Abstract
HIV-1 infection causes injury to the central nervous system (CNS) and is often associated with neurocognitive disorders. One model for brain damage seen in AIDS patients is the transgenic (tg) mouse expressing a soluble envelope protein gp120 of HIV-1 LAV in the brain in astrocytes under the control of the promoter of glial fibrillary acidic protein. These GFAP-gp120tg mice manifest several key neuropathological features observed in AIDS brains, such as decreased synaptic and dendritic density, increased numbers of activated microglia and pronounced astrocytosis. Several recent studies show that brains of GFAP-gp120tg mice and neurocognitively impaired HIV patients share also a significant number of differentially regulated genes, activation of innate immunity and other cellular signaling pathways, disturbed neurogenesis, and learning deficits. These findings support the continued relevance of the GFAP-gp120tg mouse as a useful model to investigate neurodegenerative mechanisms and develop therapeutic strategies to mitigate the consequences associated with HIV infection of the CNS, neuroAIDS and HAND.
Keywords: HIV-1, gp120, NeuroAIDS, HAND, neuroprotection, transgenic animal model
Introduction
Infection with the human immunodeficiency virus-1 (HIV-1) and acquired immunodeficiency syndrome (AIDS) continue to present a major public health problem worldwide. Besides the progressive destruction of the immune system, HIV-1 causes a range of neurological problems and neurocognitive impairments that originally were described as NeuroAIDS but are now comprehensively categorized under the term HIV-associated neurocognitive disorders (HAND) (Antinori et al, 2007). While much information has been gained over the years regarding HIV-1 infection of the periphery and the central nervous system (CNS) in general, the pathological, cellular and molecular mechanisms leading to HAND, NeuroAIDS and AIDS remain incompletely understood. However, evidence has accumulated indicating that the CNS constitutes a HIV reservoir and thus also requires attention in all viral eradication attempts (Brew et al, 2015; Churchill and Nath, 2013; Ferretti et al, 2015; Gray et al, 2014; Joseph, 2015; Kramer-Hammerle et al, 2005; Lambotte et al, 2003; Nath, 2015).
Several lines of evidence stemming from the work of numerous investigators over many years strongly suggest that at least two major pathological mechanisms contribute to the development of NeuroAIDS, HIV-1 associated neurodegeneration and consequent HAND (reviewed in (Kaul, 2008)). The first is neurotoxicity caused by toxins released by infected or immune-stimulated, inflammatory microglia and macrophages (MΦ) in the brain, constituting an indirect injurious pathway, and/or direct damaging exposure to HIV-1 and its proteins (Giulian et al, 1990; Gonzalez-Scarano and Martin-Garcia, 2005; Kaul et al, 2001; Lindl et al, 2010). The second insult by HIV in the brain comprises the impairment of neurogenesis (Krathwohl and Kaiser, 2004; Okamoto et al, 2007; Poluektova et al, 2005; Tran et al, 2005).
Since HIV-1 was discovered and linked to the development of AIDS (Barre-Sinoussi et al, 1983; Hahn et al, 1984), multiple approaches have been taken to generate suitable animal models for studying HIV disease, including NeuroAIDS (Ambrose et al, 2007; Gardner and Luciw, 1989; Klotman and Notkins, 1996; Nath et al, 2000; Toggas and Mucke, 1996; Van Duyne et al, 2009).
Animal Models Utilized in AIDS and NeuroAIDS Research
The animal models for AIDS research include a range of species, such as chimpanzees and other non-human primates, cats and rodents (rats and mice) (Ambrose et al, 2007; Gardner and Luciw, 1989; Keppler et al, 2002; Klotman and Notkins, 1996; Nath et al, 2000; Reid et al, 2001; Toggas and Mucke, 1996; Van Duyne et al, 2009). Although chimpanzees can be infected with some HIV-1 strains, they rarely develop AIDS and were in the past primarily employed in vaccine research (Nath et al, 2000). However, chimpanzees in the wild can contract Simian Immunodeficiency virus (SIV) which appears to cause an AIDS-like disease (Keele et al, 2009). In any case, chimpanzees have apparently not been employed in studies of neuroAIDS. Other non-human primates, cats and rodents are resistant to productive HIV-1 infection, but several non-human primate species are susceptible to SIV and cats are permissive to a lentivirus called Feline Immunodeficiency virus (FIV) (Ambrose et al, 2007; Clements et al, 1994; Olmsted et al, 1989). Both SIV and FIV can dependably induce AIDS-like disease and neuropathological changes or even encephalitis in a species-specific manner, and macaques and cats have been used to investigate the pathogenesis of AIDS and NeuroAIDS (Ambrose et al, 2007; Clements et al, 1994; Clements et al, 2008; Jacobson et al, 1997; Meeker et al, 1997; Olmsted et al, 1989; Williams et al, 2008). SIV is considered to be the animal virus most closely related to HIV-1, but the existing significant differences between the viruses have impeded the translation of findings into the human system, such as the development of vaccines. As an alternative approach to better adapt the SIV model for HIV research, several SIV-HIV hybrid viruses have been generated (Ambrose et al, 2007; Williams et al, 2008).
Rodents cannot be productively infected with wild-type HIV-1. However, two chimeric HIV mutants have recently been generated in which the viral envelope protein gp120 was replaced by the gp80 of ecotropic murine leukemia virus (EcoHIV) (Potash et al, 2005). This modification enabled for the first time a lasting lentiviral infection in mice that also triggered an immune response. Furthermore, one of the chimeric viruses was shown to be neuroinvasive, suggesting its potential suitability for NeuroAIDS research.
Certain immuno-compromised mouse strains can be reconstituted with a human hematopoietic system (‘humanized mouse’) which is permissive to HIV infection and thus enable small animal models for AIDS and NeuroAIDS research (Dash et al, 2011; Van Duyne et al, 2009). The intracranial injection of HIV-infected human monocyte-derived macrophages into the brains of mice with severe combined immunodeficiency (SCID) provides another model of NeuroAIDS and was used to demonstrate that HIV-infected macrophages can cause a neuropathology that shares key features with post mortem brains from HIV dementia patients (Limoges et al, 2000; Persidsky et al, 1996; Poluektova et al, 2002; Sas et al, 2007; Tyor et al, 1993). These studies also provided evidence that HIV-infection in the brain triggers a peripheral immune response.
Among the various model systems, rodents have turned out to be useful even though they cannot be productively infected with wild type HIV-1. However, one important advantage of rodents, both mice and rats, is that they can be genetically modified (Klotman and Notkins, 1996; Reid et al, 2001; Toggas and Mucke, 1996; Van Duyne et al, 2009).
Several transgenic mice and a rat have been generated that express an entire HIV genome and develop AIDS-like diseases (Hanna et al, 1998a; Hanna et al, 1998b; Iwakura et al, 1992; Leonard et al, 1988; Reid et al, 2001). The transgenic rat carries an HIV-1 provirus with functionally inactive gag and pol sequences which prevents the generation of infectious virus particles. However one HIV-transgenic mouse model and transgenic mouse microglia carrying the provirus of a macrophage-tropic HIV-1 were shown to release infectious virus (Leonard et al, 1988; Wang et al, 2003). Transgenic mouse models expressing the HIV genome in its entirety or distinct components of the virus, such as gp120, Tat or Vpr, in the brain develop various degrees of behavioral alterations as well as neuropathology, including loss of synapses, neuronal dendrites and neurons as well as glial activation, and thus recapitulate several pathological hallmarks of NeuroAIDS patients (Berrada et al, 1995; Bruce-Keller et al, 2008; D’hooge et al, 1999; Jones et al, 2007; Kim et al, 2003; Thomas et al, 1994; Toggas et al, 1994; Toneatto et al, 1999). Overall, the exact spectrum of pathological features with resemblance to AIDS and NeuroAIDS depends on the specific animal model and ranges from depletion of CD4+ T-cells to immunodeficiency to wasting disease to failure-to-thrive to neuronal injury and loss to behavioral impairment to shortened life span.
