Abstract
Lectins are proteins that are subject of intense investigations. Information on lectin from chickpea (Cicer arietinum L.) with respect to its biological activities are very limited. In this study, we purified lectin from the seeds of chickpea employing DEAE-cellulose and SP-Sephadex ion exchange chromatography and identified its molecular subunit mass as 35 kDa. The free radical scavenging activity of lectin measured by the DPPH assay has IC50 of 0.88 µg/mL. Lectin exerted antifungal activity against Candida krusei, Fusarium oxysporium oxysporium, Saccharomyces cerevisiae and Candida albicans, while antibacterial activity against E. coli, B. subtilis, S. marcescens and P. aeruginosa. The minimum inhibitory concentrations were 200, 240, 160 and 140 µg for C. krusei, F. oxysporium, S. cerevisiae and C. albicans respectively. Lectin was further examined for its antiproliferative potential against cancerous cell line. The cell viability assay indicated a high inhibition activity on Ishikawa, HepG2, MCF-7 and MDA-MB-231 with IC50 value of 46.67, 44.20, 53.58 and 37.46 µg/mL respectively. These results can provide a background for future research into the benefits of chickpea lectin to pharmacological perspective.
Keywords: Chickpea lectin, Sp-Sephadex, SRB assay, Human cancer cell lines
Introduction
Leguminous plants especially food legumes produce variety of structurally different proteins for self-defence. Characterization of such proteins is of utmost importance and lectins are one of them. Lectins are hemagglutinating proteins distributed ubiquitously in different plant species and extracted principally from seeds. The rich hydrophobic amino acid regions of lectins allow interactions with other molecules like host–pathogen interactions, cell targeting and cell–cell communications (Irlanda et al. 2017). Literature perusal confirm that plant lectins show diversity of biological activities including mitogenic (Chan et al. 2011), anti-fungal and anti-HIV-1 reverse transcriptase activities (Ye and Ng 2002) as well as anticancer properties to human cell lines (Liu et al. 2010). The isolation and purification of lectins from seeds of various taxa have been well documented. Examples include Amaranthus viridis (Kaur et al. 2006), Capsicum frutescens (Ngai and Ng 2007), Phaseolus vulgaris (Xia and Ng 2006), Withania sominifera (Ghosh 2009), Zea mays (Baker et al. 2009) and lima bean (E Lacerda et al. 2017).
Cicer arietinum L., commonly known as chickpea, is an extensively consumed pulse in India and worldwide. Seeds of chickpea provide major source of dietary proteins associated with health promoting benefits in foremost diseases like diabetes, cardiovascular diseases and some cancers (Roy et al. 2010). From chickpea, diverse class of proteins with cyclophilin-like protein (Ye and Ng 2002), chitinase (Vogelsang and Barz 1993) and some peptides displaying antifungal activities have also been identified (Chu et al. 2003). Previous reports of chickpea lectin on various facets include characterization of a clone encoding vegetative lectin from Cicer arietinum epicotyls (Esteban et al. 2002), production of stable transgene chickpea through expression of insecticidal lectin (Chakraborti et al. 2009) and root lectins (Agrawal et al. 2011). In addition, in silico studies on recombinant chickpea lectin (Wakankar et al. 2013), three dimensional structure determination (Sharma et al. 2015) and α-amylase inhibitory activity against potato beetle larvae (Wang et al. 2017) are also available in literature domain.
Lectins have been the subject of intense investigations. Chickpea lectin demonstrate diversity in molecular weights. In 1983, a 47 kDa lectin from seeds of Cicer arietinum was isolated by Kolberg et al. In 2005, our previous studies reported crystallization and preliminary X-ray characterisation of a lectin from chickpea having molecular weight of 43 kDa composed of two identical subunits (Katre et al. 2005). In 2006, Qureshi et al. isolated 120 kDa lectin from seeds of chickpea possessing high affinity for N-acetyl-d-galactosamine. Further studies related to its biological activity have not been reported for this lectin and therefore warranted. In the present paper, we describe purification of lectin from the seeds of chickpea and its biological characterization concerning antifungal, antibacterial and anticancerous activity.
Material and methods
Chemicals and reagents
SP-Sephadex and SRB was procured from Sigma-Aldrich, St. Louis, MO, USA. DEAE-cellulose was obtained from Sisco-Research Laboratories (SRL) India. Other chemicals used in the experiment were of analytical grade.
Seed material
Seeds of Cicer arietinum L. (cv-Digvijay) was obtained from Mahatma Phule Krishi Vidyapeeth, Rahuri, Maharashtra, India under MTA understanding.
Isolation and purification of lectin
Defatted 100 g chickpea seed powder was soaked in 500 mL of 10 mM Tris–HCl pH 7.2. The suspension was stirred for 16 h, centrifuged at 10,000 g for 20 min. The obtained supernatant was precipitated with 80% ammonium sulfate and recentrifuged at 10,000 g for 20 min at 4 °C. The precipitate was dissolved in 20 mM Tris–HCl buffer pH 7.2 and dialysed extensively against the same buffer. The dialysate was centrifuged at 10,000 g for 10 min and the clear supernatant was loaded onto a DEAE-cellulose column pre-equilibrated with 20 mM Tris–HCl buffer pH 7.2. The column was washed with the same buffer until the wash showed no absorbance at 280 nm. The lectin eluted as unadsorbed portion. Fractions (2 mL) of the wash with optical density > 0.2 possessing hemagglutination activities were pooled together and dialysed against 20 mM acetate buffer pH 5.0. Further, sample was loaded onto SP-Sephadex column (4 × 20 cm) pre-equilibrated with 20 mM acetate buffer (pH 5.0) and washed with the same buffer. Finally, the bound protein was eluted with acetate buffer (pH 5.0) containing NaCl in a step gradient of 0.1–0.5 M NaCl in steps of 0.1 M (Katre et al. 2005). The fractions showing hemagglutination activity were pooled and dialysed against deionized water. Protein concentrations at various stages of investigations were determined by Lowry et al. (1951).
Hemagglutination assay
Hemagglutination activity of the lectin was performed using human (A, B, AB and O) and rabbit erythrocytes. The specific activity of lectin is defined as the titre of hemagglutination per mg of protein (Liener 1962).
