Abstract
The phytohomorne methyl jasmonate (MeJA) is known to trigger extensive reprogramming of gene expression leading to transcriptional activation of many secondary metabolic pathways. However, natural rubber is a commercially important secondary metabolite and little is known about the genetic and genomic basis of jasmonate-elicited rubber biosynthesis in rubber tree (Hevea brasiliensis). RNA sequencing (RNA-seq) of H. brasiliensis bark treated with 1 g lanolin paste containing 0.02% w/w MeJA for 24 h (M2) and 0.04% w/w MeJA for 24 h (M4) was performed. A total of 2950 and 2850 differentially expressed genes in M2 and M4 compared with control (C) were respectively detected. Key genes involved in 2-C-methyl-D-erythritol 4-phosphate, rubber biosynthesis, glycolysis and carbon fixation (Calvin cycle) pathway were found to be up-regulated by MeJA treatment. Particularly, the expression of 3-hydroxy-3-metylglutaryl coenzyme A reductase in MVA pathway was down-regulated by MeJA treatment, but the expression of farnesyl diphosphate synthase (FPS) and cis-prenyltransferase (CPT, or rubber transferase) in rubber biosynthesis pathway were up-regulated by MeJA treatment. Up-regulation of critical genes in JA biosynthesis in response to MeJA treatment exhibited the self-activation of JA biosynthesis. In addition, up-regulated genes of great regulatory importance in cross-talk between JA and other hormone signaling, and of transcriptional regulation were identified. The increased expression levels of FPS and CPT in rubber biosynthesis pathway possibly resulted in an increased latex production in rubber tree treated with MeJA. The present results provide insights into the mechanism by which MeJA activates the rubber biosynthesis and the transcriptome data can also serve as the foundation for future research into the molecular basis for MeJA regulation of other cellular processes.
Electronic supplementary material
The online version of this article (10.1007/s12298-018-0529-0) contains supplementary material, which is available to authorized users.
Keywords: Rubber tree, Methyl jasmonate, Rubber biosynthesis, RNA sequencing, Transcriptome
Introduction
Although over 2500 species of higher plants have been reported to produce natural rubber, rubber tree (Hevea brasiliensis) is the only one source of commercial natural rubber (Cornish 2001; Whalen et al. 2013). With high-yielding cultivars developed and high-yielding practices with ethephon (a ethylene generator) stimulation adopted, rubber tree is extensively cultivated in tropical areas as a cash tree. Rubber is produced in the cytoplasm of specialized cells or vessels (laticifers) and stored in small subcellular rubber particles. The fluid cytoplasm of laticifers is also known as latex. Upon taping the soft bark tissues, the latex vessels are opened, the milky rubber-containing latex flows out and is periodically harvested (d’Auzac 1989; Hagel et al. 2008). Natural rubber, an important industrial and strategic material, is used extensively in many applications and products for its outstanding physical properties. In addition, the rubber wood can be used to manufacture furniture and floor after latex collected for 20 years or so.
The rubber molecule (cis-1,4-polyisoprene) is a high-molecular weight polymer composed of isoprene units derived from isopentenyl diphosphate (IPP) in the cis-configuration (Cornish 2001; Whalen et al. 2013). As the building blocks of the rubber, IPPs has been known to derive from the mevalonic acid (MVA) pathway (Hepper and Audley 1969; Skilleter and Kekwick 1971; Kekwick 1989). However, there is increasing evidence of crosstalk between the cytosolic MVA pathway and another metabolic pathway of IPP, the plastidial 1-dexoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DEX/MEP) pathway and exchange of common isoprenoid precursors to an extent (Rodríguez-Concepción et al. 2013). The MEP-derived isoprenoid precursors might be exported to the cytosol by a plastidial proton symport system (Bick and Lange 2003) and be involved in rubber biosynthesis (Ko et al. 2003; Seetang-Nun et al. 2008; Chow et al. 2007, 2012). The sequential condensation of the IPPs at the priming farnesyl pyrophosphate (FPP) into natural rubber (cis-1,4-polyisoprene) is catalyzed by cis-prenyltransferase (CPT) (Cornish and Xie 2012). CPT has been intensively investigated in rubber tree and other plants (Asawatreratanakul et al. 2003; Brasher et al. 2015; Qu et al. 2015; Schmidt et al. 2010; Spanò et al. 2015; Takahashi et al. 2012).
Free-acid jasmonic acid (JA) and JA derivatives including methyl jasmonate (MeJA) collectively referred to as jasmonates (JAs), are vital lipid-derived cellular regulators involved in diverse developmental processes and plant responses to biotic and abiotic stresses (Wasternack 2015; Wasternack and Hause 2013). Especially, JAs have been reported to transcriptionally activate secondary metabolism. Generally, secondary metabolites are both important for plant in responses to development and environmental cues, and some of them can be used by human as pharmaceuticals and other industrial materials (Cheong and Choi 2003; Pauwels et al. 2009; De Geyter et al. 2012). In rubber tree, exogenous JA and linolenic acid treatment has been reported to stimulate secondary laticifer differentiation (Hao and Wu 2000; Shi and Tian 2012; Tian et al. 2003). Moreover, natural rubber is a commercially useful secondary metabolite and exogenous applications of JAs was reported to increase latex production in a small-scale field experiment (Duan et al. 2004), but the related molecular mechanism remains elusive.
As a powerful tool for quantitatively examining gene expression changes across the transcriptome, RNA sequencing (RNA-Seq) has been applied to understand diverse biological processes in plant (Jamaluddin et al. 2017; Jasrotia et al. 2017; Spyropoulou et al. 2014; Sun et al. 2013; Wang et al. 2017). In the present study, transcriptome analysis of rubber tree in response to exogenous MeJA was conducted using Illumina RNA sequencing technique. Our objective was to identify MeJA-responsive genes in rubber tree, emphasizing the genes involved in IPP, rubber and JA biosynthesis, and the genes in phytohormone signal transduction and transcriptional regulation, which may shed light on the underlying molecular mechanism of MeJA activation of rubber biosynthesis.
