Abstract
Plant antimicrobial peptides are the interesting source of studies in defense response as they are essential components of innate immunity which exert rapid defense response. In spite of abundant reports on the isolation of antimicrobial peptides (AMPs) from many sources, the profile of AMPs expressed/identified from single crop species under certain stress/physiological condition is still unknown. This work describes the AMP signature profile of black pepper and their expression upon Phytophthora infection using label-free quantitative proteomics strategy. The differential expression of 24 AMPs suggests that a combinatorial strategy is working in the defense network. The 24 AMP signatures belonged to the cationic, anionic, cysteine-rich and cysteine-free group. As the first report on the possible involvement of AMP signature in Phytophthora infection, our results offer a platform for further study on regulation, evolutionary importance and exploitation of theses AMPs as next generation molecules against pathogens.
Keywords: Proteomics, Antimicrobial peptides, Differential expression, Host–pathogen interaction
Introduction
Antimicrobial peptides (AMP) are small peptides, size ranging from 2 to 9 kDa with broadspectrum antimicrobial activity. The percentage distribution is high in animals (74.53%) followed by plants (13.57%) (Sarika et al. 2012). On the basis of electric charge, the AMPs are either cationic or anionic (Pelegrini et al. 2011) and they are constitutively expressed or regulated upon stress (Nawrot et al. 2014). AMPs have been isolated from many plants and from various plant parts viz. leaves, stems, roots, flowers and seeds and were proved to act against phytopathogens. They belong to the families viz., defensins, thionins, lipid transfer protein (LTP), snaking, cyclotides and hevein like proteins which are cysteine rich peptides (Park et al. 2000). Except few reports (Egorov et al. 2005; Silva et al 2012; Zipfel 2009), the description of cysteine free AMPs from plants are rare. Apart from the direct action against pathogens, plant AMPs are also important molecules in MAPK (MAP Kinase) defense signaling (Scott et al. 2007), innate immunity (Rahnamaeian 2011), ROS and H2O2 accumulation (Fan et al. 2008).
Black pepper is an export oriented spice crop, rich in essential oil and oleoresin. Among the biotic /abiotic stresses, the foot rot disease caused by Phytophthora is of major concern (Anandaraj 2000) in black pepper. The investigation on presence of AMPs (both constitutive and induced) and its characterization from the resistant genotype would yield information on innate immunity, which will help in developing resistant varieties and also the candidate AMPs as possible lead molecules in future management strategies.
Chromatography based (Cammue et al. 1992) and EST based (Asiegbu et al. 2003; Ke et al. 2015) methods were used to identify and isolate the AMPs from plants. But the AMPs are underrepresented in these conventional methods, including immnunoblots due to their extreme isoelectric points and small size (Zhou et al. 2011). Weinhold et al. 2015 quantified the ectopic expression of AMPs in transgenic Nicotiana attenuata from apoplastic proteins using label-free protein quantification by nanoUPLC-MSE analysis coupled with Hi3 method. Our present study was aimed to explore the label free proteomics strategy to identify the AMPs in resistant variety of black pepper upon infection by Phytophthora. The aim of this work was to bring out the entire profile along with expression quantification of AMP signatures upon infection with Phytophthora from the total leaf protein using label free proteomics and in-silico analysis of physiochemical, biological properties of AMP signatures. For the first time, we showed the occurrence of both cysteine rich, non cysteine AMP signatures from a complex sample and some major AMPs as innate immunity factors against Phytophthora.
Materials and methods
In planta inoculation
Black pepper variety, “IISR Shakthi” resistant to Phytophthora capsici was used in this study. The plants with three to four leaves were inoculated at the abaxial side (in planta) at 3rd leaf using 72 h old mycelium of highly virulent isolate (05-06). Control plants were mock inoculated with moist cotton. The experiment was conducted in triplicates. The leaf samples were collected at 24 hpi (hours post inoculation), the necrotic spot was removed and used for protein extraction. The mock inoculated leaves were also collected for the extraction of proteins. Samples from 3 biological replicates were used for the analysis.