DPPH radical scavenging assay
The DPPH radical-scavenging capacity of samples was determined as described by Bersuder et al. (1998). A volume of 500 µl of each sample at different concentrations was added to 375 µl of 99% ethanol and 125 µl of DPPH solution (0.02% in ethanol) as free radical source. The mixtures were shaken and then incubated for 60 min in dark at room temperature. Scavenging capacity was measured spectrophotometrically by monitoring the decrease in absorbance at 517 nm. In its radical form, DPPH has an absorption band at 517 nm which disappears upon reduction by an antiradical compound. Lower absorbance of the reaction mixture indicated higher DPPH free radical-scavenging activity. Ascorbic acid was used as positive control. DPPH radical scavenging activity was calculated using the equation:
where Ablank is the absorbance of the control reaction (containing all reagents except the sample) and Asample is the absorbance of sample (with DPPH solution).
Assay of antifungal activity
Antifungal activity of lectin was assessed against various fungal species which includes Candida krusei and Fusarium oxysporum employing disc diffusion methods. Sterile petri plates (100 × 15 cm) containing 10 mL of potato dextrose agar (PDA) were used. After the mycelia colony had developed, sterile paper discs (6 mm diameter) were laid at a distance of 1 cm from the rim of mycelia colony. An aliquot of lectin dissolved in milli-Q water (1 mg/mL) was applied to a disk. The petri plates were incubated at 25 °C for 72 h until mycelia growth had enveloped peripheral disks containing control (amphotericin-B) and had produced crescents of inhibition around disks (Ye et al. 2000). The diameter of the clear zone around the well was measured as percent inhibitory influence of lectin using following equation;
where C = diameter of the fungal colony in the control petri dish, T = diameter of the fungal colony in the treated petri dish.
Fungal PDA plates were prepared for Saccharomyces cerevisiae and Candida albicans strains and different concentrations (50, 100, 150, 200, 250 and 300 µg) of dissolved lectin was placed on discs. The plates were incubated for 24 h at 28 °C until optimal growth attained and zone of inhibition was measured.
Assay of antibacterial activity
The antibacterial activity of the lectin was tested against Escherichia coli, Bacillus subtilis, Serratia marcescens and Pseudomonas aeruginosa employing agar disc diffusion method. The different concentrations (50, 100, 150, 200, 250 and 300 μg/mL) of lectin and a standard antibiotic streptomycin (1 mg/mL) was used for assay. The bacterial cultures were grown on nutrient agar medium at 37 °C and zone of inhibition was recorded.
Cell lines and culture conditions
Human endometrial adenocarcinoma cell line (Ishikawa), human hepatocellular carcinoma cell line (HepG2), Human breast cancer cell line (MCF-7), human breast adenocarcinoma cell line (MDA-MB-231) were procured from National Centre for Cell Science (NCCS), Pune, India. These cell lines were grown and maintained in a high glucose concentration (4.5 g/L) Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin in a constant humidified incubator at 37 °C and in 5% CO2. The cells were cultured (about 80% of confluents) once a week, followed by change in media one additional time in a week. Experiments were conducted using cells with < 20 passages. Cells were counted using a haemocytometer and plated according to the number of cells for each experiment for 24 h prior to addition of test compound. The test sample was solubilised in sterile filtered DMSO (< 0.5% in the culture medium) prior to addition to the culture media. Control cells were maintained in parallel and subjected to the same changes in media.
Cell viability assay
The effects of chickpea lectin on cell viability were determined by SRB assay (Skehan et al. 1990). Briefly, the cells were fixed with 10% chilled TCA for 1 h at 4 °C. To each well, 100 μl of 0.4% (w/v) SRB (1% in acetic acid) was added and incubated for 30 min at room temperature. Unbound SRB was removed by three washes with 1% acetic acid and the plates air-dried. For extracting the bound stain, 200 μl of unbuffered 10 mM Tris base, pH (10.5) was added and absorbance was read at 560 nm using microplate reader (Biorad, USA). The cell % inhibition was calculated using the following formula:
where As-absorbance of sample; Ac-absorbance of control.
Statistical analysis
The statistical analyzes were performed using the Graph Pad Prism Software (version 5) and data was expressed as mean ± SD. The IC50 concentration was calculated using concentration-effect regression line.
Result
The plants seed contain variety of proteins and lectins are one of them. The lectins studies carried out in legume species are therefore focused in seeds (Damme et al. 1998) due to 15% of lectin accessibility in the total proteins. Therefore, in the present investigation, seeds of Cicer arietinum L. were used as a source for lectin isolation. A protein with hemagglutinating activity was purified from the seeds of Cicer arietinum L. and designated as CAL. The (NH4)2SO4 precipitated crude extract of seeds gave 546.13 HAU/mg specific activity. The protein was further purified employing sequential ion exchange chromatography i.e. DEAE-cellulose and SP-Sephadex matrices. At every stage of isolation, lectin was agglutinated by trypsin-treated rabbit erythrocytes only.
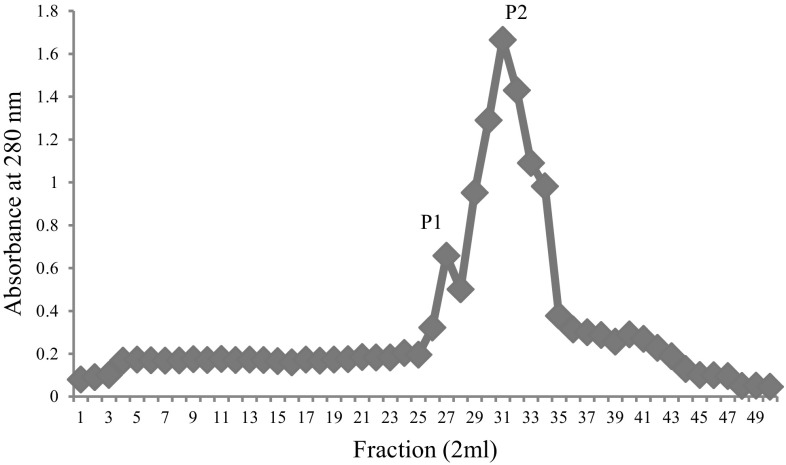
Ammonium sulfate precipitated dialysed seed extract was then chromatographed on DEAE-cellulose and protein eluted in flow- through giving hemagglutinating activity. This impure DEAE-cellulose unbound lectin was further subjected to strong cation exchange SP-Sephadex chromatography. The protein was allowed to adsorb on the cation exchange matrix containing sulfopropyl groups with 30 mL/h flow rate and elution was achieved with acetate buffer employing NaCl gradient (0.1–0.5 M). The 0.2 M eluted fractions showed highest A280 and yielded two peaks (Fig. 1) i.e. P-1 and P-2. The fractions of P-1 didn’t show any hemagglutination and hence was not taken into account for further analysis. The fractions of P-2 gave hemagglutination activity and hence pooled together. The purification chart entailing protein concentration and hemagglutinating activity values for each step are shown in Table 1. At the end of purification process, increased specific activity was found to be 2155.78 HAU/mg proteins. The purification of lectin was confirmed by the presence of single band (Fig. 2), having subunit molecular mass of 35 kDa. Our previous studies reported a 43 kDa chickpea lectin demonstrating molecular mass variation may be due to cultivars differences. The CAL mass is identical to phytohemagglutinin (PHA) of red kidney bean (Nowell 1960), soyabean (SBA) (Rizzi et al. 2003), jack bean (concanavalin-A) (Summer and Howell 1936) and a peanut lectin (PNA) (Pueppke 1979).
