Abstract
This study aimed to assess the effect of the foliar application of ascorbic acid (AA) and citric acid (CA) on total antioxidant activity (TAA), total phenolics, total flavonoids, total anthocyanin content, antioxidant enzymes, phenylalanine ammonialyase (PAL), and polyphenol oxidase (PPO) activities in apple ‘Red Spur’. The experiment was conducted on 12-years-old trees ‘Red Spur’ grafted on MM106 rootstock. The trees were sprayed with AA (0, 200 and 400 mg L−1) and/or CA (0, 200 and 400 mg L−1) at three different times during summer. Foliar application with AA and CA significantly (p < 0.01) enhanced all measured quality attributes and decreased the activity of PPO. Fruit from trees treated with AA at 400 mg L−1 and CA at 200 mg L−1 showed the highest TAA and catalase (CAT) enzyme activity. Total phenolics increased in fruits when trees were sprayed with AA and CA. Contrasting, AA treatment, CA had no significant effect on guaiacol peroxidase (G-POD). A significant decrease in PPO activity was detected in fruits when treated with both AA and CA. Both treatments significantly decreased the activity of PAL at 400 mg L−1. Considering the results, foliar application of AA and CA, either alone or in combination improved the quality and nutraceutical properties of ‘Red Spur’ apple.
Keywords: Anthocyanin content, Enzyme activity, Total flavonoids, Total phenolics
Introduction
Apple (Malus domestica Borkh.) is one of the most widely cultivated fruit crops and most commonly consumed fruits in the world (Shoji et al. 2004; Francini and Sebastiani 2013). The fruit is important for essential active bio-compounds such as antioxidants, vitamins and phenolic compounds due to the potential to decrease the risk of diseases (Francini and Sebastiani 2013). Besides the genetic, cultural practices may dramatically affect the fruit quality and its active bio-compounds. For this reason and increased public information about the benefit of fruit bio-compounds, consumers are giving more attention to nutraceutical properties of fruits. Apples are rich in antioxidants, flavonoids, anthocyanins, vitamin C, phenolics and some other important bio-compounds. Increasing fruit quality and nutraceutical properties can be important for enhancing the whole economic production for the growers (Shoji et al. 2004; Francini and Sebastiani 2013). Using chemicals in crop production systems have been highly restricted in recent years, due to adverse effects on human health and environment safety. It is necessary to introduce safe and environmental friendly compounds and their application methods to the cropping production systems (Calo et al. 2015). A new approach to enhance crop quality and bioactive compounds is the use of plant growth regulators (PGRs). Some PGRs and natural compounds such as salicylates, nitric oxide, jasmonates, brassinostroides, ascorbic acid (AA) and citric acid (CA) have been shown to improve the yield and quality of various crops (Gomez and Lajolo 2008; Asghari and Rashid Hasanlooe 2015). It has been shown that both AA and CA have substantial roles in many metabolic and physiological processes such as cell enlargement and division, resulting in increased biomass, and increased photosynthesis rate (Fayed 2010). EL-Badawy (2013) reported that AA and CA had improved vegetative growth traits such as shoot length, leaves/shoot ratio, leaf area index as well as fruit characteristics such as TSS, vitamin C, Brix˚, and fruit firmness on apricots cv. Canino.
Ascorbic acid and CA are both antioxidants and anti-stress agents, and also act as a signaling molecule in some plant physiological processes and defense mechanisms (El-Kobisy et al. 2005). Positive roles of such antioxidants in scavenging or chelating the free radicals and activating the natural resistance against different biotic and abiotic stresses have been reported in several fruit trees (Rao et al. 2000). Ascorbic acid is considered as a PGR, playing an important role in cell division and differentiation by promoting the progress from G1 to S phase of the cell cycle and also acts as a co-factor for many enzymes such as hydroxylase enzymes (Elad 1992). Due to its role as a co-factor in the biosynthesis of phytohormones such as gibberellins, ethylene and abscisic acid, AA has an important regulatory role in many physiological and biochemical process of plants. Citric acid plays an essential role in some signal transduction systems, membrane stability and function, transporter enzymes activation and metabolism, and translocation of carbohydrates (Smirnoff 1996). Since they have stimulatory effects on growth and productivity of most fruit trees, AA and CA are considered to have auxinic actions (Ragab 2002). At the same time, these antioxidants, as natural compounds are safe for human, animals, and environment. Since natural compounds and antioxidants are more readily acceptable than synthetic ones, these compounds can be considered as good alternatives to chemicals in agricultural production systems (Asghari and Rashid Hasanlooe 2015).
