Abstract
Cruciferous vegetables are rich source of glucosinolates (GSLs), which in presence of myrosinase enzyme cause hydrolytic cleavage and result in different hydrolytic products like isothiocyanates, thiocyanates, nitriles and epinitriles. The GSLs hydrolytic products are volatile compounds, which are known to exhibit bioactivities like antioxidant, fungicidal, bioherbicidal and anticancer. Among the Brassicaceae family, Brassica juncea is very well known for high content of GSLs. In the present study, the isolation of volatile oil of B. juncea var. raya was done by hydrodistillation method using clevenger apparatus and further there extraction was done by solvents ethyl acetate and dichloromethane. The volatile compounds present in the extract were analysed by gas chromatography/gas chromatography–mass spectrometry (GC/GC–MS). Fatty acid esters, sulphur and/or nitrogen compounds, carbonyl compounds and some other volatile compounds were also identified. Besides the analytical studies, the extracts were analysed for their bioactivities including radical scavenging activity by using DNA nicking assay and cytotoxic effect using different human cancer cell lines viz. breast (MCF-7 and MDA-MB-231), prostate (PC-3), lung (A-549), cervix (HeLa) and colon (HCT116) by MTT assay. The oil extracts were efficiently able to reduce the increase of cancer cells in a dose-dependent manner. Among all cell lines, the most effective anticancer activity was observed in case of breast (MCF-7) cancer cell line. So, MCF-7 cells were used for further mechanistic studies for analysing the mechanism of anticancer activity. Confocal microscopy was done for analysing morphological changes in the cells and the images confirmed the features typical of apoptosis. For evaluating the mode of cell death, spectrofluorometric determination of reactive oxygen species (ROS) and mitochondrial membrane potential (MMP) was done. The volatile oil extract treated MCF-7 cells had a significant increase in number of ROS, also there was a rise in percentage of cells with increased disruption of MMP. So, the present study marks necessary indication that B. juncea (raya) oil extracts significantly induces apoptosis in all the above mentioned cancer cells lines through a ROS-mediated mitochondrial pathway and thus play a remarkable role in death of cancer cells.
Keywords: Brassica juncea Glucosinolates, Isothiocyantes, ROS, MMP, GC–MS
Introduction
Lack of some key dietary components and incessant exposure to xenobiotics can make the human body susceptible to illness. Among all the diseases, cancer is the most dreaded disease responsible for millions of death worldwide. The medication available for its treatment is often associated with a number of complex side effects causing much more damage than cure. A dietary amendment has been long projected as an alternative approach to reduce the risk of cancer (Van Duijnhoven et al. 2009). Natural plant products found as secondary metabolites have been isolated as biologically active compounds with great curative potential as they play an imperative role in various metabolic processes such as stimulation of the immune system, free radical scavenging, regulation of gene expression in cell proliferation, cell-cycle arrest and apoptosis induction (Ramos 2008; Nzaramba et al. 2009). These different properties are due to synergistic action of vast varieties of secondary metabolities including phenols, terpenoids, alkaloids, flavonoids and glucosinolates (Newman and Cragg 2012; Galati and O’brien 2004).
Literature survey reveals that the frequent intake of cruciferous vegetables, such as cauliflower, broccoli, cabbage, turnip and Brussels sprouts is associated with a reduced incidence of cancer (Block et al. 1992). Among crucifer vegetables, Brassica juncea contains maximum amount of a volatile secondary metabolite glucosinolate which is known especially for its anticancer activity (Verhoeven et al. 1997; McNaughton and Marks 2003). Brassica juncea (B. juncea), generally known as Indian mustard, Chinese mustard, leaf mustard, or mustard green, is an important dietary species of mustard family of Brassicaceae plants. Glucosinolates (GSLs) are broken down into different hydrolytic products by the action of an important enzyme myrosinase (EC 3.2.3.1) which is a class of glycoprotein that coexist with glucosinolates in different compartment which are localized in specialized cells referred as “myrosin cells” (Drozdowska et al. 1992; Bones and Rossiter 1996). Among the different hydrolytic products, isothiocyanates especially have higher biological activity in contrast to the other products. Thus, isothiocyanate is considered to be the most significant cancer chemo-preventive phytochemical with potential health benefits (Okulicz 2010; Zhang 2010).
Till date more than 120 glucosinolates have been identified from Brassicaceae (Cruciferae) family (Fahey et al. 2001). These are basic β-d-thiogluco with variable side chain, which include alkyl, alkenyl, hydroxyalkyl, methylsulfinylalkyl, aryalkyl, methylthioalkyl and indole groups (Fahey et al. 2001). The major glucosinolates present in cruciferous vegetables are mainly categorized into aliphatic, aromatic and indole glucosinolates depending on the amino acid origin i.e. aliphatic amino acid (methionine), aromatic (tyrosine, phenylalanine or tryptophan) (Wittstock and Halkier 2002). The hydrolytic products of glucosinolates are responsible for the characteristic pungent aroma and also know for their diverse biological activites like anti microbial, antibacterial, antimutagenic and anticancer (Bones and Rosssittes 1996; Luciano and Holley 2009; Fahey et al. 2001). The cruciferous glucosinolates and their hydrolytic products can effectively inhibit tumour cell growth and, thus reduce the frequency of cancer (Martínez-Villaluenga et al. 2008). Isothiocyanates have high biological activity in comparision to other hydrolytic products of glucosinolates. The isothiocyanates are considered significant cancer chemo-preventive phytochemical with potential of modulation of phases 1 and 2 enzymes to block carcinogenesis, and also capable of inducing apoptosis to inhibit growth of malignant cells (Conaway et al. 2002; Hecht 1999; Navarro et al. 2011; Thornalley 2002; Okulicz 2010; Zhang 2010). Keeping this in mind, the present study focused on therapeutic potentials and profiling of different volatiles present in B. juncea variety raya. The volatile oils obtained were evaluated for their antioxidative potential as well as cytotoxic studies. Futher, their mechanistic studies were done using confocal imaging, ROS assay and MMP assay.
