Abstract
Vitamin A and its active metabolite retinoic acid are essential for embryonic development and adult homeostasis. Surprisingly, excess or deficiency of vitamin A and retinoic acid can cause similar developmental defects. Therefore, strict feedback and other mechanisms exist to regulate the levels of retinoic acid within a narrow physiological range. The oxidation of vitamin A to retinal has recently been established as a critical nodal point in the synthesis of retinoic acid, and over the past decade, RDH10 and DHRS3 have emerged as the predominant enzymes that regulate this reversible reaction. Together they form a codependent complex that facilitates negative feedback maintenance of retinoic acid levels and thus guard against the effects of dysregulated vitamin A metabolism and retinoic acid synthesis. This review focuses on advances in our understanding of the roles of Rdh10 and Dhrs3 and their impact on development and disease.
INTRODUCTION
Vitamin A (retinol) is an organic nutrient that is essential for human embryonic development and adult homeostasis. More specifically, during embryogenesis, sufficient levels of vitamin A are critical for brain, craniofacial, limb, and organ patterning and morphogenesis.1–3 Later, in adulthood, vitamin A plays important roles in fertility, immune function, maintenance of vision, and skeletal homeostasis.4–6 Vitamin A is available to the human diet in two distinct forms: preformed vitamin A (retinol and its esterified form, retinyl ester) and provitamin A carotenoids. Provitamin A carotenoids serve as the principle source of vitamin A within the food chain and, include α-carotene, β-carotene, and β-cryptoxanthin which are derived from plants. Preformed vitamin A is typically found in foods of animal origin, including dairy products, fish, and meat (especially liver) and most of the body’s vitamin A is stored in the liver in the form of retinyl esters.
Both provitamin A and preformed vitamin A are metabolized intracellularly via two sequential oxidation reactions to all-trans-retinoic acid (ATRA), the active form of vitamin A, which elicits essential biological functions. In the first reaction which is reversible, retinol is oxidized to form all-trans-retinal. In the second reaction, all-trans-retinal is irreversibly oxidized to ATRA. ATRA is a ligand for each of the three mammalian isotypes of the retinoic acid receptors (RARs), namely RARα, β, or γ,7–10 which form heterodimers with one of three different isotypes of the retinoid X receptor (RXR): RXRα, β, or γ.11 The RAR–RXR heterodimers associate with genomic elements consisting of direct repeats (DRs) termed retinoic acid response elements (RAREs) found in the promoter or vicinity of target genes (Figure 1).
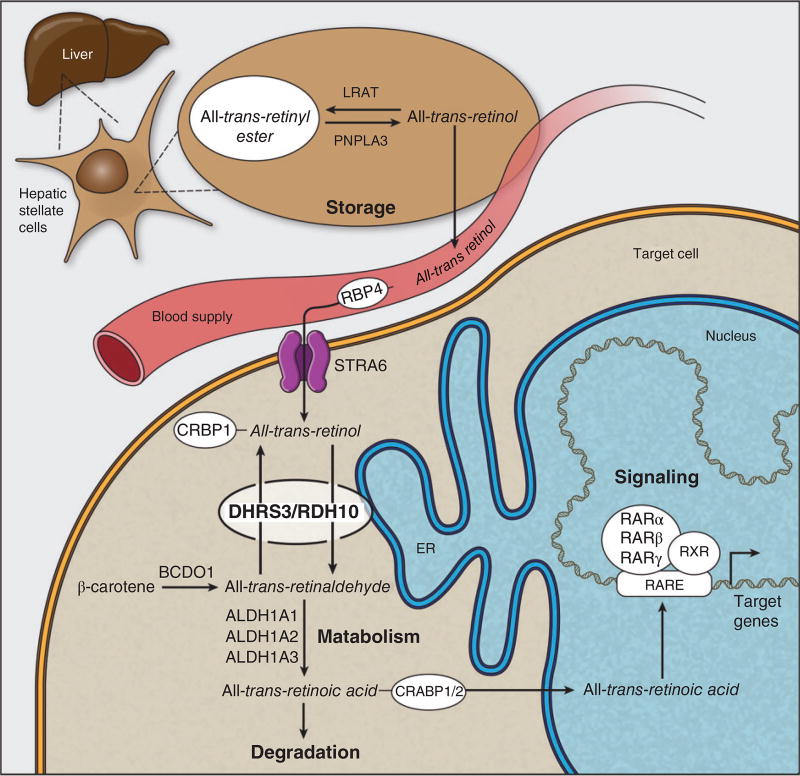
FIGURE 1.
