Abstract
The bacterium Rickettsia bellii belongs to a basal group of rickettsiae that diverged prior to the pathogenic spotted fever group and typhus group Rickettsia species. Despite a diverse representation of R. bellii across more than 25 species of hard and soft ticks in the American continent, phylogeographical relationships among strains of this basal group-Rickettsia species are unknown; the work described here explores these relationships. DNA was extracted from 30 R. bellii tick isolates: 15 from the United States, 14 from Brazil, and 1 from Argentina. A total of 2,269 aligned nucleotide sites of 3 protein coding genes (gltA, atpA, and coxA) and 2 intergenic regions (rpmE-tRNAfmet and RC1027-xthA2) were concatenated and subjected to phylogenetic analysis by Bayesian methods. Results showed a separation of almost all isolates between North and South Americas, suggesting that they have radiated within their respective continents. Phylogenetic positions of the 30 isolates could be a result of not only their geographical origin but also the tick hosts they have coevolved with. Whether R. bellii originated with ticks in North or South America remains obscure, as our analyses did not show evidence for greater genetic divergence of R. bellii in either continent.
1. Introduction
Members of the genus Rickettsia (Rickettsiales: Rickettsiaceae) are Gram-negative obligate intracellular bacteria, usually in association with arthropods, although a number of distinct genotypes have also been described from leeches, amoeba, ciliate, and hydra [1]. Phylogenetic analysis-based studies have classified the Rickettsia species into five major groups, namely, the spotted fever group (SFG), the typhus group (TG), the transitional group (TRG), the Rickettsia canadensis group, and the Rickettsia bellii group [1, 2]. This later group occupies a basal position in all major phylogenetic studies, which indicates early divergence within the genus [1, 3, 4].
Rickettsia bellii was formally described in 1983 based on isolates obtained from multiple species of ixodids (hard ticks) and argasids (soft ticks) in the United States [5]. In the United States, R. bellii has been reported to be infecting the following 8 tick species: Dermacentor variabilis, Dermacentor occidentalis, Dermacentor andersoni, Dermacentor albipictus, Dermacentor parumapertus, Haemaphysalis leporispalustris, Ornithodoros concanensis, and Argas cooleyi [5]. In recent years, investigators in Latin America have identified R. bellii in ixodid ticks throughout Latin America, including El Salvador, Costa Rica, Panama, Colombia, Brazil, Peru, and Argentina [6–13]. In Central and South America, R. bellii has been detected in at least 19 tick species, namely, Ixodes loricatus, Haemaphysalis juxtakochi, Amblyomma aureolatum, Amblyomma dubitatum, Amblyomma humerale, Amblyomma incisum, Amblyomma neumanni, Amblyomma longirostre, Amblyomma naponense, Amblyomma nodosum, Amblyomma ovale, Amblyomma oblongoguttatum, Amblyomma parvum, Amblyomma pseudoconcolor, Amblyomma rotundatum, Amblyomma sabanerae, Amblyomma scalpturatum, Amblyomma tigrinum, and Amblyomma varium [6, 7, 12, 14–16].
The wide host range and extraordinarily broad distribution of this Rickettsia species are intriguing and although R. bellii is generally considered as nonpathogenic for animals and humans [2, 17], it could play an important role in the ecology and epidemiology of other pathogenic tick-borne SFG rickettsiae in the Americas [18]. Despite the diverse representation of R. bellii across more than 25 species of hard and soft ticks in the American continent, the phylogeographical relationships of this basal group-Rickettsia species has not been examined; the work described here explores these relationships, based on a hypothesis that R. bellii could have evolved firstly in one continent or a particular tick group and then radiated to other continents or other tick groups.
2. Materials and Methods
2.1. Rickettsial Isolates
Rickettsia bellii was isolated from various species of hard ticks collected in South America and North America, as described in the original publication of each of the isolates listed in Table 1. A total of 30 isolates of R. bellii from 13 species of hard ticks from 3 countries were used for the analysis (Figure 1). These included 1 from Argentina, 14 from Brazil, and 15 from USA (Table 1). The Brazilian and Argentinean isolates are available at the Rickettsial Collection of the Laboratory of Parasitic Diseases of Faculdade de Medicina Veterinária e Zootecnia (FMVZ) of the University of São Paulo (USP), and the US isolates are available at the Rickettsial Zoonoses Branch at the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA.
Table 1.