Exogenous AA has been reported to increase the number and weight of clusters, total yield, berry juice, TSS, TSS/TA ratio, and total sugars in ‘Banatyʼ and ‘Flame seedlessʼ table grapes (Wassel et al. 2007). Also, the positive effects of exogenous AA and CA on fruit quality have been reported on ‘Anna’ apple (Ahmed et al. 1997) grapevine (Fayed 2010), and mango (Mansour et al. 2010). Application of CA on ‘Golden Delicious’ apples has been shown to improve CAT enzyme activity (Yoruk et al. 2005). It has been demonstrated that treatment of plants with AA and CA may significantly enhance fruit quality characteristics such as antioxidants, TSS, total acidity, total sugars, vitamin C level, and improve crop yield in different horticultural crops (El-Hifny and El-Sayed 2011). Ascorbic acid treatment increased the activity of antioxidant enzymes in sugarcane plants under salinity stress condition (Ejaz et al. 2012). The effects of foliar spray with ascorbic acid on antioxidant enzymes activities, lipid peroxidation and proline accumulation in canola (Brassica napus L.) under salt stress conditions has been reported (Dolatabadian et al. 2008). In last decades, fresh food consumers exhibited a superior attention to human health and the environment. These triggered enthusiastic to find alternative approaches for producing “consumer friendly” food in commercial scales. Since not much information is available regarding the effects of AA and CA on apple fruit active bio-compounds, the present study was conducted to determine the effect of foliar spray with AA and CA on fruit quality, total antioxidant activity, total phenolics, flavonoids and anthocyanin contents, CAT, G-POD, PPO and PAL enzymes activity in ‘Red Spur’ apples.
Materials and methods
Sample preparation and treatments
The study was conducted in a standard research apple orchard located at research field at the Urmia University, Iran, 12-years-old apple trees (Malus domestica cv. ‘Red Spur’) grafted on MM106. All trees were irrigated by drip irrigation system and received similar standard cultural practices adopted in the orchard. The foliar parts of trees were sprayed with ascorbic acid (AA) (Merck, KGaA, Germany) at 0, 200 and 400 mg L−1, citric acid (CA) (Merck, KGaA, Germany) at 0, 200 and 400 mg L−1) and the combinations of these treatments at three different times (on July 30, August 25 and September 20) and each tree was sprayed with 5 L of these solutions. Each treatment was conducted on four trees (each tree was considered as a replication). Control trees received no treatments. Fruits were harvested on October 5 at commercial maturity stage (20 fruits from each tree) and transported to the postharvest laboratory of Department of Horticultural Sciences, Urmia, Iran.
Determination of TAA
For determination of TAA, TP, TF and different antioxidant enzymes activity, the juice of 5 fruits including fruit flesh and peel was prepared and homogenized. Total free radicals scavenging activity was measured according to the method of Nakajima et al. (2004) with some modifications reported by Chiou et al. (2007). Fifty microliters of the diluted extracts (concentrations 2–20 mg mL−1) were added to 1 mL of 6 × 10−5 mol L−1 DPPH (free radical, 95%, Sigma Aldrich Chemie GmbH, Steinheim, Germany) in methanol. The mixture was shaked and left at room temperature for 30 min. The absorbance was measured by a spectrophotometer (UNICO UV-2100, Shanghai China) at 515 nm. Methanol was used as background correction. The percent of the reduction in DPPH was calculated according to the following equation, where Abs control is the absorbance of DPPH solution without extract.
Determination of total phenolics (TP) and total flavonoids (TF) contents
TP content was determined using Folin–Ciocalteu reagent, as described by Slinkard and Singleton (1977). Gallic acid (GAE) was used as standard and results were expressed as mg gallic acid per 100 g fresh weight (FW) basis.
Total flavonoids content of fruits was determined by a colorimetric assay according to the method described by Youngjae et al. (2007). One-milliliter aliquot of the appropriately diluted sample was added to a 15 mL tube containing 4 mL of deionized water. Then 0.3 mL of 5% NaNO2 was added to this mixture, which was allowed to stand for 5 min at room temperature, and 0.6 mL of 10% AlCl3·6H2O was added. The mixture was allowed to stand for 6 min at room temperature, and 2 mL of 1 mol L−1 NaOH was added, and the volume was made up to 10 mL with deionized water. The absorbance of the solution was measured immediately at 510 nm. The results were expressed as catechin equivalents using a standard curve prepared from authentic catechin.
