dettenhofer and Yu, 1999; Chertova et al., 2006). Parallel Gag immunoblotting was performed to ensure equivalent levels of WT or ΔMA Gag expression and release in cotransfections. Examples of representative blots are shown in Figure 6. The left two panels show cell and pelleted media signals for WT HIV-1 (PrGag, p41, and capsid [CA]), as well as EGFP and an EGFP-tagged Vpr protein, used as a positive control. Notably, whereas media and cell Gag levels were similar between the two samples, EGFP preferentially was detected in cell samples, while the tagged Vpr protein was released from cells. These results were as expected for EGFP, which did not localize with Gag (Figures 3, 5), and Vpr, which assembles into HIV-1 particles by virtue of its association with the PrGag p6 domain. The right two panels show a similar analysis with the PI5P and PI(4,5)P2 biosensors. Here again, Gag levels appeared roughly constant across samples. However, while cellular levels of the PI5P and PI(4,5)P2 biosensors appeared similar, pelleted media levels of the PI(4,5)P2 biosensor were much higher that for the PI5P biosensor. These data suggest that the PI(4,5)P2 biosensor either assembles into HIV-1 VLPs, or is induced to be released from cells by HIV-1 Gag expression. To quantify biosensor marker release from cells, we performed densitometric measurement of cell and pelleted media sample biosensor immunoblot signals from cells expressing biosensors with or without the Gag variants. As anticipated, even in the absence of Gag expression, some lipid biomarkers were released from cells as pelletable material (Figure 7, gray bars). These levels were highest for PS and PIP3, which are present at the PM, but were also significant for the PI(3,4)P2, PI(3,5)P2, and PI(4,5)P2 sensors. On co-expression with WT Gag (black bars), release levels for the PI(4,5)P2, PIP3, and PI4P biosensors jumped. This could be due either to Gag-induced shedding of membranes, or assembly of the biosensors into VLPs. Given that HIV-1 assembles at PI(4,5)P2-rich PM sites (Ono et al., 2004; Chukkapalli et al., 2008; Alfadhli and Barklis, 2014; Freed, 2015), we believe that the latter was true for the PI(4,5)P2 biosensor, and that the PIP3 and PI4P biosensors also were released by virtue of their association with lipids that were assembled into VLPs.
Figure 6. Analysis of cell and media pellet samples.
Cells were co-transfected with the WT HIV-1 Gag plus GagPol expression vector and expression vectors for EGFP, the EGFP-tagged Vpr protein (Vpr), the PI5P biosensor, or the PI(4,5)P2 biosensor. At three days post-transfection, cell lysates or VLP-containing media pellets were processed for immunoblot detection of PrGag, p41, and CA Gag proteins using an anti-HIV-1 CA antibody, or the indicated GFP-tagged proteins, using an anti-GFP antibody. The left two sets of panels were from one set of transfections, and the right two sets of panels were from a separate set of transfections. Gag cell and media pellet blots were subjected to color reactions for the same amounts of time. GFP media blot color reactions were either five times (left panels) or ten times (right panels) longer than cell lysate blots. Note that while Gag media pellet and cell lysate levels remained relatively constant, EGFP and PI5P biosensors were detected predominantly in cells, while significant amounts of EGFP-tagged Vpr and the PI(4,5)P2 biosensor were detected in media pellets.
Figure 7. Lipid biosensor release levels.
Expression vectors for the indicated lipid biosensors were co-transfected into cells either alone (+ mock; gray bars), with a WT Gag expression vector (+ WT Gag; black bars), or with a ΔMA Gag expression vector (+ ΔMA Gag; white bars). Three days post-transfection, cell and virus-like particle (VLP) samples were collected and processed for immunoblot detection of released and cellular EGFP-tagged proteins. Parallel anti-CA blots were performed to assure that similar levels of HIV-1 Gag proteins and particles were produced. Immunoblot signals were quantified densitometrically, and percentage release levels indicate the ratios of VLP EGFP signals to cellular EGFP signals times 100. Averages and standard deviations are as shown. Note that control experiments with EGFP-tagged Vpr yielded percentage release values of 0.6% (+ mock), 20% (+ WT) and 18% (+ ΔMA).