Fig. 1.
The cation exchanger SP-Sephadex chromatography of lectin eluted with 0.2 M NaCl in acetate buffer. The fractions of P-2 gave hemagglutination activity and hence pooled
Table 1.
Table depicting yield of lectin during different purification steps
| Purification steps | Total protein (mg/100 g seeds) | Hemagglutination unit (HAU) | Specific activity (HAU/mg protein) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 1600 | 8192 | 512.10 | 1 | 100 |
| 20–80% ammonium sulfate fraction | 750 | 4096 | 546.13 | 1.06 | 50 |
| DEAE-cellulose fraction | 455 | 4096 | 900.21 | 1.75 | 25 |
| SP-sephadex fraction | 95 | 2048 | 2155.78 | 4.20 | 25 |
At every stage of purification, lectin was agglutinated by trypsin-treated rabbit erythrocytes only
Fig. 2.
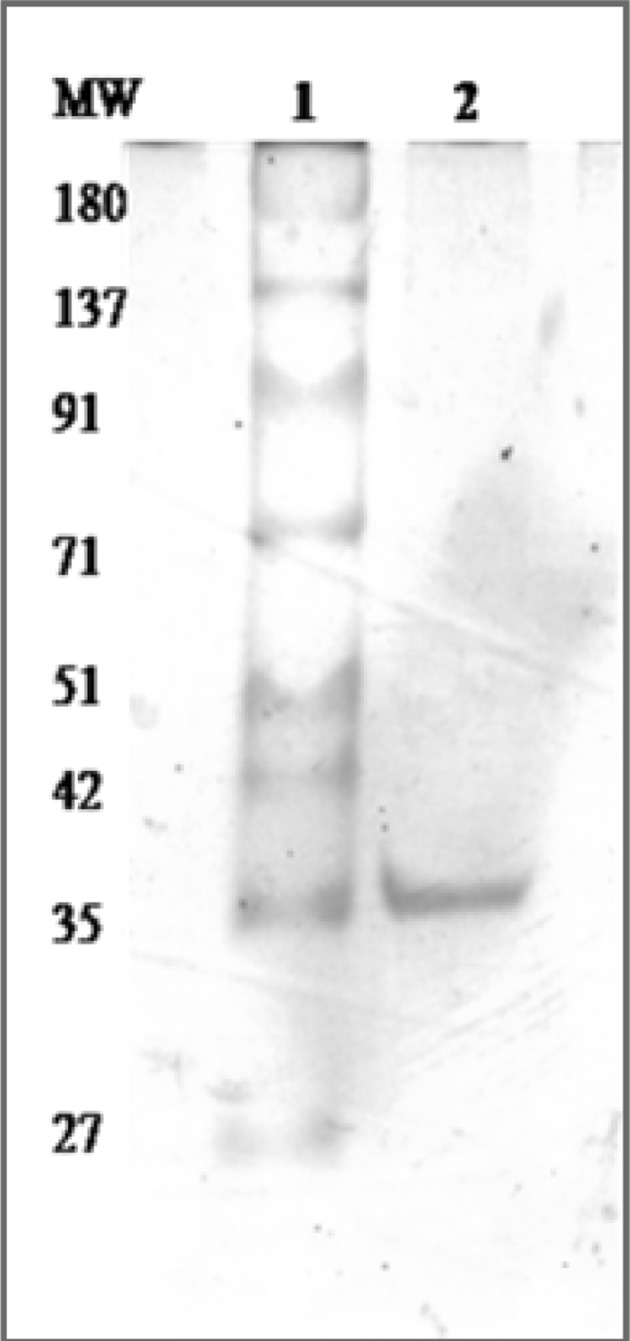
Size fractionation of purified lectin employing SDS-PAGE. Lane-1: Molecular marker, Lane-2: purified lectin
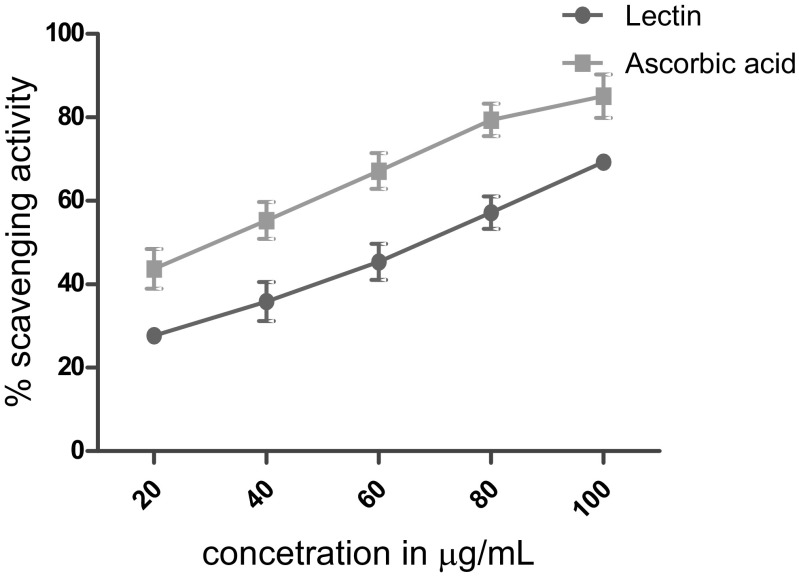
Antioxidants can donate electrons or hydrogen to radicals based on their reduction potential, power to chelate metals involved in oxidative processes and other traits (Manach et al. 2004). Legumes show the presence of bioactive proteins and phenolic compounds and are therefore rich in antioxidant compounds (Cardador-Martínez et al. 2002; Carrasco-Castilla et al. 2012). Therefore, in the present investigation in vitro antioxidant activity of lectin was evaluated employing reduction of DPPH that participates in radicle scavenging (Llorach et al. 2003). Figure 3 shows the significant differences (P < 0.05) in DPPH scavenging activities of lectin in a concentration-dependent manner (20–100 µg/mL). Chickpea lectin exhibited an IC50 of 0.88 µg/mL, compared to the antioxidant activity of ascorbic acid with IC50 of 0.52 µg/mL, which was used as a positive control.