Total anthocyanin content (TAC)
TAC in fruit peel was determined according to the method of Fuleki and Francis (1968) with minor modifications. To extract anthocyanin, 3 fruits from each replication, were chosen and peeled, 0.1 g of fruit peel tissue from each sample was ground in 10 mL acidified methanol (99:1, methanol: HCl) then the extracts were centrifuged at 6,000×g for 20 min and held at room temperature for 24 h in the darkness. After 24 h, the absorbance of the extract was measured at 510 nm. Distilled water was used as the blank.
Determination of antioxidant enzymes activity
Catalase (CAT) activity of all treated ‘Red Spur’ apple fruits was assayed according to the method of Aebi (1984) with some modifications using monitoring the disappearance of H2O2 by recording the decrease in absorbance at 240 nm. The reaction mixture contained 2.5 mL sodium phosphate buffer (50 mM, pH 7.0) and 0.3 mL of enzymatic extract. The reaction was initiated by adding 0.2 mL of 1% H2O2. One unit of enzyme activity was expressed as the amount of the enzyme catalyzing the decomposition of 1 μmol H2O2 per min at 30 °C per mg of protein under the assay conditions (Aebi 1984).
To determine guaiacol peroxidase (G-POD) activity, the reaction mixture contained 2.5 mL sodium phosphate buffer (50 mM, pH 7.0), 1 mL of 1% guaiacol, 1 mL of 1% H2O2 and 0.1 mL of enzymatic extract. Final reaction volume was 3.0 mL. The increase in absorbance at 490 nm was recorded for 1 min using a Beckman DU-7, a spectrophotometer for expressing enzyme activity (Upadhyaya et al. 1985).
Polyphenol oxidase (PPO) activity was assayed according to the method of Pizzocaro et al. (1993). 2.5 mL of buffer (100 mM sodium phosphate, pH = 4.6, containing 50 mM catechol) was added to 0.5 mL of fruit extract obtained from 5 fruits. The homogenate was placed for 5 min in water bath at 25° C, and absorption changes in 3 min were measured at 420 nm. The linear section of the activity curve as a function of time was used to determine the PPO activity.
Phenylalanine ammonialyase (PAL) enzyme was extracted according to the method of Karthikeyan et al. (2006). Fruit samples (1 g) were homogenized in 3 mL of ice-cold 0.1 M sodium borate buffer, pH 7.0, containing 2-mercaptoethanol 1.4 mM and 0.1 g insoluble polyvinyl-pyrrolidone. The extract was filtered through cheesecloth and the filtrate was centrifuged at 16,000g at 4 °C for 15 min. The supernatant was used as the enzyme source. The activity of PAL was determined as the rate of conversion of l-phenylalanine to trans-cinnamic acid. A sample containing 0.4 mL of enzyme extract was incubated with 0.5 mL of 0.1 M borate buffer, pH 8.8, and 0.5 mL of 12 mM l-phenylalanine in the same buffer for 30 min at 30 °C. The optical density (OD) value was recorded at 290 nm and the amount of trans-cinnamic acid formed calculated using its extinction coefficient of 9630 M−1. Enzyme activity was expressed as nmol trans-cinnamic acid min−1 g−1 protein.
Statistical analysis
The experiment was arranged as a randomized complete block design (CRD) with a factorial combination of treatments and four single-tree replications of each treatment combination. Treatments were AA, CA, and their application rates. SAS, version 9.3 statistical software (SAS Institute, Cary, NC, USA) was used for analysis of variance (ANOVA), to test for main treatment effects and interactions and mean treatment differences among the treatments were tested at p ≤ 0.01 by Duncanʼs multiple range test.
Results
As shown in Table 1, foliar application of AA and CA significantly enhanced TAA, TP, TF and TAC content of fruits (p < 0.01). According to the results, the highest TAA content based on 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay was recorded in fruits of trees treated with AA and CA. The tested PGRs had higher effects at higher concentrations in enhancing TAA. With increase in AA and CA concentration, TP and TF content was increased but unlike TF content, CA was more effective than AA in enhancing TP content. The highest TF content was recorded in fruits treated with 400 mg L−1 AA. Foliar spray with AA and CA significantly increased the TAC of fruits (p < 0.01). As shown in Table 1, with an increase in AA and CA concentration, fruit anthocyanin content was increased, and the highest levels were recorded when the trees were treated with 400 mg L−1 of AA or CA.
