Abstract
In the United States, newborn screening (NBS) is currently recommended for identification of 31 debilitating and potentially fatal conditions. However, individual states determine which of the recommended conditions are screened. The addition of severe combined immunodeficiency (SCID) to the recommended NBS panel has been fully instituted by 18 states, with another 11 states piloting programs or planning to begin screening in 2014. Untreated, SCID is uniformly fatal by 2 years of age. Hematopoietic stem cell transplantation is usually curative, but the success rate depends on the age at which the procedure is performed. Short-term implementation costs may be a barrier to adding SCID to states’ NBS panels. A retrospective economic analysis was performed to determine cost-effectiveness of NBS for early (<3.5 months) versus late (≥3.5 months) treatment of children with SCID at 3 centers over 5 years. Mean total charges at these centers for late treatment were 4 times greater than early treatment ($1.43 million vs $365,785, respectively). Mean charges for intensive care treatments were >5 times higher ($350,252 vs $66,379), and operating room/anesthesia charges were approximately 4 times higher ($57,105 vs $15,885). The cost-effectiveness of early treatment for SCID provides a strong economic rationale for the addition of SCID to NBS programs of other states.
Keywords: immunodeficiency, hematopoietic stem cell transplant, newborn screening, severe combined immunodeficiency
Introduction
The morbidity and/or mortality of a number of congenital disorders can be prevented if diagnosed and treated early. Because individuals with many of these conditions are asymptomatic until the onset of active disease, early detection is frequently the only means of forestalling disability or death. For example, treatment of phenylketonuria must begin shortly after birth to prevent the development of mental retardation.1 For this reason, newborn screening (NBS) programs have been implemented to identify infants affected by a number of congenital diseases, including severe combined immunodeficiency (SCID), and reduce the financial burden associated with late diagnosis.2–5 The Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) is charged with approving conditions for inclusion in a recommended uniform screening panel (RUSP) based on fulfillment of evidentiary criteria.6 An initial set of 29 disorders was selected for inclusion in the RUSP following recommendations by the American College of Medical Genetics (ACMG), with the expectation that early identification will allow those affected by these conditions to be adequately treated.7 Diseases within the general categories of organic acid disorders, amino acid disorders, fatty acid oxidation disorders, hemoglobinopathies, and “other” (including cystic fibrosis and hearing loss) are included in NBS panels.7 As of February 2013, many US states require by law, and have fully implemented, NBS for 29 core conditions.8 Most of the remaining states offer, but do not require, screening for hearing loss. Screening for secondary conditions, as well as for recently added core conditions, varies considerably among the states.8
The set of criteria formulated by the ACMG to determine the disorders to be included in the initial iteration of the RUSP included 3 broad categories (clinical characteristics of the condition; analytical characteristics of the test; and diagnosis, treatment, and management of the condition), each with a set of more specific considerations to guide suitability for inclusion. The subcategories deemed most important included clear scientific evidence of optimized outcomes from early intervention, the existence of a sensitive and specific screening test, the ability of a multiplex platform to detect multiple conditions, the potential efficacy of a treatment to prevent most or all negative consequences of the condition, and the simplicity of therapy/management at the primary care level.9
The SACHDNC continues to review conditions for inclusion into the RUSP. Following nomination, these conditions undergo a systematic review to establish suitability for recommendation of acceptability to the Secretary of Health and Human Services (HHS). Since 2007, 9 conditions have been nominated to the SACHDNC for addition into the RUSP. Although 6 of these were approved for further review, only 2 were added to the list of core conditions as of 2011: SCID and critical congenital cyanotic heart disease.10
Timing of Severe Combined Immunodeficiency Diagnosis and Treatment
Severe combined immunodeficiency is an inherited disorder resulting from at least 13 different genetic mutations11 and is broadly categorized as a deficiency in T lymphocytes with normal levels of B lymphocytes (T–B+) or a deficiency in both T and B lymphocytes (T–B–). Affected individuals are highly susceptible to bacterial, viral, and/or fungal infections. In addition, failure to thrive is often noted secondary to frequent diarrhea, which may be the presenting symptom in many patients.12 Diagnosis of SCID depends on initial exclusion of human immunodeficiency virus infection, followed by the demonstration of lymphocytopenia or, more precisely, immunophenotyping of lymphocyte subsets in comparison with age-matched controls.13 Identification of the exact form of SCID requires genetic analysis.13 The true prevalence of SCID is difficult to determine, but a minimal estimate is 1 per 40,000 to 100,000 live births.14, 15
Untreated SCID is uniformly fatal by 2 years of age. Currently, hematopoietic stem cell therapy (HSCT) is the most efficacious and potentially curative treatment;16, 17 however, efficacy rates depend on the age at which patients are diagnosed and treated. In 1 retrospective study, the survival rate in patients diagnosed at birth was 92%, compared with 61% in siblings diagnosed at a median age of 143 days.18 Another study found that survival following HSCT in patients treated before 3.5 months of age was 96%, compared with 66% in patients treated after 3.5 months.19 Thus, early diagnosis and treatment appear to have a substantial positive impact on mortality in patients with SCID.