Fig. 3.
Antioxidant activity displayed by lectin. Ascorbic acid was used as a standard
Literature perusal suggests that lectins are useful reagents for fungal inhibition (Jebali et al. 2014). The chitin and glucans are the major constituents of fungal cell wall through which an interaction of lectin antifungal activity occurs (Adams 2004). Lectins validate exploitable antifungal and biological activities. CAL demonstrated an antifungal activity to a growth reduction of 85% against C. krusei while 81% for Fusarium oxysporum (Table 2). Amphotericin B, a positive control showed 88.9% of growth inhibition. The zone of inhibition was measured against S. cerevisiae at 14 mm while C. albicans at 8 mm distance (Fig. 4).
Table 2.
Antifungal activity of chickpea lectin
| S. N. | Organism | Concentration (µg) | Growth inhibition | Positive control |
|---|---|---|---|---|
| 1 | Candida krusei | 200 | 85 ± 0.2% | 88.9 ± 0.12% |
| 2 | Fusarium oxysporium | 240 | 81 ± 0.1% | 86.2 ± 0.18% |
| 3 | Saccharomyces cerevisiae | 160 | 14 ± 0.3 mm | 23 ± 0.51 mm |
| 4 | Candida albicans | 140 | 8 ± 0.5 mm | 30 ± 0.34 mm |
Amphotericin B is used as a positive control
Fig. 4.
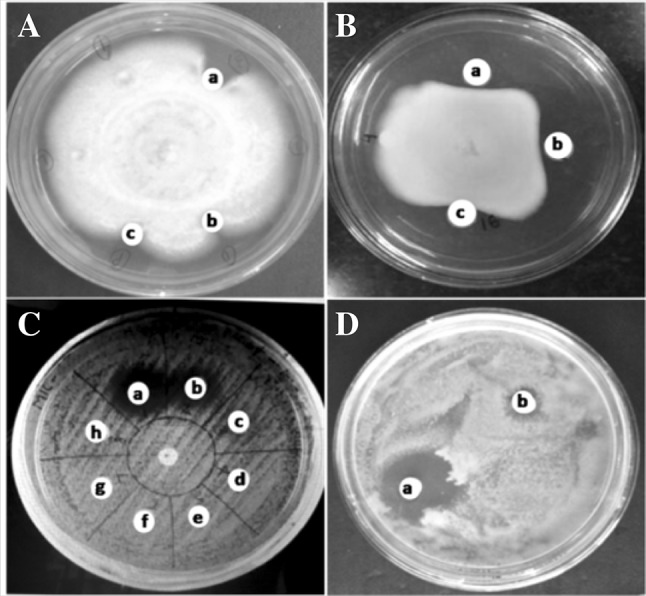
Lectin exibiting antifungal activity towards. A C. krusei (a) control-amphotericin B (20 µg), (b) 200 µg lectin and (c) 250 µg lectin, B F. oxysporium (a) control-amphotericin B (20 µg), (b) 150 µg lectin and (c) 200 µg lectin, C S. cerevisiae (a) control-amphotericin B (20 µg) and (b) 160 µg lectin, D C. albicans (a) control amphotericin B (20 µg) and (b) 140 µg lectin
Lectins inhibit the synthesis and/or deposition of chitin in the cell wall and thus, detrimental to spore germination (Lis and Sharon 1981; Selitrennikoff 2001; Trindade et al. 2006). The cell wall morphogenesis can be inhibited by small molecular weight lectins. For example hevein (4.7 kDa) and poutein (14 kDa) reach the plasma membrane of fungal cell wall by penetration and blocks the catalytic domain of enzymes (Boleti et al. 2007; Gomes et al. 2012). Recent studies have demonstrated the potential of lectins from different origin and carbohydrate specificities as antifungal agents. Plant lectins against phytopathogenic species have reported antifungal effects binding to hyphae, causing inhibition of growth and prevention of spore germination. The antifungal activity reported from plant lectins are restricted including those from Phaseolus vulgaris (Ang et al. 2014), wheat germ (Pusztai 1993), Pisum sativum (Sitohy et al. 2007) and Archidendron jiringa (Charungchitrak et al. 2011). Lectins from Phaseolus vulgaris seeds inhibited the growth of Coprinus comatus and Rhizoctonia solani (Ye et al. 2001). Lectins of many plant species for example red kidney bean and wheat germ lectin specifically binds with sugar N-acetylglucosamine and possessed antifungal activity (Sitohy et al. 2007).
Now a days antimicrobial resistant are the major problems in medical cure however, probing compounds from plant sources are still interesting. Plant lectins are projected to plug this void due to their bioactive principles. The antimicrobial activities of chickpea lectin was therefore evaluated against four species of bacteria B. subtilis, E. coli, S. marcescens and P. aeruginosa. Chickpea lectin showed antimicrobial activity with MICs in the range of 80–180 µg/mL (Table 3). Lectin performance tested against B. subtilis, E. coli, S. marcescens and P. aeruginosa showed zone of inhibition at 16, 15, 4 and 5 mm respectively. The zones of inhibition displayed by the standard drug were 30, 28, 40 and 30 mm respectively (Fig. 5). The lectin exert antimicrobial action in coherence with peptidoglycan layer and/or lipopolysaccharide of bacterial cell wall (Dziarski et al. 2000). The muramic acid and glycogen-binding sites of lectin forms hydrogen bond manifests antibacterial activity as demonstrated by lectin from Lathyrus ochrus (Bourne et al. 1994). The rhizome lectin hinder microbial growth in the four species of B. subtilis, S. aureus, E. coli and P. aeruginosa at MICs values of ≥ 0.011, 0.005, 0.092 and 0.002 mg/mL respectively (Petnual et al. 2010). Our results are in line with earlier reports signifying lectins role against bacteria.