Label free quantitative proteomics
Total leaf protein was extracted (Umadevi and Anandaraj 2015) and quantified. Three biological replicates from control and 24 hpi were used to profile the AMPs. For LC-LTQ Orbitrap MS analysis, samples were re-solubilized in 2% [v/v] acetonitrile, 0.1% [v/v] formic acid in water and injected onto an Agilent1200 (Agilent, Santa Clara, CA, USA) nano-flow LC system that was in-line coupled to the nano-electrospray source of a LTQ-Orbitrap discovery hybrid mass spectrometer(Thermo Scientific, San Jose, CA, USA). Peptides were separated on Zorbax 300SB-C18 (Agilent, Santa Clara, CA, USA) by a gradient developed from 2% [v/v]acetonitrile, 0.1% [v/v] formic acid to 80% [v/v] acetonitrile, 0.1% [v/v] formic acid in water over 70 min at a flow rate of 300 nl/min. Full MS in a mass range between m/z 300 and m/z 2000 was performed in an Orbitrap mass analyzer with a resolution of 30,000 at m/z 400 and an AGC target of 2 × 105. The strongest five signals were selected for CID–MS/MS in the LTQ ion trap at normalized collision energy of 35% using an AGC target of 1 × 105 and two micro scans. Dynamic exclusion was enabled with one repeat counts during 45 s and an exclusion period of 120 s. All the 6 samples were included in the analysis where control samples were chosen as reference and all other ion intensity maps from other samples were automatically aligned to the reference. The peptide ion detection method was high resolution. Considering the good initial alignment quality, the data set was not subjected to any further manual correction such as vector editing. Relative quantification using Hi-3 was selected for automatic processing of the software. After successful alignment, no further filtering was applied to subsequent quantification steps in the software. Parameter settings such as no protein grouping and quantitation from non-conflicting features were used for protein building. Peptide identification was performed by CID-based MS/MS of the selected precursors. For protein/peptide identification, MS/MS data were searched against the APD database using an in-house Mascot server (version 2.4) through the ProteomeDiscoverer1.4 software. The search was set up for full tryptic peptides with a maximum of three missed cleavage sites. Carbamidomethyl on cysteine, and oxidized methionine were included as variable modifications. The precursor mass tolerance threshold was 110 ppm, and the maximum fragment mass error was 0.8 Da. The significance threshold of the ion score was calculated based on a false discovery rate of < 1%, estimated by the peptide valid at or node of the Proteome Discoverer software. Ion matching requirements were two fragments per peptide, five fragments per protein, and one peptide per protein. Anova (p)* 0.05 was kept as significant in selecting the statistically significant fold change expression of AMPs.
Characterization of AMP sequences
We used APD database for the AMP signature identification (Wang et al. 2016). The AMP signatures were also queried with PhyAMP (Hammami et al. 2009) and CAMPR3 (Waghu et al. 2016). In order to characterize the AMPs in silico, we used the descriptors viz., isoelectric point, aliphatic index and grand average of hydropathy (Gasteiger et al. 2005) (GRAVY) (using Protparam tool) and the net charge using PhytoAMP database. We also characterized the AMPs as cysteine rich, cysteine free AMPs. The peptide region coding for antigenicity was predicted using Kolaskar and the secondary structures were predicted using GOR4 (Kolaskar and Tongaonkar 1990). Toxin pred (Gupta et al. 2013) and HLP tool (Sharma et al. 2014) was used to predict the toxic peptides and predict the half-life of the peptides.
Results
Label free proteomics based identification and expression dynamics of AMPs
The AMP identification was done in uninfected (control) and from the 24 hpi sample. A total of 24 black pepper AMPs (BpAMPs) was matched to the known AMPs from both the samples and the quantitative expression was also deduced using Hi3 method (Table 1). The AMPs mass ranged from 731.4332 to 2340.0385. The relative expression dynamics ranged from 1. 3 to 11.15 folds. Fourteen AMPs showed high abundance (5.88–10.59 fold) and the 10 showed low abundance. Low abundance (range) of peptides were found between 3.59 and 1.02 fold. The Hi3 quantification of peptides showed five BpAMPs with above 5 fold increase in expression were BpAMP3 (5.88 fold), BpAMP7 (11.15), BpAMP8 (6.48), BpAMP12 (10.28) and BpAMP23 (10.59).