Materials and methods
Plant material
Bark tissues were collected from the rubber trees of clone RY8813 at the Experimental Farm of the Chinese Academy of Tropical Agriculture Science (Baodaoxincun, Danzhou, Hainan Province, China). The trees were planted in 1993 and latex was harvested firstly in 2001. The tapping system was s/2 d/3 (half spiral tapped every 3 days) with 1.5%-ethephon treatment one day before tapping at intervals of 15 days.
On the 9th of April 2015 (the date for the start of tapping this year), eighteen trees of equal size in the same plot were chosen and classified into three groups (each with six trees). Sampling technique was modified with the methods described in the related literatures (Hao and Wu 2000; Shi and Tian 2012; Tian et al. 2003; Zeng et al. 2003). The stem surface from the tapping line to 5 cm below was applied with 1 g lanolin paste containing 0, 0.02 or 0.04% w/w MeJA (thus with 0, 2 mg or 4 mg MeJA in 1 g lanolin paste respectively) after the dead epidermis cuticle of treatment site was scraped with a sharp knife. After the lanolin paste application, the treatment area was covered with polyethylene membrane. Bark samples were collected from three groups 24 h after MeJA treatments, respectively. The samples were immediately frozen in liquid nitrogen and shipped on dry ice to BGI Life Tech Co., Ltd (Shenzhen, China) for Illumina sequencing.
RNA extraction, cDNA library construction and sequencing
Total RNAs were isolated from a pool of six biological replicates of the bark samples via the TRIzol® Reagent (Invitrogen) according to the protocol in the manufacturer’s instructions. RNA integrity was examined by a 2100 Bioanalyzer (Agilent Technologies). Three cDNA libraries (with no replicates), C (treatment with 1 g lanolin paste containing 0% w/w MeJA for 24 h as control), M2 (treatment 1 g lanolin paste containing with 0.02% w/w MeJA for 24 h) and M4 (treatment with 1 g lanolin paste containing 0.04% w/w MeJA for 24 h), were constructed using the mRNA-Seq 8 sample prep Kit (Illumina) following the manufacturer’s instructions.
After digestion of DNA with DNase I, 20 μg of total RNAs were purified to obtain the poly(A) mRNA molecules using poly-T oligo-attached magnetic beads. Then, the samples were fragmented into small pieces in a Thermomixer (Eppendorf, German) using divalent cations at 94 °C for 5 min and converted into the first and second-strand cDNA with the SuperScript double-stranded cDNA synthesis kit (Invitrogen, CA). End repair and adenylation of 3′ ends were performed on the resulted cDNA. Subsquently, the double stranded cDNA was purified using the QIAquick PCR Purification Kit (QIAGEN). Next, the resulting cDNA fragments were ligated with Illumina paired end adapters and used to generate cDNA libraries. Lastly, the quality of the cDNA libraries was examined by an Agilent Technologies 2100 Bioanalyzer prior to sequencing with Illumina HiSeq 4000 (Illumina Inc., San Diego, CA, USA).
Alignment, gene expression analysis and gene annotation
In order to precisely evaluate the expression levels of genes, all the RNA-seq reads were mapped against the rubber tree sequencing genome recently released (Tang et al. 2016) using TopHat on default settings (Trapnell et al. 2009). The gene expression was quantified as the count of all clean reads mapped to the sequenced genome. The expression levels of each gene were normalized by the fragments per kb per million reads (FPKM) (Mortazavi et al. 2008). The FPKM value was calculated for each protein-coding gene by Cufflinks (http://cufflinks.cbcb.umd.edu) using default parameters. Differential gene expression between the different samples was determined using Cuffdiff. In this study, we used the FDR ≤ 0.001 and the ratio (the absolute log2-fold change) larger than 2 as the threshold to judge the significance of gene expression differences. Log2 fold changes were re-calculated between C and M2, and between C and M4, using the formula of Log2(M2 + 5)/(C + 5) and Log2(M4 + 5)/(C + 5), respectively. Rubber tree genes were annotated via Blastp against peptide sequences of a minimal set of the TAIR10 release of the Arabidopsis genome (http://www.arabidopsis.org/).
Quantitative real-time PCR analysis
Six DEGs such as JAZ1, MYC2, OPR3, HDR, ERF1 and AUX2 were randomly selected to confirm the DGE results using real time quantitative PCR (qRT-PCR). The RNA samples were extracted as in the RNA sequencing and the qRT-PCR was performed according to the method described by Liu et al. (2015). The reactions of three independent biological replicates were carried out for each sample and expression levels were normalized using Eukaryotic Initiation Factor 2 (eIF2) mRNA levels. The relative expressions of the genes were calculated using the 2−∆∆Ct method. The primer sequences used in this study are listed in Table S1.
Results and discussion
Read assembly, functional annotation and classification of unigenes
Three cDNA libraries were generated with the mRNA from C, M2 and M4 bark tissues of rubber trees and sequenced with the Illumina HiSeq 4000 platform. After preprocessing and quality trimming, a total of 44.92, 44.82 and 45.12 Mb of clean reads were obtained in the C, M2 and M4 sample, respectively. The clean reads are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the accession number of SRP093864. The reads were quantitatively mapped on the rubber tree genome (or against the rubber tree genome) (Tang et al. 2016) with the Tophat software. The 80% sequences of the transcriptome could be aligned to the rubber genome, whereas the percentage of unique-mapped reads were more than 70%, showing that higher-quality transcriptome was obtained (Table S2).
Differential expression analysis of MeJA-responsive genes
To detect genes with differential expression under two MeJA treatments, we compared the transcriptome profiles of M2 and M4 with that of C, respectively. The results showed 1575 up-regulated genes and 1375 down-regulated genes existed in M2 compared with C, and 1301 up-regulated genes and 1549 down-regulated genes were obtained in M4 compared with C. The total number of differentially expressed genes (DEGs) (2950) between M2 and C is larger than that (2850) of M4 and C. To represent DEGs under MeJA treatments, we generated a heatmap through hierarchical clustering. The heat map reveals a similar pattern of up-regulated gene distribution in M2 and M4 compared with C (Fig. 1). The top 20 most up- and down-regulated genes between C and M2, and C and M4 are shown in Table S3.
Fig. 1.