Rickettsia bellii isolates from South and North America used in the present study.
| Number∗ | Isolate name | Tick host | Geographic origin | Reference |
|---|---|---|---|---|
| (1) | Mogi | Amblyomma aureolatum | Mogi das Cruzes-SP, Brazil | [19] |
| (2) | Ad-MG | Amblyomma dubitatum | Guarda-Mor-MG, Brazil | [16] |
| (3) | Ad-CORD-SP | A. dubitatum | Cordeirópolis -SP, Brazil | [20] |
| (4) | Ad-25-INT | A. dubitatum | Ribeirão Grande-SP, Brazil | [20] |
| (5) | PNSM | Amblyomma incisum | Cubatão-SP, Brazil | [21] |
| (6) | AO | Amblyomma ovale | Ribeirão Grande-SP, Brazil | [22] |
| (7) | HJ#4 | Haemaphysalis juxtakochi | Ribeirão Grande-SP, Brazil | [23] |
| (8) | IL-Mogi | Ixodes loricatus | Mogi das Cruzes-SP, Brazil | [24] |
| (9) | Ap GSV 136 | Amblyomma parvum | Chapada Gaúcha-MG, Brazil | [25] |
| (10) | IL-RS1 | I. loricatus | Derrubadas-RS, Brazil | [26] |
| (11) | RB-CL | I. loricatus | Cerro Largo-RS, Brazil | [13] |
| (12) | HJ#1 | H. juxtakochi | São Paulo-SP, Brazil | [23] |
| (13) | Ap-MS | Amblyomma pseudoconcolor | Corumbá-MS, Brazil | [16] |
| (14) | P-10 | A. ovale | Peruíbe-SP, Brazil | [27] |
| (15) | An4 | Amblyomma neumanni | Deán Funes-Córdoba, Argentina | [28] |
| (16) | 369-C | Dermacentor variabilis | Washington Co., Arkansas, USA | [5] |
| (17) | CA13-1 | Dermacentor variabilis | Yolo Co., California, USA | [29] |
| (18) | CA13-9 | D. variabilis | Yolo Co., California, USA | [29] |
| (19) | CA13-17 | D. variabilis | Yolo Co., California, USA | [29] |
| (20) | Putah Creek | D. variabilis | Solano Co., California, USA | [29] |
| (21) | Yolo | D. variabilis | Yolo Co., California, USA | [29] |
| (22) | Stevenson Bridge | D. variabilis | Yolo Co., California, USA | [29] |
| (23) | OSU 83-1223 | D. variabilis | Knox County, Ohio, USA | [30] |
| (24) | TX15-1 | Dermacentor parumapertus | Brewster County, Texas, USA | [31] |
| (25) | OSU 83-117 | D. variabilis | Licking County, Ohio, USA | [30] |
| (26) | OSU 85-1299 | D. variabilis | Morrow County, Ohio, USA | [30] |
| (27) | OSU 83-452 | D. variabilis | Coshocton County, Ohio, USA | [30] |
| (28) | Skull Valley | D. parumapertus | Tooele County, Utah, USA | [31] |
| (29) | OSU 85-389 | D. variabilis | Ohio, USA | [30] |
| (30) | CA-459 | Haemaphysalis leporispalustris | Mendocino Co., California, USA | [32] |
∗These numbers are represented in Figure 1 as the geographical location of each isolate.
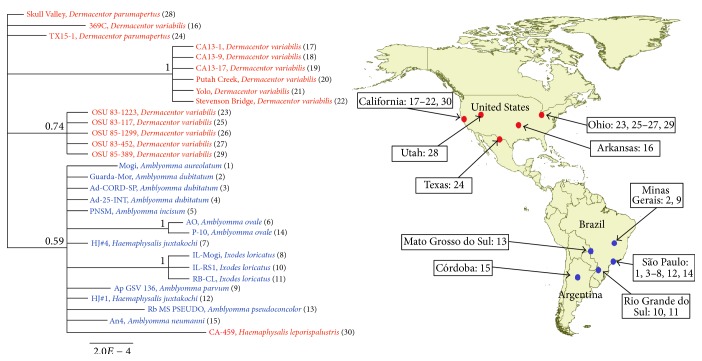
Figure 1.
Molecular phylogenetic analysis of 30 isolates of Rickettsia bellii from North and South America.
2.2. DNA Extraction and PCR
DNA from the R. bellii isolates from South America was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) and the QIAamp DNA Mini Kit (Qiagen) was used for the 15 isolates from North America, all in accordance with the manufacturer's recommendations. Amplification of fragments of five rickettsial genes and fifteen intergenic regions was attempted with the primer pairs listed in Table 2. Each PCR reaction consisted of 2 μl template DNA, 20 picomoles of each primer, and 10 μl Taq PCR Master Mix (Qiagen), while cycling conditions included a 1-minute incubation at 95°C followed by 35 cycles of a 30-second denaturation at 95°C, a 30-second annealing incubation (Table 2), and a 1-minute extension at 72°C. This was followed by a final 10-minute extension at 72°C. Gradient PCR was used to optimize the annealing temperatures in R. bellii for each primer pair.
Table 2.