Table 1.
Quantitative abundance profile of AMPs from black pepper upon 24 hpi of P. capsici
| Sequence | Peptide ion | Mass | Score | Anova | Fold change | Average normalised abundance (Control) | Average normalised abundance (24 h) | Description | Abundance | Black pepper ID |
|---|---|---|---|---|---|---|---|---|---|---|
| LGKDAVEDLESVGK | 4500 | 1458.7566 | 56.73 | 0.30 | 1.17 | 2.06e+004 | 3.17e+004 | AP00433 | High | BpAMP1 |
| AQSGKTAICKCYVKVCPR | 412 | 2011.0377 | 36.7.0 | 0.54 | 1.30 | 2.82e+005 | 3.44e+005 | AP01559 | High | BpAMP2 |
| AGLQFPVGR | 1113 | 943.5236 | 55.33 | 2.03e−005 | 5.88 | 1.24e+004 | 7.29e+004 | AP00307 | High | BpAMP3 |
| GICVPIRCPGSMRQIGTCLGAQVK | 2287 | 2340.0385 | 20.08 | 0.36 | 1.08 | 1.91e+005 | 4.40e+004 | AP00401 | Low | BpAMP4 |
| RGRCLCIGPGVK | 1012 | 1314.7148 | 21.39 | 0.92 | 1.09 | 6.03e+004 | 6.55e+004 | AP02081 | High | BpAMP5 |
| FISGLIGGLMK | 1904 | 1150.6384 | 31.14 | 2.47e−005 | 3.85 | 2.57e+004 | 6670.49 | AP02274 | Low | BpAMP6 |
| CAPKMKQIGTCGMPQVKCCK | 287 | 2226.0018 | 14.64 | 6.38e−007 | 11.15 | 8861.63 | 1.64e+005 | AP01597 | High | BpAMP7 |
| NQCINLEKAR | 535 | 1188.5906 | 27.55 | 9.19e−008 | 6.48 | 7.38e+004 | 4.78e+005 | AP00286 | High | BpAMP8 |
| GAGKAVLGK | 14 | 841.5045 | 26.89 | 9.68e−004 | 3.53 | 1.21e+006 | 4.28e+006 | AP00383 | High | BpAMP9 |
| WLERIGK | 191 | 900.5279 | 26.31 | 8.72e−004 | 2.04 | 1.65e+005 | 3.38e+005 | AP00777 | High | BpAMP10 |
| KNSPFTAKK | 1340 | 1062.5705 | 25.70 | 0.35 | 1.37 | 2.33e+004 | 3.20e+004 | AP01522 | High | BpAMP11 |
| ELENLAAMDLELQK | 8301 | 1616.8157 | 24.04 | 3.68e−010 | 10.28 | 7467.00 | 7.67e+004 | AP00612 | High | BpAMP12 |
| GTCVLVK | 4082 | 760.4121 | 23.69 | 1.64e−003 | 2.53 | 3594.22 | 9100.72 | AP00891 | High | BpAMP13 |
| SCCRSTQARNIYNAPR | 4653 | 1897.8383 | 22.56 | 0.01 | 1.70 | 2.62e+004 | 1.54e+004 | AP01585 | Low | BpAMP14 |
| ALLCKLDK | 2792 | 959.5488 | 22.39 | 0.04 | 1.25 | 1.66e+004 | 1.33e+004 | AP02299 | Low | BpAMP15 |
| TWKRPPFQTSCWGIIKE | 3255 | 2076.0737 | 21.99 | 0.01 | 3.14 | 1.72e+004 | 5481.07 | AP01940 | Low | BpAMP16 |
| GQVNDSACAANCLSLGKAGGHCEK | 979 | 2332.0176 | 21.12 | 3.10e−004 | 2.94 | 1.74e+006 | 5.93e+005 | AP00226 | Low | BpAMP17 |
| SSVVGRK | 1135 | 731.4332 | 21.05 | 1.83e−003 | 2.01 | 2.58e+004 | 1.28e+004 | AP01309 | Low | BpAMP18 |
| IQDKEGIPPDQQR | 4324 | 1522.7733 | 20.48 | 3.30e−006 | 2.49 | 3.05e+004 | 1.23e+004 | AP02030 | Low | BpAMP19 |
| NKGICVPIR | 9157 | 998.554 | 17.77 | 0.85 | 2.87 | 1651.76 | 4735.63 | AP00235 | Low | BpAMP20 |
| GIKDWIK | 4947 | 900.5037 | 15.31 | 4.59e−004 | 3.59 | 1.02e+004 | 2847.57 | AP01220 | Low | BpAMP21 |
| ALGTLLK | 2017 | 756.4833 | 12.19 | 0.16 | 1.74 | 8381.04 | 1.46e+004 | AP00909 | High | BpAMP22 |
| DAATGLVTGIQS | 536 | 1174.5724 | 11.16 | 5.23e−008 | 10.59 | 2.40e+004 | 2.54e+005 | AP00699 | High | BpAMP23 |
| WVQNYMKHLGRK | 4379 | 1575.7885 | 10.04 | 0.06 | 1.41 | 3.37e+005 | 4.74e+005 | AP02088 | High | BpAMP24 |
The identified AMP sequences were queried individually in PhytAMP database using blast tool to identify its plant origin. All the non-plant AMPs were queried against a multi-organism database (CAMP R3) and were found to have matching peptides in this database (Table 2).
Table 2.
Annotation of black pepper AMPs
| AMP | CAMP R3 | APD | PhytAMP |
|---|---|---|---|
| BpAMP1 | Crystal structure of the hexameric anti-microbial peptide channel dermcidin | Dermicidin | |
| BpAMP2 | Anti microbial peptide (Aspergillus clavatus) CAMPSQ2291 | AcAMP (Aspergillus clavatus) | Snakin |
| BpAMP3 | Buforin (CAMPSQ277) | Buforin (Toad) | |
| BpAMP4 | Lingual antimicrobial peptide (defensin family) (SQ1412) | Beta defensin | Ar-AMP Hevein |
| BpAMP5 | NMR structure of CXC chemokine CXCL11/ITAC | Chemokine | GASA-like Snakin |
| BpAMP6 | Maximin-H7 (SQ1780) | Temporin (cationic) | |
| BpAMP7 | Prepro-beta-defensin 1 (SQ2648) | Beta defensin | Ee-CBP leaves (Hevein) |
| BpAMP8 | Gamma-thionin (SQ2567) | Rs-Afp 1 plant defensin | At-AFP1 defensin |
| BpAMP9 | Ponericin-L2 (SQ218) | Ponericin | |