Of note, studies using HIV-infected humanized mice or transgenic expression of entire viral genomes or injection of HIV-infected macrophages in the brain are useful to investigate the combined pathological effects of all viral components, but cannot discern the potential contribution of a single viral factor. Therefore, approaches that use injection or transgenic expression of one viral component at a time appear very useful as well. The injection models have the advantage of deliberate and controlled timing of the application of any pathological agent into any experimental animal without requiring prior genetic modification. A limitation of many transgenic models, including the HIV-1 gp120tg mouse model that is the focus of additional sections of this article, is the constitutive expression of the transgene throughout the lifetime of the animals, which can in extreme cases prevent a normal or healthy development before onset of the desired pathological phenotype. One approach to avoid that potential caveat is the inducible expression of a transgene, such as the doxycycline-inducible HIV-1 Tat-tg mouse model (Fitting et al, 2010; Kim et al, 2003). However, doxycycline-inducible transgene expression requires continued exposure to the inducing compound and introduces an additional powerful agent into the experimental model that can have effects, at least temporarily, that are independent of the transgene expression. Moreover, expression-inducing agents could themselves modify the response of the model system to the induced transgene. Altogether, inducible and injection models seem most suitable to study acute or short–term effects of a pathological viral component. In contrast, transgenic models with constitutive expression may be more suitable to investigate long-term, chronic effects of viral proteins. On a separate note, transgenic models, such as the HIV-1 gp120tg mouse, that only express one viral protein, may miss pathological effects that originate from the combined action of different viral proteins. One approach to address this concern is the cross-breeding of transgenic models expressing different viral proteins.
Numerous studies have employed transgenic mouse models with both constitutive and inducible viral protein expression to address the pathological potential of HIV-1 components, such as gp120, Tat and Vpr, and indeed suggested that isolated viral factors can produce some of the pathological characteristics of HIV disease and NeuroAIDS (Bachis et al, 2006; Berrada et al, 1995; Bruce-Keller et al, 2008; Chatterjee et al, 2011; Hauser et al, 2009; Jones et al, 2007; Kim et al, 2003; Toggas et al, 1994; Toneatto et al, 1999). In comparison to all other animal models, mice have the additional advantage that many specific genetic knockout mutants are available. Thus, transgenic and genetic knockout mice permit studies of viral and host factors in ways that are not easily possible in other models. Therefore, it is perhaps not surprising that despite the above discussed limitations and disadvantages of constitutive transgenic expression, numerous studies have been performed with the first model that expresses HIV-1 gp120 specifically in the central nervous system.
HIV-1 gp120-Transgenic Mice as an Animal Model in NeuroAIDS Research
This article will focus on studies in the first reported HIV gp120-transgenic mouse model which expresses a soluble viral envelope gp120 of HIV-1 LAV in the brain in astrocytes under the control of the promoter of glial fibrillary acidic protein (GFAP-gp120-transgenic mouse/GFAP-gp120tg/HIVgp120tg) (Toggas et al, 1994). The transgene is expressed the highest in neocortex, olfactory bulb, hippocampus, tectum, selected white matter tracts, and along the glia limitans. Although this tg mouse only expresses viral gp120, it develops a neuropathology that is strikingly similar to human AIDS brains, including decreased synaptic and dendritic density, frank neuronal loss, increased numbers of activated microglia and pronounced astrocytosis (Toggas et al, 1994) and thus a comparable neuropathology that results when HIV-infected human macrophages are intracerebrally administered into mice with severe combined immunodeficiency (SCID) mice (Persidsky et al, 1996; Tyor et al, 1993). The founder lines described in the first study of GFAP-gp120tg mice also suggested that neuropathology required a sufficiently high expression of gp120 RNA. Moreover, a peripheral immune challenge with recombinant gp160 triggered a strong lymphocyte-mediated immune response and infiltration of the brain only in gp120tg animals, but not non-tg littermate controls or in GFAP-LacZ tg mice, thus providing indirect evidence for the presence of viral envelope protein in the CNS of gp120tg mice (Toggas and Mucke, 1998). Several subsequent studies included an additional GFAP-gp120tg founder line that expresses more easily detectable envelope protein levels and therefore is called gp120tg High Protein Expressor, (HPX) line (Garden et al, 2002; Lee et al, 2011; Maung et al, 2014; Thaney et al, 2017; Toggas and Mucke, 1996). The studies discussed here served at least one of two purposes: either improving our understanding of the neuropathological mechanism(s) of HIV infection, or exploring potential future therapies for NeuroAIDS and HAND.
The Phenotype of GFAP-HIVgp120tg Mice
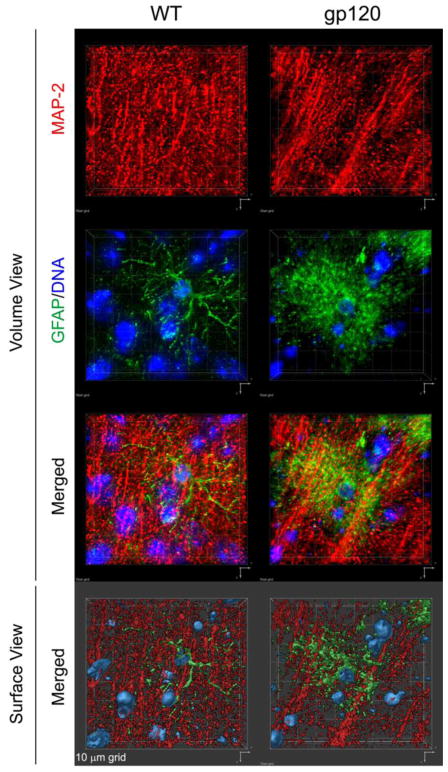
Histopathological studies of GFAP-gp120tg mice have so far primarily focused on cerebral cortex and hippocampus, because those brain structures are prominently affected in HIV patients with neurocognitive impairment (Toggas et al, 1994). Neuropathological features observed in GFAP-gp120tg mice include: 1) Loss of neuronal dendrites at 3, 4–5, 6, 10, 12 and 20 months of age (Garden et al, 2002; Kang et al, 2010; Maung et al, 2014; Thaney et al, 2017; Toggas et al, 1994)(Maung and Kaul, unpublished), (see Figure 1 for immunofluorescence staining of cerebral cortex of 6 months-old mice); 2) loss of synapses at 3, 4–5 and 6 months (Maung et al, 2014; Thaney et al, 2017; Toggas et al, 1994); 3) activated microglia at 3, 4–5, 6, 10 months (Kang et al, 2010; Maung et al, 2014; Thaney et al, 2017; Toggas et al, 1994); 4) Astrocytosis at 3, 4–5, 6 and 10 months (Kang et al, 2010; Maung et al, 2014; Thaney et al, 2017; Toggas et al, 1994); 5) Compromised neurogenesis at 2, 4 to 5 and 8 months (Avraham et al, 2015; Avraham et al, 2014b; Crews et al, 2011; Fields et al, 2014; Lee et al, 2013; Lee et al, 2011; Okamoto et al, 2007; Steiner et al, 2015); 6) Behavioral impairment: GFAP-gp120tg mice display, in comparison to non-tg littermate controls, behavioral changes or impairment. At 6 months gp120tg mice show increased anxiety-like behavior in open field, light/dark transition task, and prepulse inhibition tests (Bachis et al, 2016). At 9 to 12 months, GFAP-gp120tg mice display reduced swimming velocity, and compromised spatial learning and retention (D’hooge et al, 1999; Hoefer et al, 2015; Maung et al, 2014) as well as reduced contextual but not cued fear conditioning (Kaul et al., unpublished). GFAP-gp120tg mice also showed hyperactivity in an open field test compared to non-tg wild-type controls at 12 months of age (Fields et al, 2016a). 7) Alterations in electrophysiological function: In line with findings of behavioral changes or impairment and injury to neuronal presynaptic terminals and dendrites, electrophysiological studies detected abnormalities in short- and long-term potentiation in the CA1 region of the hippocampus in gp120tg mice compared to non-tg controls at ~ 53 days and 10.5 months of age (Hoefer et al, 2015; Krucker et al, 1998).