Preformed vitamin A is taken up from the intestinal lumen by enterocytes and transported via the blood supply as all-trans-retinol to its various target tissues. Large stores of vitamin A are kept in hepatic stellate cells of the liver by esterification of all-trans-retinol to retinyl esters. Mobilization of these stores is carried out by hydrolysis of retinyl esters back to all-trans-retinol where it is then transported by the blood supply through binding to RBP4. At target tissues, the RBP receptor, STRA6, allows for the import of retinol associated with RBP4. Once inside the cell, retinol associates with the RDH10/DHRS3 complex in the first reversible step of vitamin A metabolism to be oxidized to all-trans-retinaldehyde by RDH10. All-trans-retinaldehyde can then be reduced back to all-trans-retinol by DHRS3, or it may be further oxidized by the ALDH genes (ALDH1A1–ALDH1A3) to form ATRA. ATRA then serves as a ligand for one of three isotypes of the RARs which form a heterodimer with RXR. The RAR–RXRs associate with retinoic acid response elements (RARE) within the promoters of target genes and can induce both transcriptional activation and silencing.
In the classical model of retinoid signaling, RAR–RXR heterodimers associate with RAREs in the presence of corepressors, histone deacetylases, and methyltransferases, which maintain target genes in a transcriptionally silent state. Upon ATRA ligand binding, RAR–RXRs dissociate from corepressors and recruit coactivators resulting in transcriptional activation.12 However, deviations from this classical model have been identified in which binding of ATRA to RAR–RXRs has been shown to recruit corepressors to silence gene activation. Repression of gene expression by retinoic acid was first identified in the restriction of the homeobox B1 (Hoxb1) gene.13 Other deviations to the classical model have been found as in the case of fibroblast growth factor 8 (Fgf8),14,15 as wells as, in the differential regulation of the paired homeobox proteins, by upregulation of PHOX2A and downregulation of PHOX2B.16 Many of the genes controlled by RAR–RXRs play significant roles in cellular differentiation and developmental programming; thus, gene regulation by RAR–RXRs play critical roles in developmental patterning, differentiation of tissues, and organogenesis during embryonic development.1,3,17,18 Furthermore, following birth, RAR–RXR-based signaling continues to play important roles in postnatal life in a wide array of processes such as the immune response, germ cell function, metabolic regulation, neuronal plasticity and tissue differentiation, regeneration, and repair.19–23
The mechanisms that regulate the formation of ATRA in embryos and the plethora of developmental processes in which ATRA plays a critical role have been the subject of several excellent and recent reviews.3,24–27 However, the long established dogma that (1) oxidation of all-trans-retinol to all-trans-retinal was carried out solely by medium-chain alcohol dehydrogenase (ADH) enzymes, and did not serve an important role in the regulation of ATRA synthesis; and (2) that primary control of ATRA synthesis occurred solely at the second oxidative step has been overturned.28–30 In the current review, we therefore first describe new developments and changing paradigms in our understanding of the feedback regulation of ATRA levels in embryonic tissues. Second, we focus on the new directions and ideas regarding the role of ATRA during developmental programming.
Morphogenesis and pattern formation during embryogenesis are driven by a multitude of signaling molecule gradients that orchestrate specific programs of gene expression and ultimately determine cell fate. ATRA signaling via RAR–RXR contributes to establishing cell fate by acting as a positional morphogen.31,32 Information derived by an ATRA-responsive cell from the cellular levels of ATRA is integrated with positional and temporal information derived from other morphogen gradients allowing the cell to make complex fate decisions.33
The actions of ATRA come from its function as an autocoid, acting near its site of synthesis due to its short half-life and relatively lipophilic composition. In many developmental processes, ATRA acts in a noncell autonomous fashion whereby the developmental fate of an ATRA-responsive cell population, which cannot synthesize ATRA, is regulated by a neighboring ATRA-synthetic cell population. Noncell autonomous modes of signaling for ATRA have been proposed in the case of the developing kidney34 and brain vascular development.35 In other processes, however, ATRA also acts in a cell-autonomous, autocrine fashion, that is, by directing the expression of genes within the same cell that synthesizes ATRA from a retinaldehyde precursor. For example, such autocrine effects of ATRA have been proposed in Sertoli cells.36 This type of autocrine signaling by ATRA is particularly important in situations where ATRA regulates its own levels and metabolism within the ATRA-synthetic cell.
Regulating the levels of a diffusible molecule like ATRA requires the cooperation of binding proteins and transporters, and of synthesis and catabolism enzymes. The levels of ATRA within tissues need to be very tightly regulated to ensure proper signaling. The factors involved in ATRA metabolism and transport are modulated by both ATRA-dependent and by ATRA-independent pathways37 and are responsive to the dietary or maternal levels of retinoid precursors.