Implementation of Severe Combined Immunodeficiency Screening
The addition of SCID to the RUSP was first proposed in 2007.20 However, approval was dependent, in part, on the demonstration of a reliable screening assay using dried bloodspots, usually obtained from infant heel sticks, which serve as the source material for the screening of the other RUSP conditions.11 The selected screening assay, the T-cell receptor excision circle (TREC) test, detects small loops of DNA that arise during the T lymphocyte maturation process. Low or absent TREC counts are indicative of deficient T lymphocyte levels.21 The development of a highly sensitive, specific, reliable, and automated TREC assay has allowed for routine high-throughput screening of infants for SCID, and in 2010, the Secretary of HHS recommended the addition of SCID to the core RUSP conditions.10 As of February 2014, 18 states and the Navajo Nation have implemented screening for SCID, with another 11 states piloting programs or beginning to screen in 2014.22 The District of Columbia and 5 additional states have approved screening, which will begin in 2015 or later (Table I).23
Table I.
| Statewide and Fully Implemented | Pilots and/or Screening Planned for 2014 | Approved for Future Implementatioin |
|---|---|---|
| California | Illinois | District of Columbia |
| Colorado | Maine | Georgia |
| Connecticut | Missouri | Maryland |
| Delaware | Nebraska | New Jersey |
| Florida | North Dakota | North Carolina |
| Massachusetts | Oklahoma | Virginia |
| Michigan | Oregon | |
| Minnesota | Rhode Island | |
| Mississippi | South Dakota | |
| New York | West Virginia | |
| Ohio | ||
| Pennsylvania | ||
| Texas | ||
| Utah | ||
| Washington | ||
| Wisconsin | ||
| Wyoming |
SCID=severe combined immunodeficiency disease.
In 2008, Wisconsin was the first US state to begin widespread screening of infants for SCID.24 During the first 3 years of this program, 207,696 infants were screened. The rate of false positive results was 0.018%, the assay specificity was 99.98%, and there was a positive predictive value of 45.83% for T-cell lymphopenia of any cause.24 Five of 72 infants with abnormal initial TREC counts were diagnosed with SCID.24 By April 2011, a total of 961,925 NBSs for SCID been performed in 6 states and 1 territory, resulting in the identification of 14 cases of SCID, as well as 6 cases of SCID variants, and 40 cases of non-SCID T-cell lymphopenia.20 During the time of screening in the 6 states and 1 territory, no other cases of SCID had been reported in those locations, indicating that the addition of SCID to the RUSP enabled the positive identification of all cases at or near birth.20 These preliminary data suggest that previous estimates of the incidence SCID may have been low.