| BpAMP10 | Winter flounder 1 (Pleurocidin family) (CAMPSQ861) | Winter flounder 1 | |
| BpAMP11 | Ap (anti fungal) (CAMPSQ3306 | Ap | |
| BpAMP12 | Chrombacin (CAMP SQ2811) | Chrombacin | |
| BpAMP13 | Pilosulin 3 (CAMPSQ495) (from Insect Ant) | Pilosulin 3 | |
| BpAMP14 | Pp-AMP1 (defensin) (CAMP SQ3353) | Plant Pp-AMP1 (defensin) | Plant Pp-AMP1 (defensin) |
| BpAMP15 | Brevinin | Brevinin | |
| BpAMP16 | Nigroain-C2 (CAMPSQ3641) from frog | Nigroain C2 | |
| BpAMP17 | Defensin-1 (Apis mellifera carnica) (CAMPSQ4363) | Royalisin | |
| BpAMP18 | No hit | Odorranain | |
| BpAMP19 | CgUbiquitin (CAMPSQ3702) | Cg ubiquitin | |
| BpAMP20 | LAP-like antimicrobial peptide (fragment) (defensin) (CAMPSQ 6679) | Beta defensin | |
| BpAMP21 | Ascaphin-5(human erythrocytes) (CAMPSQ4333 | Ascaphin 5 | |
| BpAMP22 | Dermatoxin S1 (frog) (CAMPSQ 2946) | Dermatoxin | |
| BpAMP23 | Dahlein 4.3 (synthetic construct) (CAMPSQ2851) | Dahlein | |
| BpAMP24 | CCL 13 | CCL 13 chemokine |
Physiochemical and antimicrobial properties
The number of amino acids and the molecular weight of the BpAMPs ranged from 7–24 to 714–2333.5 respectively. Seven AMPs had an aliphatic index > 70, 10 AMPS < 100 and 6 AMPs < 70 to > 100. GRAVY value is calculated as the sum of hydropathy values of all the amino acids, divided by the number of amino acid residues in the query sequence. Positive and negative GRAVY is an indication of hydrophobicity and hydrophilicity respectively. Among 24 AMPS of black pepper, 12 AMPS were of hydrophobic and another 12 were hydrophilic (Table 3). The net charge of the AMPs varied from 1 to 4. Secondary structure prediction using the GOR 4 secondary structure prediction method showed 23 AMPs having extended strand and random coil in specific proportions. The only AMP (BpAMP17) from black pepper from this study was the type having alpha helix, extended strand and random coil (Table 3). The Kolaskar and Tongaonkar antigenicity prediction was used to determine sequences of antigenic determinants (epitopes) within the AMPs (Table 3). Conserved Domain search for the 24 AMPs showed using NCBI CDD tool identified conserved domains in 2 AMPs. Based on the analysis of cysteine content, the black pepper AMPs were found to have 13 cysteine free AMPs along with 11 cysteine rich AMPs. The percentage of amino acids in the cysteine free peptides is tabulated (Table 4). The Toxin pred analysis results showed that BpAMP14 was of toxic. Bowman index the protein binding potential was deduced and results in predicting half-life of peptides in the intestine like environment to find the half life for each AMPs and are tabulated (Table 3).
Table 3.
Physiochemical/antimicrobial properties of black pepper AMPs
| AMP | Number of amino acids | MW | pI | Net charge | Aliphatic index | Secondary structure (ES: extended strand; RC: random coil; AH: alpha helix) | Bowman index(kcal/mol) | Intestinal half life(sec) | Toxicity | Most antigenic region | (GRAVY) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BpAMP1 | 14 | 1459.6 | 4.32 | − 2 | 104.29 | ES (50%); RC (50%) | 1.71 | 0.766 | Non-toxin | VEDLESV | − 0.4 |
| BpAMP2 | 18 | 1955.3 | 9.42 | 4 | 65 | Extended strand (38.89%); random coil (61.11) | 1.21 | 1.620 | Non-toxin | CYVKVCP | − 0.028 |
| BpAMP3 | 9 | 944.1 | 9.79 | 1 | 86.67 | Extended strand (44.44%); random coil (55.56%) | 0.53 | 1.328 | non-toxin | AGLQFPV | 0.244 |
| BpAMP4 | 24 | 2488 | 9.22 | 3 | 93.33 | Extended strand (41.67%); random coil (58.33%) | 0.53 | 2.710 | Non-toxin | ICVPIRC | 0.446 |
| BpAMP5 | 12 | 1258.5 | 9.7 | 3 | 89.17 | Extended strand (58.33%); random coil (41.67%) | 1.34 | 2.172 | Non-toxin | CLCIGPG | 0.15 |
| BpAMP6 | 11 | 1135.4 | 8.75 | 1 | 141.82 | Extended strand (54.55%); random coil (45.45%) | − 1.71 | 1.675 | Non-toxin | FISGLIG | 1.4 |
| BpAMP7 | 20 | 2154.7 | 9.21 | 4 | 39 | Extended strand (45%); random coil (55%) | 0.66 | 2.385 | Non-toxin | CGMPQVK | − 0.15 |
| BpAMP8 | 10 | 1188.3 | 8.22 | 1 | 88 | Extended strand (20%); random coil (80%) | 3.31 | 0.617 | Non-toxin | CINLEKA | − 0.98 |
| BpAMP9 | 9 | 799.9 | 10 | 2 | 97.78 | Extended strand (55.56%); random coil (44.44%) | − 0.47 | 0.859 | Non-toxin | AGKAVLG | 0.289 |
| BpAMP10 | 7 | 901 | 8.75 | 1 | 111.43 | Extended strand (71.43%); random coil (28.57%) | 2.02 | 1.129 | Non-toxin | WLERIGK | − 0.7 |