In contrast with WT Gag, ΔMA Gag expression did not appreciably increase the release of the PI(4,5)P2 (Figure 7, white bars). This presumably reflects the fact that ΔMA Gag has lost the PI(4,5)P2 binding site and consequently does not assemble at PI(4,5)P2-rich membranes (Facke et al., 1993; Saad et al., 2006). It also is consistent with previous lipidome analyses, which showed that WT HIV-1 VLPs but not ΔMA VLPs were enriched in PI(4,5)P2 (Chan et al., 2008). As shown in Figure 7, ΔMA Gag expression also did not increase PI4P biosensor release levels, but did increase release levels of PI3P and PI(3,5)P2 biosensors. These results appeared to parallel the ΔMA colocalization results (Figures 4–5), and suggest that ΔMA VLPs may be enriched for PI3P and PI(3,5)P2. Unexpectedly, we also observed high levels of PIP3 biosensor release in the presence of ΔMA Gag. These release levels, of over 12%, potentially reflect high levels of PIP3 in ΔMA VLPs, a possibility that is supported by experiments below. However, it also is important to emphasize that all of the biosensor release levels that we observed were well below the 18–20% release levels observed for EGFP-tagged Vpr in the presence of the ΔMA or WT HIV-1 Gag expression vectors (Figure 7 legend).
2.3 Fluorescence analysis of VLPs
As an alternative approach to the analysis of lipid biosensor association with VLPs, we subjected VLPs to dual fluorescence analysis. To do so, VLP samples were adhered to coverslips, and processed for dual Gag and GFP fluorescence analysis, similar to cellular co-localization studies (Figures 3–4). Examples of our results from co-transfections of the WT Gag expression vector with expression vectors for EGFP, EGFP-tagged Vpr, and the PI(4,5)P2 biosensor are shown in Figure 8. As illustrated (Figure 8, left panels), the anti-CA primary antibody and AlexaFluor594-tagged secondary antibody detected VLPs as bright red spots. As expected from the results of Figure 7, few if any of the EGFP co-transfection particles appeared to be GFP-positive (Figure 8, top center). The EGFP-Vpr sample (Figure 8, middle row) yielded significantly different results, with a high proportion of particles that were both Gag- and GFP-positive. The PI(4,5)P2 biosensor sample (Figure 8, bottom row) gave intermediate results, in agreement with our biosensor release data (Figure 7).
Figure 8. Virus-like particle staining.
VLPs were isolated at three days post-transfection from cells co-transfected with an expression vector for WT Gag, and either EGFP, EGFP-Vpr, or the EGFP-tagged biosensor for PI(4,5)P2 (PIP45). Particles were adhered to coverslips, and processed for dual detection of HIV-1 Gag with an anti-CA primary antibody (red), or GFP (green). The rightmost columns are overlays of Gag and GFP signals. Image panels correspond to 80 × 80 microns each.
Percentages of Gag-positive VLPs that were also GFP labeled were calculated as described in the Materials and Methods. Briefly, Gag-positive spots that were small enough to be single VLPs rather than clusters or large membrane fragments were selected automatically and used to probe their partner GFP fluorescence images: particles that were of above a sufficient GFP brightness cutoff value were counted as GFP-positive. For WT and ΔMA VLPs, our EGFP-Vpr control yielded 36% and 31% GFP-positive particles (Figure 9 legend), while the EGFP control gave values of less than 1% (Figure 9). In general, our results on co-staining of VLPs reflected biosensor release results (Figure 7). Two examples are the higher WT-to-ΔMA values with the PI(4,5)P2 biosensor, and the lower WT-to-ΔMA values with the PI3P biosensor. Exceptions to the rule were PS, which gave higher GFP-positive particle percentages for ΔMA than WT VLPs, and PI(3,5)P2, which gave low values for both VLP types. These results are discussed below.
Figure 9. Percentage of virus particles labeled with lipid biosensors.