It is important to consider that in nonlaboratory settings, the levels and/or composition of ATRA precursors such as preformed vitamin A and provitamin A carotenoids, vary considerably in the diet of different populations and even among different individuals within the same population. For example, Western diets are more reliant on animal products, which provide more preformed vitamin A and less provitamin A carotenoids than the diets available to the rest of the world’s populations.38,39
Vitamin A deficiency can have serious deleterious effects ranging from loss of vision, to impaired growth and risk of infection.40 Thus optimal levels of ATRA need to be maintained despite large individual differences in the absorption and conversion of provitamin A carotenoids.41 Ensuring that embryonic tissues receive appropriate levels of ATRA at each particular stage and time of development depends on cooperation between both maternal and fetal retinoid metabolic pathways and their ability to adapt to the availability of vitamin A, as well as the need for ATRA.
TERATOGENICITY OF EXCESS OR DEFICIENCY OF ATRA
A sufficient intake of vitamin A is critical for normal embryonic development;42–45 however, administration of excess vitamin A or ATRA during early pregnancy can lead to congenital malformations of the head, heart, and limbs.46–50 Hypervitaminosis A has also been associated with abnormal conditions in adults such as an increased risk of bone fractures in the elderly,51–53 and in addition to teratogenic effects, pharmacological doses of ATRA can also cause toxicity associated with symptoms of retinoic acid syndrome.54 Paradoxically, similar congenital malformations have been observed in mothers that have ingested excess amounts of vitamin A during pregnancy or patients exposed to drugs based on ATRA or RAR-agonists, as in vitamin A-deficient patients and animal models.55,56 The overlapping spectrum of congenital malformations and conditions irrespective of excess or deficiency in vitamin A or ATRA is due to the fact that changes in the levels of embryonic ATRA alters basic developmental and homeostasis pathways.57
The spatiotemporal levels of ATRA in an embryo following adult exposure to a pharmacological dose of ATRA, is subject to the time of exposure, the efficiency of transport of ATRA across the placenta, and the pharmacokinetics and distribution of ATRA in each tissue of the fetus. While in the past, exogenous ATRA administration to pregnant female mice was used to examine the effects of excess levels of embryonic ATRA, a recent study of the immediate and late effects of ATRA administration on mouse fetal development has painted a more complex picture. Even though a pharmacological dose of ATRA can initially result in an excess of ATRA in the embryo, this excess is followed by induction of ATRA-deficiency in the recovery period.58 More specifically, within 6 h postexogenous administration of ATRA to the mother, the levels of ATRA in mouse embryos can be found in 1800-fold excess compared to untreated embryos. However, after an additional 6 h, the levels of embryonic ATRA diminish to the extent they become equal in treated versus untreated embryos. The embryonic levels of ATRA then continue to fall in treated embryos, such that by 24 h they are up to 33% lower than normally seen in untreated embryos.
The progression from short-term excess to long-term deficiency following administration of exogenous ATRA is most likely a result of the increased clearance of ATRA and compensatory enzymatic activities by maternal and embryonic tissues trying to cope with excess levels of ATRA. Similar, compensatory activity is also observed following genetic deletion of the different RARs which leads to the generation of teratogenic levels of ATRA.59 Therefore, the pharmacokinetic profile of an acute dose of ATRA consists of a short period of ATRA excess in the embryo immediately after administration, followed by a long state of ATRA deficiency.
The surprising finding that many of the deleterious effects of exogenous ATRA administration could be corrected by a subsequent dose of ATRA58 led to the conclusion that prolonged ATRA deficiency which follows an acute excess of ATRA, is responsible, at least in part, for many of the deleterious effects caused by exposure to pharmacological doses of ATRA. In fact, it is evident that ATRA deficiency induced by ATRA administration is more prolonged and has more toxic effects, than an acute change in the hours immediately following administration. Even modest changes in the embryonic levels of ATRA can result in developmental defects if these perturbations persist for a sufficiently long period of time. For example an increase in the steady state level of ATRA of only 40% in early mouse embryos results in teratogenic effects through E14.5. Similarly, decreases in the steady state levels of endogenous ATRA of comparable magnitude also elicit developmental defects.25,60,61
MOLECULAR DETERMINANTS OF RETINOID METABOLISM IN EMBRYONIC TISSUES
Uptake and Transport of Retinoids and Carotenoids
Provitamin A carotenoids are a significant source of dietary vitamin A in humans.39 Carotenoids are absorbed in the intestine and are transported in the circulation by association with lipoproteins, mostly chylomicrons.62,63 Carotenoids are taken up by several organs and tissues such as the liver, eye, ovaries, and fat. Although embryonic tissues primarily receive preformed vitamin A, there is evidence that provitamin A carotenoids can also be converted to ATRA within the fetus.60 Provitamin A carotenoids, which represent carotenoids with one unsubstituted β-ionone ring, are oxidatively cleaved to produce retinaldehyde by the enzyme β-carotene-15,15-dioxygenase 1 (BCDO1; Figure 1).64–66 BCDO1 is expressed at high levels in the enterocytes of the intestine and also in other tissues such as hepatic stellate and parenchymal cells in the liver, in mammary tissue, keratinocytes, and the kidney, as well as in embryonic tissues.67–69 The expression of BCDO1 is negatively regulated by the intestine-specific transcription factor ISX which itself is induced by ATRA via RAR.70–73