Taken together, these data suggest that adding SCID to each state’s RUSP core conditions is advantageous. Currently, however, many states do not require or have not implemented this change to their panel. In some states, a general fund supports NBS for SCID. In others, funding becomes an obstacle to implementation because legislative approval for allocation of funds within each state’s budget is required to support the introduction of SCID screening. Economic considerations, lack of advocacy, and state regulations regarding validation and pilot testing are potential obstacles to implementation. A perceived lack of cost–benefit information, and underestimation of the financial resources needed for laboratory start-up and operational costs contribute to this reluctance in adding SCID to state RUSPs.20, 25 Many states may lack adequate numbers of immunologists and/or personnel trained to perform and evaluate the TREC assay.20 Some state legislatures are constrained by existing fiscal responsibilities pertaining to the screening of previously approved disorders.25 Nevertheless, economic considerations (including the lack of SCID NBS cost-effectiveness data) are probably the most important (and common) impediment to adding SCID screening to the RUSP core conditions. Therefore, analyzing the economics of early screening and detection of SCID is essential to assess the potential for cost savings, as well as to improve survival rates among patients with SCID.
Economic analyses were not a primary consideration in the development of recommendations for the inclusion of disorders in the RUSP by the ACMG.7 Worldwide, several studies have demonstrated the cost-effectiveness of NBS,26–28 although the idiosyncrasies of individual healthcare systems make it difficult to translate the results of these studies across national borders. A number of models indicate that NBS for individual disorders, such as medium-chain acyl-CoA dehydrogenase deficiency29 and cystic fibrosis,30 and for multiple conditions covered by the RUSP,28, 31 is cost-effective in the United States.
Cost analysis of SCID screening, and in particular the analysis of the difference between early and late diagnosis, should help allay the concerns that other state legislatures may have in deciding whether to incorporate SCID in their state NBS panel. To address these potential concerns, we conducted an economic analysis that assessed differences in hospital charges between early- and late-diagnosed SCID cases and evaluated the associated short-term economic benefits attributable to SCID. This analysis may provide important data that can be used by state healthcare organizations to make judgments on whether SCID should be added to state RUSPs.
Economic Outcomes of Management of Severe Combined Immunodeficiency
To approximate the cost savings associated with early versus late SCID diagnosis, a retrospective analysis of the actual charges for early (defined as <3.5 months of age) versus late (≥3.5 months) diagnosis and treatment of SCID was conducted at 3 centers (in California, Florida, and Pennsylvania). This multisite review, approved by each center’s local institutional review board, included data from 5 participating centers, although only 3 centers had complete data. All cases of SCID diagnosed and treated at the 3 centers in the 6 years from November 2005 to November 2011 were included in this analysis. Hospital charges were retrospectively collected for all subjects diagnosed with SCID during the time of the study, regardless of outcome. Investigators at each site met with hospital decision support analysts to collect data using a standardized query of hospital databases, as well as associated data, such as SCID phenotype, genotype, comorbidities, length of hospital care, and breakdown of charges where available (Table II). Data were captured for total charges, charges from diagnosis to transplantation, and transplantation to 180 days. Descriptive statistics (mean, standard deviation) were provided for comparison between early and late transplantations. Significant differences between charges were analyzed using the Mann-Whitney test.
Table II.
Characteristics of Subjects With SCID Who Underwent Hematopoietic Stem Cell Transplantation
| Early Transplantation (<3.5 months) (n=7) |
Late Transplantation (≥3.5 months) (n=13) |
|
|---|---|---|
| Study site | ||
| California | 2 | 0 |
| Florida | 1 | 5 |
| Pennsylvania | 4 | 8 |
| SCID type | ||
| XSCID | 3 | 5 |
| Jak3 | 0 | 1 |
| IL-7rα | 1 | 0 |
| Omenn | 1 | 1 |
| ADA | 0 | 2 |
| Artemis | 0 | 1 |
| Unknown | 2 | 3 |
| Outcomes | ||
| Death before transplantation | 0 | 1 |
| Complications | 4 | 8 |
| Alive and well | 3 | 3 |
| Death after transplantation | 0 | 1 |
| Admitted at outside hospital before diagnosis | 0 | 11 |
| Insurance type | ||
| Private | 2 | 7 |
| Government subsidized | 3 | 6 |
| Other/unknown | 2 | 0 |
| Average number of hospital days billed | 63.4 | 113.2 |
| Average charges/diagnosis to transplant | $101,385.13* | $864,774.67** |
| Average charges/transplant to 180 days | $211,597.46 | $904,797.93** |
| Average total charges | $365,784.89 | $1,458,045.90** |
| Average total charges per day billed | $5,769.47 | $12,880.25 |
ADA=adenosine deaminase; IL-7ra=interleukin-7 receptor α; Jak3=Janus kinase 3; SCID=severe combined immunodeficiency; XSCID=X-linked severe combined immunodeficiency.