VLPs, isolated from cells co-transfected with the indicated GFP-tagged biosensors and either WT (black) or ΔMA (white) Gag expression vectors, were processed for dual GFP plus Gag fluorescence detection as in Figure 6. VLPs were picked based on their sizes and Gag fluorescence signals. Percentages of GFP-positive VLPs were calculated as detailed in the Materials and Methods section. Values represent averages and standard deviations from at least ten separate images, and at least 5000 VLPs. Pairwise WT versus ΔMA comparisons determined to be significant (** P < 0.01) or highly significant (** * P < 0.001) are indicated. Note that control experiments with EGFP-tagged Vpr yielded 36% GFP positive VLPs for WT Gag, and 31% GFP positive VLPs for ΔMA Gag.
3. DISCUSSION
In an effort to examine HIV-1 Gag interactions with phospholipids, we have undertaken the analysis of WT and ΔMA Gag associations with a panel of GFP-tagged acidic phospholipid biosensors. This approach takes advantage of the lipid biosensors that have been developed recently (Varnai and Balla, 1998; Kanai et al., 2001; Yeung et al., 2008; Yip et al., 2008; Gozani et al., 2003; Hammond et al., 2013; Ariotti et al., 2015; Platre and Jaillais, 2016), but has a number of caveats. One is that the specificities of different biosensors for their lipid ligands can vary, can be affected by expression levels, and may perturb Gag-lipid binding. A second is that the partial nuclear localization of PI3P, PI4P, PI(3,4)P2, and PI(3,5)P2 biosensors may impact their associations with Gag proteins. Stabilities and trafficking patterns of lipid ligands also may be perturbed by biosensor binding (Yip et al., 2008; Platre and Jaillais, 2016). We also acknowledge that the resolution of our fluorescence work is diffraction-limited, with a calculated resolution of about 260 nm in the x-y plane for red fluorophores. Additionally, as noted above, our studies on the effects of Gag proteins on biosensor release may score either for direct assembly into VLPs, or indirect effects on membrane shedding. Finally, it is important to point out that assembly of a phospholipid-bound biosensor automatically implies that the MA domain of PrGag can not be binding the same molecule. Thus, we envision that biosensor incorporation into VLPs is a consequence of the lipid-bound biosensor being a bystander in the assembly process.
Despite the aforementioned caveats, we believe our results to be informative and consistent with what previous observations are available. In particular, previous studies have shown that HIV-1 MA binds PI(4,5)P2, that WT HIV-1 assembly occurs at PI(4,5)P2-enriched sites, and that PI(4,5)P2 is enriched in WT versus ΔMA VLPs (Chan et al., 2008). Our results showing higher levels of PI(4,5)P2 biosensor colocalization, release, and VLP staining with WT versus ΔMA Gag are in agreement with these previous observations. At the other end of the spectrum, it is also no surprise that EGFP, and the PI5P biosensor that localizes to cell nuclei (Platre and Jaillais, 2016) neither colocalized with either Gag protein, nor assembled into VLPs.
The PI3P and PI4P biosensors appeared to show contrasting behaviors. PI4P has been shown to have a role at the Golgi apparatus in the sorting of proteins to endosomes or the PM, and serves as a substrate to (PI4P) 5-kinase (PIP5K), which converts PI4P to PI(4,5)P2 (Ono et al., 2004; van den Bout and Divecha, 2009; Platre and Jaillais, 2016). Our observations showed slightly higher association levels of the PI4P biosensor with WT Gag versus ΔMA Gag based on subcellular localization, Gag induced release, and VLP labeling experiments Figures 5, 7, 9). We infer that these data correlate with the partial localization of PI4P to the PM, where WT HIV-1 assembles (Ono et al., 2004; Alfadhli and Barklis, 2014; Freed, 2015), relative to the apparent intracellular assembly sites (Facke et al., 1993; Figure 3) of ΔMA Gag proteins. PI3P has been shown to reside at early endosomes, but also can be produced in the endoplasmic reticulum (ER; Platre and Jaillais, 2016; Nascimbeni et al., 2017). Our results demonstrated higher PI3P biosensor colocalization, Gag-induced release, and VLP labeling levels with ΔMA Gag than with WT Gag (Figures 5, 7, 9). These results are consistent with the observed ΔMA Gag assembly on ER or other intracellular membranes with a slightly different ΔMA construct (Facke et al., 1998), and support the notion that ΔMA VLPs are released from cells via a vesicular transport pathway.