Table 3.
Antibacterial activity of lectin
| S. N. | Organism | Concentration of lectin (µg/mL) | Zone of inhibition (mm) | Positive control (mm) |
|---|---|---|---|---|
| 1 | Bacillus subtilis | 140 µg | 16 ± 0.14 | 30 ± 0.4 |
| 2 | Escherichia coli | 180 µg | 15 ± 0.09 | 28 ± 0.2 |
| 3 | Serratia marcescens | 80 µg | 4 ± 0.30 | 40 ± 0.4 |
| 4 | Pseudomonas aeruginosa | 120 µg | 5 ± 0.19 | 30 ± 0.6 |
Streptomycin is used as a positive control
Fig. 5.
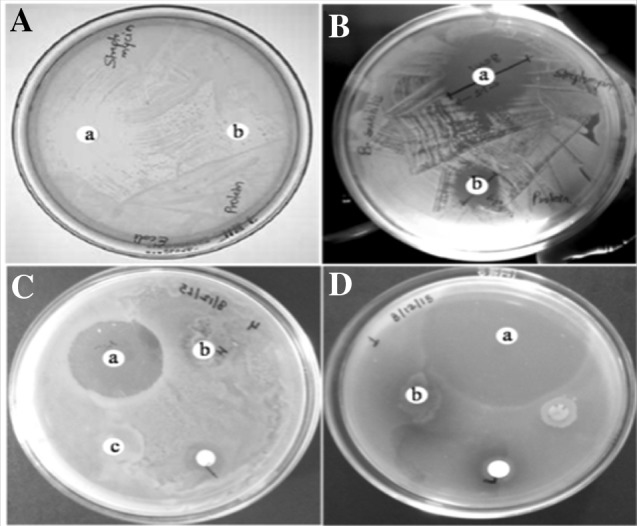
Lectin exibiting antibacterial activity towards. A E. coli (a) control-streptomycin (20 µg), (b) 180 µg lectin, B B. Subtilis (a) control-streptomycin (20 µg) and (b) 140 µg lectin, C S. marcescens (a) control-streptomycin (20 µg) and (b) 120 µg lectin, D P. aeruginosa (a) control-streptomycin (20 µg) and (b) 80 µg lectin
The potential of lectins displaying anticancer activity are well documented. Many studies are underway exploring different cell surface sugar moieties of cancerous cells. Literature perusal revealed different modes of cytotoxic effect by plant lectins (Lin et al. 2008; Li et al. 2010). Earlier studies revealed ethanol/acetone extract from Cicer arietinum L. inhibited Caco-2 cells (Giron-Calle et al. 2004) while C-25, an antifungal protein exerted reduction in human oral carcinoma cell proliferation (Kumar et al. 2014). The other legume lectin family examples include concanavalin A (Schwarz et al. 1999), Canavalia ensiformis (Faheina-Martins et al. 2012), Typhonium divaricatum L. (Luo et al. 2007), phytohaemagglutinin-L (Schwarz et al. 1999), Griffonia simplicifolia agglutinin and wheat germ agglutinin (Kiss et al. 1997).
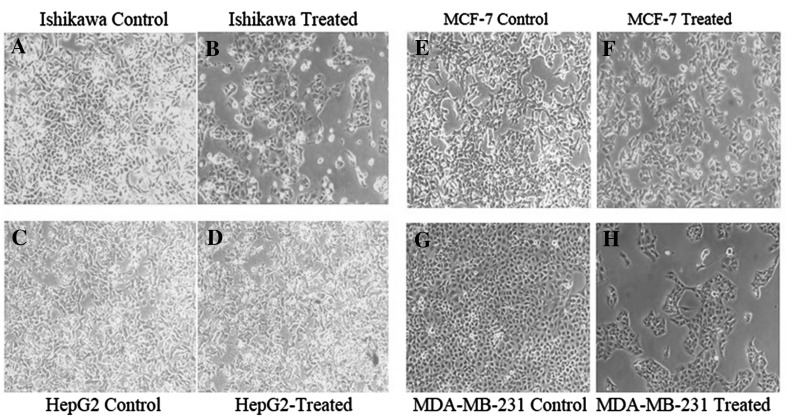
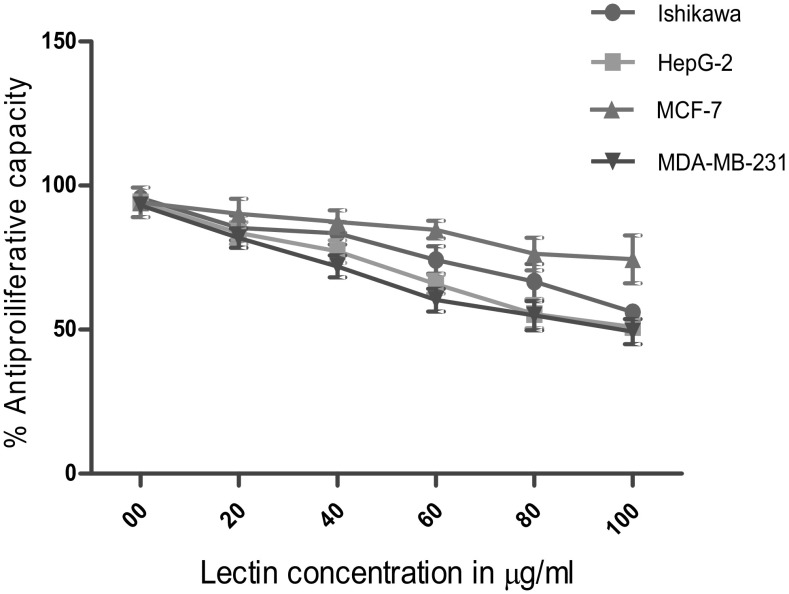
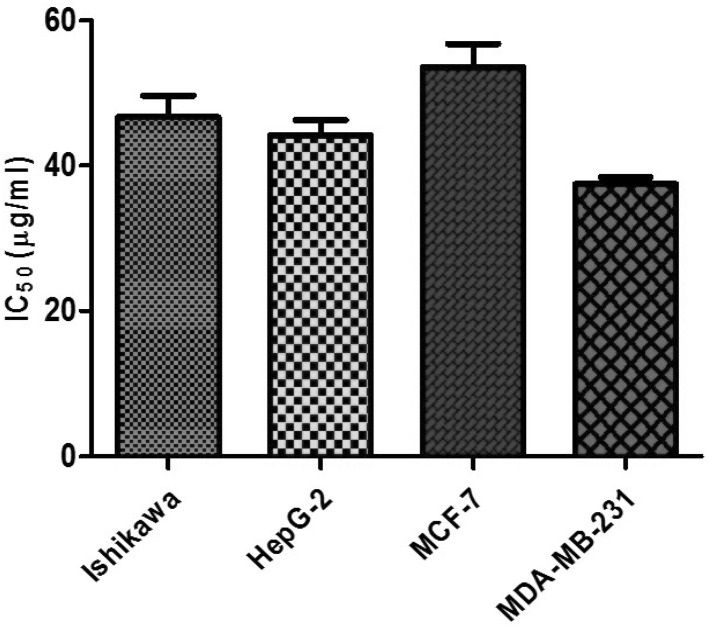
The growth inhibitory effects of CAL on human cancer cell lines were tested. This included Ishikawa, HepG2, MCF-7 and MDA-MB-231 employing SRB assay. In this assay, the fixed cells having basic amino acid binds electrostatically to a dye sulforhodamine-B. This assay is sensitive at large-scale application evaluating cytotoxicity. The MTT assay requires cellular metabolic activity to convert the colorless tetrazolium to the purple colored formazone dye. Therefore, it detects only viable cells, whereas the SRB method does not distinguish between viable and dead cells. This difference however does not compromise the ability of the SRB assay to detect cytotoxic effects of a drug. The morphological changes due to different concentrations of lectin revealed (Fig. 6) marked inhibition on cell viability in a dose-dependent manner. A spectrum of lectin concentration 20, 40, 60, 80 100 µg/mL was employed to test its efficacy on these cell lines (Fig. 7). After 24 h treatment, Ishikawa, HepG2, MCF-7 and MDA-MB-231 cell line possess the IC50 value of 46.67, 44.20, 53.58 and 37.46 µg/mL respectively (Fig. 8). The previous report on other plant hemagglutinins finds similar observation validating anticancer activity (Ye and Ng 2011). Lectins induce apoptosis and regulate the expression of apoptotic genes in cancer cells (Lam and Ng 2010; Ramnath et al. 2009). The present study manifest chickpea lectins as bactericide and fungicide in addition to an antiproliferative agent against cancer cell lines. These results can provide a background for future research into the benefits of chickpea lectin to pharmacological perspective.
Fig. 6.
Effect of chickpea lectin on the viability of human cancer cell lines
Fig. 7.
Cell viability of cancer cells treated with different concentration of lectin
Fig. 8.
The inhibitory concentration of lectin on Ishikawa, HepG2, MCF-7 and MDA-MB-231 cancerous cell line
Acknowledgement
The authors are thankful to University Grants Commission (UGC), New Delhi for funding and specially Mr. Ajay Gautam, for providing research fellowship. The authors are indebted to Dr. M. Yasin, Senior Breeder, RAK Agriculture College, Sehore for providing chickpea seed material.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Adams DJ. Fungal cell wall chitinases and glucanases. Microbiology. 2004;150:2029–2035. doi: 10.1099/mic.0.26980-0. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Kumar S, Jaiswal YK, Das HR, Das RH. A mesorhizobium lipopolysaccharide (LPS) specific lectin (CRL) from the roots of nodulating host plant, Cicer arietinum. Biochimie. 2011;93:440–449. doi: 10.1016/j.biochi.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Ang ASW, Cheung RCF, Dan X, Chan YS, Pan W, Ng TB. Purification and characterization of a glucosamine binding antifungal lectin from Phaseolus vulgaris cv. Chinese Pinto Beans with antiproliferative activity towards nasopharyngeal carcinoma cells. Appl Biochem Biotech. 2014;172(2):672–686. doi: 10.1007/s12010-013-0542-2. [DOI] [PubMed] [Google Scholar]