Materials and methods
Chemicals and reagents
Roswell park memorial institute medium (RPMI-1640), Dulbecco’s Modified Eagle’s medium (DMEM), penicillin, streptomycin, foetal bovine serum (FBS), Rohdamine-121 and the fluorescent probes 2,7-dichlorofluorescin diacetate (DCFH-DA) were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). Gentamicin was purchased from Abbott Healthcare Pvt. Ltd. pBR322 plasmid DNA was purchased from GeNeiTM. Mumbai, India. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and trypan blue dye were purchased from HiMedia, Mumbai, India. All the remaining reagents used were of analytical grade.
Preparation of plant extract
Brassica juncea (raya) seeds were procured from Punjab Agricultural University (PAU), Ludhiana. They were washed and dried to remove any dust. The extraction of glucosinolate hydrolytic products (GHPs) was done using modified hydrodistillation method (Arora et al. 2016). For this, 200 g seeds of raya were crushed and added to 1000 mL of distilled water. The crushed mixture was added in a flat bottom flask and placed on a magnetic stirrer with hot plate. Further, Clevenger apparatus was attached to the flask. The hot plate was then set at 500 °C and rotation at 500 rpm until it reached boiling point and then the hot plate was adjusted to 400 °C and 380 rpm. The distillate was collected as a mixture of water and oil. The glucosinolate hydrolytic products in the mixture were then extracted using ethyl acetate (EA) and dichloromethane (DCM) simultaneously. The upper oily layer was separated and lower one was discarded. Excess of solvent in this oil layer was evaporated by using rota evaporator. The final residue was then collected and stored at (− 80 °C) until further use.
Gas chromatography mass spectrometry
Analysis of extract was done using a Shimadzu gas chromatography (GC) (model 2010) coupled with a mass spectrometry (MS) detector equipped with a capillary column. Helium gas was used as carrier gas which was maintained at flow rate of 2 mL/min and injection volume of 1 μL. The column used was equipped with DB-5MS capillary column. The temperature of column was programmed at 60 °C at the start for 5 min and then slowly raised up to 280 °C for 5 min. The spectrum was scanned from m/z 250–500 amu. The components of ethyl acetate and DCM extract were identified using Wiley’s online computer library and with published data.
DNA protection assay
The ability of extracted volatile oils (ethyl acetate and DCM extracts) to protect pBR322 (super coiled) DNA from damage caused by hydroxyl free radicals was carried out using Fenton reagent as described by Lee et al. 2002. The super coiled plasmid pBR322 DNA (5 µg) was added to 10 µL of freshly prepared Fenton’s reagent (50 mM ascorbic acid, 30 mM H2O2 and 80 mM FeCl3) followed by addition of different concentration of ethyl acetate and DCM extracts. Rutin, a standard compound was taken as a positive control. For negative control, Fenton’s reagent was replaced by distilled water in equal amount. Finally, the total amount of reaction mixture was raised to the 20 μL by adding distilled water. The reaction mixture was incubated at 37 °C for 30 min. Following incubation, 3 µL of loading buffer (50% glycerol and 0.25% bromophenol blue) was added. The reaction mixture containing loading buffer was subjected to agarose gel electrophoresis at 50 v (1.5–2 v/cm) for 1.54 h using 1% agarose gel dissolved in TBE buffer (40 mM tris buffer, 16 mM acetic acid and 1 m EDTA, pH 8.0) along with ethidium bromide. DNA bands were then analyzed and quantified using gel documentation system (Gel DOCXR, Bio Rad, USA) and quantity one software v 4.5.2 (Bio Rad).
Anti proliferative studies
Procurement and maintenance of Cancer cell lines
For cytotoxic activity, six different human cancer cell lines of different origin viz. MCF-7 and MDAMB (breast cancer cell lines), HCT 116 (colon cancer), A549 (lung cancer), PC-3 (prostate cancer) and HeLa (cervix cancer) were used. These cell lines were procured from the National Centre for Cell Science (NCCS, Pune, India) and were grown in DMEM and RPMI-1640 medium supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin (10 µg/mL). The cell cultures were maintained under controlled lab conditions of 37 °C under humidity conditions of 5% CO2.
Measurement of cell viability
Cell viability was determined using method described by Militão et al. 2006 with slight modifications. Cells were trypsinised using trypsinization medium (PBS + Trypsin) and then centrifuged at 2000 rpm. The cell pellet was resuspended in its maintaining medium and cells were stained with trypan blue dye (0.4% in PBS) to assess total viable cells number. The viabile cells were further used for different experiments.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The colorimetric MTT assay was used to determine cell proliferation with slight modifications (Mosmann 1983; Twentyman and Luscombe 1987). Cells were collected by trypsinization and plated for 24 h in a 96 multiwell plate in the concentration of 103 cells per well before treatment. Following incubation, they were treated with different concentrations of test material (plant extracts) in serial dilution for next 24 h. Cells were incubated with MTT dye (100 µL/well) for 4 h. After incubation, the medium was discarded and 100 µL of DMSO was added. Finally, the reading was taken at 595 nm wavelength using microplate reader (Biotek synergy HT).
Nuclear morphology studies
Nuclear morphology studies were done using Confocal microscopy method proposed by White et al. 1987; Bassan et al. 2013. MCF-7 cells were seeded in concentration of 1x106cells/well on cover slip and incubated for 24 h. The cells were treated with IC50 concentration of 1 mL/well ethyl acetate and DCM extracts for 12 h at 37 °C and washed thrice with PBS. Treated cells were fixed with paraformaldehyde and washed with chilled PBS. Then cells permeabilized using 3% PBS were placed over mounting fluid (PBS: Glycerol, 1:1) and stained in dark with DAPI stain (1 mg/mL in PBS). The cells were analysed and photographed under Nikon air laser scanning confocal microscope system (Nikon Corp. Japan).