We did not see compelling Gag-related differences in the behavior of the PS biosensor. Part of this may be due to the fact that release levels in the absence of Gag expression were high for this to lipid biosensor. This simply may reflect the general abundance of PS (Vance, 2015). The high levels of virus-independent PS biosensor release from cells (Figure 7) makes it difficult to interpret the slightly increased PS biosensor colocalization and VLP staining levels with ΔMA versus WT Gag. Recent evidence indicates that PS is enriched in WT HIV-1 relative to the PMs of producer cells (Lorizate et al., 2013), while our data suggest the higher percentage of ΔMA versus WT VLPs that incorporate the PS biosensor may pertain an intracellular localization that may overlap the ER (Facke et al., 1993), where PS synthesis occurs (Vance, 2015).
Our results with the PI(3,4)P2, PI(3,5)P2, and PIP3 biosensors also did not lead to simple interpretations. PI(3,5)P2 is a minor phospholipid that may reside in late endosomes or lysosomes (Hasegawa et al., 2017): we observed that the PI(3,5)P2 biosensor colocalized better with ΔMA Gag than WT Gag (Figure 5), but did not effectively assemble into VLPs (Figure 9). PI(3,4)P2 can be generated through the actions of a variety of kinases and phosphatases, and appears to regulate cytoskeletal arrangements at the PM: its biosensor did not localize well with WT or mutant Gag, was not induced to be released from cells by either Gag, and stained WT and ΔMA VLPs at the same levels. Finally, the biosensor for PIP3, a signaling phospholipid often generated by [PI(4,5)P2] 3 kinase (PI3K) action at the PM, did indeed localize to the PMs of cells (Figures 2–4), and showed high levels of Gag-independent release from cells (Figure 7). However, PIP3 biosensor release from cells was increased by both WT and ΔMA Gag expression, and approximately 12% of ΔMA VLPs were labeled with it. These results may be a consequence of PIP3 biosensor perturbation of PIP3 levels and/or activities in cells (Yip et al., 2008; Platre and Jaillais, 2016), or may indicate a potentially interesting association of PIP3 with the HIV-1 Gag particle assembly machinery. Further investigations with complementary approaches are warranted to distinguish these possibilities.
4. MATERIALS AND METHODS
4.1 Constructs
The vector for expression of WT Gag and GagPol proteins, psPAX2, was obtained from the NIH AIDS Reagent Program, and has been described before (Zuffery et al., 1997; Lopez et al., 2014). The psPAX2-ΔMA construct is similar to our previously described ΔMA Gag constructs (Wang et al., 1993; Ritchie et al., 2015) in that it retains the N-terminal myistoylation signal and C-terminal MA-CA juncture sequence but deletes the remainder of MA. The psPAX2-ΔMA deletion removes MA residues 17–118, and retains the juncture sequence gat cga tgg / gca gct gac where the codons before and after the slash are MA codons 16 and 119. The expression plasmids for EGFP (pEGFP-c1; Clontech) and EGFP-tagged Vpr (EGFP-Vpr) have been described previously (Schaeffer et al., 2001). The lipid biosensors for PS (Lact-C2-GFP), PI3P (p40PX-EGFP), PI4P (GFP-P4M-SidM), and PI5P (GFP-ING2[pHD]) were all from Addgene, and have been described previously (Yeung et al., 2008; Kanai et al., 2001; Hammond et al., 2014; Gozani et al., 2003). The lipid biosensors for PI(4,5)P2 (pLNCX2-PH-PLCδ1-GFP) and PIP3 (pLNCX2-PH-Btk-GFP) were both from Dr. Fikadu Tafesse (OHSU). These were constructed respectively by insertion of XhoI-NotI fragments from PH-PLCδ1-GFP and PH-Btk-GFP (Addgene; Varnai and Balla, 1998) into the XhoI and NotI sites of pLNCX2 (Clontech), downstream of the CAG enhancer and CMV promoter. The lipid biosensors for PI(3,4)P2 (EGFP-C2Tapp1PH) and PI(3,5)P2 (EGFP-C1ML1N*2) respectively were constructed by insertion of SalI-XbaI fragments from mKate2-P2A-APEX2-TAPP1PH and mKate2-P2A-APEX2-ML1N*2 (Addgene; Ariotte et al., 2015) into the SalI-XbaI fragments of pEGFP-c1 (Clontech), in frame and downstream from the EGFP coding region.