Fig. 1. Immunofluorescence staining of MAP-2-positive neuronal dendrites and GFAP-positive astrocytes in cerebral cortex of HIV gp120-transgenic and non-transgenic, wild type control mice.
Sagittal brain sections of 6 months-old gp120-transgenic and wild-type (WT) littermate controls were immune-stained for neuronal MAP-2 (red) and astrocytic GFAP (green). DNA (blue) was labeled with H33342 and is shown to indicate nuclei. Fluorescence-labeled sections were analyzed using a Zeiss Axiovert 100 M inverted microscope and Slidebook software (Intelligent Imaging Innovations, Denver, CO) to record Z-stacks and perform deconvolution and 3D reconstruction. The upper six panels show 3D volume views, the bottom two panel are 3D surface views. Representative areas of mid-frontal cortex, layer 3, are shown. Note the difference in the density of MAP-2 immunoreactive neuropil and astrocyte morphology between WT and gp120tg samples, and the dimensions of the 10 μm grid.
Research Topics and Studies Using GFAP-HIVgp120tg Mice
1) Mechanism(s) of HIV-associated neurotoxicity and compromised neurogenesis
In comparison to non-tg controls, young but not 6 months-old GFAP-gp120tg mice presented with increased plasma corticosterone, and plasma and pituitary adreno-corticotropic hormone (ACTH) levels, indicating activation of the hypothalamic-pituitary axis (HPA) (Raber et al, 1996). The stimulation of the endocrine system depended on activation of N-methyl-D-asparate-type glutamate receptors (NMDAR), neuronal nitric oxide synthase (nNOS) and reactive oxygen species (ROS) as it was inhibited by the non-competitive NMDAR inhibitor memantine, the nNOS blocker NG-methyl-L-arginine (LNMA) and a superoxide dismutase (SOD)-transgene (Raber et al, 1996). Hence, excitotoxic and oxidative stress appeared to be major contributors to HIVgp120-induced brain injury and possibly NeuroAIDS.
Along those lines, another study analyzed the brain’s glutamate uptake systems, system XAG (sodium-dependent) and xc- (sodium-independent), in striatum and hippocampus. This investigation found (30–35 %) reductions in the kinetics of systems XAG and xc- in both neurons and glia of the striatum, but not hippocampus, of HIVgp120tg mice compared to non-tg wild-type controls (Melendez et al, 2016). Also, an increased number of spines in the amygdala and higher levels of brain-derived neurotrophic factor and tissue plasminogen activator were associated with an elevated level of anxiety-like behavior in 6 months-old GFAP-gp120tg mice in comparison to age-matched wild-type controls (Bachis et al, 2016). A proteomics study of synaptosomes found that the ratio of activated, phosphorylated to total Akt was diminished in forebrain of GFAP-gp120tg mice compared to non-tg wild-type controls, indicating compromised signaling of the pro-survival protein kinase pathway (Banerjee et al, 2012). This finding is in line with an earlier study showing that neuroprotection against neurotoxicity of HIVgp120 by physiological CCR5 ligands requires signaling of the Akt pathway (Kaul et al, 2007).
GFAP-gp120tg mice display clear signs of immune activation and neuroinflammation, including activated microglia and pronounced astrocytosis (Toggas et al, 1994). One early study reported increased amounts of the chemokines CXCL10 and CCL2 in brains of GFAP-gp120tg mice compared to non-tg controls (Asensio et al, 2001). The same study also observed an elevated number of CD3+ T cells in GFAP-gp120tg brains.
Crosses of GFAP-gp120tg with CCR5KO mice led to several novel findings regarding the role of innate immunity in HIV-associated brain injury and behavioral impairment (Maung et al, 2014): 1) CCR5 was crucial in vivo for neuronal damage and behavioral impairment triggered by a CXCR4-using HIV/gp120; 2) CCR5 deficiency protected GFAP-gp120tg mice against impairment of spatial learning and memory; 3) Astrocytosis occurred independently of neuronal damage and behavioral impairment; 4) The acute phase protein lipocalin-2 (LCN2) was identified as a novel potential player in HIV-associated neuronal injury. Combining LCN2 with inhibition of CCR5 signaling provided a novel mechanism to abrogate microglial activation and neurotoxicity of a CXCR4-using HIVgp120; 5) A genome-wide CNS gene expression analysis showed that brains of HIVgp120tg mice share a significant fraction of differential gene regulation with brains of HIV- and HIV encephalitis (HIVE) patients with neurocognitive impairment (Maung et al, 2014). The study revealed that GFAP-gp120tg brains mounted an immune response involving besides pro-inflammatory stimulation of macrophages, activation of nuclear factor κB (NFκB) by virus, Toll-like receptors (TLRs) and interferons (IFNβ and γ). The study might have translational implications by suggesting that HIV patients who are not elite controllers may benefit from pharmacological CCR5 inhibition even if they are infected with a CXCR4-preferring virus. The gene expression pattern indicative of a type I IFN signature was in accordance with an earlier report of IFN-stimulated gene (ISG)-15 in the brain of HIVgp120tg mice (Wang et al, 2012). A recent follow-up study confirmed that GFAP-gp120tg mice mounted a transient IFNβ response around 1.5 months of age (Thaney et al, 2017). While the mRNA expression for IFNβ had returned to baseline in 3 and 6 months-old animals, the signature of IFN-stimulated genes remained detectable.
Another earlier study suggested that activation of protein kinase C (PKC) may contribute to astrocytosis, a hallmark of neuroinflammation observed in HIVE and GFAP-gp120tg mice (Wyss-Coray et al, 1996). Similar to other neurodegenerative diseases, increased expression of matrix metalloproteinase (MMP)-2 has been associated with inflammation and neurodegeneration in brains of GFAP-gp120tg animals compared to non-tg littermate controls, (Marshall et al, 1998). Several studies found evidence of neuronal apoptosis in post-mortem brains of HIV patients with dementia (Adle-Biassette et al, 1995; Petito and Roberts, 1995; Shi et al, 1996). Introduction of a dominant negative interfering mutant of Caspase 1 (Casp1DN), an intracellular enzyme linked to regulation of apoptosis, into GFAP-gp120tg mice by cross-breeding protected neuronal dendrites, suggesting a critical role for the caspase system in HIV neurotoxicity (Garden et al, 2002).
The gp120 tg mouse model also presents with mitochondrial abnormalities like those observed in postmortem brain tissues from HIV-infected decedents (Fields et al, 2013; Fields et al, 2016b). Specifically, proteins controlling mitochondrial fission and fusion, dynamin-related protein (DRP) 1 and mitofusin (MFN) 1, respectively, are altered in brains of gp120 tg mice and in HAND decedents (Avdoshina et al, 2016; Fields et al, 2016b). In brains of humans and HIVgp120tg mice, decreased DRP1 and increased MFN1 are associated with enlarged and damaged mitochondria in neurons (Avdoshina et al, 2016; Fields et al, 2016b). Furthermore, in these translational studies, gp120 recombinant protein induced mitochondrial fusion through a reduction in DRP1 and an induction of MFN1 expression in neurons, in vitro (Avdoshina et al, 2016; Fields et al, 2016b). Potential therapeutic strategies to rectify these gp120-induced mitochondrial alterations will be discussed below.