Estimation of reactive oxygen species
The estimation of intercellular peroxides was measured by using spectrofluorometry method given by Wei et al. 2013 with slight modification. An oxidative sensitive fluorescent dye 2,7-dichlorodihydro fluoroscein diacetate (DCF-DA) was used. The cancer cells were seeded in 26 well plate in the concentration of 1 × 106 cells/well for 24 h. Following incubation for 24 h, the cells were treated with IC30, IC50, and IC70 concentration of plant extracts and positive control camptothecin for 12 h at 37 °C. Then, the cells were washed thrice with phosphate buffered saline (PBS) to remove any extra plant extracts. To each well, DCF-DA with the concentration 10 µg/mL was added and cells were incubated for 30 min. After the period of incubation, cells were washed with PBS to remove any extra dye. They were then analyzed by spectrofluorometry at the excitation and emission wavelength of 480 nm and 530 nm, respectively.
Measurement of mitochondrial membrane potential
The changes in mitochondrial membrane potential (MMP) were measured following the method described by Wang et al. 2003. The cancer cells were incubated in the 24 well plate at a concentration of 106 for 24 h at 37 °C. After 24 h, cells were incubated with plant extracts (IC30, IC50 and IC70) as well as with positive control camptothecin and incubated for 24 h at temperature of 37 °C and 5% level of CO2. Thereafter, cells were washed thrice with PBS. Cells were then treated with Rhodamine-123 at concentration of 10 µg/mL and kept for 1 h incubation. The optical density was measured using microplate reader at 485/20 nm excitation and 528/20 nm emission.
Statistical analysis
The experimental data of MMP and ROS were expressed as mean ± SE along with their F-ratios. To determine mean values and significant differences between the mean, one way analysis of variance (ANOVA) was done at p ≤ 0.05.
Result and discussion
Extraction and profiling
Hydrodisitillation of B. juncea seeds in a Clevenger-type apparatus ensures a higher concentration of volatile compounds and thus eases its isolation process. The high temperature and simultaneous action of myrosinase enzyme result in thermal degradation of parent glucosinolates into their hydrolytic products (Fahey et al. 2001; Bones and Rossiter 1996). The quality and quantity of different hydrolytic products of glucosinolates showed variation with different conditions like temperature, plant part and extraction method (Blaževic and Mastelic 2008; Holst and Williamson 2004). GSLs are generally present in almost all parts of plant viz roots, stem, leaves, fruit (pod), leaves and seeds (Vig et al. 2009). For the present study, seeds of B. juncea var raya were used because seeds are known to be richest source of glucosinolates (Fahey et al. 2001; Bassan et al. 2013; Bhandari and Kwak 2015) and also most frequently used in food. Conventional hydrodistillation method had number of drawbacks like low yield and burning of seed material, which was solved by using modified hydrodistillation method (Arora et al. 2016).
The modified hydrodistillation-method of seeds of raya was done to obtain maximum volatiles of interest. Further, for better recovery, the mixture (water and oil) was passed through solvent ethyl acetate and dichloromethane (DCM) (Kore et al. 1993; Al-Gendy 2008). The compositions of oil fractions were analyzed by comparing their relative retention times and the mass spectra with the available data of authentic samples in online Wiley data library. The ethyl acetate oil fraction of seeds was profiled using GC–MS and represented the percentage total yield about 0.0392% (m/m) from 250 g of seeds. Total 39 major and minor peaks were found out of which, 13 fractions were found to be dominated by the nitrogen and sulfur groups and 12 volatiles were fatty acid and ester covering major compounds and remaining were minor peaks. The GSLs hydrolytic products present in EA oil fraction were identified by comparing their mass data with the wiley’s online computer library and with published data. The Table 1 showed GHPs as 3-butenyl isothiocyanate, allyl isothiocyanate, 3-(Methylthio)Propyl isothiocyanate and phenethyl isothiocyanate. Among GHPs, major compound was Allyl isothiocyanate (23%) derived from GSL sinigrin followed by 2-phethyl isothiocyanate (~ 20%) and 3-butenyl isothiocyanate (18%). GSL gluconapin degradation resulted in 3-butenyl isothiocyanate and 2-phethyl isothiocyanate was derived from parent GSL gluconasturiin. These nitrogen and sulphur compounds results from tissue disruption and myrosinase activity, cleaves glucose molecule into instable intermediate. This unstable aglycone rearranges to produce isothiocyanate depending on parameter like pH, temperature, ferrous ions and storage (Blaževic and Mastelic 2008).
Table 1.
List of hydrolytic products of glucosinolates of B. juncea var. raya ethyl acetate seeds extract, based on MS and GC retention data
| S. no. | Hydrolytic product of glucosinolate | Area % | RT | Mass data [M+] |
|---|---|---|---|---|
| 1 | Allyl isothiocyanate | 0.623 | 5.61 | 117[M+]72 58 45 44 39 |
| 2 | Allyl thiocyanate | 1.359 | 10.30 | 99[M+]72 71 58 44 41 |
| 3 | 3-butenylIsothiocyanate (C5H7NS) | 76.548 | 16.507 | 113[M+] 112 85 72 55 45 |
| 4 | 1-isothiocyanato-3-methyl butane | 0.365 | 20.729 | 129[M+]—— |
| 5 | 3-(Methylthio)propyl isothiocyanate | 0.898 | 33.393 | 147[M+]86 73 72 46 41 |
| 6 | Phenethyl isothiocyanate (C9H9NS) | 2.569 | 40.197 | 163[M+]91 77 65 51 |
The DCM fraction oil was characterised by the presence of total 24 peaks and the percentage total yield obtained was 0.028% (m/m) from 250 g of seeds, which was lower in comparison with ethyl acetate fraction. The analysis of DCM fraction oil gave a diverse number of constituents. Among them major were sulphur-nitrogen compounds and fatty acid/esters. The most of sulphur compounds are hydrolytic products of glucosinolates shown in Table 2 like Sec-Butyl isothiocyanate, Allyl Isothiocyanate, Isothiocyanic acid, Phenethyl isothiocyanate (15.15%) and 4-pentenyl isothiocyanate (12.548%). In both the oil fractions, major fatty acids and esters found were butanedioic acid (1.6–16%), hexaicenoic acid (4.98–20%), nonanedioic acid (4.73%) and octadecenoic acid (5.67–23.3%). Some alkanes, aliphatic and aromatic alcohol were also detected. These aliphatic volatile compounds (alcohols, aldehydes, acids and esters) were degraded products of fatty acid catabolism (Mastelic et al. 2008).