Primer pairs used for amplification of rickettsial genes or intergenic regions in the present study.
| Primer pair | Target | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Annealing temperature (°C) | Amplicon size (nt) | Reference |
|---|---|---|---|---|---|---|
| (1) | mppA-purC | GCAATTATCGGTCCGAATG | TTTCATTTATTTGTCTCAAAATTCA | 45 to 551 | 160 | [33] |
| (2) | nusG-rplK | CAGTTGCAATATTGGTAAAGCA | CAGCAGCTGGAATTATCAAGTT | 45 to 551 | 270 | [33] |
| (3) | murG-RC0563 | GAAGAAAAGAAGGGCATAAGCTA | CAAGCTGAAAGTAAAAACATTCC | 40 to 521 | 293 | [33] |
| (4) | ntrY-rpsU | AGCTGCTGTTGCTAAAGTAAAAA | CAAGAAGCAGCAAGAAGACAGA | 52 to 641 | 363 | [33] |
| (5) | dksA-xerC | TCCCATAGGTAATTTAGGTGTTTC | TACTACCGCATATCCAATTAAAAA | 45 to 551 | 416 | [33] |
| (6) | spo0J-abcT1 | AAAGATTTGGAAGAATTAGACTTGAT | TTTGCTTAAACCAACCATTTCA | 45 to 551 | 259 | [33] |
| (7) | fabZ-lpxD | TGTTAGGATCGATTTTAAGTACTCTATCT | TGGATTGGCATAGACAATCTATTA | 45 to 551 | 195 | [33] |
| (8) | RC1137-tlc-5 | CGGGATAACGCCGAGTAATA | ATGCCGCTCTGAATTTGTTT | 45 to 551 | 264 | [33] |
| (9) | RC0230-RC0231 | TGCACCCGCCTAAAACTAAC | ATGGTCGGCCGTAGAAAAA | 45 to 551 | 232 | [33] |
| (10) | groES-RC0970 | CTTGCATCGGCTTTTCTTTT | AGCTTTGAGCTGATGGGCTA | 45 to 551 | 215 | [33] |
| (11) | rrf-pyrH | GAGCTTTCTCCATCTTTTCTTG | AAAGGGGAATATACGACAATTGAG | 45 to 551 | 238 | [33] |
| (12) | tRNAGly-tRNATyr | AGCTTGGAAGGCTGGAACTC | ATCCTTCTCCCTCCACCACT | 45 to 551 | 148 | [33] |
| (13) | pal-RC1201 | TGCAAGCACACATAATGCAA | TCAAAATCGATTCCTCTTTTCC | 45 to 551 | 216 | [33] |
| (14) | atpA | ATCAAGCGTTGCACAGATAG | GGAAGTGCCGTAAGTGAACC | 58 | Unknown∗ | [1] |
| (15) | rpmE-tRNAfMet | TTCCGGAAATGTAGTAAATCAATC | TCAGGTTATGAGCCTGACGA | 54 | 144 | [33] |
| (16) | RC1027-xthA2 | GGTATGTAAATGAGCCTTATCAATACT | TCAGTAGTATAAGTAGCTCCTGCTGTC | 54 | 351 | [33] |
| (17) | gltA | GTCTACTGCTTCGTGTAGATCAAC | GGCTGACCTATAGAATATTTATAAGAC | 54 | 408 | [25] |
| (18) | coxA | ACAGCCGTTGATATGGCTA | CATATTCCAACCGGCAAAAG | 58 | Unknown∗ | [1] |
| (19) | coxA | GGTGCTCCTGATATGGCATT | CATATTCCAGCCGGCAAAAG | 58 | Unknown∗ | [1] |
| (20) | atpA | ATCAAGCGTTGCACAGATAG | CGACTTACCGAAATACCGAC | 56 | 449 | [1, 34] |
∗Data not given in the original publication; 1tested in gradient PCR.
2.3. DNA Purification and Sequencing
DNA fragments amplified by PCR were visualized using a UV lamp in a 1.5% agarose gel containing 0.1 μg/ml ethidium bromide. PCR products of the appropriate size were cut from the gel and then purified using the Wizard SV gel and PCR clean-up system (Promega, Madison, WI). Sequencing reactions were prepared using one microliter of purified PCR product and the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions and then sequenced using an ABI 3100 genetic analyzer (Applied Biosystems). Each PCR amplicon was sequenced at least once in both directions.
2.4. Sequence Alignment and Phylogenetic Inferences
The resulting sequences were assembled using Geneious® 9.1.4 software (Biomatters Ltd., Auckland, New Zealand) and aligned using ClustalW [35] and MEGA 6.0.6 software [36]. The resulting alignment was examined by eye to ensure proper alignment of the sequences and the simple indel-coding method [37] was used to remove insertions/deletions. MrBayes 3.2.6 [38] was used in Geneious® to perform a Bayesian phylogenetic analysis. The Jukes-Cantor model was used in an analysis consisting of 10,000,000 generations (1,000,000 burn-ins). The analysis included 3 heated chains, and an effective sample size (ESS) of 901 was achieved for all parameters.
2.5. Accession Numbers
The GenBank accession numbers for the DNA sequences generated in this study for the 30 R. bellii isolates shown in Table 1 are the following: gltA gene (MF154866–MF154895), atpA gene (MF154926–MF154955), coxA gene (MF154896–MF154925), rpmE-tRNAfmet intergenic region (MF154956–MF154985), and RC1027-xthA2 intergenic region (MF154986–MF155015).