Heatmap of DEGs between M2 and M4, C and M2, and C and M4 based on RNA-seq results. Red and blue colors indicate the up- and down-regulated genes, respectively (color figure online)
Identification of DEGs involved in IPP and rubber biosynthesis
MeJA has been reported to be as an effective elicitor for enhancement of the production of some secondary metabolites in plants (Gundlach et al. 1992; Singh et al. 1998; van der Fits and Memelink 2000; Kim et al. 2004; Zhao et al. 2004; Thanh et al. 2005; Zhao et al. 2010). In rubber tree, jasmonic acid has been suggested to play a role in laticifer differentiation (Hao and Wu 2000; Shi and Tian 2012; Tian et al. 2003). But very limited information about the effect of JAs on rubber biosynthesis is available. Our study showed that the expression of 3-hydroxy-3-metylglutaryl coenzyme A reductase (HMGR) in MVA pathway was down-regulated by MeJA treatment, but the expression of farnesyl diphosphate synthase (FPS) and cis-prenyltransferase (CPT, or rubber transferase) in rubber biosynthesis pathway were up-regulated by MeJA treatment (Fig. 2). In addition, a systemic up-regulation of key genes by exogenous MeJA in MEP pathway, including genes encoding 1-deoxy-d-xylulose 5-phosphate (DXP) synthase (DXS), DXP reductoisomerase (DXR), 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol (CDP-ME) synthase (CMS), 2C-methyl-d-erythritol 2,4-cyclodiphosphate (MEcPP) synthase (MCS), 4-hydroxy-3-methylbut-2-enyl diphosphate (HMBPP) synthase (HDS) and HMBPP reductase (HDR), was observed (Fig. 2). In fact, main pathways of primary metabolism were also induced by MeJA application. Notably, some genes encoding ribulose bisphosphate carboxylase small chain 1A (RBCS1A), phosphofructokinase (PFK), pyruvate kinase (PK) and triose-phosphate isomerase (TPI or TIM), in carbon fixation (Calvin cycle) and glycolysis pathway (the upstream long-range biosynthesis pathway of natural rubber) were also up-regulated by MeJA treatment (Table S4).
Fig. 2.
DEGs involved in IPP and rubber biosynthesis in rubber tree bark treated with MeJA. HMG-CoA 3-hydroxy-3-metylglutaryl coenzyme A, MVA mevalonate, MVP 5-phosphomevalonate, MVPP 5-diphosphomevalonate, IPP isopentenyl diphosphate, DMAPP dimethylallyl diphosphate, FPP farnesyl diphosphate, G3P glyceraldehyde 3-phosphate, DXP 1-deoxy-d-xylulose 5-phosphate, MEP 2- C- methyl-d-erythritol 4-phosphate, CDP-ME 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol, CDP-MEP 4-(cytidine 5′-diphospho)-2- C- methyl-d-erythritol phosphate, MEcPP 2C-methyl-d-erythritol 2,4-cyclodiphosphate, HMBPP 4-hydroxy-3-methylbut-2-enyl diphosphate. Enzymes include AACT acetoacetyl-CoA thiolase, HMGS HMG-CoA synthase, HMGR HMG-CoA reductase, MVK MVA kinase, PMVK MVP kinase, DPMD MVPP decarboxylase, IDI IPP isomerase, FPS FPP synthase, CPT rubber transferase or cis-prenyltransferase, DXS DXP synthase, DXR DXP reductoisomerase, CMS CDP-ME synthase, CMK CDP-ME kinase, MCS MEcPP synthase, HDS HMBPP synthase; HDR HMBPP reductase. Pale pink and pale green cells represent up- and down-regulated DEGs respectively (color figure online)
HMGR has been reported to play only limiting role in plant IPP biosynthesis of MVA pathway (Rodríguez-Concepción et al. 2013; Vranová et al. 2012). Although HMGR expression could be up-regulated by treatment with MeJA in different species (Schmidt and Baldwin 2006; Wang et al. 2007; Dai et al. 2011; Wang et al. 2014; Spyropoulou et al. 2014; Shi et al. 2015; Sharma et al. 2015), transcriptional suppression or dose-dependent regulation of HMGR expression by MeJA treatment has also been reported (Burnett et al. 1993; Maldonado-Mendoza et al. 1994; Choi et al. 1994). In rubber tree, exogenous applications of JAs could increase latex production in a small-scale field experiment (Duan et al. 2004). But it has been shown that the transcriptional regulation of HMGR seemed not to be important for enhancing latex productivity and the down-regulation of HMGR by MeJA was reminiscent of the case of ethephon stimulation (Zhu and Zhang 2009; Adiwilaga and Kush 1996; Wang et al. 2016; Liu et al. 2016; Liu 2016). However, in a recent study, the expression of HMGR was enhanced with jasmonic acid (JA), linolenic acid (LA) (Loh et al. 2016). The exact reason for this variation is unclear to us, but the different sampling strategies could partly explain the difference. In their study, bark samples were harvested from the young rubber plants (3 layer stage) after 2 months of the treatments with JA and LA. In present study, bark tissues were collected from the mature rubber trees (22-year-old rubber trees with 14 years of tapping) 24 h after MeJA treatments.
Up-regulated genes in MEP pathway included genes encoding the major rate-determining enzymes (DXS and HDR, the first and the last enzyme of the MEP pathway) and other two potential control points (DXR and HDS) of the metabolic flux to plastidial isoprenoids (Vranová et al. 2012). Additionally, our result is consistent with the increase of transcript abundance of genes such as DXS, DXR, MCS and HDS in MEP pathway following elicitation of MeJA (Sun et al. 2013).
The increased expression levels of FPS and CPT in rubber biosynthesis pathway possibly resulted in an increased latex production in rubber tree treated with MeJA.
Contrary to our results, it has been documented that genes for primary metabolism such as glycolysis and ribulose bisphosphate carboxylase/oxygenase (Rubisco) in carbon fixation were repressed with MeJA treatment (Schmidt and Baldwin 2006; Jung et al. 2007). Even in barley (Hordeum vulgare L.) leaf, a decrease in content and activity of Rubisco treated with JA or MeJA were reported (Popova and Vaklinova 1988; Weidhase et al. 1987). Whether the up-regulated expression of the genes with exogeneous application of MeJA can cause the enhancement of photosynthetic capacity and glycolytic flux in rubber tree, need to be investigated.