Table 2.
List of hydrolytic products of glucosinolates of B. juncea var. raya dichloromethane (DCM) seeds extract, based on MS and GC retention data
| S. no. | Hydrolytic product of glucosinolate [M+] | Area | RT | Mass data |
|---|---|---|---|---|
| 1. | Sec-butyl isothiocyanate (C5H9NS) | 0.523 | 3.618 | 115[M+]43 55 57 72 |
| 2. | Allyl isothiocyanate | 1.423 | 7.61 | 117[M+]72 58 45 44 39 |
| 3. | 4-Pentenyl isothiocyanate (C6H9NS) | 12.548 | 14.134 | 127[M+] 99 85 72 67 |
| 4. | Isothiocyanic acid (CHNS) | 15.15 | 17.81 | 73[M+]— |
| 5. | Phenethyl isothiocyanate (C9H9NS) | 2.569 | 40.197 | 163[M+]91 77 65 51 |
DNA protection assay
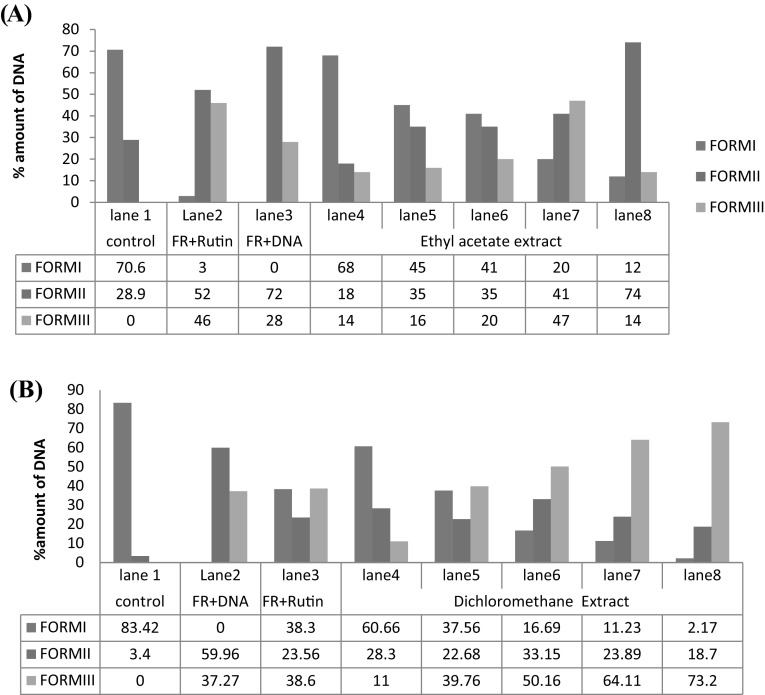
The hydroxyl radical protective effect of both EA and DCM extracts on pBR322 plasmid was done using DNA nicking assay. These hydroxyl radicals produced by stress are harmful, unstable, and thus have the ability to react with biomolecules (Balasubramanian et al. 1998; Agnihotri and Mishra 2009). Hydroxyl radicals produced by the Fenton reaction are the main reason for oxidative nicks in supercoiled DNA FORM I strands which results in its open circular FORM II and relaxed or liner FORM III (Lloyd et al. 1998). For positive control, rutin was used which helps in protecting supercoiled DNA from fenton’s reagent generated free radical attack. The exposure of pBR322 DNA to Fenton’s reagent eventually results in nicks in the strand, mainly due to the generation of reactive species-hydroxyl radical (Golla and Bhimathati 2014). The increase in percentage of double stranded nicked (II) and linear (III) DNA was observed on incubation with Fenton’s reaction mixture. However, amount of supercoiled, open circular and linear DNA varied when reaction mixture was incubated with different concentration of oil extracts. Densitometric analysis showed a higher amount of integrated supercoiled DNA at 1.0 µL/mL concentration of both extracts and confirmed the ˙OH scavenging potential of the extracts and active constituents isolated from B. juncea oil (Fig. 1). The percentage of supercoiled DNA was 68% in EA extract and 60% in case of DCM extract at highest concentration of 1.0 µL/mL of concentration. It was noticed that EA extract showed least protective activity of 12% at 0.2 µL/mL. At lower most concentration of DCM extract 0.2 µL/mL showed 6% which is almost considered as insignificant protection. From the result, it was apparent that the both the oil extracts exhibited significant Fenton reagent radical scavenging activity only at high concentration which is comparable to that of the standard rutin. The amount of protection of DNA proceeds in a concentration-dependent manner. Although both extract have shown remarkable activity at different concentration, but overall ethyl acetate oil extract showed unsurpassed activity in protecting DNA damage against hydroxyl free radicals generated by Fenton’s reagent in comparison to DCM extract. This difference in activity might be due to the presence of high percentage content of allyl isothiocyanates in case of EA extract. A study report by Manesh and Kuttan (2003) explained a sturdy hydroxyl radical scavenging ability of phenyl and allyl isothiocyanates.