| BpAMP11 | 9 | 1020.2 | 10.3 | 3 | 11.11 | Extended strand (44.44%); random coil (55.56%) | 2.71 | 1.111 | Non-toxin | SPFTAKK | − 1.522 |
| BpAMP12 | 14 | 1616.8 | 4 | − 3 | 125.71 | Extended strand (21.43%); random coil (42.86%) | 1.51 | 0.106 | Non-toxin | AAMDLEL | − 0.3 |
| BpAMP13 | 7 | 718.9 | 8.22 | 1 | 138.57 | Extended strand (71.43%); random coil (28.57%) | − 1.01 | 0.746 | Non-toxin | GTCVLVK | 1.386 |
| BpAMP14 | 16 | 1840 | 9.69 | 3 | 36.88 | Extended strand (31.25%); random coil (68.75%) | 3.87 | 0.427 | Toxin | CCRSTQA | − 1.006 |
| BpAMP15 | 8 | 903.1 | 8.24 | 1 | 158.75 | Extended strand (25%); random coil (75%) | 0.24 | 2.604 | Non-toxin | ALLCKLD | 0.55 |
| BpAMP16 | 17 | 2077.4 | 9.3 | 2 | 45.88 | Extended strand (23.53%); random coil (76.47%) | 1.6 | 1.834 | Non-toxin | PPFQTSC | − 0.741 |
| BpAMP17 | 24 | 2333.5 | 6.72 | 1 | 61.25 | Alpha helix (25%); Extended strand (8.33%); random coil (75%) | 1.17 | 2.740 | Non-toxin | ACAANCL | − 0.217 |
| BpAMP18 | 7 | 731.8 | 11 | 2 | 82.86 | Extended strand (57.14%); random coil (42.86%) | 2.02 | 0.594 | Non-toxin | SSVVGRK | − 0.286 |
| BpAMP19 | 13 | 1523.6 | 4.56 | − 1 | 60 | Extended strand (15.38%); random coil (84.62%) | 3.88 | 1.097 | Non-toxin | GIPPDQQ | − 1.846 |
| BpAMP20 | 9 | 999.2 | 9.51 | 2 | 118.89 | Extended strand (44.44%); random coil (55.56%) | 1.22 | 1.107 | Non-toxin | KGICVPI | 0.2 |
| BpAMP21 | 7 | 859 | 8.59 | 1 | 111.43 | Extended strand (28.57%); random coil (71.43%) | 0.95 | 1.143 | Non-toxin | GIKDWIK | − 0.514 |
| BpAMP22 | 7 | 714.9 | 8.8 | 1 | 181.43 | Extended strand (57.14%); random coil (42.86%) | − 1.34 | 1.738 | Non-toxin | ALGTLLK | 1.171 |
| BpAMP23 | 12 | 1132.2 | 3.8 | − 1 | 105.83 | Extended strand (58.33%); random coil (41.67%) | 0.28 | 0.543 | Non-toxin | LVTGIQS | 0.508 |
| BpAMP24 | 12 | 1559.8 | 10.29 | 4 | 56.67 | Extended strand (25%); random coil (75%) | 2.36 | 1.612 | Non-toxin | VQNYMKH | − 1.267 |
Table 4.
Cysteine free AMPs with the amino acid composition and regulation
| AMP | Amino acid composition (%) | Regulation upon Phytophthora 24h (fold change) |
|---|---|---|
| BPAMP1 | Ala, Ser (7.14); ASP, Glu, Gly, Val, Lys, Leu (14.29) | Up (1.17) |
| BPAMP3 | Ala, Phe, Pro, Gln, Arg, Val (11.11); Gly (22.22) | Up (5.88) |
| BPAMP6 | Phe, Lys, Met, Ser (9.09;)Ile, Leu (18.18); Gly (27.27) | Down (3.85) |
| BPAMP9 | Ala, Lys (22.22); Gly (33.33); Leu, Val (11.11) | Up (3.53) |
| BPAMP10 | Glu, Gly, Ile, Leu, Lys, Arg, Try (14.29) | Up (2.04) |
| BPAMP11 | Ala, Phe, Asn, Pro, Ser, Thr (11.11); Lys (33.33) | Up (10.28) |
| BPAMP12 | Ala (14.29); Asp, Lys, Met, Asn, Gln (7.14); Glu (21.43); Leu (28.47) | Up (2.53) |
| BPAMP18 | Gly, Lys, Arg (14.29); Ser, Val (28.57) | Down (2.01) |
| BPAMP19 | Asp, Ile, pro (15.38); Gln(23.08); Glu, Gly, Lys, Arg (7.69) | Down (2.49) |
| BPAMP21 | Asp, Gly, Trp (14.29); Ile, Lys (28.57) | Down (3.59) |
| BPAMP22 | Ala, Gly, Lys, Thr (14.29); Leu (42.86) | Up (1.74) |
| BPAMP23 | Ala, Gly, Thr (16.67); Asp, Ile, Leu, Gln, Ser, Val (8.33) | Up (10.59) |
| BPAMP24 | Gly, His, Leu, Met, Asn, Gln, Arg, Val, Trp, Tyr (8.33); Lys (16.67) | Up (1.41) |
Discussion
In spite of abundant reports on the isolation of AMPs from many sources, the profile of AMPs expressed/identified from single crop species under certain stress/physiological condition is still unknown. In this study, we aimed to identify AMP using label free proteomic analysis of protein extract from black pepper leaf upon infection with P. capsici. The 24 hpi samples were taken from resistant genotype as there is no visible symptom expressed in this cultivar, where as in case of susceptible variety, visible symptoms expresses in 24 hpi (Unpublished data). The peptide data from antimicrobial peptide (APD) database (Wang et al. 2016) was used to query the peptide sequences from control and 24 hpi using Progenesis IQ software. A total of 5 AMPs was found to have similarity to plant peptides and the rest 16 AMPs failed to find a match with the available entries in the PhytAMP database. The search in CAMPR3, the multi-organism database showed the identity for all 16 non-plant AMPs. We suppose them to be homologs of animal /insect AMP signatures. These AMP homologs may be of evolutionary novelty in black pepper.