To further investigate the roles of mitochondrial dysfunction, which leads to questions about altered autophagy, and persistent inflammation in the CNS, the GFAP-gp120tg mouse has proven an effective and relevant model that mirrors key features of the neuropathology observed in human NeuroAIDS. Original observations suggested that autophagy was increased in postmortem brain tissues of HIV patients (Zhou et al, 2011), however, later studies revealed autophagy markers are decreased in post mortem brain tissues of HIV patients over the age of 50 (Fields et al, 2013). Remarkably similar to human brains, autophagy markers were also decreased in aged HIVgp120tg mice (Fields et al, 2013). Furthermore, decreased autophagy was associated with increased neuroinflammation and neurodegeneration. These effects were reversed by inducing autophagy function via gene delivery of beclin 1 (see below and (Fields et al, 2013)).
Since cART has transformed HIV infection into a chronic disease, evidence has emerged suggesting that long-term survival can be associated with an Alzheimer’s Disease (AD)-like brain pathology (Brew et al, 2009; Green et al, 2005). Interestingly, normal human amyloid precursor protein expressed as transgene protected neurons of GFAP-gp120tg and control mice at ~ 5 months of age against acute or chronic excitotoxic injury (Masliah et al, 1997; Mucke et al, 1995). However, a triple transgenic mouse model expressing APP/PS1 mutants associated with AD and HIVgp120 displayed intraneuronal deposition of Aβ similar to brains of HIV patients (Bae et al, 2014; Green et al, 2005).
Accumulation of phosphorylated protein Tau (pTau) is another key feature of AD brains that was found in brain specimen of NeuroAIDS patients in comparison to age-matched healthy controls and GFAP-gp120tg mice, thus revealing another pathological feature of HIV-infected brains that is present in the tg mouse model (Kang et al, 2010). However, it remains to be elucidated if pTau is a contributing cause or mere consequence of HIVgp120-initiated CNS injury.
The GFAP-gp120tg mouse has been studied in another context related to cART. HIV-associated sensory neuropathy (HIV-SN) is a frequent neurological complication of the periphery in patients treated with dideoxynucleoside anti-retrovirals. GFAP-gp120tg mice exposed to didanosine (DDI) developed a distal degeneration of unmyelinated sensory axons, recapitulating the ‘dying back’ process associated with C-fiber loss seen in patients with HIV-SN (Keswani et al, 2006).
While HIV-1 seems unable to productively infect neurons, it has been reported that neural progenitor cells are permissive to the virus (Lawrence et al, 2004; Mattson et al, 2005; Schwartz and Major, 2006). However, independently of viral infection, HIV-1/gp120 was found to affect human and rodent neural progenitor cells (Krathwohl and Kaiser, 2004; Okamoto et al, 2007). Comparable to what has been observed in brain specimen from HIV dementia patients, the hippocampal dentate gyrus of GFAP-gp120tg mice presents with a reduction of proliferating neural progenitors in in comparison to non-transgenic controls (Okamoto et al, 2007). In vitro studies indicated that gp120 inhibited proliferation of neural progenitor cells via activation of a pathway comprising mitogen activated protein kinase p38 (p38MAPK), MAPK-activated protein kinase 2, a cell-cycle check-point kinase, and Cdc25B/C which in turn caused an arrest of the cell cycle in the G1 phase. Less neural progenitor proliferation in the presence of viral gp120 resulted in a smaller pool of progenitor cells to differentiate into neurons, thus impairing neurogenesis (Okamoto et al, 2007). Inhibition of hippocampal neurogenesis in GFAP-gp120tg mice was confirmed in subsequent studies, which implied roles for serotonin, cyclin-dependent kinase 5 (CDK5), fibroblast growth factor (FGF), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), cannabinoid (CB)2 receptor, fatty acid amide hydrolase (FAAH), Cox2 and prostaglandin E2 (PGE2) (Avraham et al, 2015; Avraham et al, 2014b; Fields et al, 2014; Lee et al, 2013; Steiner et al, 2015). One study found in brain specimen of HIVE patients and HIVgp120tg mouse brains evidence of aberrant activity of CDK5 and hyperphosphorylation of collapsin-response mediator protein-2 (CRMP2) which in turn can impair neurite outgrowth (Crews et al, 2011).
2) Effect of HIV in CNS on Behavioral Performance
GFAP-gp120-transgenic mice display in comparison to non-transgenic littermate controls an increased anxiety-like behavior at 6 months of age and additional behavioral changes or impairment at 9 to 12 months, such as altered escape latency, reduced swimming velocity, and impaired spatial learning and retention (Bachis et al, 2016; D’hooge et al, 1999; Hoefer et al, 2015; Maung et al, 2014; Patrick et al, 2011) as well as diminished contextual but not cued fear conditioning at 9 to 13 months (Kaul et al., unpublished).
3) Interplay of HIV/NeuroAIDS and Drug Abuse
Abuse of drugs constitutes a major comorbidity of HIV infection and the associated neurocognitive impairment (Carey et al, 2006; Chang et al, 2014; Kapadia et al, 2005; Mitchell et al, 2006; Urbina and Jones, 2004) and the GFAP-gp120tg mouse model has been employed to investigate the combined effects of psychostimulant drugs and HIV-1 on the brain and behavior (Bandaru et al, 2011; Roberts et al, 2010; Soontornniyomkij et al, 2016).
GFAP-gp120tg mice showed in comparison to non-tg wild-type controls an altered acute response to Methamphetamine (METH) that was detectable as changes in stereotypic behavior (Roberts et al, 2010). In another study, transgenic gp120 expression in the brain was also associated with an increased preference for both METH and a highly palatable non-drug reinforcer (saccharin) as well as increased sensitivity to METH-induced conditioned reward (Kesby et al, 2012). These findings suggested an increased sensitivity to METH as a potentially underlying reason for a frequent abuse by HIV-infected individuals.
The potential additive or interactive effects of HIV proteins and drug abuse on neurocognitive deficits can be quantified using translational studies of mouse models such as the GFAP-gp120tg mouse. Such studies are more powerful with the implementation of behavioral paradigms that can be translated across species, e.g., to humans as well as rodents. In this manner, deficits that are observed in humans with HIV can also be examined in mice, contributing to the validity of the murine model and illuminating neurobiological substrates that are difficult to isolate in humans. One example of a cross-species paradigm is the behavioral pattern monitor (BPM), an extension of the traditional rodent open field that has been adapted for testing in humans (Perry et al, 2009; Young et al, 2016). Using the mouse BPM, one study assessed behavioral inhibition deficits in male and female mice, manifesting as increased motor activity, inappropriate perseverative behavior, and elevated exploratory response to novel stimuli (Henry et al, 2013). Female gp120tg mice who had been exposed to a chronic regimen of METH showed the highest level of exploration (hole-poking) compared to the other female mouse groups. The gp120tg mice exhibited less rearing and slightly less locomotion, relative to the wild-type mice. The findings suggested both gp120 expression and chronic METH exposure modified behavioral inhibition, but such effects might be sex-dependent. Although robust gender differences have not been reported in human studies using the analogous behavioral paradigm (known as the human BPM), the findings in gp120tg mice underscored the importance of examining gender differences in HIV given potential gender or estrogen-related differences in HIV infection of the CNS (Wilson et al, 2006).