Early: average charges dx-tx calculated for n=6 instead of 7 (only post tx charges applied in 1 case)
Late: average total charges calculated for n=13 (total cases); charges dx-tx calculated for n=8 (2 referred in for transplant only, and 1 patient died before transplant; previous charges incurred at an outside hospital for all 3 of these as well); average charges tx-180d calculated for n=12 because 1 patient died before transplant.
During the review period, 25 cases of SCID were identified across the 3 institutions. Seven subjects underwent early transplantation (<3.5 months; 2 in California, 1 in Florida, and 4 in Pennsylvania), 13 underwent late transplantation (≥3.5 months; 5 in Florida and 8 in Pennsylvania), and 5 received gene therapy (all in California). In 2 of the early transplantation cases, SCID was diagnosed by NBS, whereas in the late transplantation cases, SCID was diagnosed after the child presented with infection, and typically after previous hospitalization. For early diagnoses, time to transplant ranged from 3wks to 3m. For late diagnoses, time to transplant ranged from 2m to 7m.The 2 early cases in California were identified by NBS. During the study period, NBS for SCID was not being performed in Florida or Pennsylvania. One subject diagnosed late died before transplantation, and 1 diagnosed early was transferred to the referral hospital only for the period from transplantation to 180 days post-transplantation. No subjects undergoing early transplantation had prior hospitalizations, compared with 11 of 13 subjects (85%) who were diagnosed and underwent late transplantation. Of subjects who underwent transplantation, 9 of 20 (45%) were covered under government-subsidized insurance. Table II summarizes the cases by SCID type, outcomes, insurance, average number of hospital days billed, and whether the subjects were admitted to an outside hospital before diagnosis. Charges occurring at outside hospitals were not accessible and therefore were not captured as part of this study, although in some subjects, the charges were known to be considerable.
Total hospital charges, charges from diagnosis to transplantation, and charges from transplantation to 180 days post-transplantation were all significantly less for early versus late transplantation across the 3 institutions (Figure 1). Mean charges for early transplantation ($365,785) were approximately one-quarter of the charges for late transplantation ($1.43 million). Therefore, early treatment charges were approximately $1 million less than late treatment charges. Mean charges for individual revenue codes (where available) were also less for early transplantation versus late transplantation (pharmacy, $153,450 vs $326,592; intravenous therapy, $3,518 vs $8,261; supplies, $63,814 vs $81,246; and laboratory, $146,013 vs $226,607). Notably, mean charges for intensive care and operating room/anesthesia charges for late treatment were approximately 5 times and 4 times higher than for early treatment ($350,252 vs $66,379 and $57,105 vs $15,885 respectively; Figure 2). Higher costs for late transplantation may in part be because these patients are generally sicker and require longer ICU stays.
Figure 1.