Table 1.
Effect of foliar spray with AA and CA on fruit firmness, TAA, TP, TF, TAC and PAL enzyme activity of ‘Red Spur’ apple fruit
| AA (mg L−1) | CA (mg L−1) | TAA (% DPPH) | TP (mg GAE/100 g FW) | TF (mg CAT/100 g FW) | TAC (mg/100 g Fw) |
|---|---|---|---|---|---|
| 0 | 0 | 26.08d | 337.80c | 1.19c | 5.13c |
| 200 | 29.05cd | 424.66a | 1.27ab | 6.31bc | |
| 400 | 31.90bc | 432.29a | 1.26ab | 7.71a | |
| 200 | 0 | 33.27b | 396.13ab | 1.25ab | 7.22ab |
| 200 | 36.66a | 398.93ab | 1.26ab | 6.46ab | |
| 400 | 33.50b | 414.96a | 1.25ab | 7.09ab | |
| 400 | 0 | 36.78a | 364.52bc | 1.28a | 6.23bc |
| 200 | 38.97a | 422.02a | 1.26ab | 6.99ab | |
| 400 | 37.79a | 362.52bc | 1.25b | 6.75ab |
Means indicated with different lowercase letters in a column are significantly different at p < 0.01 according to the Duncan’s multiple range test
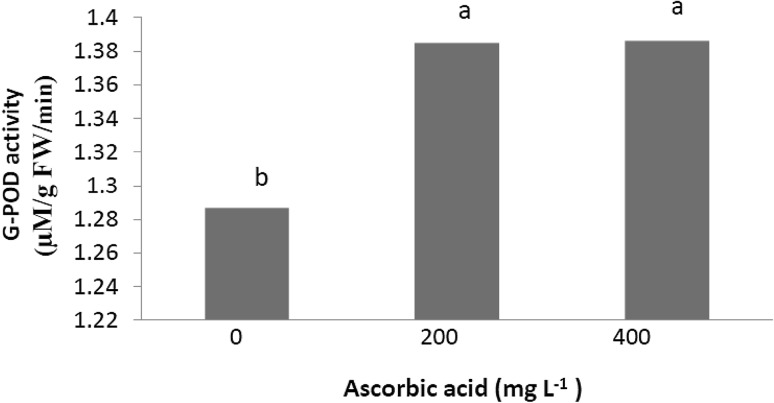
As shown in Fig. 1, increase in CAT activity was observed with AA and CA treatments and AA was more effective than CA (p < 0.01, Fig. 2). No synergistic effect was observed between PGRs in enhancing CAT activity. The highest CAT activity (4.41 U mg−1 protein) was recorded in combination treatment of 200 mg L−1 AA and 400 mg L−1 CA. According to data presented in Fig. 2, AA at 200 and 400 mg L−1 enhanced G-POD activity in ‘Red Spur’ apple fruits (p < 0.01) (1.39 μM/g FW/min). CA had no significant effect on G-POD activity (data not shown). As shown in Fig. 3, significant differences were recorded between the PPO activities of treated and control apples (p < 0.01). PPO activity was effectively decreased in all treatments. The lowest PPO activity was recorded in fruit treated with 200 mg L−1 AA. The maximum level of PPO activity was recorded in control fruit (Fig. 3). PAL enzyme activity was significantly increased by both tested compounds but as shown in Fig. 4, no significant differences were observed between same applied concentrations of AA and CA alone or in combination of them.
Fig. 1.
Interaction effect of AA and CA on CAT enzyme activity of ‘Red Spur’ apples. Means indicated with different lowercase letters are significantly different at p < 0.01 according to Duncan’s multiple range test
Fig. 2.
Effect of AA on G-POD enzyme activity of ‘Red Spur’ apples. Means indicated with different lowercase letters are significantly different at p < 0.01 according to Duncan’s multiple range test
Fig. 3.
Interaction effect of AA and CA on PPO enzyme activity of ‘Red Spur’ apples. Means indicated with different lowercase letters are significantly different at p < 0.01 according to Duncan’s multiple range test
Fig. 4.