3. Results
Among the 20 primer pairs used for amplification of rickettsial DNA fragments, only the primer pairs # 15, 16, 17, 19, and 20 (Table 2) were successful in amplifying DNA from the R. bellii isolates. These primers corresponded to 3 protein coding genes (gltA, atpA, and coxA) and 2 intergenic regions (rpmE-tRNAfmet and RC1027-xthA2). The nucleotide sequences from the five loci for each of the 30 isolates were concatenated and used to perform a Bayesian analysis of their phylogeny. There was a separation of the isolates into at least three clades: one representing all South American isolates and the United States isolate CA-459 cultivated from a H. leporispalustris tick collected in northern California [39], one comprising 6 isolates from D. variabilis collected in northern California [29], and a third clade comprising 5 isolates from D. variabilis collected in Ohio [30] (Figure 1). North American isolates from D. parumapertus and the 369-C strain from D. variabilis did not group within any of the above clades. Within the South American clade, there were two smaller well-supported clades (each with a posterior probability of 1.0), one consisting of three isolates associated with I. loricatus ticks and one consisting of two A. ovale-associated isolates.
Overall, there were very few polymorphisms (<0.5%) between the 30 R. bellii isolates. In the 2,269-nucleotide alignment, identity values between the isolates varied from 99.56 to 100% (Table S1). DNA sequences of the isolates composing the main South American clade were 99.69–100% identical and at the same time 99.56–99.74% identical to the clade containing D. variabilis isolates from California and 99.74–99.91% identical to the clade containing D. variabilis isolates from Ohio. Within-clade identities were 99.74–100% for the D. variabilis isolates from Ohio and 100% for the D. variabilis isolates from California; the Ohio clade was 99.74% identical to the California clade. Finally, sequences of the three North American isolates (two from D. parumapertus and one from D. variabilis) that did not group within any main clade were 99.87–99.96% identical to each other and 99.60–99.91% identical to the remaining isolates.
4. Discussion
Here we performed for the first time a phylogenetic analysis of multiple isolates of R. bellii. Because these isolates represented a number of different locations in North and South America, our intention was to infer phylogeographical relationships. Indeed, there was a separation of almost all isolates between the two continents, suggesting that they have radiated within their respective continents. Although the posterior probability of the separation of the South American clade from the North American isolates was not strong (PP = 0.59), this topology was also achieved using both the GTR and HKY85 evolutionary models (supplemental material), giving additional evidence for this separation. At the same time, it is noteworthy that while the North American clades represented isolates exclusively from Dermacentor ticks, the South American clade was composed of isolates from the genera Amblyomma, Haemaphysalis, and Ixodes. Under such circumstances, the phylogenetic positions of the 30 isolates of the present study could be a result of not only their geographical origin but also the tick hosts in which they were isolated from. This later assumption is corroborated by the position of the isolate from H. leporispalustris, which, despite being from North America, grouped within the South American clade, where at least two other isolates from Haemaphysalis ticks were present.
To our knowledge, the number and diversity of tick species infected with R. bellii are the largest and broadest described among species in the genus Rickettsia. The magnitude of different host species indicates horizontal transmission among tick populations, especially in South America, where isolates from different tick species and genera grouped together in the same clade. It is generally accepted that R. bellii is not pathogenic for vertebrates; in fact, R. bellii has never been isolated from a vertebrate host [2]. On the other hand, there has been serological evidence of animal natural infection or exposure by R. bellii [40, 41]. While R. bellii horizontal transmission among ticks via vertebrate host cannot be discarded, another likely mechanism could be via tick parasitoids, since the parasitism of the hymenopteran Ixodiphagus spp. is relatively common among different tick genera in the Americas [42, 43]. In fact, a recent study provided molecular detection of tick-borne rickettsiae in Ixodiphagus wasps that had emerged from ticks [44], highlighting the possibility that rickettsial organisms could be shared by ticks and their parasitoids. Regardless of the mechanisms of horizontal transmission, the tendency of each R. bellii genotype to be associated with a different tick species (Figure 1) suggests that horizontal transmission was more efficient at earlier times; thereafter, most of the R. bellii isolates are likely to have coevolved specifically with their specific tick species host, possibly towards a symbiotic association. In fact, analysis of the genome of the type strain of R. bellii has shown features compatible with various symbiotic bacteria, such as a large genome size with high coding capacity, in contrast to the reduced genome size with low coding capacity of the pathogenic rickettsiae [4, 45].