Preformed vitamin A (retinol and retinyl esters) is hydrolyzed in the intestinal lumen by several lipases with broad specificity and then is taken up by enterocytes through facilitated transport or passive diffusion (reviewed in Refs 74 and 75). Retinol is re-esterified within the enterocytes primarily through the actions of lecithin retinol acyltransferase (LRAT).76 Within the enterocyte, esterification of retinol is also enhanced in the presence of cellular retinol-binding protein II (CRBPII), which binds retinol with high affinity at low nanomolar concentrations.76–79 Postprandial retinyl esters and carotenoids are secreted from the intestine as part of chylomicrons, the bulk of which are taken up by liver as chylomicron remnants. However, chylomicrons can also deliver retinoids to some target tissues including the embryo via the placenta.80–82 Circulating carotenoids and retinyl esters are also found associated with lipoproteins including very low density lipoproteins.83,84 Retinyl ester-containing chylomicron remnants are endocytosed by hepatocytes and hydrolysed through the activity of several carboxylesterases and lipases.84,85 Other ester hydrolases found in target tissues, such as lipoprotein lipase, hydrolyze retinyl esters from chylomicrons and allow cells to take up the free retinol.82,86 It is important to note that a significant amount of unesterified retinol is secreted in the portal circulation independent of apolipoproteins and that this pathway may be important in certain pathological conditions.74
The main mode of transport for retinoids is via blood circulation and the most efficient delivery system for retinoids to embryonic tissues is in the form of retinol associated with serum retinol binding protein (RBP), or more specifically RBP4 (Figure 1). During postnatal life, RBP4 is secreted by hepatocytes which transport retinol to most target tissues including the placenta. Within the fetus, RBP4 is secreted by both fetal hepatocytes and the visceral yolk sac which facilitates the transport of retinol from the placenta to the embryonic sites of ATRA synthesis.87,88 The importance of RBP4 was demonstrated in adult mice carrying RBP4 null mutations as they displayed decreased fertility as well as severe structural changes to the retina with accompanying decreases in vision.89,90 The role of RBP4 in delivering retinol to the placenta and from the placenta to embryonic tissues is evident in the developmental delay and abnormalities associated with impaired synthesis of either maternal and/or the fetal-derived RBP.81
RBP interacts with several surface receptors on target tissues. The best known RBP receptor is stimulated by retinoic acid 6 (STRA6) which is found in many organ–blood barriers, in the retinal pigment epithelium (RPE), choroid plexus, and Sertoli cells, as well as epidermal keratinocytes, dermal fibroblasts, and the placenta.91–94 STRA6 plays a critical role in allowing tissues such as the RPE and the choroid plexus to import retinol from the retinol–RBP complex referred to as holo-RBP.93,95–97 Although it was previously suggested that STRA6 couples with CRBP1 to internalize retinol,98,99 a recently determined cryo-EM structure of STRA6 suggests that STRA6 mediates internalization of retinol by diffusion through the cellular membrane.100 In cases where there is an excess of serum RBP unbound to retinol, or apo-RBP, and unesterified retinol within the cytoplasm, STRA6 can mediate efflux of cellular retinol to apo-RBP (Figure 1).99,101,102 A second RBP4 receptor (RBPR2) is found in the small intestine and hepatocytes, and is thought to potentially play a role in the reverse transport of retinol to the liver.103
The exact mechanism for maternal-fetal transfer of retinol from holo-RBP4 across the placenta to the developing fetus remains to be determined. Importantly, however, it has been shown that neither maternal, nor fetal RBP4 can cross the placenta.104 There is clear evidence that the delivery of retinol via holo-RBP4 to the placenta is receptor-mediated105– 107 and that STRA6 is one possible placental RBP receptor.108 But what mediates the transfer of retinol from the maternal placenta to the embryonic circulation is currently unknown. Similarly, it is also not known whether this transfer involves a transcellular or a paracellular pathway, or both.
Among the many issues and unanswered questions regarding the targeting and transport of RBP4 across the placenta, one of the most important is the relevance of extrapolating findings derived from mouse models to human biology. For example, there are important differences between rodents and humans in terms of placental development and even in terms of the role of the visceral yolk sac which functions throughout murine pregnancy but is mostly a vestigial structure in humans after the first trimester.109,110 There are also species-specific differences in the extent of retinoid delivery to the fetus mediated by lipoprotein-based transport of retinyl esters. Therefore beyond initial reports,111,112 there has been a paucity of studies investigating the mechanisms of retinol delivery across the human placenta.