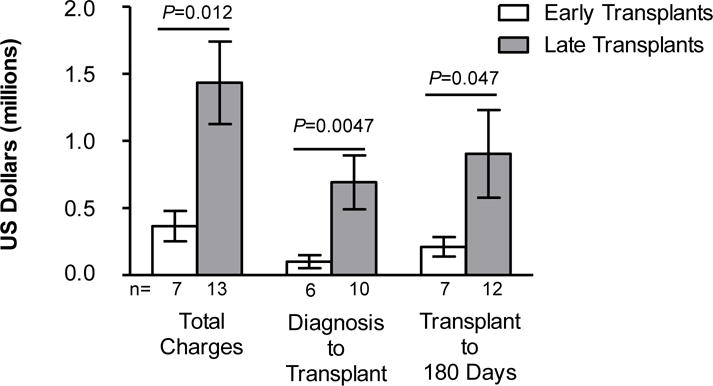
Mean hospital charges of subjects with SCID who underwent HSCT early (<3.5 months) or late (≥3.5 months). Total charges, charges from diagnosis to transplantation, and charges from transplantation to 180 days post-transplantation are shown and represent combined data from the 3 centers. Error bars indicate standard error of the mean. HSCT=hematopoietic stem cell transplantation; SCID=severe combined immunodeficiency.
Figure 2.
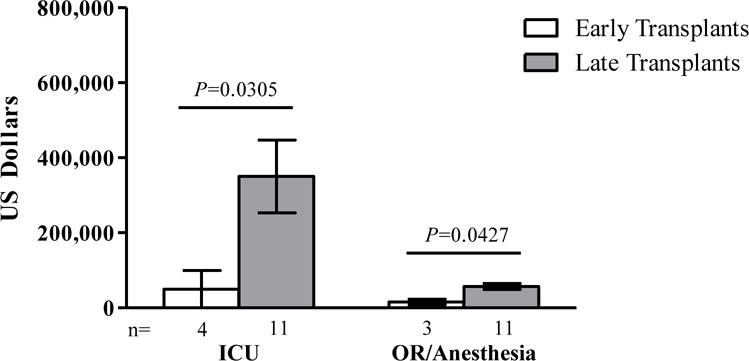
Mean intensive care unit and operating room/anesthesia charges for subjects with SCID who underwent HSCT early (<3.5 months) or late (≥3.5 months). Results represent combined data from the 3 centers. Error bars indicate standard error of the mean. HSCT=hematopoietic stem cell transplantation; ICU=intensive care unit; OR=operating room; SCID=severe combined immunodeficiency.
Cost-effectiveness of Newborn Screening for Severe Combined Immunodeficiency
The question of the cost benefits of early diagnosis of SCID and T-cell lymphopenia is often raised as states move toward nationwide compliance with the recommendation by the US Secretary of HHS to include SCID in the NBS standard panel. Previous reports have addressed the question of the cost benefits of NBS for SCID using models that study the impact of early detection on SCID natural history.2 However, the present study, which assessed actual hospital charges over a 6-year period, provides state governments with critical data about the cost benefits associated with early detection and management of SCID. Although charges are not actual costs to the hospital or payer, they serve as a useful proxy to estimate the positive economics in the short run of early screening for SCID. The data provided in this report suggest that hospital charges, including charges for ICU and OR/anesthesia, play a role in the increased costs associated with late SCID diagnosis. Taking into consideration the pilot data from Florida, along with a predicted incidence of SCID of 1 in 40,000 live births and an estimated 250,000 births per year in this state, we predicted that 40 NBSs would show low or absent levels of T lymphocytes that would require further confirmation by flow cytometry. Of these possible cases, 28 to 30 would be confirmed as actual T lymphocyte deficiencies, and 6 to 7 would qualify as SCID requiring HSCT per year. This information was then used to put the cost of NBS for SCID in perspective relative to the burden of associated charges for late diagnosis of SCID without NBS in place. The cost estimates presented below were provided in testimony before the Florida NBS advisory committee, and were instrumental in educating legislators about the potential cost savings to the state.