Fig. 1.
a Effect of ethyl acetate (EA), b dichloromethane (DCM) extracts of B. juncea (raya) seeds on hydroxyl free radical induced degradation of supercoiled pBR322 plasmid using plasmid DNA nicking assay. 1–8 Represent Lanes (Lane 1: negative control; Lane 2: FR + Rutin; Lane 3: FR; Lane 4–8: FR + concentration of compounds (1.0, 0.8, 0.6, 0.4 and 0.2 µL/mL); A represent FORM II (NICKED); B represent FORM III (LINEAR) and C represent FORM I (SUPERCOILIED). The DNA degradation was analyzed using an agarose-gel electrophoresis. (II) Densitometric analysis of different forms of untreated (CONTROL) and treated DNA
Antiproliferative studies
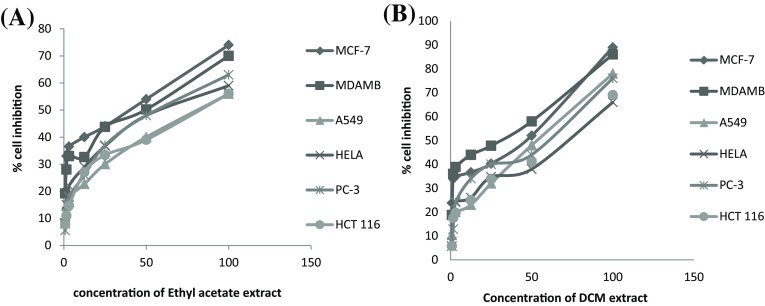
MTT assay is most commonly applicable for the purpose of measuring the cytotoxic potential of drug in case of cell lines (Twentyman and Luscombe 1987). It is a tetrazolium-based colorimetric assay in which MTT dye is reduced by metabolically active cells only as they have ability to convert water insoluble violet-blue formazan crystals which are soluble in organic solvents and measured spectrophotometrically at 545 (van Meerloo et al. 2011; Stockert et al. 2012). Both the oil extracts were tested for cytotoxic potential against six different human cancer cell lines at different concentrations (6.25, 12.5, 25, 50 and 100 µL/mL) by using MTT assay. It was observed that both extracts have shown dose dependent inhibition i.e. as we increase concentration of extract it will increase inhibition in growth of cancer cells. Over all ethyl acetate extract was able to inhibit all cancer cell lines effectively in comparison to DCM extract at same concentration. Their IC30, IC50 and IC70 values were calculated by using best regression model (Fig. 2) and compared with positive control camptothecin in conc. of 1 µ/M (Table 3). It was found that minimum IC50 value of ethyl acetate extract was found to be 32.93 µg/mL in case of MCF-7 cell line and maximum IC50 value is 67.25 µg/mL in case of HeLa cell line. Almost similar results were found in DCM extract with only difference in the minimum IC50value of 43.10 µg/mL in case of MCF-7 and maximum IC50 value of 80.162 µg/mL in case of A549. The hydrolytic products as well as combined effect of volatiles present in the extracts are responsible for the anticancer activity. Al-Gendy et al. 2010 have observed that the hydrolytic product of glucosinolates have shown marked in vitro cytotoxicity against erythioleukaenic K562 cells. Also the hydrolytic products of glucosinolates are known as antimitotic substances and have antiproliferative activity (Nastruzzi et al. 1996). The changes in the pathways of apoptosis may results in many pathological abnormalities which may lead to diseases such as cancer and neurodegenerative diseases etc. (Kroemer and Reed 2000). Present study results showed differential cytotoxic effect of extracts as well as the isolated compounds against different cell lines may be attributed to the chemical nature of different volatiles present or absent in extracts of B. juncea. From GC–MS analysis, the major hydrolytic compound in ethyl acetate extract were allyl isothiocyanate, butenyl thiocyanate and phenethyl isothiocyanate and in DCM extract, major glucosinolates were allyl isothiocyanate. Allyl isothiocyannate are reported to be known for their anticancer property as it inhibits prostate cancer cells (Srivastava et al. 2003) as well as LNCaP human prostate cancer cells (Xiao et al. 2003). The highly significant cancer risk reduction is observed with increasing intake in crucifer (Jain et al. 1999 and Kolonel et al. 2000) and it was also reported that reduction in breast cancer risk is related to crucifer consumption (Terry et al. 2001).
Fig. 2.
Graph showing concentration dependent growth inhibition (%) of human cancer cell line on treatment of ethyl acetate extract (a), dichloromethane (DCM) extract (b) of B. juncea seed
Table 3.
The IC50 values of B. juncea oil extract {ethyl acetate and dichloromethane (DCM)} on different cell lines by using best fit regression model
| Extract | Cell line | Equation | Extracts IC50 |
|---|---|---|---|
| Ethyl acetate | Mcf-7 | y = 0.574x + 28.54 | 32.93 |
| MDA-MB | y = 0.546x + 32.02 | 37.16 | |
| PC-3 | y = 0.632x + 15.41 | 54.73 | |
| A549 | y = 0.597x + 17.55 | 54.35 | |
| HeLa | y = 0.480x + 17.72 | 67.25 | |
| HCT116 | y = 0.518x + 18.14 | 61.50 | |
| Dichloro-methane (DCM) | Mcf-7 | y = 0.447 x + 30.73 | 43.10 |
| PC-3 | y = 0.531x + 15.36 | 65.23 | |
| MDA-MB | y = 0.444x + 27.29 | 51.14 | |
| A549 | y = 0.430x + 15.53 | 80.16 | |
| HeLa | y = 0.461x +18.39 | 68.56 | |
| HCT116 | y = 0.449x + 15.59 | 78.86 |
Nuclear morphology studiess
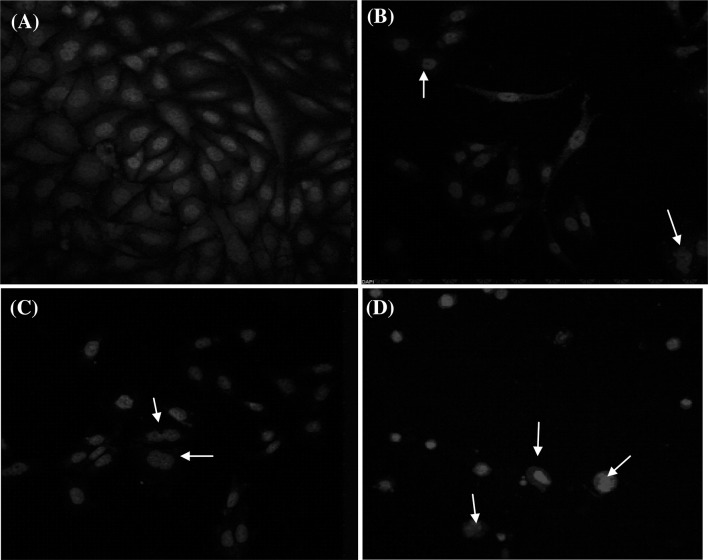
4′,6-Diamidino-2-phenylindole (DAPI) stain selectively fasten to the AT rich regions of DNA and allows monitoring of nuclear damage and morphological changes by confocal microscopy. The images in Fig. 3 show nuclear condensation in MCF-7 cells treated with IC50 value of extracts of ethyl acetate and DCM compared with untreated control cells. For positive control, cells were treated with camptothecin (Fig. 3b) and this result in remarkable nuclear shrinkage. The untreated cells showed no nuclear deformity as shown in Fig. 3a while treated cells demonstrated cell shrinkage, chromatin condensation, chromatin aggregation, and nuclear fragmentation further indicated that these compounds triggered cell death by apoptosis (Ahmad et al. 1997). Liu et al. (2011) reported that isothiocyanates causes the nuclear condensation in case of prostate cancer cells, which lead to apopotsis which further supported these results. The apoptotic cell shows characteristic properties like membrane blebbing, cell shrinking, nuclear condensation, degradation of chromosomal DNA and formation of apoptotic bodies (Pulido and Parrish 2003; Cummings et al. 2004).