It has been proposed that the R. bellii group diverged prior to the division between the SFG and the TG, forming a basal group that also includes various herbivorous arthropod symbionts [1, 46]. While the genus Rickettsia was considered to be approximately 150 million years old, the divergence of the R. bellii group was estimated to have occurred much more recently, around 50 million years ago [1]. Analyses of the oldest tick fossils, from the Cretaceous, indicate that extant tick genera (Amblyomma, Ornithodoros) were soundly established around 100 million years ago [47–49]. From these data, it can be inferred that R. bellii likely radiated with its principal hosts approximately 50 million years ago. While ticks and rickettsiae are distributed in all continents, it is noteworthy that, under natural conditions and without human interference, tick species from the New World do not occur in the Old World and vice versa; the only exceptions are a few tick species that are associated with transcontinental marine birds [50]. Indeed, this scenario has accounted for the presence of R. bellii-infected ticks restricted to the New World, regardless of the ability of this bacterium to infect a vast array of tick genera and species. On the other hand, whether R. bellii originated with ticks in North or South America remains obscure, since our phylogenetic analyses did not show any evidence for greater genetic divergence of R. bellii in any of the two continents. Further analyses encompassing more R. bellii isolates from different tick genera and species, encompassing more molecular markers, should provide cues for the origin and radiation of R. bellii in the New World.
5. Conclusion
Phylogeographical analysis of 30 strains (15 from North America and 15 from South America) isolated from 13 species of 4 genera shows a clear differentiation between most of the North and South American isolates, indicating geographic isolation between isolates of these two continents. Additionally, this analysis separated isolates of R. bellii by the species of tick from which these were isolated, indicating that the isolates could have coevolved with their tick vectors over time.
Acknowledgments
This work received financial support from the São Paulo Research Foundation (FAPESP, Grant #2015/10060-6).
Disclosure
The findings and conclusions are those of the authors and do not necessarily reflect the views of the US Department of Health and Human Services or the official position of the Centers for Disease Control and Prevention.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Supplementary Materials
An identity matrix table of the 2,269-nucleotide alignment used for the phylogeny if R. bellii is available in the supplemental material for this article (Table S1). The final alignment comprising 2,269 nucleotides was concatenated in the following order: rpmE-tRNAfmet (414-nt), gltA (357-nt), coxA (898-nt), atpA (449-nt), and RC1027-xthA2 (151-nt); and it is also available in the supplementary material (Rbellii final alignment.fas). Two additional phylogenetic trees are also available in the supplementary material (Rbellii GTR tree.pdf and Rbellii HKY tree.pdf). Table S1: identity matrix of a 2,269-nucleotide alignment of the 30 Rickettsia bellii isolates from Brazil (BRA), Argentina (ARG), and the United States (USA) used in the present study. Supplementary Figure 1: molecular phylogenetic analysis of 30 isolates of Rickettsia bellii from North and South America. A total of 2,269 aligned nucleotide sites of 3 protein coding genes (gltA, atpA, and coxA) and 2 intergenic regions (rpmE-tRNAfmet and RC1027-xthA2) were concatenated and subjected to Bayesian analysis. A total of 10,000,000 generations were run using the GTR model with a sample frequency of 10,000. The analysis was run with 3 heated chains, and the first 1,000,000 generations were discarded as burn-in. Numbers at nodes are support values derived from posterior probability. The scale bar is in units of expected substitutions per site. Supplementary Figure 2: molecular phylogenetic analysis of 30 isolates of Rickettsia bellii from North and South America. A total of 2,269 aligned nucleotide sites of 3 protein coding genes (gltA, atpA, and coxA) and 2 intergenic regions (rpmE-tRNAfmet and RC1027-xthA2) were concatenated and subjected to Bayesian analysis. A total of 10,000,000 generations were run using the HKY85 model with a sample frequency of 10,000. The analysis was run with 3 heated chains, and the first 1,000,000 generations were discarded as burn-in. Numbers at nodes are support values derived from posterior probability. The scale bar is in units of expected substitutions per site.