4.2 Cell culture
HEK 293T cells were maintained as described previously (Lopez et al., 2014; Ritchie et al., 2015, 2017) in Dulbecco’s modified Eagle’s medium (DMEM) plus 10% fetal calf sera (FCS), 10 mM Hepes pH 7.4, penicillin and streptomycin at 37°C in 5% CO2. For transfections, confluent 10 cm plates of cells were split 1:4 onto 10 cm plates the day prior to transfections. Transfections were performed using either calcium phosphate (Lopez et al., 2014; Ritchie et al., 2015, 2017) or polyethyleneimine (PEI; Longo et al., 2013) protocols with 24 μg of lipid biosensor plasmids, or with mixtures of 9 μg lipid biosensor plasmids plus 15 μg of either the WT or ΔMA psPAX2 HIV-1 Gag plus GagPol expression plasmids. For PEI transfections, cells were pretreated 4 h with 25 μM chloroquine plus 50 μg/ml gentamycin in 10 ml growth media. PEI-DNA complexes were formed by adding 0.5 ml of 144 μg/ml PEI (Polysciences, Inc., #23966, linear, MW 25,000) in Opti-MEM (Thermo Fisher) media dropwise to 24 μg of DNA in 0.5 ml Opti-MEM. For each plate of cells, 1 ml PEI-DNA mixes were incubated 15–20 min at 25°C, and then added dropwise to cells in media plus chloroquine and gentamycin. Cells were incubated overnight at 37°C/5%CO2, after which cells were refed with 10 ml growth media plus gentamycin.
For standard collection of cell and virus samples (Wang et al., 1993; Lopez et al., 2014; Ritchie et al., 2015), cells in 10 cm dishes were incubated an additional 48 h. Cell pellets were collected and washed in phosphate-buffered saline (PBS; 9.5 mM sodium potassium phosphate [pH 7.4], 137 mM NaCl, 2.7 mM KCl) and snap frozen at −80°C. Media containing VLPs were filtered through 0.45-μm-pore filters (Gelman) and concentrated by centrifugation through 20% sucrose in PBS cushions (1–2 h at 197,000 × g [40,000 rpm, SW41 rotor, 2-ml cushions]). Virus pellets were resuspended in 0.1 ml PBS per cell plate and stored in aliquots at −80 °C. For cellular immunofluorescence localization experiments (Scholz et al., 2008; Ritchie et al., 2015, 2017), 40–42 h post-transfection, cells were split 1:10–1:40 onto 22×22 mm coverslips that had been pretreated with poly-L-lysine solution (Sigma P4707; MW 75,000–100,000, 0.01%; 20–30 min at 25°C) in wells of six well plates supplemented with 2 ml growth media plus gentamycin. Cells were incubated an additional 24–30 h prior to immunofluorescence processing.