The differential expression of 24 AMPs suggests that a combinatorial strategy is working in the defense network in black pepper against P. capsici. BpAMP3 (AGLQFPVGR) was found to be the Buforin homolog. Burofin isolated from frog showed broad spectrum antimicrobial activity including fungi by penetrating the cell membrane (Park et al. 2000) BpAMP7 (CAPKMKQIGTCGMPQVKCCK) was the Hevein type AMP showed similarity to AMP from Euonymus europaeus (European spindle tree). This small hevein like chitin binding protein possesses antifungal property and active against Phytophthora cryptogea. The chitin-binding hevein-type polypeptides were identified with three (Ac-AMP), four (hevein), five (Eucommia ulmoides AMPs) and 10 disulphide bonds (Ee-CBP) from E. europaeus (Van den Bergh et al. 2002). The BpAMP7 identified from black pepper was found to have 4 cysteine residues. BpAMP8 (NQCINLEKAR) was identified as Rs-Afp1of Raddish. This type causes membrane permealisation and formation of reactive oxygen species (Matejuk et al. 2010) It was demonstrated that that transgenic tomato plants with this AMP was resistant to Phytophthora infestans (Parashina et al. 2000). BpAMP12 (ELENLAAMDLELQK) was the chrombacin analog. These peptides have the ability to induce chemotaxis and initiation of release of cytokines (Salzet and Stefano 2003). BpAMP23 (DAATGLVTGIQS) was found to be an analog of Dahlein, bioactive peptides from frog Litoria dahlia which was found to have wide spectrum activity (Wegener et al. 2001).
Isoelectric point is an important factor as it affects the solubility of the AMP. The black pepper AMPs had pI range from 3.8 to 10.3 denoting the presence of most acidic and alkaline range having peptides. Aliphatic index shows the thermal stability of the AMPs. The aliphatic index of 16 black pepper AMPs were with 70 -100 showing greater thermal stability.
Studies demonstrated that at least net charge 2 is required for the amphipathic nature (Hancock 1997). The net charge of most of the black pepper AMPs was found to be 2 and above, which indicated their antimicrobial potential. The total of ionizable amino acid residues at a particular pH determines the anionic or cationic net surface charge to AMPs. Anionic /cationic AMPs are constitutive or inducible defense barriers against microbial infections and also they might have the ability to improve host immunity by acting as immune modulators (Robinson et al. 2012). We found 4 anionic AMPS (BpAMP1, 12, 19 and 23) while another 18 AMPS were cationic in nature. The plant derived anionic AMPs are attractive molecule against cancer. This group of AMPs is reported as host defense peptides from plants and is shown to have anticancer property (Song et al. 2012). On the other hand, the plant cationic AMPs are shown to have activity against negatively charged microbial membranes.
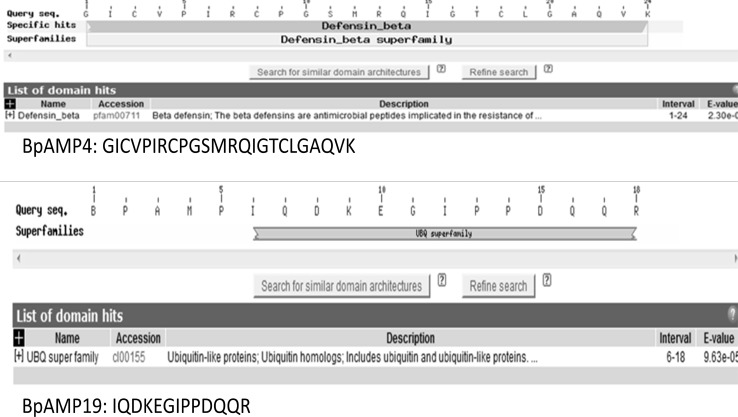
The majority of black pepper AMPs were found to have extended strand and random coil secondary structures. The reports state that the anionic peptides should have extended strand and random coil (Powers and Hancock 2003). The extended class of peptides is rich in proline and/or glycine contents and lacks classical secondary structures. The random coils are found to be involved in cell permeation in case of Indolicidin AMP (Zhang et al. 2001). The conserved domain search yielded BpAMP4 (GICVPIRCPGSMRQIGTCLGAQVK) with defensin beta superfamily and BpAMP (IQDKEGIPPDQQR) with UBQ super family conserved domain (Fig. 1). These results further strengthen that the label free proteomics approach as reliable and quick method to identify AMPs even from complex samples and possibility to find the gene fragment coding for AMPs.