A further example of translation between species can be illustrated with the prepulse inhibition (PPI) paradigm. PPI is a measure of sensorimotor gating and is regulated by a network of neural structures including the dopaminergic circuitry implicated in inhibitory function. PPI can be assessed across species. Impaired sensorimotor inhibition, measured by PPI of the eyeblink startle response, was observed in HIV-infected persons with neurocognitive impairment compared to cognitively intact HIV-infected persons (Minassian et al, 2013), suggesting that early inhibition deficits accompany or may even precede downstream cognitive impairment in HIV-infected individuals. In rodents, PPI is quantified using the whole-body startle response, and PPI in GFAP-gp120tg and METH-exposed mice was studied (Henry et al, 2014). Prior to chronic METH exposure, female gp120tg mice exhibited decreased PPI compared with female wild-type mice, whereas male gp120tg mice showed increased acoustic startle response compared with the other groups. These findings in rodents, along with the results in humans indicating PPI deficits in HIV-infected persons with neurocognitive impairment (Minassian et al, 2013), suggest that inhibition deficits, which likely reflect HIV- and METH-related alterations in dopaminergic signaling, may not occur as a global phenomenon but may emerge in association with higher-order cognitive deficits or biological variations, e.g., those affected by sex.
In another study, GFAP-gp120tg mice were exposed to an escalating-dose, multiple-binge METH regimen at 3–4 months of age (Hoefer et al, 2015). Potential long-term effects were investigated after 6–7 months of drug abstinence employing behavioral tests and analyzing neuropathology, electrophysiology and gene expression. Behavioral assessment revealed impaired learning and memory in both gp120tg and wild-type animals treated with METH. Neuropathological analysis showed that METH triggered similarly to HIVgp120 a significant loss of pre-synaptic terminals and neuronal dendrites in hippocampus and cerebral cortex of wild-type animals. Electrophysiology experiments with hippocampal slices revealed that METH exposed GFAP-gp120tg animals displayed reduced post-tetanic potentiation, whereas both METH and gp120 expression led to reduced long-term potentiation. Arrays of quantitative reverse transcription-polymerase chain reaction exposed a significant dysregulation of specific components of GABAergic and glutamatergic neurotransmission systems in response to gp120 expression, METH and their combination, providing a potential mechanism for synaptic dysfunction and behavioral impairment. Thus, both HIVgp120 and METH caused lasting behavioral impairment in association with altered gene expression and neuropathology. However, the combination of METH exposure and HIVgp120 expression produced the most pronounced, long lasting pre- and post-synaptic alterations in conjunction with impaired learning and memory (Hoefer et al, 2015). The same conclusion regarding learning and memory was drawn in a separate behavioral study using the same METH regimen in GFAP-gp120tg mice (Kesby et al, 2015b). A cross-species study investigated in both humans and GFAP-gp120tg mice the separate and combined effects of METH and HIVgp120 on learning and executive functions (Kesby et al, 2015a). The results demonstrated that HIV in humans and viral gp120 in mice each impaired learning. Furthermore, a history of METH exposure appeared to aggravate HIV-associated neurocognitive deficits in both species. Overall, the similar pattern of outcomes in both species suggested that the viral envelope protein gp120 could significantly contribute to learning deficits in HIV patients.
Morphine exposure was found to increase oxidative stress and post-synaptic damage in brains of GFAP-gp120tg and non-tg control mice (Bandaru et al, 2011). Upon withdrawal the altered response to oxidative stress and synaptic damage was largely reversed in non-tg but not gp120tg brain. Moreover, GFAP-gp120tg brains displayed compared to non-tg controls an altered Sphingomyelin metabolism with elevated levels of ceramide. Ceramide has been implicated in neuronal injury and cell death pathways and although morphine reduced ceramide levels, withdrawal restored the elevated concentrations in GFAP-gp120tg mice (Bandaru et al, 2011).
4) Exploration of treatments or prevention of HIV-associated brain injury
Numerous studies have employed the GFAP-gp120tg mouse model to explore potential therapeutic strategies for HIV-induced brain injury and the associated neurocognitive disorders. Since it was recognized that HIV damages the brain not only through neurotoxicity but also by compromising neurogenesis and thus affects basic homeostasis and repair, the studies have targeted both pathological mechanisms (Kaul, 2008; Krathwohl and Kaiser, 2004; Okamoto et al, 2007). Several studies found evidence that regulation or inhibition of CDK5 using pharmacological or genetic approaches or exercise rescued hippocampal neurogenesis in GFAP-gp120tg mice. The successful approaches included CDK5 knockout, the cell cycle inhibitor roscovitine, the tyrosine kinase inhibitor sunitinib and an exercise-induced increase of BDNF expression (Fields et al, 2014; Lee et al, 2013; Patrick et al, 2011; Wrasidlo et al, 2014). However, exercise and selective serotonin re-uptake inhibitors (SSRI) also restored hippocampal neurogenesis in GFAP-gp120tg mice (Lee et al, 2011; Steiner et al, 2015). The exact mechanism(s) remain to be elucidated but appear to involve reduced susceptibility to excitotoxic injury. The genetic knockout of FAAH which results in increased expression of Cox2 and production of PGE2 and activation of the CB2 receptor were also found to enhance neurogenesis in GFAP-gp120tg mice while concomitantly diminishing astrogliosis (Avraham et al, 2014a; Avraham et al, 2015). These two studies suggested that a reduction or modulation of initially pro-inflammatory pathways promoted neurogenesis specifically in GFAP-gp120tg/FAAH-KO mice since, in contrast, non-tg FAAH-KO animals showed impaired neurogenesis (Avraham et al, 2015).
An early study aiming to protect the brain and specifically neurons from HIV-1 induced excitotoxic injury treated GFAP-gp120tg mice with the non-competitive NMDAR inhibitor memantine (Toggas et al, 1996). Memantine applied over a six-week period prevented damage to neuronal dendrites and presynaptic terminals in the mouse model. A subsequent clinical study of the NMDAR antagonist suggested an improvement of the pathologically disturbed neuronal metabolism but produced no significant improvement of neurocognitive performance in impaired HIV patients (Schifitto et al, 2007). A more recent study used a modified version of memantine, called nitro-memantine, as a pharmacological tool in comparison with genetic manipulation of NMDAR activity through overexpression of the modulatory subunit GluN3A in the GFAP-gp120tg mouse (Nakanishi et al, 2016). Although both nitro-memantine and GluN3A inhibited NMDAR activity, only nitro-memantine protected pre-synaptic terminals in HIVgp120tg mice, whereas overexpression of GluN3A caused itself significant neuronal damage. It was hypothesized that nitro-memantine predominantly interacted with pathologically activated extrasynaptic activated NMDARs, whereas GluN3A overexpression may have disturbed normal activity of synaptic and extrasynaptic NMDARs (Okamoto et al, 2009).
As mentioned above, the brains of both humans with HAND/dementia and GFAP-gp120tg mice present with hyperphosphorylated tau protein, which has been implicated in neuronal damage and loss. Intranasal treatment of 6 months-old GFAP-gp120tg for 4 months with a combination of erythropoietin and insulin-like growth factor I (EPO+IGF-I) provided neuroprotection in association with increased phosphorylation/activation of Akt (PKB) and phosphorylation/inhibition of glycogen synthase kinase (GSK)-3β, which notably reduced downstream hyperphosphorylation of tau (Kang et al, 2010).
The GFAP-gp120tg mouse provides a relevant model to test therapeutic strategies targeting dysfunctional autophagy and mitochondrial function in the brain. As proof of concept, gene delivery to the brains of aged gp120tg mice of Beclin 1, a key protein for the initiation of autophagy, restored levels of autophagosomes and reduced neurodegeneration and neuroinflammation (Fields et al, 2013). The beneficial effects of increased autophagy may result from increased clearance of protein aggregates and/or through recycling of damaged mitochondria via mitophagy. Further evidence for the neuroprotective effect of increasing the recycling of damaged mitochondria was illustrated in subsequent studies in which DRP1 gene delivery promoted mitochondrial fission, a prerequisite for mitophagy, and reduced neurodegeneration (Fields et al, 2016b).