- Baker RL, Brown RL, Chen ZY, Cleveland TE, Fakhoury AM. A maize lectin-like protein with antifungal activity against Aspergillus flavus. J Food Prot. 2009;72:120–127. doi: 10.4315/0362-028X-72.1.120. [DOI] [PubMed] [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine glucose model system I: investigation of the antioxidant role of histamine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Food Agr. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Boleti APA, Freire MGM, Coelho MB, Silva W, Baldasso PA, Gomes VM, Marangoni S, Novello JC, Macedo ML. Insecticidal and antifungal activity of a protein from Pouteria torta seeds with lectin-like properties. J Agric Food Chem. 2007;55:2653–2658. doi: 10.1021/jf0636317. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Ayouba A, Rouge P, Cambillau C. Interaction of a legume lectin with two components of the bacterial cell wall. J Biol Chem. 1994;269:9429–9435. [PubMed] [Google Scholar]
- Cardador-Martínez A, Loarca-Piña G, Oomah BD. Antioxidant activity in common beans (Phaseolus vulgaris L.) J Agric Food Chem. 2002;50:6975–6980. doi: 10.1021/jf020296n. [DOI] [PubMed] [Google Scholar]
- Carrasco-Castilla J, Hernández-Álvarez AJ, Jiménez-Martínez C, Jacinto-Hernández C, Alaiz M, Girón-Calle J, Vioque J, Dávila-Ortiz G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012;131:1157–1164. doi: 10.1016/j.foodchem.2011.09.084. [DOI] [PubMed] [Google Scholar]
- Chakraborti D, Sarkar A, Mondal HA, Das S. Tissue specific expression of potent insecticidal, Allium sativum leaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora. Transgenic Res. 2009;18:529–544. doi: 10.1007/s11248-009-9242-7. [DOI] [PubMed] [Google Scholar]
- Chan YS, Wong JH, Ng TB. A glucuronic acid binding leguminous lectin with mitogenic activity toward mouse splenocytes. Protein Pept Lett. 2011;18:194–202. doi: 10.2174/092986611794475110. [DOI] [PubMed] [Google Scholar]
- Charungchitrak S, Petsom A, Sangvanich P, Karnchanatat A. Antifungal and antibacterial activities of lectin from the seeds of Archidendron jiringa Nielsen. Food Chem. 2011;126(3):1025–1032. doi: 10.1016/j.foodchem.2010.11.114. [DOI] [Google Scholar]
- Chu KT, Liu KH, Ng TB. Cicerarin, a novel antifungal peptide from the green chickpea. Peptides. 2003;24:659–663. doi: 10.1016/S0196-9781(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Damme EJMV, Peumans WJ, Barre A, Rougé P. Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci. 1998;17(6):575–692. doi: 10.1080/07352689891304276. [DOI] [Google Scholar]
- Dziarski R, Rasenick MM, Gupta D. Bacterial peptidoglycan binds to tubulin. Biochim Biophys Acta. 2000;1524:17–26. doi: 10.1016/S0304-4165(00)00137-9. [DOI] [PubMed] [Google Scholar]
- E Lacerda RR, do Nascimento ES, de Lacerda JT, Pinto LD, Rizzi C, Bezerra MM, Pinto IR, Filho SM, Pinto VP, Filho GC, Gadelha CA, Gadelha TS. Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int J Biol Macromol. 2017;95:1072–1081. doi: 10.1016/j.ijbiomac.2016.10.097. [DOI] [PubMed] [Google Scholar]
- Esteban R, Dopico B, Muñoz FJ, Romo S, Labrador E. A seedling specific vegetative lectin gene is related to development in Cicer arietinum. Physiol Plant. 2002;114:619–626. doi: 10.1034/j.1399-3054.2002.1140416.x. [DOI] [PubMed] [Google Scholar]
- Faheina-Martins GV, da Silveira AL, Cavalcanti BC, Ramos MV, Moraes MO, Pessoa C, Araújo DAM. Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines. Toxicol In Vitro. 2012 doi: 10.1016/j.tiv.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Ghosh M. Purification of a lectin-like antifungal protein from the medicinal herb, Withania somnifera. Fitoterapia. 2009;80:91–95. doi: 10.1016/j.fitote.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Giron-Calle J, Vioque J, DelMarYust M, Pedroche J, Alaiz M, Millán F. Effect of chickpea aqueous extracts, organic extracts, and protein concentrates on cell proliferation. J Med Food. 2004;7(2):122–129. doi: 10.1089/1096620041224175. [DOI] [PubMed] [Google Scholar]
- Gomes FS, Procópio TF, Napoleão TH, Coelho LCBB, Paiva PMG. Antimicrobial lectin from Schinus terebinthifolius leaf. J Appl Microbiol. 2012;114:672–679. doi: 10.1111/jam.12086. [DOI] [PubMed] [Google Scholar]