The data obtained in this study enabled estimation of the net burden of late SCID diagnosis and treatment on Medicaid compared with NBS and subsequent treatment. This assessment raised awareness about NBS and contributed to the decision to include NBS for SCID in the Florida panel. Legislation requiring screening for SCID in Florida was enacted in October 2012.32 According to data from the Florida Department of Health, about 50% of live births in the state of Florida are covered by Medicaid.33 Furthermore, the average number of live births per year in Florida from 2006 to 2011 was approximately 224,000.34 Thus, approximately 112,000 births per year in Florida are covered by Medicaid. Although the true incidence of SCID is unknown, early data from pilot studies in several states and in California’s first 6 months of screening estimated an incidence between 1 per 22,00025 and 1 per 46,00010 live births. Based on these estimates, it may be expected that approximately 5 to 10 SCID cases per year will occur in Florida. Recently published results for the first 2 years of screening for SCID in California show a combined incidence of 1 case per 49,700 live births for all types of SCID.35
Further, based on a cost of $16.67 per screen (includes staff time, equipment, colocation costs, and referral center contracts) and approximately 263,000 screens/year (which factors in extra screens required for repeat testing), the annual cost to Medicaid would be approximately $2.2 million in total; this should be compared with an estimated hospital charge of at least $2 million per child who needs late transplantation. Assuming 10 cases of SCID per year in Florida, these estimates indicate an accrued cost savings of up to $4.8 million annually if SCID is added to the state’s RUSP. This figure is larger if the estimate is based soley on cost of the actual screening test, which is approximately $4.50 per test.
Although the cut-off point for early transplantation in the patients described here was 3.5 months, transplantations, in general, should be performed as early as possible.36, 37 Data from siblings diagnosed with SCID at birth because of a positive family history would provide an informative comparative cohort. However, the small number of patients in this study made it difficult to obtain those data, and some SCID types may have different associated morbidities that can impact both the transplant and post-transplant course The group of patients who were transplanted at >3.5 months included 2 patients with ADA deficiency and 1 with Artemis.There was one patient with ADA in the early transplantation group who died prior to transplant and had required extensive medical care including intensive care. This may have biased the data somewhat, because transplantation in patients with these conditions is usually associated with poorer clinical outcomes.38–40 It should also be noted that the charges described here are underestimates, as charges were calculated for up to only 180 days post-transplantation. In addition, the hospital query did not include charges by some affiliated physicians who used a separate billing system. Because a hospital-based query was used, charges accrued for prior hospitalizations and medical care were also not included. These were likely to be more significant in the late diagnosis and treatment group. For example, known charges not captured for 1 late-group patient exceeded $600,000 (personal communication). The late-group total charges are further underestimated because 2 of the study subjects in Florida were still accruing charges at the time of completion of this study since they were still hospitalized and within 180 days post-transplantation. The charges for one of the early patients may be overestimated because the primary care physician waited for genetic confirmation before immunology referral, and in the interim, the patient developed cytomegalovirus infection and hepatitis.
Our data support the view that later diagnosis and treatment of SCID is associated with a greater financial burden to the healthcare system than early diagnosis and treatment. This is in agreement with previous studies that have demonstrated that early diagnosis and treatment of SCID before 3.5 months results in greater survival and decreased treatment costs.20 Without NBS, children with SCID are usually not referred to a specialist until approximately 6 months of age and the mean age at treatment is about 34 weeks (8 months).41 For children who survived following treatment, the mean age at diagnosis was 29 weeks; for children who did not survive following treatment, the mean age at diagnosis was 57 weeks. Survival in children diagnosed neonatally or prenatally was 85% compared with 58% in children diagnosed later (P=0.026).42 Gathering more data for children with SCID identified by NBS will facilitate future prospective studies of SCID. Furthermore, more data from Florida and other states will allow for additional cost analyses to be performed. Nevertheless, with the addition of SCID to the Florida RUSP, all cases should be detected early, and HSCT may be performed in a time period that will lead to cost savings and better outcomes for patients. It is hoped that these results can be used to assist state legislators in making informed decisions regarding budgeting and support for implementing the addition of SCID to local RUSPs.
Acknowledgments
Medical editing support was provided by Daniel McCallus, PhD, and Roderick H. Sayce, BSc, MBA, at Complete Publication Solutions, LLC; this support was funded by CSL Behring, LLC. The authors were fully responsible for the content of the manuscript, and approved submission.