A more recent study using the gp120 tg mouse suggests that anti-inflammatory molecules may also provide protection from gp120-induced neurotoxicity (Fields et al, 2016a). The immunosuppressive compound FK506 reduced neuroinflammation and neurodegeneration in the gp120 tg mouse model. FK506 also partially restored mitochondrial abnormalities in the gp120 tg mice, however, treatment did not restore DRP1 levels to those of control mice (Fields et al, 2016a). These data suggest that reducing neuroinflammation is a promising therapeutic strategy for HAND, though, more mechanism-directed approaches, such as targeting autophagy or mitochondrial dynamics, may prevent the initiation of neuroinflammation.
HIV-1 infection triggers an innate immune response including type I interferons (IFNα and β)(Doyle et al, 2015; Poli et al, 1994; Xiang et al, 2004). However, chronic expression of IFNα in the HIV-1 infected CNS has been linked to cognitive impairment and inflammatory neuropathology (Sas et al, 2009; Sas et al, 2007). In contrast, IFNβ has been implicated in the control of HIV and SIV infection in the brain (Barber et al, 2004; Kitai et al, 2000). A recent study showed that IFNβ confers neuronal protection against HIVgp120 toxicity and GFAP-gp120tg mice mount a transient IFNβ response with IFNβ mRNA significantly increased in brains at 1.5, but not 3 or 6 months of age (Thaney et al, 2017). Neuroprotection by IFNβ against toxicity of HIVgp120 required IFNα receptor 1 (IFNAR1) and the β-chemokine CCL4. Moreover, a four-week intranasal IFNβ treatment of HIVgp120tg mice starting at 3.5 months of age increased expression of CCL4 and concomitantly abrogated gp120-induced damage to neuronal dendrites and pre-synaptic terminals in hippocampus and cerebral cortex (Thaney et al, 2017). Altogether, the results suggested exogenous IFNβ as a neuroprotective factor that has potential to ameliorate in vivo HIV-induced brain injury.
Future Studies Utilizing the HIVgp120tg Mouse Model
The body of published studies that used the GFAP-gp120tg mouse model suggests its suitability for investigating several open questions in neuroAIDS research, including: 1) The role of host factors in HIV-associated brain injury; 2) Eradication of viral sequences – the transgenic HIV gp120 constitutes an original viral sequence integrated in the host genome. Although the viral sequence is expressed only in the CNS, the integrated DNA sequence is present in the genome of all tissues; 3) Investigation of effects of abused drugs and cART on HIV-associated brain injury, behavior and neural circuitries. The published studies on the effects of METH and morphine in GFAP-gp120tg mice strongly suggest that this mouse model will be useful to investigate the impact of other drugs as well, such as nicotine, cannabis, cocaine and prescription painkillers; 4) The effect of Aging on HIV-associated brain injury – GFAP-gp120tg mice can be aged to reach 20+ months of age (Maung et al, 2014); and 5) The continuing exploration of strategies for treatment or prevention of HIV-associated brain injury, and behavioral impairment that characterizes HAND. All current animal models have limitations, but an in-depth analysis of existing models for HIV disease of the CNS, such as the GFAP-gp120tg mouse discussed here, and their utilization for pre-clinical intervention studies still has potential to provide invaluable information.
Acknowledgments
This work was supported by NIH grants R01 MH087332, MH104131, MH105330 (to M.K.) and P50 DA026306 (Project 1 to A.M. and J.W.Y.; Project 5 to M.K.). JAF was supported by R01 MH105319 (PI: Dr. C.L. Achim).
Footnotes
Conflict of Interest Disclosure
The authors state that they have no conflict of interest.
References
- Adle-Biassette H, Levy Y, Colombel M, Poron F, Natchev S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Ambrose Z, Kewalramani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: in search of an animal model. Trends Biotechnol. 2007;25:333–337. doi: 10.1016/j.tibtech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS, Campbell IL. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75:7067–7077. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Fields JA, Castellano P, Dedoni S, Palchik G, Trejo M, Adame A, Rockenstein E, Eugenin E, Masliah E, Mocchetti I. The HIV Protein gp120 Alters Mitochondrial Dynamics in Neurons. Neurotox Res. 2016;29:583–93. doi: 10.1007/s12640-016-9608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Wood J, Wang L, Masliah E, Avraham S. Impaired Neurogenesis by HIV-1-Gp120 is Rescued by genetic deletion of Fatty Acid Amide Hydrolase Enzyme. Br J Pharmacol. 2014a doi: 10.1111/bph.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Wood J, Wang L, Masliah E, Avraham S. Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme. Br J Pharmacol. 2015;172:4603–14. doi: 10.1111/bph.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Zvonok A, Masliah E, Avraham S. The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol. 2014b;171:468–479. doi: 10.1111/bph.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Forcelli P, Masliah E, Campbell L, Mocchetti I. Expression of gp120 in mice evokes anxiety behavior: Co-occurrence with increased dendritic spines and brain-derived neurotrophic factor in the amygdala. Brain Behav Immun. 2016;54:170–7. doi: 10.1016/j.bbi.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M, Patel N, Xu H, Lee M, Tominaga-Yamanaka K, Nath A, Geiger J, Gorospe M, Mattson MP, Haughey NJ. Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. J Neurosci. 2014;34:11485–11503. doi: 10.1523/JNEUROSCI.0210-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol. 2011;6:640–649. doi: 10.1007/s11481-011-9289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Liao L, Russo R, Nakamura T, McKercher SR, Okamoto S, Haun F, Nikzad R, Zaidi R, Holland E, Eroshkin A, Yates JR, III, Lipton SA. Isobaric tagging-based quantification by mass spectrometry of differentially regulated proteins in synaptosomes of HIV/gp120 transgenic mice: implications for HIV-associated neurodegeneration. Exp Neurol. 2012;236:298–306. doi: 10.1016/j.expneurol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SA, Herbst DS, Bullock BT, Gama L, Clements JE. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neurovirol. 2004;10(Suppl 1):15–20. doi: 10.1080/753312747. [DOI] [PubMed] [Google Scholar]
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Berrada F, Ma D, Michaud J, Doucet G, Giroux L, Kessous-Elbaz A. Neuronal expression of human immunodeficiency virus type 1 env proteins in transgenic mice: distribution in the central nervous system and pathological alterations. J Virol. 1995;69:6770–6778. doi: 10.1128/jvi.69.11.6770-6778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Robertson K, Wright EJ, Churchill M, Crowe SM, Cysique LA, Deeks S, Garcia JV, Gelman B, Gray LR, Johnson T, Joseph J, Margolis DM, Mankowski JL, Spencer B. HIV eradication symposium: will the brain be left behind? J Neurovirol. 2015;21:322–34. doi: 10.1007/s13365-015-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Chang SL, Connaghan KP, Wei Y, Li MD. NeuroHIV and use of addictive substances. Int Rev Neurobiol. 2014;118:403–440. doi: 10.1016/B978-0-12-801284-0.00013-0. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Callen S, Seigel GM, Buch SJ. HIV-1 Tat-mediated neurotoxicity in retinal cells. J Neuroimmune Pharmacol. 2011;6:399–408. doi: 10.1007/s11481-011-9257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS. 2013 doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Anderson MG, Zink MC, Joag SV, Narayan O. The SIV model of AIDS encephalopathy. Role of neurotropic viruses in diseases. Res Publ Assoc Res Nerv Ment Dis. 1994;72:147–157. [PubMed] [Google Scholar]
- Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol. 2008;14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Ruf R, Patrick C, Dumaop W, Trejo-Morales M, Achim CL, Rockenstein E, Masliah E. Phosphorylation of collapsin response mediator protein-2 disrupts neuronal maturation in a model of adult neurogenesis: Implications for neurodegenerative disorders. Mol Neurodegener. 2011;6:67. doi: 10.1186/1750-1326-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, Epstein AA, Gelbard HA, Boska MD, Poluektova LY. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31:3148–3157. doi: 10.1523/JNEUROSCI.5473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Goujon C, Malim MH. HIV-1 and interferons: who’s interfering with whom? Nat Rev Microbiol. 2015;13:403–13. doi: 10.1038/nrmicro3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep. 2015;12:280–8. doi: 10.1007/s11904-015-0267-7. [DOI] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E. Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol. 2014;9:102–116. doi: 10.1007/s11481-013-9520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Rockenstein E, Mante M, Spencer B, Grant I, Ellis R, Letendre S, Patrick C, Adame A, Masliah E. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol. 2013;19:89–101. doi: 10.1007/s13365-012-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Overk C, Adame A, Florio J, Mante M, Pineda A, Desplats P, Rockenstein E, Achim C, Masliah E. Neuroprotective effects of the immunomodulatory drug FK506 in a model of HIV1-gp120 neurotoxicity. J Neuroinflammation. 2016a;13:120. doi: 10.1186/s12974-016-0585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Serger E, Campos S, Divakaruni AS, Kim C, Smith K, Trejo M, Adame A, Spencer B, Rockenstein E, Murphy AN, Ellis RJ, Letendre S, Grant I, Masliah E. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol Dis. 2016b;86:154–69. doi: 10.1016/j.nbd.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D’Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MB, Luciw PA. Animal models of AIDS. FASEB J. 1989;3:2593–2606. doi: 10.1096/fasebj.3.14.2556312. [DOI] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gray LR, Roche M, Flynn JK, Wesselingh SL, Gorry PR, Churchill MJ. Is the central nervous system a reservoir of HIV-1? Curr Opin HIV AIDS. 2014;9:552–558. doi: 10.1097/COH.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Hahn BH, Shaw GM, Arya SK, Popovic M, Gallo RC, Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984;312:166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Hanna Z, Kay DG, Cool M, Jothy S, Rebai N, Jolicoeur P. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J Virol. 1998a;72:121–132. doi: 10.1128/jvi.72.1.121-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998b;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A Translational Methamphetamine ARCG. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav Brain Res. 2013;236:210–20. doi: 10.1016/j.bbr.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell MR, Perry W, Young JW, Minassian A Translational Methamphetamine ARCG. Prepulse inhibition in HIV-1 gp120 transgenic mice after withdrawal from chronic methamphetamine. Behav Pharmacol. 2014;25:12–22. doi: 10.1097/FBP.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer MM, Sanchez AB, Maung R, de Rozieres CM, Catalan IC, Dowling CC, Thaney VE, Pina-Crespo J, Zhang D, Roberts AJ, Kaul M. Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp Neurol. 2015;263:221–234. doi: 10.1016/j.expneurol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Shioda T, Tosu M, Yoshida E, Hayashi M, Nagata T, Shibuta H. The induction of cataracts by HIV-1 in transgenic mice. AIDS. 1992;6:1069–1075. doi: 10.1097/00002030-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Henriksen SJ, Prospero-Garcia O, Phillips TR, Elder JH, Young WG, Bloom FE, Fox HS. Cortical neuronal cytoskeletal changes associated with FIV infection. J Neurovirol. 1997;3:283–289. doi: 10.3109/13550289709029469. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, Jhamandas J, Power C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–3711. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J. Eradication of HIV-1 from CNS reservoirs: current strategies and future priorities. J Neurovirol. 2015;21:219–21. doi: 10.1007/s13365-015-0337-z. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Digicaylioglu M, Russo R, Kaul M, Achim CL, Fletcher L, Masliah E, Lipton SA. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 2010;68:342–352. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41:1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- Kaul M. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler OT, Welte FJ, Ngo TA, Chin PS, Patton KS, Tsou CL, Abbey NW, Sharkey ME, Grant RM, You Y, Scarborough JD, Ellmeier W, Littman DR, Stevenson M, Charo IF, Herndier BG, Speck RF, Goldsmith MA. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J Exp Med. 2002;195:719–736. doi: 10.1084/jem.20011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, Markou A, Grant I, Semenova S. Methamphetamine Exposure Combined with HIV-1 Disease or gp120 Expression: Comparison of Learning and Executive Functions in Humans and Mice. Neuropsychopharmacology. 2015a;40:1899–909. doi: 10.1038/npp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Hubbard DT, Markou A, Semenova S. Expression of HIV gp120 protein increases sensitivity to the rewarding properties of methamphetamine in mice. Addict Biol. 2012 doi: 10.1111/adb.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S Translational Methamphetamine ARCG. Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. Eur Neuropsychopharmacol. 2015b;25:141–50. doi: 10.1016/j.euroneuro.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai R, Zhao ML, Zhang N, Hua LL, Lee SC. Role of MIP-1beta and RANTES in HIV-1 infection of microglia: inhibition of infection and induction by IFNbeta. J Neuroimmunol. 2000;110:230–239. doi: 10.1016/s0165-5728(00)00315-5. [DOI] [PubMed] [Google Scholar]
- Klotman PE, Notkins AL. Transgenic models of human immunodeficiency virus type-1. Curr Top Microbiol Immunol. 1996;206:197–222. doi: 10.1007/978-3-642-85208-4_11. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2003;13:95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19:418–31. doi: 10.1007/s13365-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Song H, Nath A, Venkatesan A. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis. 2011;41:678–687. doi: 10.1016/j.nbd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JM, Abramczuk JW, Pezen DS, Rutledge R, Belcher JH, Hakim F, Shearer G, Lamperth L, Travis W, Fredrickson T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988;242:1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- Limoges J, Persidsky Y, Poluektova L, Rasmussen J, Ratanasuwan W, Zelivyanskaya M, McClernon DR, Lanier ER, Gendelman HE. Evaluation of antiretroviral drug efficacy for HIV-1 encephalitis in SCID mice. Neurology. 2000;54:379–389. doi: 10.1212/wnl.54.2.379. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DC, Wyss-Coray T, Abraham CR. Induction of matrix metalloproteinase-2 in human immunodeficiency virus-1 glycoprotein 120 transgenic mouse brains. Neurosci Lett. 1998;254:97–100. doi: 10.1016/s0304-3940(98)00674-0. [DOI] [PubMed] [Google Scholar]
- Masliah E, Westland CE, Rockenstein EM, Abraham CR, Mallory M, Veinberg I, Sheldon E, Mucke L. Amyloid precursor proteins protect neurons of transgenic mice against acute and chronic excitotoxic injuries in vivo. Neuroscience. 1997;78:135–146. doi: 10.1016/s0306-4522(96)00553-2. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Maung R, Hoefer MM, Sanchez AB, Sejbuk NE, Medders KE, Desai MK, Catalan IC, Dowling CC, de Rozieres CM, Garden GA, Russo R, Roberts AJ, Williams R, Kaul M. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J Immunol. 2014;193:1895–1910. doi: 10.4049/jimmunol.1302915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Thiede BA, Hall C, English R, Tompkins M. Cortical cell loss in asymptomatic cats experimentally infected with feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1997;13:1131–1140. doi: 10.1089/aid.1997.13.1131. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Roman C, Capo-Velez CM, Lasalde-Dominicci JA. Decreased glial and synaptic glutamate uptake in the striatum of HIV-1 gp120 transgenic mice. J Neurovirol. 2016;22:358–65. doi: 10.1007/s13365-015-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W Translational Methamphetamine ARCG. Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2013;19:709–17. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Morris SR, Kent CK, Stansell J, Klausner JD. Methamphetamine use and sexual activity among HIV-infected patients in care--San Francisco, 2004. AIDS Patient Care STDS. 2006;20:502–510. doi: 10.1089/apc.2006.20.502. [DOI] [PubMed] [Google Scholar]