- Irlanda LD, Ana Maria GP, Luz VM. Legume lectins: proteins with diverse applications. Int J Mol Sci. 2017;18:1–18. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebali J, Fakhfekh E, Morgen M, Srairi-Abid N, Majdoub H, Gargouri A, El Ayeb M, Luis J, Marrakchi N, Sarray S. Lebecin, a new C-type lectin like protein from Macrovipera lebetinavenom with antitumor activity against the breast cancer cell line MDA-MB231. Toxicon. 2014;86:16–27. doi: 10.1016/j.toxicon.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Katre UV, Gaikwad SM, Bhagyawant SS, Deshpande UD, Khan MI, Suresh CG. Crystallization and preliminary X-ray characterization of a lectin from Cicer arietinum (chickpea) Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61:141–143. doi: 10.1107/S1744309104032166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N, Dhuna V, Kamboj SS, Agrewala JN, Singh J. A novel antiproliferative and antifungal lectin from Amaranthus viridis Linn seeds. Protein Pept Lett. 2006;13:897–905. doi: 10.2174/092986606778256153. [DOI] [PubMed] [Google Scholar]
- Kiss R, Camby I, Duckworth C, De Decker R, Salmon I, Pasteel JL, Danguy A, Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simpliciflia, concanavalin A, wheat germ, and peanut agglutinin on HCT-15, Lovo, d SW 837 human colorectal cancer cell growth. Gut. 1997;40:253–261. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolberg J, Michaelsen TE, Sletten K. Properties of a lectin purified from the seeds of Cicer arietinum. Hoppe Seylers Z Physiol Chem. 1983;364(6):655–664. doi: 10.1515/bchm2.1983.364.1.655. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kapoor V, Gill K, Singh K, Xess I, Das SN, Dey S. Antifungal and antiproliferative protein from Cicer arietinum: a bioactive compound against emerging pathogens. Biomed Res Int. 2014;2014:387203. doi: 10.1155/2014/387203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Ng TB. First report of a haemagglutinin induced apoptotic pathway in breast cancer cells. Biosci Rep. 2010;30(5):307–317. doi: 10.1042/BSR20090059. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang G, Ng TB, Wang H. A novel lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. J Biomed Biotech. 2010;2010:716515. doi: 10.1155/2010/716515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liener IE. Toxic factors in edible legumes and their elimination. Am J Clin Nutr. 1962;11:281–298. doi: 10.1093/ajcn/11.4.281. [DOI] [PubMed] [Google Scholar]
- Lin P, Ye X, Ng TB. Purification of melibiose-binding lectins from two cultivars of chinese black soybeans. Acta Biochim Biophys Sin. 2008;40(12):1029–1038. doi: 10.1111/j.1745-7270.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H, Sharon N. Lectins in higher plants. In: Abraham M, editor. Proteins and nucleic acids: the biochemistry of plants. New York: Academic Press; 1981. pp. 371–447. [Google Scholar]
- Liu B, Bian HJ, Bao JK. Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 2010;287(1):1–12. doi: 10.1016/j.canlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Llorach R, Espín JC, Tomás-Barberán FA, Ferreres F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J Agric Food Chem. 2003;51:2181–2187. doi: 10.1021/jf021056a. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Resebrough JJ, Farel L, Randal RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luo Y, Xu X, Liu J, Li J, Sun Y, Liu Z, Liu J, Van Damme E, Balzarini J, Bao J. A novel mannose-binding tuber lectin from Typhonium divaricatum (L.) decne (family Araceae) with antiviral activity against HSV-II and anti-proliferative effect on human cancer cell lines. J Biochem Mol Biol. 2007;40(3):358–367. doi: 10.5483/bmbrep.2007.40.3.358. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy S, Jimenez L. Polyphenols: food source and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Ngai PH, Ng TB. A lectin with antifungal and mitogenic activities from red cluster (Capsicum frutescens) seeds. Appl Microbiol Biotechnol. 2007;74:366–371. doi: 10.1007/s00253-006-0685-y. [DOI] [PubMed] [Google Scholar]
- Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20(4):462–466. [PubMed] [Google Scholar]
- Petnual P, Sangvanich P, Karnchanatat A. A lectin from the rhizomes of turmeric (Curcuma longa L.) and its antifungal, antibacterial, and alpha-glucosidase inhibitory activities. Food Sci Biotechnol. 2010;19:907–916. doi: 10.1007/s10068-010-0128-5. [DOI] [Google Scholar]