Fig. 3.
Nuclear alterations observed in MCF-7cells. a Untreated cells, b cells treated with positive control camptothecin, c cells after treatment with IC50 concentration of ethyl acetate extract, d cells after treatment with IC50 concentration of dichloromethane (DCM) extract. Cells were stained with DAPI stain. Arrows mark the cells, which exhibit blebbing, nuclear condensation, fragmentation and formation of apoptotic bodies
Effect of extracts on intercellular ROS
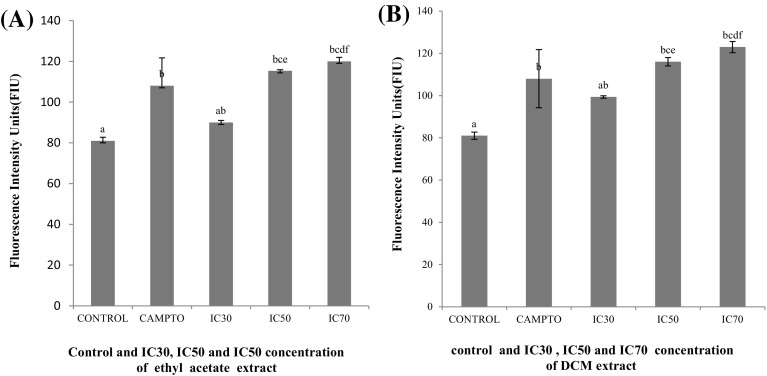
Earlier investigations have shown that treatment of cancer cells with natural plant product results in production of ROS (Efferth et al. 2007; Martín et al. 2009; Luo et al. 2010). Mitochondria produce ROS (reactive oxygen species) which has a role in depolarizing oxidative stress. This excessive production of ROS results in cell apoptosis (Chen et al. 2008; Nigam et al. 2009). ROS can inhibit the mitochondrial respiration chain and initiate apoptotic signaling (Choi et al. 2008). Pelicano in 2003 have shown that increasing ROS generation in mitochondria can effectively kill cancer cells. In the present study MCF-7 cells were treated with different IC (IC30, IC50 and IC70) concentration of both extracts and camptothecin was used as positive control. The ROS generation ability was assessed using a peroxide sensitive fluorescent probe (DCFH-DA), which is converted into 2,7-dichlorofluorescein (DCF fluorescence) and analyzed spectrophotometerically. Both the extracts at IC70 concentration act as effective generators of ROS intermediates and enhances its production, the increased ROS and thus DCF fluorescence was higher than that in control untreated cells. It is also observed from Fig. 4 that IC30, IC50 concentrations are weak elicitors of intracellular ROS. Both extracts were found to alter its generation intracellularly and significantly elevated ROS at IC30, IC50 and IC70 respectively.
Fig. 4.
ROS level analyzed in MCF-7 cells treated with ethyl acetate (a), DCM (b) extracts (IC30, IC50, and IC70) in comparison to untreated cells (control). Error bars indicating standard error mean (n = 4). The concentration with same alphabet shows no significant difference, while the other with different alphabets shows significant difference at p ≤ 0.05
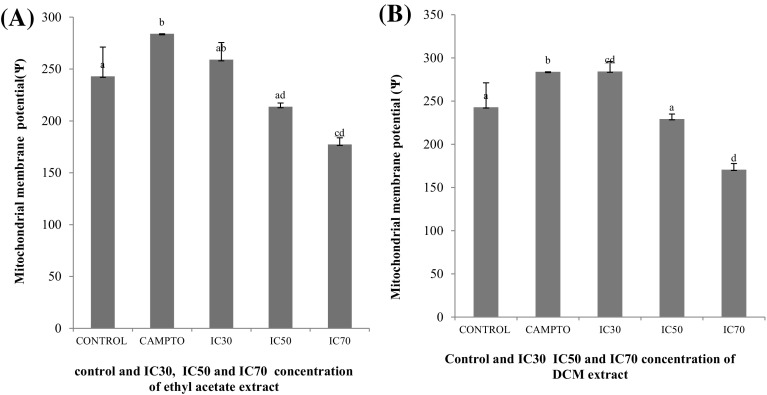
Disruption of mitochondrial membrane potential
One of the key cellular event taking place during apoptosis is disruption of mitochondrial membrane potential (ΔΨmt). The reduction in membrane potential resulted in increased depolarization of mitochondrial membrane that causes increase in permeability and release of factors responsible for apoptosis which eventually leads to cell death (Zhang and Callaway 2002). To analyze the involvement of mitochondria in treated as well as non-treated MCF-7 cells, change in MMP using Rhodamin-123 was measured. The Fig. 5 depicts marked changes in mitochondrial membrane potential (ΔΨmt) in cells treated with both oil extracts in comparison to untreated cells. Both extracts have shown shift in MMP result in a concentration-dependent depolarization in mitochondria i.e. cells treated with the IC70 concentration have showed maximum shift in mitochondrial membrane potential as compared with untreated cells. The stimulus like ROS overproduction or direct DNA damage induce changes in the inner mitochondrial membrane and that results in the reduction of mitochondrial transmembrane potential and thus causes liberation of apoptogenic factors from the intermembrane space into the cytoplasm (Yang et al. 1997; Elmore 2007).