DEGs involved in JA biosynthesis
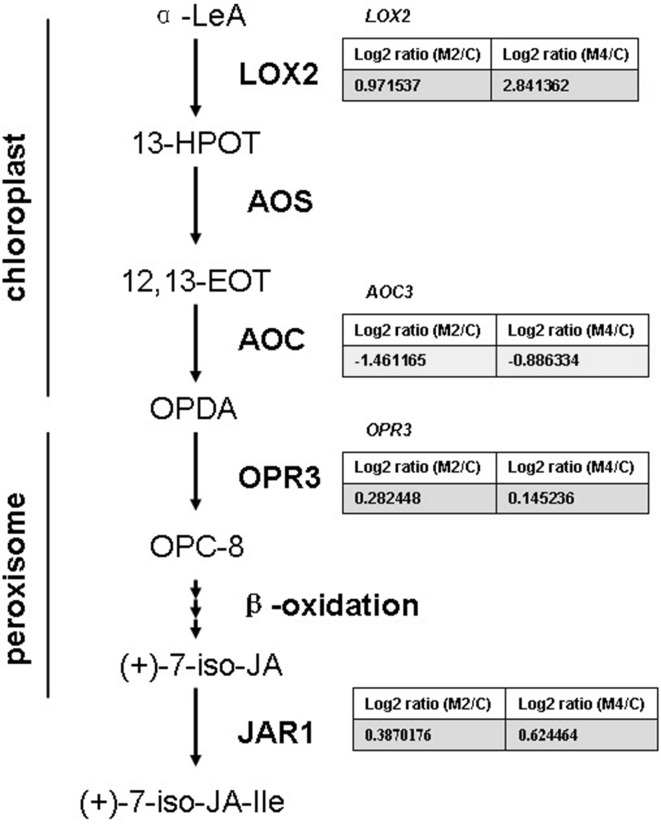
Two critical genes encoding lipoxygenase 2 (LOX2), first enzyme of JA biosynthesis, and 12-oxophytodienoate reductase 3 (OPR3), first enzyme of peroxisomal JA biosynthesis, were up-regulated by MeJA treatment (Fig. 3). In addition, the transcript of JA-amino acid synthetase (JAR1), the enzyme which catalyses the final step in the production of the bioactive JA compound, were found to be accumulated after MeJA treatment (Fig. 3). The increase of transcript abundance of genes in JA biosynthesis induced by exogenous MeJA is consistent with the self-activation of JA biosynthesis reported in the literature (Sun et al. 2013;Yan et al. 2013;Wasternack 2007; Pauwels et al. 2008; Men et al. 2013). The positive feedback loop is one of important regulations of JA biosynthesis and can be explained by SCFCOI1-JAZ regulatory module (Wasternack and Hause 2013).
Fig. 3.
DEGs involved in JA biosynthesis pathway in rubber tree bark treated with MeJA. α-LeA a-linolenic acid, 13-HPOT (13S)-hydroperoxyoctadecatrienoic acid, 12,13-EOT 12,13-epoxyoctadecatrienoic acid, OPDA 12-oxophytodienoic acid, OPC-8 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid, JAs jasmonates. The enzymes are LOX2 lipoxygenase 2, AOC allene oxide cyclase, OPR3 12-oxophytodienoate reductase 3, JAR1 JA-amino acid synthetase. Pale pink and pale green cells represent up- and down-regulated DEGs respectively (color figure online)
DEGs of hormone signaling components and transcription factors
After MeJA application, not only the genes encoding JA signaling components such as JASMONATE ZIM DOMAIN (JAZ) and MYC2 were differentially expressed, but also the genes encoding signaling components of other major plant hormones such as auxin, cytokinin (CK), ethylene (ET), abscisic acid (ABA), brassinosteroid (BR), salicylic acid (SA) and gibberellin (GA) were up- or down-regulated (Table S5). Notably, MYC2, the most prominent JAZ-interacting transcription factor and a master switch in JA signaling which regulates the majority of JA responses (Wasternack and Hause 2013; Kazan and Manners 2013), were shown to be up-regulated. Some genes encoding the components involved in the cross-talk between JA and other hormones were obviously up-regulated, such as ETHYLENE RESPONSE FACTOR 1 (ERF1) and ETHYLENE INSENSITIVE 3 (EIN3), which are required for the both JA-ET synergistic and antagonistic interaction (Zhu 2014; Zhu et al. 2011; Zhang et al. 2012; Song et al. 2014a, b; Zhang et al. 2014), were found to be up-regulated. NONEXPRESSOR OF PR GENES1 (NPR1), the central regulator in SA signaling and an essential factor which mediates the antagonistic interaction between JA and SA (Spoel et al. 2003; Dong 2004; Leon-Reyes et al. 2009), was observed to be up-regulated in M2 but down-regulated in M4 compared with C. The transcripts of ABA receptor PYR/PYL proteins which are linked to ABA-JA cross-talk (Lackman et al. 2011; Aleman et al. 2016), and ARF and IAA proteins which are related to JA-auxin (Nagpal et al. 2005; Tabata et al. 2010; Reeves et al. 2012), showed enhancement after MeJA treatment. It seems that the output of JA signaling depends on the complex cross-talk between JAs and other hormones, and ultimately mediates transcription regulation of secondary metabolic pathways (Pauwels et al. 2009).
JAs regulate a wide variety of biological processes in plants via triggering a transcription cascade (Pauwels et al. 2009). In our study, a large number of DEGs of putative TFs were identified (Table S6). We found that gene expression of many TFs of different families shown to be as regulators of the biosynthesis of different groups of secondary metabolites such as AP2/ERF (APETALA2/Ethylene-Response Factors), bHLH (basic Helix-Loop-Helix), MYB, and WRKY were induced by MeJA treatment, consistent with the previous studies (De Geyter et al. 2012; Zhou and Memelink 2016; Chezem and Clay 2016). However, their detailed regulatory role and mode in IPP and rubber biosynthesis remained to be solved. The real time quantitative PCR (qRT-PCR) results validated the authenticity of the expression patterns obtained in this transcriptome analysis (Fig. 4).