- Mucke L, Abraham CR, Ruppe MD, Rockenstein EM, Toggas SM, Mallory M, Alford M, Masliah E. Protection against HIV-1 gp120-induced brain damage by neuronal expression of human amyloid precursor protein. J Exp Med. 1995;181:1551–1556. doi: 10.1084/jem.181.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N, Kang YJ, Tu S, McKercher SR, Masliah E, Lipton SA. Differential Effects of Pharmacologic and Genetic Modulation of NMDA Receptor Activity on HIV/gp120-Induced Neuronal Damage in an In Vivo Mouse Model. J Mol Neurosci. 2016;58:59–65. doi: 10.1007/s12031-015-0651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21:227–34. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath BM, Schumann KE, Boyer JD. The chimpanzee and other non-human-primate models in HIV-1 vaccine research. Trends Microbiol. 2000;8:426–431. doi: 10.1016/s0966-842x(00)01816-3. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted RA, Barnes AK, Yamamoto JK, Hirsch VM, Purcell RH, Johnson PR. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989;86:2448–2452. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E. Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. Am J Pathol. 2011;178:1646–1661. doi: 10.1016/j.ajpath.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Poli G, Biswas P, Fauci AS. Interferons in the pathogenesis and treatment of human immunodeficiency virus infection. Antiviral Res. 1994;24:221–233. doi: 10.1016/0166-3542(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Poluektova LY, Munn DH, Persidsky Y, Gendelman HE. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J Immunol. 2002;168:3941–3949. doi: 10.4049/jimmunol.168.8.3941. [DOI] [PubMed] [Google Scholar]
- Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Toggas SM, Lee S, Bloom FE, Epstein CJ, Mucke L. Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: evidence for involvement of NMDA receptors and nitric oxide synthase. Virology. 1996;226:362–373. doi: 10.1006/viro.1996.0664. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Maung R, Sejbuk NE, Ake C, Kaul M. Alteration of Methamphetamine-induced stereotypic behaviour in transgenic mice expressing HIV-1 envelope protein gp120. J Neurosci Methods. 2010;186:222–5. doi: 10.1016/j.jneumeth.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29:3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson HA, Tyor WR. Cognitive dysfunction in HIV encephalitic SCID mice correlates with levels of Interferon-alpha in the brain. AIDS. 2007;21:2151–2159. doi: 10.1097/QAD.0b013e3282f08c2f. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, Jarvik JG, Miller EN, Singer EJ, Ellis RJ, Kolson DL, Simpson D, Nath A, Berger J, Shriver SL, Millar LL, Colquhoun D, Lenkinski R, Gonzalez RG, Lipton SA. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- Shi B, De GU, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, Grant I Translational Methamphetamine ARCG. Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. J Neuroimmune Pharmacol. 2016;11:495–510. doi: 10.1007/s11481-016-9699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JP, Bachani M, Wolfson-Stofko B, Lee MH, Wang T, Li G, Li W, Strayer D, Haughey NJ, Nath A. Interaction of paroxetine with mitochondrial proteins mediates neuroprotection. Neurotherapeutics. 2015;12:200–216. doi: 10.1007/s13311-014-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaney VE, O’Neill AM, Hoefer MM, Maung R, Sanchez AB, Kaul M. IFNβ Protects Neurons from Damage in a Murine Model of HIV-1 Associated Brain Injury. Scientific Reports. 2017;7:46514. doi: 10.1038/srep46514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas FP, Chalk C, Lalonde R, Robitaille Y, Jolicoeur P. Expression of human immunodeficiency virus type 1 in the nervous system of transgenic mice leads to neurological disease. J Virol. 1994;68:7099–7107. doi: 10.1128/jvi.68.11.7099-7107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Mucke L. Transgenic models in the study of AIDS dementia complex. Curr Top Microbiol Immunol. 1996;206:223–241. doi: 10.1007/978-3-642-85208-4_12. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Mucke L. Transgenic models to assess the pathogenic potential of viral products in HIV-1-associated CNS disease. In: Gendelman HE, Lipton SA, Epstein L, Swindells S, editors. The neurology of AIDS. Chapman & Hall; New York: 1998. pp. 156–167. [Google Scholar]
- Toneatto S, Finco O, van der PH, Abrignani S, Annunziata P. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS. 1999;13:2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Miller RJ. The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol. 2005;160:68–76. doi: 10.1016/j.jneuroim.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci U S A. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina A, Jones K. Crystal methamphetamine, its analogues, and HIV infection: medical and psychiatric aspects of a new epidemic. Clin Infect Dis. 2004;38:890–894. doi: 10.1086/381975. [DOI] [PubMed] [Google Scholar]
- Van Duyne R, Pedati C, Guendel I, Carpio L, Kehn-Hall K, Saifuddin M, Kashanchi F. The utilization of humanized mouse models for the study of human retroviral infections. Retrovirology. 2009;6:76. doi: 10.1186/1742-4690-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EJ, Sun J, Pettoello-Mantovani M, Anderson CM, Osiecki K, Zhao ML, Lopez L, Lee SC, Berman JW, Goldstein H. Microglia from mice transgenic for a provirus encoding a monocyte-tropic HIV type 1 isolate produce infectious virus and display in vitro and in vivo upregulation of lipopolysaccharide-induced chemokine gene expression. AIDS Res Hum Retroviruses. 2003;19:755–765. doi: 10.1089/088922203769232557. [DOI] [PubMed] [Google Scholar]
- Wang RG, Kaul M, Zhang DX. Interferon-stimulated gene 15 as a general marker for acute and chronic neuronal injuries. Sheng Li Xue Bao. 2012;64:577–83. [PMC free article] [PubMed] [Google Scholar]
- Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S. Nonhuman primate models of NeuroAIDS. J Neurovirol. 2008;14:292–300. doi: 10.1080/13550280802074539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Dimayuga FO, Reed JL, Curry TE, Anderson CF, Nath A, Bruce-Keller AJ. Immune modulation by estrogens: role in CNS HIV-1 infection. Endocrine. 2006;29:289–297. doi: 10.1385/ENDO:29:2:289. [DOI] [PubMed] [Google Scholar]
- Wrasidlo W, Crews LA, Tsigelny IF, Stocking E, Kouznetsova VL, Price D, Paulino A, Gonzales T, Overk CR, Patrick C, Rockenstein E, Masliah E. Neuroprotective effects of the anti-cancer drug sunitinib in models of HIV neurotoxicity suggests potential for the treatment of neurodegenerative disorders. Br J Pharmacol. 2014;171:5757–5773. doi: 10.1111/bph.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Masliah E, Toggas SM, Rockenstein EM, Brooker MJ, Lee HS, Mucke L. Dysregulation of signal transduction pathways as a potential mechanism of nervous system alterations in HIV-1 gp120 transgenic mice and humans with HIV-1 encephalitis. J Clin Invest. 1996;97:789–798. doi: 10.1172/JCI118478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, George SL, Wunschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet. 2004;363:2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- Young JW, Minassian A, Geyer MA. Locomotor Profiling from Rodents to the Clinic and Back Again. Curr Top Behav Neurosci. 2016;28:287–303. doi: 10.1007/7854_2015_5015. [DOI] [PubMed] [Google Scholar]
- Zhou D, Masliah E, Spector SA. Autophagy Is Increased in Postmortem Brains of Persons With HIV-1-Associated Encephalitis. J Infect Dis. 2011;203:1647–1657. doi: 10.1093/infdis/jir163. [DOI] [PMC free article] [PubMed] [Google Scholar]