References
- 1.Weinert L. A., Werren J. H., Aebi A., Stone G. N., Jiggins F. M. Evolution and diversity of Rickettsia bacteria. BMC Biology. 2009;7, article no. 6 doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parola P., Paddock C. D., Socolovschi C., et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clinical Microbiology Reviews. 2013;26(4):657–702. doi: 10.1128/cmr.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stothard D. R., Clark J. B., Fuerst P. A. Ancestral divergence of Rickettsia bellii from the spotted fever and typhus groups of Rickettsia and antiquity of the genus Rickettsia. International Journal of Systematic Bacteriology. 1994;44(4):798–804. doi: 10.1099/00207713-44-4-798. [DOI] [PubMed] [Google Scholar]
- 4.Merhej V., Raoult D. Rickettsial evolution in the light of comparative genomics. Biological Reviews. 2011;86(2):379–405. doi: 10.1111/j.1469-185X.2010.00151.x. [DOI] [PubMed] [Google Scholar]
- 5.Philip R. N., Casper E. A., Anacker R. L. Rickettsia bellii sp. nov.: A tick-borne Rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. International Journal of Systematic Bacteriology. 1983;33(1):94–106. doi: 10.1099/00207713-33-1-94. [DOI] [Google Scholar]
- 6.Labruna M. B., Mattar V S., Nava S. Rickettsioses in Latin America, Caribbean, Spain and Portugal. Revista MVZ Córdoba. 2011;16(2):2435–2457. doi: 10.21897/rmvz.282. [DOI] [Google Scholar]
- 7.Ogrzewalska M., Literak I., Cardenas-Callirgos J. M., Capek M., Labruna M. B. Rickettsia bellii in ticks Amblyomma varium Koch, 1844, from birds in Peru. Ticks and Tick-borne Diseases. 2012;3(4):254–256. doi: 10.1016/j.ttbdis.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Ogrzewalska M., Saraiva D. G., Moraes-Filho J., et al. Epidemiology of Brazilian spotted fever in the Atlantic Forest, state of Sao Paulo, Brazil. Parasitology. 2012;139(10):1283–1300. doi: 10.1017/s0031182012000546. [DOI] [PubMed] [Google Scholar]
- 9.Miranda J., Mattar S. Molecular detection of Rickettsia bellii and Rickettsia sp. strain Colombianensi in ticks from Cordoba, Colombia. Ticks and Tick-borne Diseases. 2014;5(2):208–212. doi: 10.1016/j.ttbdis.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Andoh M., Sakata A., Takano A., et al. Detection of Rickettsia and Ehrlichia spp. In ticks associated with exotic reptiles and amphibians imported into Japan. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0133700.e0133700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogrzewalska M., Literák I., Capek M., et al. Bacteria of the genus Rickettsia in ticks (Acari: Ixodidae) collected from birds in Costa Rica. Ticks and Tick-borne Diseases. 2015;6(4):478–482. doi: 10.1016/j.ttbdis.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Barbieri A. R. M., Romero L., Labruna M. B. Rickettsia bellii infecting Amblyomma sabanerae ticks in El Salvador. Pathogens and Global Health. 2012;106(3):188–189. doi: 10.1179/2047773212Y.0000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krawczak F. S., Binder L. C., Oliveira C. S., et al. Ecology of a tick-borne spotted fever in southern Brazil. Experimental and Applied Acarology. 2016;70(2):219–229. doi: 10.1007/s10493-016-0070-1. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh D., Bezerra R. A., Luz H. R., et al. Detection of Rickettsia bellii and Rickettsia amblyommii in Amblyomma longirostre (Acari: Ixodidae) from Bahia state, Northeast Brazil. Brazilian Journal of Microbiology. 2015;46(3):879–883. doi: 10.1590/S1517-838246320140623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares H. S., Barbieri A. R. M., Martins T. F., et al. Ticks and rickettsial infection in the wildlife of two regions of the Brazilian Amazon. Experimental and Applied Acarology. 2015;65(1):125–140. doi: 10.1007/s10493-014-9851-6. [DOI] [PubMed] [Google Scholar]
- 16.Costa F. B., Barbieri A. R. M., Szabó M. P. J., Ramos V. N., Piovezan U., Labruna M. B. New records of Rickettsia bellii-infected ticks in Brazil. Brazilian Journal of Veterinary Research and Animal Science. 2017;54(1):92–95. doi: 10.11606/issn.1678-4456.bjvras.2017.114141. [DOI] [Google Scholar]
- 17.Labruna M. B., Whitworth T., Horta M. C., et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian Spotted Fever is endemic. Journal of Clinical Microbiology. 2004;42(1):90–98. doi: 10.1128/jcm.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macaluso K. R., Sonenshine D. E., Ceraul S. M., Azad A. F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. Journal of Medical Entomology. 2002;39(6):809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 19.Pinter A., Labruna M. B. Isolation of Rickettsia rickettsii and Rickettsia bellii in cell culture from the tick Amblyomma aureolatum in Brazil. Annals of the New York Academy of Sciences. 2006;1078:523–529. doi: 10.1196/annals.1374.103. [DOI] [PubMed] [Google Scholar]