Fig. 4.
qRT-PCR validation of six DEGs involved in H. brasiliensis bark tissues treated with MeJA compared with the control. The axis indicates treatments; the y-axis indicates relative expression level. a JAZ1 b MYC2; c OPR3; d HDR; e ERF1; f AUX2
Conclusions
Our results suggested that activation of the expression of rubber biosynthesis genes such as FPS and CPT possibly leads to increase in latex production in rubber tree with MeJA application. MeJA induced up-regulation of key genes in glycolysis and carbon fixation (Calvin cycle) pathway might also play an important role in the stimulation of latex production. Elevation of transcript levels of critical genes in JA biosynthesis revealed that a JA autoregulatory loop existed. Identification of MeJA-responsive components in cross-talk between JA and other hormone signaling, and MeJA-responsive TFs demonstrated a complex control of MeJA-mediated downstream processes. Overall, our transcriptome data provides a valuable start point to identifying key JA-responsive TFs and enzymes that control the metabolic flow through the biosynthetic pathway of IPP and rubber, and can serve as a resource for unraveling the molecular basis of MeJA regulation of other cellular processes. For example, besides injuries inflicted by plant-eating organisms and typhoon, tapping represents a routine injury of rubber tree. JA, a wound hormone, mediate systemic wound responses which depend on large-scale changes in gene expression (Koo and Howe 2009).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1: The forward and reverse primers used in validation experiment of gene expression by qRT-PCR (DOC 37 kb)
Table S2: Overview of the sequencing and mapping in this study (DOC 34 kb)
Table S3: The top 20 most up- and down-regulated genes between C and M2, and C and M4. (XLS 34 kb)
Table S4: DEGs were significantly enriched in glycolysis and carbon fixation (Calvin cycle) pathway in M2 and M4 compared with C. (XLS 23 kb)
Table S5: DEGs in phytohormone signaling in M2 and M4 compared with C. (XLS 24 kb)
Table S6: DEGs in transcriptional regulation in M2 and M4 compared with C. (XLS 46 kb)
Acknowledgements
We would like to thank Prof. Tang and Dr. Fang (Rubber Research Institute, Chinese Academy of Tropical Agricultural Sciences) for their support with the genome sequencing data of rubber tree. This work was funded by the National Natural Science Foundation of China (No. 31560573) and the Hainan Province Major Science and Technology Project (ZDZX2013023).
Authors contributions
J.P.L. supervised the experiments and wrote the manuscript. J. H. conducted the bioinformatics studies and carried out qRT-PCR validation. L.S.Z supervised the he bioinformatics studies and revised the paper. Y.H.L participated in the bioinformatics studies. C.P.Y. and Y.F.Z. participated in the qRT-PCR validation. X.L.G and Y.J.L. participated in collection of plant materials. All the authors read and approved the manuscript for publication.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
This study was conducted according to compliance with ethical standards. This study does not involve the use of any human, animal and endangered or protected plant species as materials.
Footnotes
Jin-Ping Liu, Jin Hu, and Liangsheng Zhang authors contributed equally to this work.
Electronic supplementary material
The online version of this article (10.1007/s12298-018-0529-0) contains supplementary material, which is available to authorized users.
Contributor Information
Jin-Ping Liu, Email: liu3305602@163.com.
Liangsheng Zhang, Email: fafuzhang@163.com.
References
- Adiwilaga K, Kush A. Cloning and characterization of cDNA encoding farnesyl diphosphate synthase from rubber tree (Hevea brasiliensis) Plant Mol Biol. 1996;30:935–946. doi: 10.1007/BF00020805. [DOI] [PubMed] [Google Scholar]
- Aleman F, Yazaki J, Lee M, Takahashi Y, Kim AY, Li Z, Kinoshita T, Ecker JR, Schroeder JI. An ABA-increased interaction of the PYL6 ABA receptor with MYC2 transcription factor: a putative link of ABA and JA signaling. Sci Rep. 2016;6:28941. doi: 10.1038/srep28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asawatreratanakul K, Zhang YW, Wititsuwannakul D, Wititsuwannakul R, Takahashi S, Rattanapittayaporn A, Koyama T. Molecular cloning, expression and characterization of cDNA encoding cis-prenyltransferases from Hevea brasiliensis: a key factor participating in natural rubber biosynthesis. Eur J Biochem. 2003;270:4671–4680. doi: 10.1046/j.1432-1033.2003.03863.x. [DOI] [PubMed] [Google Scholar]
- Bick JA, Lange BM. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys. 2003;415:146–154. doi: 10.1016/S0003-9861(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Brasher MI, Surmacz L, Leong B, Pitcher J, Swiezewska E, Pichersky E, Akhtar TA. A two-component enzyme complex is required for dolichol biosynthesis in tomato. Plant J. 2015;82:903–914. doi: 10.1111/tpj.12859. [DOI] [PubMed] [Google Scholar]
- Burnett RJ, Maldonado-Mendoza IE, McKnight TD, Nessler CL. Expression of a 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Camptotheca acuminate is differentially regulated by wounding and methyl jasmonate. Plant Physiol. 1993;103:41–48. doi: 10.1104/pp.103.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J-J, Choi YD. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003;19:409–413. doi: 10.1016/S0168-9525(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Chezem WR, Clay NK. Regulation of plant secondary metabolism and associated specialized cell development by MYBs and bHLHs. Phytochemistry. 2016;131:26–43. doi: 10.1016/j.phytochem.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Bostock RM, Avdiushko S, Hildebrand DF. Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants: methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc Natl Acad Sci USA. 1994;91:2329–2333. doi: 10.1073/pnas.91.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KS, Wan KL, Isa MNM, Bahari A, Tan SH, Harikrishna K, Yeang HY. Insights into rubber biosynthesis from transcriptome analysis of Hevea brasiliensis latex. J Exp Bot. 2007;58:2429–2440. doi: 10.1093/jxb/erm093. [DOI] [PubMed] [Google Scholar]