Funding Source: CSL Behring LLC
EEP has received grants from CSL Behring and has acted as a consultant to CSL Behring and Baxter. MJD has acted as a consultant to CSL Behring and Baxter. AZ is an employee of CSL Behring.
Abbreviations
- ACMG
American College of Medical Genetics
- HHS
Health and Human Services
- HSCT
hematopoietic stem cell therapy
- ICU
intensive care unit
- NBS
newborn screening
- OR
operating room
- RUSP
recommended uniform screening panel
- SACHDNC-
Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children
- SCID
severe combined immunodeficiency
- TREC
T-cell receptor excision circle
Footnotes
Conflict of Interest/Disclosure Information:
The other authors have no conflicts to declare.
References
- 1.Pitt JJ. Newborn screening. Clin Biochem Rev. 2010;31:57–68. [PMC free article] [PubMed] [Google Scholar]
- 2.Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID) Mol Genet Metab. 2011;104:383–9. doi: 10.1016/j.ymgme.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andermann A, Blancquaert I, Beauchamp S, Costea I. Guiding policy decisions for genetic screening: developing a systematic and transparent approach. Public Health Genomics. 2011;14:9–16. doi: 10.1159/000272898. [DOI] [PubMed] [Google Scholar]
- 4.McGhee SA, Stiehm ER, McCabe ER. Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Pediatr. 2005;147:603–8. doi: 10.1016/j.jpeds.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JMG, Jungner G. The principles and practice of screening for disease. World Health Organization. Available at: http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. Accessed February 12, 2014.
- 6.US Department of Health and Human Services. Secretary’s advisory committee on heritable diseases in newborns and children. Available at: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/nominatecondition/index.html. Accessed February 12, 2014.
- 7.American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: toward a uniform screening panel and system–executive summary. Pediatrics. 2006;117:S296–307. doi: 10.1542/peds.2005-2633I. [DOI] [PubMed] [Google Scholar]
- 8.National Newborn Screening & Global Resource Center. National newborn screening status report. Available at: http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/nbsdisorders.pdf. Accessed February 12, 2014.
- 9.American College of Medical Genetics. Newborn screening: toward a uniform screening panel and system. doi: 10.1097/01.gim.0000223891.82390.ad. Available at: http://mchb.hrsa.gov/programs/newbornscreening/screeningreportpdf.pdf. Accessed February 12, 2014. [DOI] [PMC free article] [PubMed]
- 10.Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Annual Report to Congress. 2011 Available at: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/reportsrecommendations/reports/sachdnc2011report.pdf. Accessed February 12, 2014.
- 11.Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49:25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hague RA, Rassam S, Morgan G, Cant AJ. Early diagnosis of severe combined immunodeficiency syndrome. Arch Dis Child. 1994;70:260–3. doi: 10.1136/adc.70.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Burg M, Gennery AR. Educational paper. The expanding clinical and immunological spectrum of severe combined immunodeficiency. Eur J Pediatr. 2011;170:561–71. doi: 10.1007/s00431-011-1452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:687–8. [PubMed] [Google Scholar]
- 15.Lindegren ML, Kobrynski L, Rasmussen SA, Moore CA, Grosse SD, Vanderford ML, et al. Applying public health strategies to primary immunodeficiency diseases: a potential approach to genetic disorders. MMWR Recomm Rep. 2004;53:1–29. [PubMed] [Google Scholar]
- 16.Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 17.Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295:508–18. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 18.Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117:3243–6. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- 19.Puck JM, SCID Newborn Screening Working Group Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120:760–8. doi: 10.1016/j.jaci.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Buckley RH. The long quest for neonatal screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2012;129:597–604. doi: 10.1016/j.jaci.2011.12.964. quiz 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115:391–8. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Immune Deficiency Foundation. SCID Newborn Screening: current status of implementation map. Available at: http://primaryimmune.org/idf-advocacy-center/idf-scid-newborn-screening-campaign/. Accessed February 12, 2014.