- 20.De Campos Pacheco R., Horta M. C., Pinter A., et al. Survey of Rickettsia spp in the ticks Amblyomma cajennense and Amblyomma dubitatum in the State of São Paulo. Journal of the Brazilian Society of Tropical Medicine. 2009;42(3):351–353. doi: 10.1590/S0037-86822009000300023. [DOI] [PubMed] [Google Scholar]
- 21.Sabatini G. S. Pesquisa de Carrapatos e Riquétsias no Parque Estadual da Serra do Mar, Núcleo Itutinga – Pillões, São Paulo [Dissertation, thesis] São Paulo, SP.: University of São Paulo; 2010. [Google Scholar]
- 22.Pacheco R., Rosa S., Richtzenhain L., Szabó M. P. J., Labruna M. B. Isolation of Rickettsia bellii from Amblyomma ovale and Amblyomma incisum ticks from southern Brazil. Revista MVZ Cordoba. 2008;13(2):1273–1279. [Google Scholar]
- 23.Labruna M. B., Pacheco R. C., Richtzenhain L. J., Szabó M. P. J. Isolation of Rickettsia rhipicephali and Rickettsia bellii from Haemaphysalis juxtakochi ticks in the state of São Paulo, Brazil. Applied and Environmental Microbiology. 2007;73(3):869–873. doi: 10.1128/AEM.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horta M. C., Pinter A., Schumaker T. T. S., Labruna M. B. Natural infection, transovarial transmission, and transstadial survival of Rickettsia bellii in the tick Ixodes loricatus (Acari: Ixodidae) from Brazil. Annals of the New York Academy of Sciences. 2006;1078:285–290. doi: 10.1196/annals.1374.053. [DOI] [PubMed] [Google Scholar]
- 25.Barbieri A. R. M. Ecologia de carrapatos e rickettsias transmitidas por carrapatos em uma reserva natural de Cerrado brasileiro [Ph.D. thesis] São Paulo, SP.: University of São Paulo; 2016. [Google Scholar]
- 26.Krawczak F. S. PhD thesis. University of São Paulo, São Paulo, SP. Pesquisa de infecção por riquétsias do grupo da febre maculosa em cães, pequenos mamíferos e carrapatos em área endêmica e não endêmicas nos biomas Pampa e Mata Atlântica no estado do Rio Grande do Sul [Ph.D. thesis] São Paulo, SP.: University of São Paulo; 2016. [Google Scholar]
- 27.Szabó M. P. J., Nieri-Bastos F. A., Spolidorio M. G., Martins T. F., Barbieri A. M., Labruna M. B. In vitro isolation from Amblyomma ovale (Acari: Ixodidae) and ecological aspects of the Atlantic rainforest Rickettsia, the causative agent of a novel spotted fever rickettsiosis in Brazil. Parasitology. 2013;140(6):719–728. doi: 10.1017/s0031182012002065. [DOI] [PubMed] [Google Scholar]
- 28.Labruna M. B., Pacheco R. C., Nava S., Brandão P. E., Richtzenhain L. J., Guglielmone A. A. Infection by Rickettsia bellii and Candidatus "Rickettsia amblyommii" in Amblyomma neumanni ticks from Argentina. Microbial Ecology. 2007;54(1):126–133. doi: 10.1007/s00248-006-9180-3. [DOI] [PubMed] [Google Scholar]
- 29.Hecht J. A., Allerdice M. E. J., Krawczak F. S., Labruna M. B., Paddock C. D., Karpathy S. E. Development of a Rickettsia bellii-specific TaqMan assay targeting the citrate synthase gene. Journal of Medical Entomology. 2016;53(6):1492–1495. doi: 10.1093/jme/tjw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poetter K. PhD thesis. Molecular population and evolutionary genetics of Rickettsia. Columbus, OH, USA: Ohio State University; 1989. [Google Scholar]
- 31.Paddock C. D., Allerdice M. E. J., Karpathy S. E., et al. Unique strain of Rickettsia parkeri associated with the hard tick Dermacentor parumapertus Neumann in the western United States. Applied and Environmental Microbiology. 2017;83(9) doi: 10.1128/AEM.03463-16.e03463-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philip R. N., Casper E. A., Lane R. S. Serotypes of tick-borne spotted fever group Rickettsiae from western California. The American Journal of Tropical Medicine and Hygiene. 1981;30(3):722–727. doi: 10.4269/ajtmh.1981.30.722. [DOI] [PubMed] [Google Scholar]
- 33.Fournier P.-E., Zhu Y., Ogata H., Raoult D. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. Journal of Clinical Microbiology. 2004;42(12):5757–5766. doi: 10.1128/JCM.42.12.5757-5766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitorino L., Chelo I. M., Bacellar F., Zé-Zé L. Rickettsiae phylogeny: A multigenic approach. Microbiology. 2007;153(1):160–168. doi: 10.1099/mic.0.2006/001149-0. [DOI] [PubMed] [Google Scholar]
- 35.Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogden T. H., Rosenberg M. S. How should gaps be treated in parsimony? A comparison of approaches using simulation. Molecular Phylogenetics and Evolution. 2007;42(3):817–826. doi: 10.1016/j.ympev.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Huelsenbeck J. P., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 39.Emmons R. W., Dondero D. V., Nelson B. C., Lane R. S. Ecology of tick-borne agents in California. The American Journal of Tropical Medicine and Hygiene. 1981;30(1):239–252. doi: 10.4269/ajtmh.1981.30.239. [DOI] [PubMed] [Google Scholar]