- Chow KS, Mat-Isa MN, Bahari A, Ghazali AK, Alias H, Mohd-Zainuddin Z, Hoh CC, Wan KL. Metabolic routes affecting rubber biosynthesis in Hevea brasiliensis latex. J Exp Bot. 2012;63:1863–1871. doi: 10.1093/jxb/err363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K. Biochemistry of natural rubber, a vital raw material, emphasizing biosynthetic rate, molecular weight and compartmentalization, in evolutionarily divergent plant species. Nat Prod Rep. 2001;18:182–189. doi: 10.1039/a902191d. [DOI] [PubMed] [Google Scholar]
- Cornish K, Xie W. Natural rubber biosynthesis in plants: rubber transferase. Methods Enzymol. 2012;515:63–82. doi: 10.1016/B978-0-12-394290-6.00004-5. [DOI] [PubMed] [Google Scholar]
- Dai Z, Cui G, Zhou SF, Zhang X, Huang L. Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J Plant Physiol. 2011;168:148–157. doi: 10.1016/j.jplph.2010.06.008. [DOI] [PubMed] [Google Scholar]
- d’Auzac J. Factors involved in the stopping of latex flow after tapping. In: d’Auzac J, Jacob L, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton: CRC Press; 1989. pp. 257–280. [Google Scholar]
- De Geyter N, Gholami A, Goormachtig S, Goossens A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012;17:349–359. doi: 10.1016/j.tplants.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Dong X. NPR1, all things considered. Curr Opin Plant Biol. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Duan C, Zeng R, Li Y. Regulation of plant hormones on biosynthesis of natural rubber in Hevea brasiliensis. Chin J Trop Agric. 2004;24:61–68. [Google Scholar]
- Gundlach H, Muller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagel JM, Yeung EC, Facchini PJ. Got milk? The secret life of laticifers. Trends Plant Sci. 2008;13:631–639. doi: 10.1016/j.tplants.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Hao BZ, Wu JL. Laticifer differentiation in Hevea brasiliensis: induction by exogenous jasmonic acid and linolenic acid. Ann Bot. 2000;85:37–43. doi: 10.1006/anbo.1999.0995. [DOI] [Google Scholar]
- Hepper CM, Audley BG. The biosynthesis of rubber from hydroxyl-methyl-glutaryl coenzyme A in Hevea brasiliensis latex. Biochem J. 1969;114:379–386. doi: 10.1042/bj1140379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaluddin ND, Noor NM, Goh HH. Genome-wide transcriptome profiling of Carica papaya L. embryogenic callus. Physiol Mol Biol Plants. 2017;23(2):357–368. doi: 10.1007/s12298-017-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasrotia RS, Iquebal MA, Yadav PK, Kumar N, Jaiswal S, Angadi UB, Rai A, Kumar D. Development of transcriptome based web genomic resources of yellow mosaic disease in Vigna mungo. Physiol Mol Biol Plants. 2017;23(4):767–777. doi: 10.1007/s12298-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Lyou SH, Yeu S, Kim MA, Rhee S, Kim M, Lee JS, Choi YD, Cheong JJ. Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep. 2007;26:1053–1063. doi: 10.1007/s00299-007-0311-1. [DOI] [PubMed] [Google Scholar]
- Kazan K, Manners JM. MYC2: the master in action. Mol Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- Kekwick RGO. The formation of isoprenoids in Hevea latex. In: d’Auzac J, Jacob L, Chrestin H, editors. Physiology of rubber tree latex. Boca Raton: CRC Press; 1989. pp. 145–164. [Google Scholar]
- Kim YS, Hahn EJ, Murthy HN, Paek KY. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett. 2004;26:1619–1622. doi: 10.1007/s10529-004-3183-2. [DOI] [PubMed] [Google Scholar]
- Ko JH, Chow KS, Han KH. Transcriptome analysis reveals novel features of the molecular events occurring in the laticifers of Hevea brasiliensis (para rubber tree) Plant Mol Biol. 2003;53:479–492. doi: 10.1023/B:PLAN.0000019119.66643.5d. [DOI] [PubMed] [Google Scholar]
- Koo AJ, Howe GA. The wound hormone jasmonate. Phytochemistry. 2009;70:1571–1580. doi: 10.1016/j.phytochem.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackman P, González-Guzmán M, Tilleman S, Carqueijeiro I, Pérez AC, Moses T, Seo M, Kanno Y, Häkkinen ST, Van Montagu MC, Thevelein JM, Maaheimo H, Oksman-Caldentey KM, Rodriguez PL, Rischer H, Goossens A. Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc Natl Acad Sci USA. 2011;108:5891–5896. doi: 10.1073/pnas.1103010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Reyes A, Spoel SH, De Lange ES, Abe H, Kobayashi M, Tsuda S, Millenaar FF, Welschen RA, Ritsema T, Pieterse CM. Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009;149:1797–1809. doi: 10.1104/pp.108.133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-P. Molecular mechanism underlying ethylene stimulation of latex production in rubber tree (Hevea brasiliensis) Trees. 2016;30:1913–1921. doi: 10.1007/s00468-016-1455-9. [DOI] [Google Scholar]
- Liu J-P, Xia Z-Q, Tian X-Y, Li Y-J. Transcriptome sequencing and analysis of rubber tree (Hevea brasiliensis Muell.) to discover putative genes associated with tapping panel dryness (TPD) BMC Genom. 2015;16:398. doi: 10.1186/s12864-015-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-P, Zhuang Y-F, Guo X-L, Li Y-J. Molecular mechanism of ethylene stimulation of latex yield in rubber tree (Hevea brasiliensis) revealed by de novo sequencing and transcriptome analysis. BMC Genom. 2016;17:257. doi: 10.1186/s12864-016-2587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh SC, Thottathil GP, Othman AS. Identification of differentially expressed genes and signalling pathways in bark of Hevea brasiliensis seedlings associated with secondary laticifer differentiation using gene expression microarray. Plant Physiol Biochem. 2016;107:45–55. doi: 10.1016/j.plaphy.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Maldonado-Mendoza IE, Burnett RJ, Lòpez-Meyer M, Nessler CL. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by wounding and methyl jasmonate: implications for the production of anti-cancer alkaloids. Plant Cell Tiss Organ Cult. 1994;38:351–356. doi: 10.1007/BF00033896. [DOI] [Google Scholar]