What we do know is that LRAT is critically required for the uptake of retinol from plasma by target tissues such as RPE cells through binding to Stra6.113 LRAT also plays an important role in the formation of retinyl esters in quiescent hepatic stellate cells, the body’s principal vitamin A storage site under normal physiological conditions (Figure 1). Although stellate cells represent approximately 8% of the total cells in a healthy liver, they store 80–90% of the liver’s retinoid content which is equivalent to 50–80% of the total retinoid stores of the body.114 As in the intestine, esterification of retinol in the liver and in the RPE is enhanced in the presence of cellular RBPs, in this case, CRBP1.115–117 Hydrolysis of retinyl esters stored in the liver or in target tissues can be carried out by several potential enzymes. Of note, hormone-sensitive lipase and patatin-like phospholipase domain-containing 3 - (PNPLA3) have been implicated in the hydrolysis of retinyl esters in adipose tissue and hepatic stellate cells, respectively (Figure 1).118–120 However, it is likely that there are several other enzymes capable of such activity in adipose tissue, hepatic stellate cells, and RPE.
Regulation of Vitamin A Uptake, Transport, and Storage
In the case of circulating forms of retinoids, ATRA controls the expression levels of the receptors of serum RBP, namely, STRA6 and RBPR2. While the expression of STRA6 is induced by ATRA via RARγ,121–123 the expression of the liver RBP4 receptor, RBPR2, is repressed by ATRA and is negatively correlated with liver retinoid status.103 ATRA also controls the expression levels of CRBP1 and CRBP2 which play important roles in channeling ligands to and from cellular receptors and/or enzymes.124,125 In general, the expression of both CRBP1 and CRBP2 is increased in response to ATRA acting via RAR/RXR on several well-characterized RARE sequences found in their promoters. However, the levels of expression of CRBP2 in the small intestine are increased in the case of vitamin A deficiency, suggesting additional mechanisms may result in differential expression of these accessory proteins in specific tissues.126
Several mechanisms allow for cells to regulate the levels of retinyl esters to respond to vitamin A deficiency or to accommodate an intake of dietary vitamin A. Feedback regulation by ATRA signals vitamin A status and affects the uptake, delivery, and storage of retinol as retinyl esters.74 ATRA augments the formation of retinyl esters through upregulation of LRAT.127,128 These changes allow tissues to increase their storage of vitamin A in times of supply and also help tissues avoid forming excess ATRA from free retinol. The activity of BCDO1 also appears to regulate the activity and expression of LRAT and other acyltransferases and thereby influence the formation of retinyl esters in the fetus. Interestingly, the mechanism by which this occurs does not involve cleavage of its known substrate β-carotene.60,129 Our knowledge of the intracellular retinyl ester hydrolases operating within the liver, RPE, adipose tissue, and other tissues to store retinoids remains rudimentary. However, retinyl ester hydrolases play a critical role in the uptake of retinol from retinyl esters as well as in determining the availability of retinol for ATRA. Thus, this process is likely to be tightly regulated. In support of this idea, the expression of the retinyl ester hydrolase, PNPLA3 in hepatic stellate cells, is inversely correlated with unesterified retinol availability.120
Interconversion of Retinol and Retinaldehyde
The first step in the metabolism of vitamin A to ATRA is the oxidation of all-trans-retinol to all-trans-retinal (Figure 1). For many years, it was commonly thought that cytosolic ADHs were primarily responsible for this reaction during embryogenesis and that they carried out the reaction ubiquitously and redundantly. The primary reasons for this idea lay firstly in the observation that Adh7 was ubiquitously expressed during embryogenesis. Secondly, although Adh1 and Adh4 exhibit tissue specific domains of activity,130 Adh1−/−, Adh4−/−, and Adh7−/− loss-of-function mouse models survive into adulthood without evidence of developmental defects.131–133 The lack of a discernible developmental phenotype in Adh loss-of-function models therefore led to the idea that primary control for the synthesis of ATRA occurred at the second step through retinaldehyde dehydrogenase (RALDH) oxidation of retinal to ATRA. In support of this idea, Raldh2 loss-of-function mouse mutants exhibited diminished ATRA synthesis in association with considerable developmental defects in heart, limb, and axial development.134
However, the long established dogma that (1) oxidation of all-trans-retinol to all-trans-retinal during embryogenesis was carried out solely by ADH enzymes and did not serve an important role in the regulation of ATRA synthesis; and (2) that primary control of ATRA synthesis occurred solely at the second oxidative step was overturned by the identification of the short-chain dehydrogenase, RDH10, and its role in the first oxidation step of vitamin A metabolism.28–30 The importance of RDH10 in embryonic development and ATRA synthesis and signaling was clearly revealed in hypomorphic Rdh10trex/trex and null Rdh10−/− mouse models which are embryonic lethal between E10.5 and E14.5 in association with insufficient ATRA production.28–30,129 Analysis of in vivo ATRA activity in null Rdh10−/− mutants carrying the RARE-lacZ transgene reporter, revealed a complete absence of RA production prior to E9.5.29 Similarly, In Rdh10trex/trex embryos, ATRA activity was completely absent in the craniofacial, trunk, and limb regions. Only a considerably diminished domain of activity remained within the neural tube (Figure 2).28,136,137 Furthermore, in enzymatic assays, the Rdh10trex mutant allele was shown to be incapable of oxidizing vitamin A (retinol) to retinal.28 A further indication of RDH10’s critical role in this process is the substantial reduction of NAD-dependent retinol oxidase activity in Rdh10 loss-of-function embryos.30 RDH10 is membrane bound in target cells and carries out the oxidation of all-trans-retinol within pools of the cellular membrane that are free of inhibitory RBP1.138
FIGURE 2.