- Pueppke SG. Distribution of lectins in the Jumbo Virginia and Spanish varieties of the peanut, Arachis hypogaea L. Plant Physiol. 1979;64:575–580. doi: 10.1104/pp.64.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai A. Antinutritive effects of wheat-germ agglutinin and other N- acetylglucosamine-specific lectins. Br J Nutri. 1993;70:313–321. doi: 10.1079/BJN19930124. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Dash P, Srivastava PS, Koundal KR. Purification and characterization of an N-acetyl-D-galactosamine-specific lectin from seeds of chickpea (Cicer arietinum L.) Phytochem Anal. 2006;17:350–356. doi: 10.1002/pca.925. [DOI] [PubMed] [Google Scholar]
- Ramnath V, Rekha PS, Kuttan G, Kuttan R. Regulation of caspase-3 and Bcl-2 expression in Dalton’s lymphoma ascites cells by Abrin. Evid Based Complement Altern Med. 2009;6(2):233–238. doi: 10.1093/ecam/nem099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi C, Galeoto L, Zoccatelli G, Vincenzi S, Chignola R, Peruffo ADB. Active soybean lectin in foods: quantitative determination by ELISA using immobilised asialofetuin. Food Res Int. 2003;36:815–821. doi: 10.1016/S0963-9969(03)00076-0. [DOI] [Google Scholar]
- Roy F, Boye JI, Simpson BK. Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res Int. 2010;43:432–442. doi: 10.1016/j.foodres.2009.09.002. [DOI] [Google Scholar]
- Schwarz RE, Wojciechowicz DC, Picon AI, Schwarz MZ, Paty PB. Wheat germ agglutinin-mediated toxicity in pancreatic cancer cells. J Cancer. 1999;80:1754–1762. doi: 10.1038/sj.bjc.6690593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selitrennikoff CP. Antifungal proteins. Appl Environ Microbiol. 2001;67(7):2883–2894. doi: 10.1128/AEM.67.7.2883-2894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Katre UV, Suresh CG. Crystal structure of a plant albumin from Cicer arietinum (chickpea) possessing hemopexin fold and hemagglutination activity. Planta. 2015;241(5):1061–1073. doi: 10.1007/s00425-014-2236-6. [DOI] [PubMed] [Google Scholar]
- Sitohy M, Doheim M, Badr H. Isolation and characterization of a lectin with antifungal activity from Egyptian Pisum sativum seeds. Food Chem. 2007;104(3):971–979. doi: 10.1016/j.foodchem.2007.01.026. [DOI] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Summer JB, Howell SF. The identification of the hemagglutinin of the Jack bean with Concanavalin A. J Bacteriol. 1936;32:227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade MB, Lopes JL, Soares-Costa A, Monteiro-Moreira AC, Moreira RA, Oliva ML, Beltramini LM. Structural characterization of novel chitin-binding lectins from the genus Artocarpus and their antifungal activity. Biochim Biophys Acta. 2006;1764:146–152. doi: 10.1016/j.bbapap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Vogelsang R, Barz W. Purification, characterization and differential hormonal regulation of a β-1, 3-glucanase and two chitinases from chickpea (Cicer arietinum L.) Planta. 1993;189:60–69. doi: 10.1007/BF00201344. [DOI] [PubMed] [Google Scholar]
- Wakankar MS, Krishnasastry MV, Jaokar TM, Patel KA, Gaikwad SM. Solution and in silico studies on the recombinant lectin from Cicer arietinum seeds. Int J Biol Macromol. 2013;56:149–155. doi: 10.1016/j.ijbiomac.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen M, Zhu Y, Qian P, Zhou Y, Wei J, Shen Y, Mijiti A, Gu A, Wang Z, Zhang H, Ma H. Isolation, identification and characterization of a new type of lectin with α-amylase inhibitory activity in chickpea (Cicer arietinum L.) Protein Pept Lett. 2017 doi: 10.2174/0929866524666170711120501. [DOI] [PubMed] [Google Scholar]
- Xia L, Ng TB. A hemagglutinin with mitogenic activity from dark red kidney beans. J Chromatogr B Anal Technol Biomed Life Sci. 2006;844:213–216. doi: 10.1016/j.jchromb.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Ye XY, Ng TB. Isolation of a new cyclophilin-like protein from chickpeas with mitogenic, antifungal and anti-HIV-1 reverse transcriptase activities. Life Sci. 2002;70:1129–1138. doi: 10.1016/S0024-3205(01)01473-4. [DOI] [PubMed] [Google Scholar]
- Ye XJ, Ng TB. Antitumor and HIV-1 reverse transcriptase inhibitory activities of a hemagglutinin and a protease inhibitor from mini-black soybean. Evid Based Complement Altern Med. 2011;2011:851396. doi: 10.1155/2011/851396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XY, Wang HX, Ng TB. Dolichin, a new chitinase-like antifungal protein isolated from field beans (Dolichos lablab) Biochem Biophys Res Commun. 2000;269:155–159. doi: 10.1006/bbrc.2000.2115. [DOI] [PubMed] [Google Scholar]
- Ye XY, Ng TB, Tsang PWK, Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J Protein Chem. 2001;20(5):367–375. doi: 10.1023/A:1012276619686. [DOI] [PubMed] [Google Scholar]