Fig. 1.
Black pepper AMPs with conserved domains
The cysteine free AMPs are more common in animal, insects and they were found to be linear peptides without cysteine with a high proportion of certain residues. In plant, till now only 3 reports are available on linear cysteine free AMPs (Egorov et al. 2005; Zipfel 2009; Silva et al. 2012). Out of 13 cysteine free BpAMPs, 9 AMPs were found to be in up-regulation (BpAMP1, 3, 9, 10, 11, 12, 22, 23 and 24) and the remaining 4 were with down-regulation. The cysteine rich AMPs are very common to plant kingdom with varying number of cysteine residues. In this study, we identified AMPs in black pepper with 4 cysteine residues (BpAMP7), 3 cysteine residues (BpAMP2, 4 and 17), 2 cysteine residues (BpAMP5 and 14) and 1 cysteine residue (BpAMP8, 13, 15, 16 and 20). Among the 11 cysteine rich AMPs, two were up-regulated upon Phytophthora infection, BpAMP7 (11.15 fold) that is similar to hevein type AMP and BpAMP8 (6.48 fold) similar to Rs-Afp-1, suggesting them to be the effective candidate AMPs as molecule against Phytophthora.
In addition to this, the analysis to detect the toxic nature and half-life of the AMPs which are important for any drug development. This information would be important for any researcher to use the peptides towards drug development.
By using label free proteomics strategy, we established for the first time the black pepper peptidome associated with the innate immunity against Phytophthora. We showed the occurrence of both cysteine rich, cysteine free AMPs from a complex sample and some major AMP signatures as innate immunity factors against Phytophthora. However, whether all the AMPs or some major AMPs are contributing to the pathogen resistance in this genotype still needs to be worked out. Our work presented here will offer a basic platform for further studying the immunology and evolutionary significance of these newly discovered AMPs in black pepper and also utilizing some of the AMPs as next generation fungicide molecules.
Acknowledgements
We thank the Indian Council of Agricultural Research, New Delhi for funding through Outreach program on Phytophthora, Fusarium and Ralstonia diseases of horticultural and field crops (PhytoFuRa) and mass spectrometry facility, C-CAMP, NCBS, Bangalore for the LC/MS analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Anandaraj M. Diseases of black pepper. In: Ravindran PN, editor. Black pepper (Piper nigrum L.) New York: Harwood Academic Publishers; 2000. pp. 239–268. [Google Scholar]
- Asiegbu FO, Choi W, Li G, Nahalkova J, Dean RA. Isolation of a novel antimicrobial peptide gene (SpAMP) homologue from Pinus sylvestris (Scots pine) following infection with the root rot fungus Heterobasidion annosum. FEMS Microbiol Lett. 2003;228:27–31. doi: 10.1016/S0378-1097(03)00697-9. [DOI] [PubMed] [Google Scholar]
- Cammue BPA, De Bolle MFC, Terras FRG, Proost P, Van Damme J, Rees SB, Vanderleyden J, Broekaert WF. Isolation and characterization of a novel class of plant antimicrobial peptides from Mirabilis jalapa L. seeds. J Biol Chem. 1992;267:2228–2233. [PubMed] [Google Scholar]
- Egorov TA, Odintsova TI, Vitaliy A, Pukhalsky VA, Grishin EV. Diversity of wheat anti-microbial peptides. Peptides. 2005;26:2064–2073. doi: 10.1016/j.peptides.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Fan B, Shen L, Liu K, Zhao D, Yu M, Sheng J. Interaction between nitric oxide and hydrogen peroxide in post harvest tomato resistance response to Rhizopus nigricans. J Sci Food Agric. 2008;88:1238–1244. doi: 10.1002/jsfa.3212. [DOI] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The proteomics protocols handbook. New York: Humana Press; 2005. pp. 571–607. [Google Scholar]
- Gupta S, Kapoor P, Chaudhary K, Gautam A, Kumar R, Raghava GPS. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE. 2013;8(9):e73957. doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami R, Ben Hamida J, Fliss I. PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res D. 2009;963:8. doi: 10.1093/nar/gkn655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE. Peptide antibiotics. Lancet. 1997;349(9049):418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- Ke T, Cao H, Huang J, Hu F, Huang J, Dong C, Ma X, Yu J, Mao H, Wang X, Niu Q, Hui F, Liu S. EST-based in silico identification and in vitro test of antimicrobial peptides in Brassica napus. BMC Genom. 2015;16:653. doi: 10.1186/s12864-015-1849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276(1–2):172–174. doi: 10.1016/0014-5793(90)80535-Q. [DOI] [PubMed] [Google Scholar]