Interaction effect of AA and CA on PAL enzyme activity of ‘Red Spur’ apples. Means indicated with different lowercase letters are significantly different at p < 0.01 according to Duncan’s multiple range test
Discussion
Multiple antioxidant systems are involved in plants for scavenging reactive oxygen species (ROS) and free radicals (Foyer and Noctor 2000). AA acts as a secondary antioxidant during reductive recycling of oxidized form of α-tocopherol, another lipophilic antioxidant molecule (Noctor and Foyer 1998). In detoxification process, superoxide dismutase (SOD) converts superoxide radicals to H2O2 at a very fast rate. The toxic H2O2 should be eliminated through the action of CAT and other peroxidases by ascorbate–glutathione cycle (Mishra et al. 2006). AA has been proved to increases the activity of ascorbate–glutathione cycle and CAT in detoxification process pathway (Dixit et al. 2001). AA and CA as antioxidants have anti-stress effects leading to the protection of photosynthetic pigments and photosynthesis systems of the leaves (Fayed 2010). The role of AA in protecting plasma membrane and preventing of lipid oxidation during plant metabolism has been well demonstrated (Hegab 2000). Also, AA and CA has been proposed to have roles in some signal transduction pathways leading to activation of secondary metabolism routes in plants and fruits (Smirnoff 1996). The present study showed that foliar application of AA and CA, enhanced TAA and antioxidant enzymes activity. Our result is in agreement with Shalata and Neumann (2001) who reported that antioxidant activity and resistance of tomato seedlings (Lycopersicon esculentum Mill. cv. M82) increased under salt stress with AA treatment. The positive roles of antioxidants in maintaining the stability of cell membranes and walls may lead to increased fruit quality (Elad 1992; Ezz et al. 2012; El-Badawy 2013; Shazly et al. 2013).
Phenolic compounds are the most important secondary metabolites in plants which act as natural antioxidants, free radical scavengers, and inhibitors of free radical production as well as stimulators for antioxidant synthesis (Hossain et al. 2009). Increase in total phenolics by AA and CA treatment might be due to the important role of these antioxidants in decreasing respiration process (Elad 1992). Also, it has been demonstrated that both AA and CA could enhance the activity of PAL, the key enzyme responsible for phenolics biosynthesis in plants (Winkle-Shirley 2001). Our results confirm the findings of Ezz et al. (2012) who reported an increase of phenolics in mango trees after treatment with AA and CA. Flavonoids are the most common and widely distributed group of plant phenolic compounds, produced in phenylpropanoid pathway. The major flavonoids in apple fruits are quercetin 3-glycosides, catechin, epicatechin, procyanidins, phloridzin and cyanidin-3-glycosides (Lancaster et al. 1994). Our results showed that foliar spray with AA and CA significantly increased fruit TF content. AA and CA may enhance specific secondary metabolism including phenolics and flavonoids in plant tissues (Fayed 2010). The positive effect of these antioxidants on total flavonoids production could attribute to an increase in PAL enzyme activity (Dutilleul et al. 2003). Previous research indicated that AA has a positive effect on photosynthesis, stomatal conductance, respiration decrease, chlorophyll, leaf area and leaf weight (Zulaikha 2013). AA has auxinic function and effective role in the biosynthesis of carbohydrates (Ragab 2002; Shazly et al. 2013). Therefore, it may be possible soluble sugars, which are main substrates for biosynthesis of different secondary metabolites like flavonoids and phenolics, increase by AA and CA treatment.
Apple fruit contains considerable amounts of anthocyanins, chlorophylls, carotenoids, and flavonols, which in combination together play the most important role in fruit color. In red apple cultivars such as ‘Red Spur’, anthocyanin is the main coloring agent (Honda et al. 2002). The red color of the apple is due to the flavonoid cyanidin-3-galactoside located in the vacuoles of skin cells (Lancaster et al. 1994). Because carbohydrates are the main substrate for anthocyanin synthesis, AA and CA have a positive effect on carbohydrate biosynthesis (Zulaikha 2013; Shazly et al. 2013). Increase in anthocyanin production is the result of carbohydrate synthesis and PAL activity and both AA, and CA may have positive roles in increasing carbohydrate production and PAL activity (Farag and Nagy 2012). CAT and G-POD, as important enzymatic antioxidants, can scavenge the ROS and prevent oxidative burst (Zhang et al. 2009). The ROS when left unchecked cause oxidative damage resulting in lipid peroxidation, protein denaturation. ROS is also involved in natural and induced senescence and cell death in plants (Lai et al. 2011). Our results showed that AA and CA significantly increased CAT and G-POD activity. AA is a major metabolite in plants that have antioxidant properties. It has been demonstrated that glutathione-ascorbate cycle and CAT activity may increase in the presence of ascorbic acid to cope with oxidative stress (Dixit et al. 2001). An increased activity of catalase and peroxidase enzymes by AA treatment has been shown in soybean under drought stress conditions (Haddadchi and Gerivani 2009). It has been proposed that AA may enhance the CAT activity by decreasing pH of the cells (Yoruk et al. 2005).