- 40.Pacheco R. C., Horta M. C., Moraes-Filho J., Ataliba A. C., Pinter A., Labruna M. B. Rickettsial infection in capybaras (Hyrochoerus hydrochaeris) from São Paulo Brazil: Serological evidence for infection by Rickettsia bellii and Rickettsia parkeri. Biomédica. 2008;27(3):364–371. [PubMed] [Google Scholar]
- 41.Coelho M. G., do Nascimento Ramos V., Limongi J. E., et al. Serologic evidence of the exposure of small mammals to spotted-fever Rickettsia and Rickettsia bellii in Minas Gerais, Brazil. The Journal of Infection in Developing Countries. 2016;10(3):275–282. doi: 10.3855/jidc.7084. [DOI] [PubMed] [Google Scholar]
- 42.Hu R., Hyland K. E., Oliver A review on the use of ixodiphagus wasps (Hymenoptera: Encyrtidae) as natural enemies for the control of ticks (Acari: Ixodidae) Systematic and Applied Acarology. 1998;3:19–28. doi: 10.11158/saa.3.1.3. [DOI] [Google Scholar]
- 43.Lopes A. J. O., Nascimento-Júnior J. R. S., Silva C. G., Prado Á. P., Labruna M. B., Costa-Júnior L. M. Parasitism by ixodiphagus wasps (Hymenoptera: Encyrtidae) in Rhipicephalus sanguineus and Amblyomma ticks (Acari: Ixodidae) in three regions of Brazil. Journal of Economic Entomology. 2012;105(6):1979–1981. doi: 10.1603/EC12012. [DOI] [PubMed] [Google Scholar]
- 44.Bohacsova M., Mediannikov O., Kazimirova M., Raoult D., Sekeyova Z. Arsenophonus nasoniae and rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0149950.e0149950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogata H., La Scola B., Audic S., et al. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genetics. 2006;2(5):733–744. doi: 10.1371/journal.pgen.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlman S. J., Hunter M. S., Zchori-Fein E. The emerging diversity of Rickettsia. Proceedings of the Royal Society B Biological Science. 2006;273(1598):2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klompen H., Grimaldi D. First mesozoic record of a parasitiform mite: A larval argasid tick in Cretaceous Amber (Acari: Ixodida: Argasidae) Annals of the Entomological Society of America. 2001;94(1):10–15. doi: 10.1603/0013-8746(2001)094[0010:FMROAP]2.0.CO;2. [DOI] [Google Scholar]
- 48.Grimaldi D. A., Engel M. S., Nascimbene P. C. Fossiliferous cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. American Museum Novitates. 2002;3361:1–71. doi: 10.1206/0003-0082(2002)361<0001:FCAFMB>2.0.CO;2. [DOI] [Google Scholar]
- 49.Nava S., Guglielmone A. A., Mangold A. J. An overview of systematics and evolution of ticks. Frontiers in Bioscience. 2009;14(8):2857–2877. doi: 10.2735/3418. doi: 10.2735/3418. [DOI] [PubMed] [Google Scholar]
- 50.Guglielmone A. A., Apanaskevich D. A., Estrada-Peña A., Robbins R. G., Petney T. N., Horak I. G. The Hard Ticks of the World: (Acari: Ixodida: Ixodidae) 1st. Vol. 1. Dordrecht: Springer Netherlands press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An identity matrix table of the 2,269-nucleotide alignment used for the phylogeny if R. bellii is available in the supplemental material for this article (Table S1). The final alignment comprising 2,269 nucleotides was concatenated in the following order: rpmE-tRNAfmet (414-nt), gltA (357-nt), coxA (898-nt), atpA (449-nt), and RC1027-xthA2 (151-nt); and it is also available in the supplementary material (Rbellii final alignment.fas). Two additional phylogenetic trees are also available in the supplementary material (Rbellii GTR tree.pdf and Rbellii HKY tree.pdf). Table S1: identity matrix of a 2,269-nucleotide alignment of the 30 Rickettsia bellii isolates from Brazil (BRA), Argentina (ARG), and the United States (USA) used in the present study. Supplementary Figure 1: molecular phylogenetic analysis of 30 isolates of Rickettsia bellii from North and South America. A total of 2,269 aligned nucleotide sites of 3 protein coding genes (gltA, atpA, and coxA) and 2 intergenic regions (rpmE-tRNAfmet and RC1027-xthA2) were concatenated and subjected to Bayesian analysis. A total of 10,000,000 generations were run using the GTR model with a sample frequency of 10,000. The analysis was run with 3 heated chains, and the first 1,000,000 generations were discarded as burn-in. Numbers at nodes are support values derived from posterior probability. The scale bar is in units of expected substitutions per site. Supplementary Figure 2: molecular phylogenetic analysis of 30 isolates of Rickettsia bellii from North and South America. A total of 2,269 aligned nucleotide sites of 3 protein coding genes (gltA, atpA, and coxA) and 2 intergenic regions (rpmE-tRNAfmet and RC1027-xthA2) were concatenated and subjected to Bayesian analysis. A total of 10,000,000 generations were run using the HKY85 model with a sample frequency of 10,000. The analysis was run with 3 heated chains, and the first 1,000,000 generations were discarded as burn-in. Numbers at nodes are support values derived from posterior probability. The scale bar is in units of expected substitutions per site.