- Men L, Yan S, Liu G. De novo characterization of Larix gmelinii (Rupr.) Rupr. transcriptome and analysis of its gene expression induced by jasmonates. BMC Genom. 2013;14:548. doi: 10.1186/1471-2164-14-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, Ecker JR, Reed JW. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development. 2005;132:4107–4118. doi: 10.1242/dev.01955. [DOI] [PubMed] [Google Scholar]
- Pauwels L, Morreel K, De Witte E, Lammertyn F, Van Montagu M, Boerjan W, Inzé D, Goossens A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci USA. 2008;105:1380–1385. doi: 10.1073/pnas.0711203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Inze D, Goossens A. Jasmonate-inducible gene: what does it mean? Trends Plant Sci. 2009;14:87–91. doi: 10.1016/j.tplants.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Popova LP, Vaklinova SG. Effect of jasmonic acid on the synthesis of ribulose-1,5-bisphosphate carboxylase-oxygenase in barley leaves. J Plant Physiol. 1988;133:210–215. doi: 10.1016/S0176-1617(88)80139-1. [DOI] [Google Scholar]
- Qu Y, Chakrabarty R, Tran HT, Kwon EJ, Kwon M, Nguyen TD, Ro DK. A lettuce (Lactuca sativa) homolog of human Nogo-B receptor interacts with cis-prenyltransferase and is necessary for natural rubber biosynthesis. J Biol Chem. 2015;290:1898–1914. doi: 10.1074/jbc.M114.616920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PH, Ellis CM, Ploense SE, Wu MF, Yadav V, Tholl D, Chételat A, Haupt I, Kennerley BJ, Hodgens C, Farmer EE, Nagpal P, Reed JW. A regulatory network for coordinated flower maturation. PLoS Genet. 2012;8:e1002506. doi: 10.1371/journal.pgen.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Concepción M, Campos N, Ferrer A, Boronat A. Biosynthesis of isoprenoid precursors in Arabidopsis. In: Bach TJ, Rohmer M, editors. Isoprenoid synthesis in plants and microorganisms: new concepts and experimental approaches. New York: Springer; 2013. pp. 439–456. [Google Scholar]
- Schmidt DD, Baldwin IT. Transcriptional responses of Solanum nigrumto methyl jasmonate and competition: a glasshouse and field study. Funct Ecol. 2006;20:500–508. doi: 10.1111/j.1365-2435.2006.01122.x. [DOI] [Google Scholar]
- Schmidt T, Hillebrand A, Wurbs D, Wahler D, Lenders M, Schulze Gronover C, Prüfer D. Molecular cloning and characterization of rubber biosynthetic genes from Taraxacum koksaghyz. Plant Mol Biol Rep. 2010;28:277–284. doi: 10.1007/s11105-009-0145-9. [DOI] [Google Scholar]
- Seetang-Nun Y, Sharkey TD, Suvachittanont W. Molecular cloning and characterization of two cDNAs encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase from Hevea brasiliensis. J Plant Physiol. 2008;165:991–1002. doi: 10.1016/j.jplph.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Sharma SN, Jha Z, Sinha RK, Geda AK. Jasmonate-induced biosynthesis of andrographolide in Andrographis paniculata. Physiol Plant. 2015;153:221–229. doi: 10.1111/ppl.12252. [DOI] [PubMed] [Google Scholar]
- Shi M-J, Tian W-M. Effect on the induction of the secondary laticifer differentiation by the transportation of exogenous JA in Hevea brasiliensis. Chin J Trop Crops. 2012;33:1647–1653. [Google Scholar]
- Shi J, Ma C, Qi D, Lv H, Yang T, Peng Q, Chen Z, Lin Z. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biol. 2015;15:233. doi: 10.1186/s12870-015-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Gavrieli J, Oakey JS, Curtis WR. Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyanus muticus in root cultures. Plant Cell Rep. 1998;17:391–395. doi: 10.1007/s002990050412. [DOI] [PubMed] [Google Scholar]
- Skilleter DN, Kekwick RGO. The enzymes forming isopentenyl pyrophosphate from 5-phosphomevalonate (mevalonate-5-phosphate) in the latex of Hevea brasiliensis. Biochem J. 1971;124:407–415. doi: 10.1042/bj1240407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Huang H, Gao H, Wang J, Wu D, Liu X, Yang S, Zhai Q, Li C, Qi T, Xie D. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell. 2014;26:263–279. doi: 10.1105/tpc.113.120394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Wasternack C, Xie D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr Opin Plant Biol. 2014;21:112–119. doi: 10.1016/j.pbi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Spanò D, Pintus F, Esposito F, Loche D, Floris G, Medda R. Euphorbia characias latex: micromorphology of rubber particles and rubber transferase activity. Plant Physiol Biochem. 2015;87:26–34. doi: 10.1016/j.plaphy.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CM. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulou EA, Haring MA, Schuurink RC. RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters. BMC Genom. 2014;15:402. doi: 10.1186/1471-2164-15-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Yang Y, Xie F, Wen JF, Wu J, Wilson IW, Tang Q, Liu H, Qiu D. Deep sequencing reveals transcriptome re-programming of Taxus x media cells to the elicitation with methyl jasmonate. PLoS ONE. 2013;8:e62865. doi: 10.1371/journal.pone.0062865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, Ueno Y, Yamamoto KT, Machida Y, Nakamura K, Ishiguro S. Arabidopsis AUXIN RESPONSE FACTOR6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol. 2010;51:164–175. doi: 10.1093/pcp/pcp176. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Lee H-J, Yamashita S, Koyama T. Characterization of cis-prenyltransferases from the rubber producing plant Hevea brasiliensis heterologously expressed in yeast and plant cells. Plant Biotech. 2012;29:411–417. doi: 10.5511/plantbiotechnology.12.0625a. [DOI] [Google Scholar]