The presence of PPO in plant tissues causes a significant decrease in anthocyanin, leading to loss of color, flavor and nutritional value (Smirnoff 1995). PPO is often activated during the ripening and senescence stage or stress conditions when the membrane is damaged (Mayer 2006). Acidic properties of ascorbic and citric acid enable them to reduce tissue pH and PPO enzyme activity (Lamikanra and Watson 2003; He and Luo 2007). The active site of PPO includes two copper atoms. In addition to lowering the pH, citric acid acts by chelating the copper at the active site of the enzyme (Ibrahim et al. 2004). Our results showed that PPO activity was decreased in all AA and CA treatments. Another possible role of AA and CA in decreasing the PPO activity may be due to their antioxidative roles, which may decrease the membrane deterioration rate and prevent PPO gene expression (Elad 1992).
Conclusions
The results of this study indicated that foliar spray with AA and/or CA significantly improved quality and nutraceutical properties of ‘Red Spur’ apples. Also, increase in PAL enzyme activity, as a key enzyme involved in phenolics, anthocyanins, and flavonoids biosynthesis, results in improved fruit color and nutritional quality. In this study, foliar application of AA and CA effectively reduced PPO activity. Therefore, foliar spray with 400 mg L−1 CA and/or 200 or 400 mg L−1 CA during growth season can apply as a safe and environmentally friendly agrotechnical tool to improve fruit quality, nutraceutical properties, and color of ‘Red Spur’ apples.
Acknowledgement
Authors are thankful to vice chancellor research office at the Urmia University for supporting of this study.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahmed FF, Akl AM, Gobara AA, Monsour AEM. Yield and quality of Anna apple trees (Malusdomestica L.) in response to foliar application of ascobine and citrine fertilizers. Hort Sci. 1997;32:486. [Google Scholar]
- Asghari M, Rashid Hasanlooe A. Interaction effects of salicylic acid and methyl jasmonate on total antioxidant content, catalase and peroxidase enzymes activity in Sabrosa strawberry fruit during storage. Sci Hort. 2015;197:490–495. doi: 10.1016/j.scienta.2015.10.009. [DOI] [Google Scholar]
- Calo JR, Crandall PG, O’Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems—a review. Food Control. 2015;54:111–119. doi: 10.1016/j.foodcont.2014.12.040. [DOI] [Google Scholar]
- Chiou A, Karathanos VT, Mylona A, Salta FN, Preventi F, Andrikopoulos NK. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 2007;102:516–522. doi: 10.1016/j.foodchem.2006.06.009. [DOI] [Google Scholar]
- Dixit V, Pandey V, Shyam R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum) J Exp Bot. 2001;52:1101–1109. doi: 10.1093/jexbot/52.358.1101. [DOI] [PubMed] [Google Scholar]
- Dolatabadian A, Sanavy SAMM, Chashmi NA. The effects of foliar application of ascorbic acid (vitamin C) on antioxidant enzymes activities, lipid peroxidation and proline accumulation of canola (Brassica napus L.) under conditions of salt stress. J Agron Crop Sci. 2008;194:206–213. doi: 10.1111/j.1439-037X.2008.00301.x. [DOI] [Google Scholar]
- Dutilleul C, Garmier M, Noctor G, Mathieu C, Chetrit P, Foyer C, Paepe R. Leaf mitochondria modulate whole cell redox homeostasis set antioxidant capacity and determine stress resistance through altered signaling and during regulation. Plant Cell. 2003;15:1212–1226. doi: 10.1105/tpc.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz B, Sajid ZA, Aftab F. Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp. hybrid cv. HSF-240 under salt stress. Turk J Biol. 2012;36:630–640. [Google Scholar]
- Elad Y. The use of antioxidants (free radical scavengers) to control grey mould (Botrytis cinerea) and white mould (Sclerotiniasclerotiomm) in various crops. Plant Pathol. 1992;41:417–426. doi: 10.1111/j.1365-3059.1992.tb02436.x. [DOI] [Google Scholar]