Fig. 5.
MMP level analyzed in MCF-7 cells treated with ethyl acetate (a), DCM (b) extracts (IC30, IC50, and IC70) in comparison to untreated cells (control). Error bars indicating standard error mean (n = 4). The concentration with same alphabet shows no significant difference, while the other with different alphabets shows significant difference at p ≤ 0.05
Summary
In conclusion, our present study provides evidences that the volatile compounds present in extract of ethyl acetate and dichloromethane of B. juncea (raya) seeds are characterised by the presence of hydrolytic products of glucosinolates. Beside glucosinolate hydrolytic products, volatile oil also contains fatty acids and lipoxygease pathway. These extracts provide effective protection to plasmid DNA against hydroxyl ions. However, the strongest activity was exhibited by ethyl acetate extract. The anticancer activity of both extracts was tested against six cancer cell lines by using MTT assay. Both oil fractions have effective anticancer activity and induces cell death in all the six cancer cell lines. However, most effective cytotoxicity results were seen in case of MCF-7 cell line with minimum IC50 value. Mechanistic studies suggested that extract treatment to MCF-7 cells causes morphological changes like membrane blebbing and nuclear condensation in cells that are critical events of apoptosis or cell death. The ability of extracts to generate ROS and MMP was also analysed. Both extracts treatment results in effective cytotoxicity that might be via mitochondria-dependent pathway possibly dependent on ROS generation. Thus, they can further be exploited and their volatiles can be used for the development of chemo curative agents after their further mechanistic studies. Glucosinolate degradation products and other volatiles present in oil fractions may be responsible for the observed bioactivities.
Acknowledgements
Present work was funded by the Department of Science and Technology (DST) Promotion of University Research and Scientific Excellence (PURSE) and the UPE programme of University Grants Commission (UGC), New Delhi.
References
- Agnihotri N, Mishra PC. Mechanism of scavenging action of N-acetylcysteine for the OH radical: a quantum computational study. J Phys Chem B. 2009;113(35):12096–12104. doi: 10.1021/jp903604s. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Feyes DK, Agarwal R, Mukhtar H, Nieminen AL. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- Al-Gendy AA. Phytochemical and biological screening of glucosinolates and volatile constituents of different Brassicaceae plants growing in Egypt. Bull Fac Pharm. 2008;46:235–244. [Google Scholar]
- Al-Gendy AA, El-Gindi OD, Hafez AS, Ateya AM. Glucosinolates, volatile constituents and biological activities of Erysimum corinthium Boiss. (Brassicaceae) Food Chem. 2010;118(3):519–524. doi: 10.1016/j.foodchem.2009.05.009. [DOI] [Google Scholar]
- Arora R, Singh B, Vig AP, Arora S. Conventional and modified hydrodistillation method for the extraction of glucosinolate hydrolytic products: a comparative account. SpringerPlus. 2016;5(1):1–4. doi: 10.1186/s40064-016-2021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci. 1998;95(17):9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassan P, Sharma S, Arora S, Vig AP. Antioxidant and in vitro anti-cancer activities of Brassica juncea (L.) Czern. seeds and sprouts. Int J Pharma Sci. 2013;3:343–349. [Google Scholar]
- Bhandari SR, Kwak JH. Chemical composition and antioxidant activity in different tissues of Brassica vegetables. Molecules. 2015;20(1):1228–1243. doi: 10.3390/molecules20011228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blažević I, Mastelić J. Free and bound volatiles of rocket (Eruca sativa Mill.) Flavour Fragr J. 2008;23(4):278–285. doi: 10.1002/ffj.1883. [DOI] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18(1):1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant. 1996;97(1):194–208. doi: 10.1111/j.1399-3054.1996.tb00497.x. [DOI] [Google Scholar]
- Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181(7):1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WY, Choi BT, Lee WH, Choi YH. Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed Pharmacother. 2008;62(9):637–644. doi: 10.1016/j.biopha.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Conaway C, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3(3):233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Kinsey GR, Bolchoz LJ, Schnellmann RG. Identification of caspase-independent apoptosis in epithelial and cancer cells. J Pharmacol Exp Ther. 2004;310(1):126–134. doi: 10.1124/jpet.104.065862. [DOI] [PubMed] [Google Scholar]
- Drozdowska M, Thangstad OP, Beisvaag T, Evjen K, Bones A, Iversen A. Myrosinase and myrosin cell development DUR embryogenesis and seed maturation. Isr J Bot. 1992;41(4–6):213–223. [Google Scholar]
- Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS ONE. 2007;2(8):e693. doi: 10.1371/journal.pone.0000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Galati G, O’brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Golla U, Bhimathati SS. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci World J. 2014;2014:8. doi: 10.1155/2014/215084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and lung cancer. JNCI J Natl Cancer Inst. 1999;91(14):1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- Holst B, Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat Product Rep. 2004;21(3):425–447. doi: 10.1039/b204039p. [DOI] [PubMed] [Google Scholar]
- Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34(2):173–184. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Prev Biomark. 2000;9(8):795–804. [PubMed] [Google Scholar]