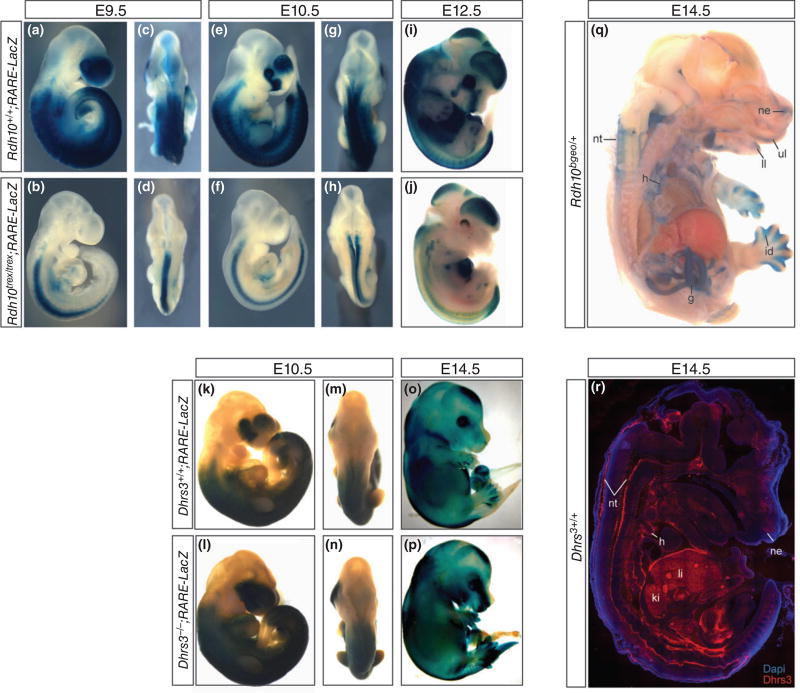
(a–p) Lateral and dorsal views of E9.5, E10.5, E12.5, and E14.5 control RARE-LacZ, Rdh10trex/trex; RARE-LacZ, and Dhrs3−/−;RARE-LacZ embryos illustrating diminished retinoic acid signaling in the craniofacial and trunk regions of Rdh10trex/trex embryos and expanded regions of signaling in the frontonasal region and tail of Dhrs3−/− embryos. (q) Lateral view of LacZ expression in a bisected E14.5 Rdh10bgeo/+ embryo revealing RDH10 activity in the upper and lower lip, nasal epithelium, neural tube, heart, gut, and interdigital zone of the limbs. (r) Lateral view of an E14.5 embryo section immunostained with anti-DHRS3 (red) and DAPI (blue) illustrating DHRS3 activity in the neural tube, heart, liver, kidney, and nasal epithelium; g, gut; h, heart; id, interdigital zone of the limbs; ki, kidney; li, liver; ul, upper lip; ll, lower lip; nt, neural tube; ne, nasal epithelium. Panels (g)–(i) were adapted from Ref 135.