- El-Badawy HEM. Effect of some antioxidants and micronutrients on growth, leaf mineral content, yield and fruit quality of Canino apricot trees. J Appl Sci Res. 2013;9:1228–1237. [Google Scholar]
- El-Hifny IMM, El-Sayed MAM. Response of sweet pepper plant growth and productivity to application of ascorbic acid and biofertilizer under saline conditions. Aust J Basic Appl Sci. 2011;5:1273–1283. [Google Scholar]
- El-Kobisy DS, Kady KA, Medani RA. Response of pea plant Pisum sativum L. to treatment with ascorbic acid. Egypt J Appl Sci. 2005;20:36–50. [Google Scholar]
- Ezz TM, Aly MA, Awad RM (2012) Storage ability of mango fruits improvement by some natural preharvest applications. In: ATINER’S conference paper series No. AGR 2012-0238, Athens
- Farag KM, Nagy NMN. Effect of pre and post-harvest calcium and magnesium compounds and their combination treatments on Anna apple fruit quality and shelf life. J Hort Sci Ornam Plants. 2012;4:155–168. [Google Scholar]
- Fayed TA. Effect of some antioxidants on growth, yield and bunch characteristics of Thompson seedless grapevine. Am Eur J Agric Environ Sci. 2010;8:322–328. [Google Scholar]
- Foyer CH, Noctor G. Oxygen processing in photosynthesis: regulation and signaling. N Phytol. 2000;146:359–388. doi: 10.1046/j.1469-8137.2000.00667.x. [DOI] [Google Scholar]
- Francini A, Sebastiani L. Phenolic compounds in apple (Malus x domestica Borkh.): compounds characterization and stability during postharvest and after processing. Antioxidants. 2013;2:181–193. doi: 10.3390/antiox2030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins. J Food Sci. 1968;33:266–274. doi: 10.1111/j.1365-2621.1968.tb01365.x. [DOI] [Google Scholar]
- Gomez ML, Lajolo FM. Ascorbic acid metabolism in fruits: activity of enzymes involved in synthesis and degradation during ripening in mango and guava. J Sci Food Agric. 2008;88:756–762. doi: 10.1002/jsfa.3042. [DOI] [Google Scholar]
- Haddadchi GR, Gerivani Z. Effects of phenolic extracts d canola (Brassica napus) on germination and physicological responese of soybean (Glycin max) seedlings. Int J Plant Prod. 2009;3(1):63–74. [Google Scholar]
- He Q, Luo Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 2007;3:1–7. doi: 10.2212/spr.2007.6.3. [DOI] [Google Scholar]
- Hegab YM. Response of Balady mandrin trees to application of citric and ascorbic acid in combined with iron and zinc. Egypt J App Sci. 2000;15(1):50–70. [Google Scholar]
- Honda C, Kotoda N, Wada M, Kondo S, Kobayashi Sh, Soejima J, Zhang Z, Tsuda T, Moriguchi T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. J Plant Physiol Biochem. 2002;40:955–962. doi: 10.1016/S0981-9428(02)01454-7. [DOI] [Google Scholar]
- Hossain MA, Salehuddin SM, Kabir MJ, Rahman SMM, Rupasinghe HV. Sinensetin, rutin, 3′-hydroxy-5, 6, 7, 4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of the skin of apple fruit. Food Chem. 2009;113:185–190. doi: 10.1016/j.foodchem.2008.07.085. [DOI] [Google Scholar]
- Ibrahim R, Osman A, Saari N, Abdul-Rahman RA. Effects of anti-browning treatments on the storage quality of minimally processed shredded cabbage. J Food Agric Environ. 2004;2:54–58. [Google Scholar]
- Karthikeyan M, Radhika K, Mathiyazhagan S, Bhaskaran R, Samiyappan R, Velazhahan R. Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agent. Braz J Plant Physiol. 2006;18:367–377. doi: 10.1590/S1677-04202006000300003. [DOI] [Google Scholar]
- Lai T, Wang Y, Li B, Qin G, Tian S. Defense responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biol Technol. 2011;62:127–132. doi: 10.1016/j.postharvbio.2011.05.011. [DOI] [Google Scholar]
- Lamikanra O, Watson MA. Biochemical changes associated with fresh-cut fruit processing and storage. Fresh Shelf Life Food. 2003;836:52–68. doi: 10.1021/bk-2003-0836.ch004. [DOI] [Google Scholar]