- Kore AM, Spencer GF, Wallig MA. Purification of the ω-(methylsulfinyl) alkyl glucosinolate hydrolysis products: 1-isothiocyanato-3-(methylsulfinyl) propane, 1-isothiocyanato-4-(methylsulfinyl) butane, 4-(methylsulfinyl) butanenitrile, and 5-(methylsulfinyl) pentanenitrile from broccoli and Lesquerella fendleri. J Agric Food Chem. 1993;41(1):89–95. doi: 10.1021/jf00025a019. [DOI] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50(22):6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- Liu KC, Huang YT, Wu PP, Ji BC, Yang JS, Yang JL, Chung JG. The roles of AIF and Endo G in the apoptotic effects of benzyl isothiocyanate on DU 145 human prostate cancer cells via the mitochondrial signaling pathway. Int J Oncol. 2011;38(3):787–796. doi: 10.3892/ijo.2010.894. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Carmichael PL, Phillips DH. Comparison of the formation of 8-hydroxy-2′-deoxyguanosine and single-and double-strand breaks in DNA mediated by fenton reactions. Chem Res Toxicol. 1998;11(5):420–427. doi: 10.1021/tx970156l. [DOI] [PubMed] [Google Scholar]
- Luciano FB, Holley RA. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157: H7. Int J Food Microbiol. 2009;131(2):2405. doi: 10.1016/j.ijfoodmicro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Luo M, Liu X, Zu Y, Fu Y, Zhang S, Yao L, Efferth T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem Biol Interact. 2010;188(1):151–160. doi: 10.1016/j.cbi.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Manesh C, Kuttan G. Anti-tumour and anti-oxidant activity of naturally occurring isothiocyanates. J Exp Clin Cancer Res CR. 2003;22(2):193–199. [PubMed] [Google Scholar]
- Martín R, Ibeas E, Carvalho-Tavares J, Hernández M, Ruiz-Gutierrez V, Nieto ML. Natural triterpenic diols promote apoptosis in astrocytoma cells through ROS-mediated mitochondrial depolarization and JNK activation. PLoS ONE. 2009;4(6):e5975. doi: 10.1371/journal.pone.0005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Villaluenga C, Frías J, Gulewicz P, Gulewicz K, Vidal-Valverde C. Food safety evaluation of broccoli and radish sprouts. Food Chem Toxicol. 2008;46(5):1635–1644. doi: 10.1016/j.fct.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Mastelic J, Jerkovic I, Blažević I, Poljak-Blaži M, Borović S, Ivančić-Baće I, Smrečki V, Žarković N, Brčić-Kostic K, Vikić-Topić D, Müller N. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J Agric Food Chem. 2008;56(11):3989–3996. doi: 10.1021/jf073272v. [DOI] [PubMed] [Google Scholar]
- McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr. 2003;90(03):687–697. doi: 10.1079/BJN2003917. [DOI] [PubMed] [Google Scholar]
- Militão GC, Dantas IN, Pessoa C, Falcão MJ, Silveira ER, Lima MA, Curi R, Lima T, Moraes MO, Costa-Lotufo LV. Induction of apoptosis by pterocarpans from Platymiscium floribundum in HL-60 human leukemia cells. Life Sci. 2006;78(20):2409–2417. doi: 10.1016/j.lfs.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nastruzzi C, Cortesi R, Esposito E, Menegatti E, Leoni O, Iori R, Palmieri S. In vitro cytotoxic activity of some glucosinolate-derived products generated by myrosinase hydrolysis. J Agric Food Chem. 1996;44(4):1014–1021. doi: 10.1021/jf9503523. [DOI] [PubMed] [Google Scholar]
- Navarro SL, Li F, Lampe JW. Mechanisms of action of isothiocyanates in cancer chemoprevention: an update. Food Funct. 2011;2(10):579–587. doi: 10.1039/c1fo10114e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam N, Bhui K, Prasad S, George J, Shukla Y. [6]-Gingerol induces reactive oxygen species regulated mitochondrial cell death pathway in human epidermoid carcinoma A431 cells. Chem Biol Interact. 2009;181(1):77–84. doi: 10.1016/j.cbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Nzaramba MN, Reddivari L, Bamberg JB, Miller JC., Jr Antiproliferative activity and cytotoxicity of Solanum jamesii tuber extracts on human colon and prostate cancer cells in vitro. J Agric Food Chem. 2009;57(18):8308–8315. doi: 10.1021/jf901567k. [DOI] [PubMed] [Google Scholar]
- Okulicz M. Multidirectional time-dependent effect of sinigrin and allyl isothiocyanate on metabolic parameters in rats. Plant Foods Hum Nutr. 2010;65(3):217–224. doi: 10.1007/s11130-010-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido MD, Parrish AR. Metal-induced apoptosis: mechanisms. Mutat Res/Fundam Mol Mech Mutagen. 2003;533(1):227–241. doi: 10.1016/j.mrfmmm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52(5):507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Xiao D, Lew KL, Hershberger P, Kokkinakis DM, Johnson CS, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24(10):1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- Stockert JC, Blázquez-Castro A, Cañete M, Horobin RW, Villanueva Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012;114(8):785–796. doi: 10.1016/j.acthis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA. 2001;285(23):2975–2977. doi: 10.1001/jama.285.23.2975. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Isothiocyanates: mechanism of cancer chemopreventive action. Anticancer Drugs. 2002;13(4):331–338. doi: 10.1097/00001813-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56(3):279. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, Thorlacius-Ussing O. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89(5):1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Cancer Cell Cult Methods Protoc. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997;103(2):79–129. doi: 10.1016/S0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- Vig AP, Rampal G, Thind TS, Arora S. Bio-protective effects of glucosinolates—a review. LWT Food Sci Technol. 2009;42(10):1561–1572. doi: 10.1016/j.lwt.2009.05.023. [DOI] [Google Scholar]
- Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, Agarwal A. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003;80:844–850. doi: 10.1016/S0015-0282(03)00983-X. [DOI] [PubMed] [Google Scholar]
- Wei J, Liu M, Liu H, Wang H, Wang F, Zhang Y, Han L, Lin X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J Appl Toxicol. 2013;33(8):756–765. doi: 10.1002/jat.2725. [DOI] [PubMed] [Google Scholar]
- White JG, Amos WB, Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U, Halkier BA. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002;7(6):263–270. doi: 10.1016/S1360-1385(02)02273-2. [DOI] [PubMed] [Google Scholar]
- Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24(5):891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol Nutr Food Res. 2010;54(1):127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364(Pt 1):301. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]