During embryogenesis, Rdh10 is dynamically expressed both temporally and spatially commencing around E8.0 within the prospective hindbrain and lateral mesoderm tissues.28 Between E8.25 to E10.5, Rdh10 continues to be expressed in the lateral plate mesoderm, and dramatically expands to the somites, limb buds, head mesenchyme, primitive olfactory, optic, otic, lung, and foregut tissues.28 The spatiotemporal pattern of Rdh10 expression closely correlates with the spatiotemporal pattern of developmental defects observed in RDH10 loss-of-function mouse embryos. E9.5 Rdh10trex/trex mutants display duplicated or displaced otic vesicles and agenesis of the third to sixth caudal pharyngeal arches.28 By E10.5, Rdh10trex/trex mutant embryos exhibit truncated forelimbs28,29 with interdigital webbing that persists at E14.5136 (Table 1). Somitogenesis, or the process of forming somites, which are precursors of metameric vertebrae and muscle, is also perturbed in Rdh10trex/trex mutants.140 Furthermore, variable orofacial cleft phenotypes are evident in Rdh10trex/trex mutant embryos between E12.5 and E13.0 including a midline facial cleft of the frontonasal process in which the nasal septum and cavities fail to form.28 In addition, these mutants exhibit disruptions in organogenesis with defective development of the heart, lungs, liver, stomach, pancreas, and gastrointestinal tract (Table 1).28 Rdh10−/− null mutant embryos exhibit a more severe phenotype than Rdh10trex/trex embryos, which is consistent with a more complete loss of RA signaling.29 By E9.5, null Rdh10−/− mutants exhibit defective turning, axial elongation, and overall growth. Furthermore, the first pharyngeal arch is reduced in size and arches 2–6 fail to form. Rdh10−/− null mutants exhibit embryonic lethality by E10.5, likely as a consequence of severe cardiac defects including failure to undergo looping and failure to form the proper heart chambers.29 Other more recently generated Rdh10 loss-of-function mouse models have revealed critical roles for Rdh10 and RA signaling in salivary gland development, brain vascular formation, juvenile spermatogenesis, and dark vision adaptation35,145–147 (Table 1).
TABLE 1.
Recorded Developmental Defects for Animal Models of Rdh10 and Dhrs3 loss-of-function
| Phenotype | Rdh10 | Dhrs3 |
|---|---|---|
| Lethality | Rdh10trex/trex E10.5 to E14.528 | Dhrs3−/− E17.5135 |
| Rdh10−/− E10.528 | ||
| Craniofacial | Clefting | Palatogenesis |
| Rdh10m366Asp E10.5 midline facial cleft61 | Dhrs3−/−: | |
| Rdh10trex/trex: | E14.5 palatal shelves fail to elevate135 | |
| E12.5–E13.0 frontonasal process displays variable clefting28 | E18.5 clefting of the secondary palate139 | |
| E12.5–E13.0 defective formation of the nasal septum and chambers28 | ||
| Somites | Somitogenesis | Somitogenesis |
| Rdh10trex/trex 8–15 somite stage consistently smaller somites 1–6; somite size normalizes from somite 7 onward140 | Xenopus defective somitogenesis141 | |
| Skeletal | Rdh10trex/trex E14.5 lack atlas and axis vertebrae140 | Axial skeleton |
| Dhrs3−/− E14.5/E17.5 abnormal axial skeleton development; vertebral fusions and delayed ossification135 | ||
| Limb development | Forelimb growth | No recorded developmental defects |
| Rdh10trex/trex and Rdh10−/− E10.5–E13.0 stunted forelimb growth, however, normal hindlimb morphology3,28,29,61,142 | ||
| Interdigital webbing | ||
| E14.5 fail to lose interdigital mesenchyme61,136 | ||
| Brain | Hindbrain patterning Rdh10−/− E7.75 defective hindbrain patterning142,143 | No recorded developmental defects |
| Brain Vasculature | ||
| Rdh10 ENU induced mutant E14.5 long, thin neocortex with reduced numbers of blood vessels. Large diameter blood vessels in PNVP vasculature35 | ||
| Organogenesis | Heart | Heart |
| Rdh10trex/trex E9.5 heart edema and looping effects28 | Dhsr3−/− defective cardiac outflow tract formation; defects in atrial and ventricular separation135 | |
| Rdh10−/− fail to undergo looping and chamber formation29 | ||
| Xenopus enlarged heart with increased cardiomyocyte number144 | ||
| Eyes | ||
| RPE-specific Rdh10 knockout at 6wks impairments in 11-cis-retinal regeneration and effected dark adaptation after bright illumination145 | ||
| Lungs | ||
| Rdh10trex/trex: | ||
| E10.5 lung bud agenesis28 | ||
| E12.5 to E13.0 hypoplasia28,142 | ||
| Liver | ||
| Rdh10trex/trex E12.5 to E13.0 hypoplastic with fewer lobes28,142 | ||
| Pancreas | ||
| Rdh10trex/trex E12.5 to E13.0 structures not found28,142 | ||
| Salivary gland | ||
| Rdh10trex/trex E14.5 size reduction in submandibular salivary glands; normal morphology146 | ||
| Stomach/Midgut | ||
| Rdh10trex/trex E12.5 to E13.0 hypoplastic28 | ||
| Testes | ||
| Rdh10fl/fl,Amh-Cre+ juvenile male mice at 2–3 weeks defective sertoli cell differentiation of spermatogonial cells147 |
Nonmammalian models have also contributed to our understanding of the role Rdh10 plays during embryogenesis and in the production and regulation of ATRA. The characterization of these models has helped to demonstrate the conserved role of RDH10 within several vertebrate species during embryogenesis. In zebrafish, rdh10a, is expressed in the paraxial mesoderm in early somite stage embryos148 and in the absence of rdh10a, zebrafish embryos exhibit a decrease in ATRA production and signaling. At 24 h postfertilization, this results in anteriorization of the nervous system, together with enlarged hearts and increased numbers of cardiomyocytes.144 The phenotype is considerably worsened when rdh10a is depleted in combination with aldh1a2 (raldh2), the primary enzyme responsible for the second oxidation step of vitamin A metabolism.144
Interestingly, depletion or inhibition of RA synthesis results in an increase in Rdh10 expression.29,148,149 Conversely, exposure to exogenous RA has the opposite effect of decreasing Rdh10 expression. These findings are indicative of a negative feedback mechanism in which Rdh10 is inactivated when sufficient levels of ATRA have been produced. In further support of this idea, Rdh10 possesses a putative RARE in its 5′ upstream enhancer control region suggesting this feedback may be direct.29 Thus, loss-of-function studies in animal models support a role for RDH10 as the predominant and earliest acting enzyme in the oxidation of retinol to retinal during embryonic development. The interconversion of all-trans-retinol to all-trans-retinal by RDH10 is therefore now recognized as an important spatiotemporal control or nodal point in the synthesis of ATRA, which assists in regulating the levels of ATRA during embryogenesis to help guard against vitamin A toxicity.