4.3 Protein analysis
For protein analysis, cell samples (20% of pellets from each10-cm plate) were suspended in IPB (20 mM Tris–HCl [pH7.5], 150 mM NaCl, 1 mM ethylenediamine tetraacetic acid [EDTA], 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1.0% Triton X-100, 0.02% sodium azide), incubated on ice for 5 min, vortexed, and subjected to centrifugation 13,000 × g for 15 min at 4 °C. Soluble material was collected, mixed with 1 volume of 2× sample buffer (12.5 mM Tris–HCl [pH 6.8], 2% SDS, 20% glycerol, 0.25% bromophenol blue) and 0.1 volume of β-mercaptoethanol (β-Me). For virus samples, 40 μl aliquots of resuspended virus pellets were mixed with 1 volume of 2× sample buffer and 0.1 volume of β-Me. Cell and virus samples were heated to 95 °C 3–5 min, and subjected to SDS-polyacrylamide electrophoresis (SDS-PAGE). After SDS-PAGE fractionation, proteins were electroblotted, and immunoblotted following previously described methods (Wang et al., 1993; Scholz et al., 2008; Lopez et al., 2014; Ritchie et al., 2015, 2017). The primary antibody used for detection of HIV-1 CA was Hy183 (kindly provided by Bruce Chesebro), used at 1:15 dilution from the hybridoma culture medium; while the primary antibody for GFP was a rabbit antibody (Life Technologies, A11122, 2 mg/ml), used at a 1:3000 dilution. Secondary antibodies (Promega) were antimouse or antirabbit IgG alkaline phosphatase-conjugated secondary antibodies used at 1:15,000. Color reactions for visualization of antibody-bound bands employed nitrobluetetrazolium plus 5-bromo-4-chloro-3-indolyl phosphate in AP buffer (100 mM Tris–hydrochloride [pH 9.5], 100 mM NaCl, 5 mM MgCl2). For quantitation, immunoblots were air-dried and scanned using an Epson Perfection V600 scanner. Band intensities of scanned TIFF images were determined using NIH Image J software (Schneider et al., 2012). For calculation of lipid biosensor (GFP) release values, only samples with similar cell and VLP Gag levels were compared, and the formula 100 × (VLP GFP intensity/VLP GFP color reaction time)/(cellular GFP intensity/cellular GFP color reaction time) was used. Release values were averaged from two to three independent experiments.
4.4 Cell fluorescence microscopy
For dual fluorescent detection of HIV-1 Gag and GFP-tagged biosensors, cells were washed once in PBS, fixed in 4% paraformaldehyde (PFA; Sigma) in PBS at room temperature for 30 min, washed in PBS, permeabilized in 0.2% Triton X-100 in PBS at room temperature for 10 min, washed once, and incubated in culture medium for 10 min. Subsequently, anti-HIV-1 CA primary antibody (Hy183) in culture medium was added, and cells were incubated at 37 °C for 1 h, rocking every 15 min. After primary antibody incubations, coverslips were washed three times in culture medium, then secondary antibody (anti-mouse Alexa-Fluor 594; Invitrogen) diluted 1:1000 in media was added, and the samples were incubated at 37 °C for 30 min, rocking once at 15 min. Following incubations, the cells were washed twice in culture medium and three times in PBS, followed by mounting onto microscope slides in Fluoro-G mounting medium. It is worthwhile to note, that although it has been recommended that the PI4P biosensor be used with unfixed cells (Hammond et al., 2014), we did not observe differences in PI4P biosensor staining of unfixed versus fixed cells, and thus performed our analyses with fixed cells.
Samples were viewed on a Zeiss AxioObserver fluorescence microscope using a 63× (Planapochromat) objective and a Zeiss filter set 10 (excitation bandpass, 450–490; beamsplitter Fourier transform, 510; emission bandpass, 515–565) for green fluorophores, or Zeiss filter set 20 (excitation bandpass, 546/12; beamsplitter Fourier transform, 560; emission bandpass, 575–640) for red fluorophores. Images were collected with Zeiss Axiovision software with a fixed gain setting of 100 and exposures taken to maximize brightness levels without overexposure. For the purpose of presenting color images, gray-scale 16 bit TIF images were converted to green or red images using the Image J Image/Lookup Tables function, and then saved as 8 bit RGB images. For overlays of red and green images, RGB TIF images were opened in Adobe Photoshop, layered using the screen option, and flattened. Colocalization analysis of HIV-1 Gag (CA) and biosensor (GFP) signals was performed by determining Pearson’s Correlation Coefficient (PCC) values, which vary from -1 (inversely correlated) to +1 (completely correlated) (Adler and Parmryd, 2010). To do so, matched images in Image J were stacked, single cell areas without background regions were boxed and cropped, destacked, and then used as input for the Image J JACoP PCC Plugin (Schneider et al., 2012). Colocalization values were averaged from at least 12 pairs of cell images for each Gag/lipid biosensor combination. Values were evaluated via one-way ANOVA analyses and Tukey comparisons, using GraphPad Prism 5 software.