- Lancaster JE, Grant JE, Lister CE, Taylor MC. Skin color in apples-influence of co-pigmentation and plastid pigments on shade and darkness of red color in five genotypes. J Am Soc Hortic Sci. 1994;119:63–69. [Google Scholar]
- Mansour AEM, El-Shammaa MS, Shaaban EA, Maksoud MA. Influence of some antioxidants on yield and fruit quality of four mango cultivars. Res J Agric Biol Sci. 2010;6:962–965. [Google Scholar]
- Mayer AM. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry. 2006;67:2318–2331. doi: 10.1016/j.phytochem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Mishra S, Srivastava S, Tripathi RD, Govindrajan R, Kuriakose SV, Prasad MNV. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopamonnieri L. Plant Physiol Biochem. 2006;44:25–37. doi: 10.1016/j.plaphy.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Nakajima JI, Tanaka I, Seo S, Yamazaki M, Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. Biomed Res Int. 2004;2004:241–247. doi: 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pizzocaro F, Torreggiani D, Gilardi G. Inhibition of apple polyphenoloxidase (PPO) by ascorbic acid, citric acid and sodium chloride. J Food Process Preserv. 1993;17:21–30. doi: 10.1111/j.1745-4549.1993.tb00223.x. [DOI] [Google Scholar]
- Ragab MM (2002) Effect of spraying urea, ascorbic acid and NAA on fruiting of Washington Navel orange trees. M.Sc. thesis, Faculty of Agriculture, Minia University Egypt
- Rao MV, Koch JR, Davis KR. Ozone: a tool for probing programmed cell death in plants. Plant Mol Biol. 2000;44:346–358. doi: 10.1023/A:1026548726807. [DOI] [PubMed] [Google Scholar]
- Shalata A, Neumann PM. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot. 2001;52:2207–2211. doi: 10.1093/jexbot/52.364.2207. [DOI] [PubMed] [Google Scholar]
- Shazly SM, Eisa AM, Moatamed AMH, Kotb HRM. Effect of some agrochemical pre harvest foliar application on yield and quality of Swelling peach trees. Alexander J Agric Res. 2013;58(3):219–229. [Google Scholar]
- Shoji T, Akazome Y, Kanda T, Ikeda M. The toxicology and safety of apple polyphenolic extract. Food Chem Toxicol. 2004;42:959–967. doi: 10.1016/j.fct.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- Smirnoff H. Antioxidant systems and plant response to the environment. In: Smirnoff H, editor. Environment and plant meta bolism. Oxford: BIOS Scientific Publishers; 1995. pp. 217–244. [Google Scholar]
- Smirnoff N. Botanical briefing: the function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. doi: 10.1006/anbo.1996.0175. [DOI] [Google Scholar]
- Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smidth BN. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol. 1985;121:453–461. doi: 10.1016/S0176-1617(85)80081-X. [DOI] [Google Scholar]
- Wassel AH, Hameed MA, Gobara A, Attia M. Effect of some micronutrients, gibberellic acid and ascorbic acid on growth, yield and quality of white Banaty seedless grapevines. Afr Crop Sci Soc. 2007;8:547–553. [Google Scholar]
- Winkle-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoruk IH, Demir H, Ekici K, Sarvan A. Purification and properties of catalase from Van Apple (Golden Delicious) Pak J Nutr. 2005;4:8–10. doi: 10.3923/pjn.2005.8.10. [DOI] [Google Scholar]
- Youngjae S, Rui HL, Jacqueline FN, Darryl H, Christopher B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Technol. 2007;45:349–357. [Google Scholar]
- Zhang Z, Nakano K, Maezawa S. Comparison of the antioxidant enzymes of broccoli after cold or heat shock treatment at different storage temperatures. Postharvest Biol Technol. 2009;54:101–105. doi: 10.1016/j.postharvbio.2009.05.006. [DOI] [Google Scholar]
- Zulaikha R. Effect of foliar spray of ascorbic acid, Zn, seaweed extracts force and bio fertilizers vegetative growth and root growth of olive (Olea Europea L.) transplants cv. Hog Blanca. Int J Pure Appl Sci Technol. 2013;17:79–89. [Google Scholar]