- 23.Newborn Screening Translational Research Network. NBSTRN SCID National Call. 2013 Jan 24; Available at: https://www.nbstrn.org/sites/default/files/SCID%20National%20Monthly%20January%202013%20Final(1).pdf. Accessed February 12, 2014.
- 24.Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008–2011) J Clin Immunol. 2012;32:82–8. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 25.Brower A. SCID report to the secretary and committee discussion. Available at: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/meetings/twentyfourth/sachdncscid.pdf. Accessed February 12, 2014.
- 26.Hamers FF, Rumeau-Pichon C. Cost-effectiveness analysis of universal newborn screening for medium chain acyl-CoA dehydrogenase deficiency in France. BMC Pediatr. 2012;12:60. doi: 10.1186/1471-2431-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norman R, Haas M, Chaplin M, Joy P, Wilcken B. Economic evaluation of tandem mass spectrometry newborn screening in Australia. Pediatrics. 2009;123:451–7. doi: 10.1542/peds.2008-0911. [DOI] [PubMed] [Google Scholar]
- 28.Tiwana SK, Rascati KL, Park H. Cost-effectiveness of expanded newborn screening in Texas. Value Health. 2012;15:613–21. doi: 10.1016/j.jval.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Venditti LN, Venditti CP, Berry GT, Kaplan PB, Kaye EM, Glick H, et al. Newborn screening by tandem mass spectrometry for medium-chain Acyl-CoA dehydrogenase deficiency: a cost-effectiveness analysis. Pediatrics. 2003;112:1005–15. doi: 10.1542/peds.112.5.1005. [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, Rosenberg MA, Peterson A, Makholm L, Hoffman G, Laessig RH, et al. Analysis of the costs of diagnosing cystic fibrosis with a newborn screening program. J Pediatr. 2003;142:617–23. doi: 10.1067/mpd.2003.209. [DOI] [PubMed] [Google Scholar]
- 31.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117:S287–95. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 32.Cookro DV. Florida Department of Health Announcement Letter. Available at: http://www.floridahealth.gov/healthy-people-and-families/childrens-health/newborn-screening/_documents/scidannouncement.pdf. Accessed February 12, 2014.
- 33.Florida Department of Health. Births covered by Medicaid; FloridaCHARTS. Available at: http://www.floridacharts.com/charts/DataViewer/BirthViewer/BirthViewer.aspx?cid=595. Accessed February 12, 2014.
- 34.Florida Department of Health. Florida birth query system; FloridaCHARTS. Available at: http://www.floridacharts.com/FLQUERY/Birth/BirthRpt.aspx. Accessed February 12, 2014.
- 35.Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: Results of the first 2 years. J Allergy Clin Immunol. 2013;132:140–50e7. doi: 10.1016/j.jaci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 37.Railey MD, Lokhnygina Y, Buckley RH. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. J Pediatr. 2009;155:834–40e1. doi: 10.1016/j.jpeds.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzolari E, de Martiis D, Forino C, Lanfranchi A, Giliani S, Marzollo R, et al. Single-center analysis of long-term outcome after hematopoietic cell transplantation in children with congenital severe T cell immunodeficiency. Immunol Res. 2009;44:4–17. doi: 10.1007/s12026-008-8022-4. [DOI] [PubMed] [Google Scholar]
- 39.Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113:4114–24. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 40.Hassan A, Booth C, Brightwell A, Allwood Z, Veys P, Rao K, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120:3615–24. doi: 10.1182/blood-2011-12-396879. quiz 26. [DOI] [PubMed] [Google Scholar]
- 41.Adeli MM, Buckley RH. Why newborn screening for severe combined immunodeficiency is essential: a case report. Pediatrics. 2010;126:e465–9. doi: 10.1542/peds.2009-3659. [DOI] [PubMed] [Google Scholar]
- 42.Chan A, Scalchunes C, Boyle M, Puck JM. Early vs delayed diagnosis of severe combined immunodeficiency: a family perspective survey. Clin Immunol. 2011;138:3–8. doi: 10.1016/j.clim.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