4.5 VLP fluorescence analysis
To adhere VLPs to coverslips, 18 mm circular coverslips in wells of six well tissue culture plates first were incubated 5 min at room temperature in 0.01% poly-L-lysine (Sigma P4707), wicked with filter paper and dried 5 min. VLP samples (4 ul) were applied to the centers of the coverslips, incubated 10 min, and then wicked. VLPs were fixed by the gentle addition of 4% PFA in PBS and incubation for 5 min at room temperature. After fixation, coverslips were washed 3 min in PBS, and processed for dual detection of HIV-1 Gag and GFP-tagged biosensors as described for cell samples. VLP samples were viewed and imaged as for cell images, except that gain and exposure settings were identical for all pairs of images.
To determine the percentages of Gag-positive particles that also were GFP-positive, we analyzed VLP images using a combination of Image J and Excel or R software. As a first step, images were normalized in Image J to include the full range (0–65535) of brightness values, and then inverted. Following these steps, images were thresholded to include only the top 15% of pixel brightness values (above 55705), and converted to Gag and GFP binary masks. To remove large, GFP+ artifacts from consideration, GFP binary masks were filtered using the Image J Analyze Particle command with a size range of >10 pixels, and then subtracted from the Gag images. VLP positions then were picked from the Gag masks and data for these particles were extracted from the original Gag and background-subtracted GFP images using a combination of the Set Measurements and Analyze Particles (size range 0–4 pixels) commands. Particle data were analyzed using Excel or R, and GFP+ VLPs were defined as VLPs (from Gag images) that had average normalized GFP brightness values of at least 1000. Values represent averages and standard deviations from at least ten separate images, and at least 5000 VLPs. Values were evaluated via one-way ANOVA analyses and Tukey comparisons, using GraphPad Prism 5 software. Note that control experiments with EGFP-tagged Vpr yielded 36% EGFP positive VLPs for WT Gag, and 31% EGFP positive VLPs for ΔMA Gag.
Highlights.
Lipid biosensors have been used to characterize HIV-1 assembly sites.
Wild type and deletion matrix HIV-1 viruses assemble at different sites.
The lipid biosensor for PI(4,5)P2 is enriched in wild type HIV-1.
The lipid biosensor for PI3P is enriched in deletion matrix HIV-1.
Acknowledgments
We are grateful to Chris Ritchie and Dr. Fikadu Tafesse for advice and assistance during the course of this research, to Dr. Bruce Chesebro for originally providing us with the anti-Gag Hy183 cell line, and to Addgene and the NIH AIDS Reagent Program for plasmids. These investigations were supported by NIH grants R01 GM060170 and R01 GM101983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A. 2010;77:733–742. doi: 10.1002/cyto.a.20896. [DOI] [PubMed] [Google Scholar]
- Alfadhli A, Barklis E. The roles of lipids and nucleic acids in HIV-1 assembly. Frontiers in Microbiology. 2014;5:1–11. doi: 10.3389/fmicb.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadhli A, Still A, Barklis E. Analysis of human immunodeficiency virus type 1 matrix binding to membranes and nucleic acids. J Virol. 2009;83:12196–12203. doi: 10.1128/JVI.01197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti N, Hall TE, Rae J, Ferguson C, McMahon KA, Martel N, Webb RE, Teasdale RD, Parton RG. Modular detection of GFP-labeled proteins for rapid screening by electron microscopy in cells and organisms. Developmental Cell. 2015;35:513–525. doi: 10.1016/j.devcel.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Bess JW, Jr, Gorelick RJ, Bosche WJ, Henderson LE, Arthur LO. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- Brugger B, Glass B, Haberkant P, Liebrecht I, Wieland F, Krausslich HG. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol. 2008;82:11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley MA, Luttge B, Freed EO. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol. 2011;410:582–608. doi: 10.1016/j.jmb.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Jr, Sowder RC, 2nd, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. Interaction between HIV-1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient Gag-membrane binding. J Virol. 2008;82:2405–2417. doi: 10.1128/JVI.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenhofer M, Yu XF. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäcke M, Janetzko A, Shoeman R, Krausslich HG. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J Virol. 1993;67:4972–4980. doi: 10.1128/jvi.67.8.4972-4980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed EO. HIV-1 assembly, release and maturation. Nat Rev Microbiology. 2015;13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluschankof P, Mondor I, Gelderblom HR, Sattentau QJ. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenor J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell. 2003;114:99–111. doi: 10.1016/s0092-8674(03)00480-x. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Machner MP, Balla T. A novel probe for phosphatdylinositol 4-phosphate reveals multiple pools beyond the golgi. J Cell Biol. 2014;205:113–126. doi: 10.1083/jcb.201312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa J, Strunk BS, Weisman LS. PI5P and PI(3,5)P2: minor but essential phosphoinositides. Cell Structure and Function. 2017;42:49–60. doi: 10.1247/csf.17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- Lemmon MA. Membrane recognition by phospholipid-binding domains. Nature Reviews Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- Longo PA, Kavran JM, Kim MS, Leahy DH. Transient mammalian cell transfection with polyethyleneimine (PEI) Methods Enzymol. 2013;529:227–240. doi: 10.1016/B978-0-12-418687-3.00018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CS, Sloan R, Cylinder I, Kozak SL, Kabat D, Barklis E. RRE-dependent HIV-1 Env RNA effects on Gag protein expression, assembly and release. Virology. 2014;462–463:126–134. doi: 10.1016/j.virol.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorizate M, Sachsenheimer T, Glass B, Habermann A, Gerl MJ, Krausslich HG, Brugger B. Comparative lipidomics analysis of HIV-1 particles and their producer cell membrane in different cell lines. Cellular Microbiology. 2013;15:292–304. doi: 10.1111/cmi.12101. [DOI] [PubMed] [Google Scholar]
- Nascimbeni AC, Codogno P, Morel E. Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 2017;284:1267–1278. doi: 10.1111/febs.13987. [DOI] [PubMed] [Google Scholar]
- Olety B, Veatch SL, Ono A. Phosphatidylinositol-(4,5)-bisphosphate acyl chains differentiate membrane binding of HIV-1 Gag from that of the phospholipase C01 pleckstrin homology domain. J Virol. 2015;89:7861–7873. doi: 10.1128/JVI.00794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol-(4,5)-bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platre MP, Jaillais Y. Guidelines for the use of protein domains in acidic phospholipid imaging. Methods Mol Bio. 2016;1376:175–194. doi: 10.1007/978-1-4939-3170-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reil H, Bukovsky AA, Gelderblom HR, Gottlinger HG. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 1998;17:2699–2708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C, Cylinder I, Platt EJ, Barklis E. Analysis of HIV-1 Gag protein interactions via biotin ligase tagging. J Virol. 2015;89:3988–4001. doi: 10.1128/JVI.03584-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C, Mack A, Harper L, Alfadhli A, Stork JSP, Nan X, Barklis E. Analysis of K-Ras interactions by biotin ligase tagging. Cancer Genomics Proteomics. 2017;14:225–239. doi: 10.21873/cgp.20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci USA. 2006;103:11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E, Geleziunas R, Greene WC. Human immunodeficiency virus type I Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75:2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz I, Still A, Dhenub TC, Coday K, Webb M, Barklis E. Analysis of human immunodeficiency virus matrix domain replacements. Virology. 2008;371:322–335. doi: 10.1016/j.virol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedbury P, Ablan SD, Freed EO. Global rescue of defects in envelope glycoprotein incorporation: implications for matrix structure. PLoS Pathogens. 2013;9:1–13. doi: 10.1371/journal.ppat.1003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-(3H)-inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Zhang Y, McDermott J, Barklis E. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J Virol. 1993;67:7067–7076. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- Yip SC, Eddy RJ, Branch AM, Pang H, Wu H, Yan Y, Drees BE, Neilsen PO, Condeelis J, Backer JM. Quantitation of PI(3,4,5)P3 dynamics in EGF-Stimulated carcinoma cells: a comparison of PH domain-mediated versus immunological methods. Biochem J. 2008;411:441–448. doi: 10.1042/BJ20071179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuffery R, Nagy D, Mandel R, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]