- Tang C, Yang M, Fang Y, Luo Y, Gao S, Xiao X, An Z, Zhou B, Zhang B, Tan X, Yeang HY, Qin Y, Yang J, Lin Q, Mei H, Montoro P, Long X, Qi J, Hua Y, He Z, Sun M, Li W, Zeng X, Cheng H, Liu Y, Yang J, Tian W, Zhuang N, Zeng R, Li D, He P, Li Z, Zou Z, Li S, Li C, Wang J, Wei D, Lai CQ, Luo W, Yu J, Hu S, Huang H. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat Plants. 2016;2:16073. doi: 10.1038/nplants.2016.73. [DOI] [PubMed] [Google Scholar]
- Thanh NT, Murthy HN, Yu KW, Hahn EJ, Paek KY. Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl Microbiol Biotechnol. 2005;67:197–201. doi: 10.1007/s00253-004-1759-3. [DOI] [PubMed] [Google Scholar]
- Tian WM, Shi MJ, Yu FY, Wu JL, Hao BZ, Cui KM. Localized effects of mechanical wounding an exogenous jasmonic acid on the induction of secondary laticifer diferentiation in Hevea brasiliensis. Acta Bot Sin. 2003;45:1366–1372. [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Memelink J. ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science. 2000;289:295–297. doi: 10.1126/science.289.5477.295. [DOI] [PubMed] [Google Scholar]
- Vranová E, Coman D, Gruissem W. Structure and dynamics of the isoprenoid pathway network. Mol Plant. 2012;5:318–333. doi: 10.1093/mp/sss015. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo B, Zhang F, Yao H, Miao Z, Tang K. Molecular cloning and functional analysis of the gene encoding 3-hydroxy-3-methylglutaryl coenzyme Areductase from hazel (Corylus avellana L. Gasaway) J Biochem Mol Biol. 2007;40:861–869. doi: 10.5483/bmbrep.2007.40.6.861. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Zheng LP, Zhao PF, Zhao YL, Wang JW. Cloning and characterization of an elicitor-responsive gene encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase involved in 20-hydroxyecdysone production in cell cultures of Cyanotis arachnoidea. Plant Physiol Biochem. 2014;84:1–9. doi: 10.1016/j.plaphy.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang D, Sun Y, Yang Q, Chang L, Wang L, Meng X, Huang Q, Jin X, Tong Z. Comprehensive proteomics analysis of laticifer latex reveals new insights into ethylene stimulation of natural rubber production. Sci Rep. 2016;5:13778. doi: 10.1038/srep13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang F, Chen H, Liang X, Huang Y, Yi J. Comparative genomic hybridization and transcriptome sequencing reveal that two genes, OsI_14279 (LOC_Os03g62620) and OsI_10794 (LOC_Os03g14950) regulate the mutation in the γ-rl rice mutant. Physiol Mol Biol Plants. 2017;23(4):745–754. doi: 10.1007/s12298-017-0460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. How jasmonates earned their laurels: past and present. J Plant Growth Regul. 2015;34:761–794. doi: 10.1007/s00344-015-9526-5. [DOI] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhase RA, Lehmann J, Kramell H, Sembdner G, Parthier B. Degradation of ribulose-1,5-bisphosphate carboxylase and chlorophyll in senescing barley leaf segments triggered by jasmonic acid methylester, and counteraction by cytokinin. Physiol Plantarum. 1987;69:161–166. doi: 10.1111/j.1399-3054.1987.tb01961.x. [DOI] [Google Scholar]
- Whalen M, McMahan C, Shintani D. Development of crops to produce industrially useful natural rubber. In: Bach TJ, Rohmer M, editors. Isoprenoid synthesis in plants and microorganisms: new concepts and experimental approaches. New York: Springer; 2013. pp. 329–345. [Google Scholar]
- Yan Y, Borrego E, Kolomiets MV (2013) Jasmonate biosynthesis, perception and function in plant development and stress response. In: Baez RV (ed) Lipid metabolism, chap. 16, (Rijeka: InTech), pp 393–442
- Zeng RZ, Duan CF, Li Y. Construction of the SSH library of latex cDNA and sequence analyses of JA-stimulated rubber tree. Chin J Trop Crops. 2003;24:1–6. [Google Scholar]
- Zhang X, Wang C, Zhang Y, Sun Y, Mou Z. The Arabidopsis mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell. 2012;24:4294–4309. doi: 10.1105/tpc.112.103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu Z, An F, Hao D, Li P, Song J, Yi C, Guo H. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell. 2014;26:1105–1117. doi: 10.1105/tpc.113.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zheng SH, Fujita K, Sakai K. Jasmonate and ethylene signalling and their interaction are integral parts of the elicitor signalling pathway leading to {beta}-thujaplicin biosynthesis in Cupressus lusitanica cell cultures. J Exp Bot. 2004;55:1003–1012. doi: 10.1093/jxb/erh127. [DOI] [PubMed] [Google Scholar]
- Zhao CL, Cui XM, Chen YP, Liang Q. Key enzymes of triterpenoid saponin biosynthesis and the induction of their activities and gene expressions in plants. Nat Prod Commun. 2010;5:1147–1158. [PubMed] [Google Scholar]
- Zhou M, Memelink J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol Adv. 2016;34:441–449. doi: 10.1016/j.biotechadv.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Zhu Z. Molecular basis for jasmonate and ethylene signal interactions in Arabidopsis. J Exp Bot. 2014;65:5743–5748. doi: 10.1093/jxb/eru349. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Zhang ZL. Ethylene stimulation of latex production in Hevea brasiliensis. Plant Signal Behav. 2009;4:1072–1074. doi: 10.4161/psb.4.11.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, An F, Feng Y, Li P, Xue L, Mu A, Jiang Z, Kim JM, To TK, Li W, Zhang X, Yu Q, Dong Z, Chen WQ, Seki M, Zhou JM, Guo H. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: The forward and reverse primers used in validation experiment of gene expression by qRT-PCR (DOC 37 kb)
Table S2: Overview of the sequencing and mapping in this study (DOC 34 kb)
Table S3: The top 20 most up- and down-regulated genes between C and M2, and C and M4. (XLS 34 kb)
Table S4: DEGs were significantly enriched in glycolysis and carbon fixation (Calvin cycle) pathway in M2 and M4 compared with C. (XLS 23 kb)
Table S5: DEGs in phytohormone signaling in M2 and M4 compared with C. (XLS 24 kb)
Table S6: DEGs in transcriptional regulation in M2 and M4 compared with C. (XLS 46 kb)