- Matejuk A, Leng Q, Begum MD, Woodle MC, Scaria P, Chou S-T, Mixson AJ. Peptide-based antifungal therapies against emerging infections. Drugs Future. 2010;35(3):197. doi: 10.1358/dof.2010.035.03.1452077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot R, Barylski J, Nowicki G, Broniarczyk J, Buchwald W, Gozdzicka-Jozefiak A. Plant antimicrobial peptides. Folia Microbiol. 2014;59(3):181–196. doi: 10.1007/s12223-013-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashina EV, Serdobinskii LA, Kalle EG, Lavorova NV, Avetnov VA, Lumin VG, Naroditskii BS. Genetic engineering of oilseed rape and tomato plants expressing a radish defensin gene. Russ J Plant Physiol. 2000;47:417–423. [Google Scholar]
- Park CJ, Park CB, Hong SS, Lee HS, Lee SY, Kim SC. Characterization and cDNA cloning of two glycine- and histidine-rich antimicrobial peptides from the roots of shepherd_spurse, Capsella bursa-pastoris. Plant Mol Biol. 2000;44:187–197. doi: 10.1023/A:1006431320677. [DOI] [PubMed] [Google Scholar]
- Pelegrini PB, Del Sarito RP, Silva ON, Franco OL, Grossi-de-sa MF. Antimicrobial peptides from plants: what they are and how they probably work. Biochem Res Int. 2011;2011:250349. doi: 10.1155/2011/250349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JP, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24(11):1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Rahnamaeian M. Antimicrobial peptides: modes of mechanism, modulation of defense responses. Plant Signal Behav. 2011;6:1325–1332. doi: 10.4161/psb.6.9.16319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MW, Hutchinson AT, Donnelly S. Antimicrobial peptides: utility players in innate immunity. Front Immunol. 2012;3:325. doi: 10.3389/fimmu.2012.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzet M, Stefano G. Chromacin-like peptide in leeches. Neuro Endocrinol Lett. 2003;24(3/4):227–232. [PubMed] [Google Scholar]
- Sarika, Iquebalb MA, Rai A. Biotic stress resistance in agriculture through antimicrobial peptides. Peptides. 2012;36:322–330. doi: 10.1016/j.peptides.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Scott MG, Dullaghan E, Mookherjee N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamil P, Yu JJ, Li Y, Donini O, Guarna MM, Finlay BB, North JR, Hancock RE. An anti infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- Sharma A, Singla D, Rashid M, Raghava GPS. Designing of peptides with desired half-life in intestine-like environment. BMC Bioinformatics. 2014;15:282. doi: 10.1186/1471-2105-15-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva ON, Porto WF, Migliolo L, Mandal SM, Gomes DG, Holanda HH, Silva RS, Dias SC, Costa MP, Costa CR, Silva MR, Rezende TM, Franco OL. Cn-AMP1: a new promiscuous peptide with potential for microbial infections treatment. Biopolymers. 2012;98(4):322–331. doi: 10.1002/bip.22071. [DOI] [PubMed] [Google Scholar]
- Song R, Wei R, Luo H, Wang D. Isolation and characterization of an antibacterial peptide fraction from the pepsin hydrolysate of half-fin anchovy (Setipinna taty) Molecules. 2012;17:2980–2991. doi: 10.3390/molecules17032980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umadevi P, Anandaraj M. An efficient protein extraction method for proteomic analysis of black pepper (Piper nigrum L.) and generation of protein map using nano LC-LTQ Orbitrap mass spectrometry. Plant Omics. 2015;8(6):500–507. [Google Scholar]
- Van den Bergh KPB, Proost P, Van Damme J, Coosemans J, Van Damme EJM, Peumans WJ. Five disulfide bridges stabilize a hevein-type antimicrobial peptide from the bark of spindle tree (Euonymus europaeus L.) FEBS Lett. 2002;530(1–3):181–185. doi: 10.1016/S0014-5793(02)03474-9. [DOI] [PubMed] [Google Scholar]
- Waghu FH, Barai RS, Gurung P, Idicula-Thomas S. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016;44:D1094–D1097. doi: 10.1093/nar/gkv1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener KL, Brinkworth CS, Bowie JH. Bioactive dahlein peptides from the skin secretions of the Australian aquatic frog Litoria dahlii: sequence determination by electrospray mass spectrometry. Rapid Commun Mass Spectrom. 2001;15(18):1726–1734. doi: 10.1002/rcm.429. [DOI] [PubMed] [Google Scholar]
- Weinhold A, Wielsch N, Svatos A, Baldwin IT. Label-free nanoUPLC-MSE based quantification of antimicrobial peptides from the leaf apoplast of Nicotiana attenuate. BMC Plant Biol. 2015;15:18. doi: 10.1186/s12870-014-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rozek A, Hancock REW. Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem. 2001;276(38):35714–35722. doi: 10.1074/jbc.M104925200. [DOI] [PubMed] [Google Scholar]
- Zhou M, Hu Q, Li Z, Li D, Chen C, Luo H. Expression of a novel antimicrobial peptide Penaeidin 4-1 in creeping bentgrass (Agrostis stolonifera L.) enhances plant fungal disease resistance. PLoS ONE. 2011;6(9):e24677. doi: 10.1371/journal.pone.0024677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. Early molecular events in PAMP-triggerd immunity. Curr Opin Plant Biol. 2009;12:414–420. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]