Although RDH10 is currently considered the most important retinol dehydrogenase present in the early embryo, there are other enzymatic activities that contribute to retinaldehyde formation and retinoid function later in life. For example, two additional retinol dehydrogenases, RDH1 and DHRS9, play important roles in postnatal retinoid metabolism in adipose tissue and astrocytes, respectively.150,151 Furthermore, several soluble enzymes belonging to classes I, III, and IV ADH enzymes have been shown to play a role in postnatal retinol metabolism by protecting against vitamin A toxicity.131,152–154
It is important to note that the oxidation of retinol to retinal is reversible and the reduction of all-trans-retinal to retinol is particularly important in the conversion of β-carotene to retinol for storage and transport to target tissues (Figure 1). Many retinaldehyde reductases have been identified in the eye (reviewed in Ref 155) and the reduction of all-trans-retinaldehyde is critical for vision.156 However, despite the well-recognized roles for retinaldehyde reductases, the identity of individual reductases with physiological relevance in tissues, besides the eye, remain to be determined.
Recently, we and others described a role for the short-chain dehydrogenase/reductase (SDR) family, member 3 (DHRS3) in reducing retinaldehyde to retinol.135,139,141,157,158 Dhrs3 is expressed in a dynamic spatiotemporal pattern that overlaps with many tissues in which vitamin A metabolism and signaling are known to occur during mouse embryogenesis. The highest levels of DHRS3 protein in E14.5 embryos are observed in the liver, kidney, nasal epithelium, and interdigital zones of limbs (Figure 2). In the heart, high levels are found in the pericardial mesothelium, while in the neural tube, high levels of activity are found along the dorsal and ventral midline. In the brain, DHRS3 also localizes to the developing pituitary, cerebellum, and choroid plexus and has been observed in the retina, thyroid, lung, and intestine (Figure 2).135 In addition to mouse embryos, DHRS3 is known to be active in human testes, liver, and small intestine tissues.157
Loss-of-function studies in mice have demonstrated that Dhrs3−/− is critical for normal embryogenesis as mutant embryos dye during late gestation, around E17.5 to E18.5.135 Similar to models of Rdh10 loss-of-function, Dhrs3−/− mutant embryos display perturbed craniofacial, heart, and axial development (Figure 2 and Table 1).135 In the head, the palatal shelves fail to elevate and then also fail to fuse, resulting in cleft palate. Cardiac anomalies present in the form of ventricular septal defects, atrial septal defects, and double-outlet right ventricle. In the axial skeleton, vertebral fusions, specifically at C1 and C2 occur, as wells as delayed ossification within the jaw, and skull.135 In Dhrs3−/− mice containing the RARE-lacz transgenic reporter, ATRA activity was shown to be increased in the majority of tissues compared to wild-type littermates with expanded domains particularly in the frontonasal region and tail145,146 (Figure 2). Notably, the Dhrs3−/− mutants which displayed the largest increase in ATRA activity tended to exhibit the most severe developmental defects.
In addition to mice, other species-specific models have validated the role of DHRS3 in vitamin A metabolism and embryogenesis providing evidence for its conserved role between vertebrate species. For example, a reduction of dhrs3a in zebrafish leads to increased ATRA-reporter activity and activation of ATRA-target genes.148 More specifically, the knockdown of dhrs3a results in an expanded domain of ATRA signaling which encompasses the anterior portion of the spinal cord and part of the hindbrain including rhombomeres 7/8. This impacts nervous system patterning and results in a reduced number of neurons known as T-interneurons in the hindbrain.148 Similarly, morpholino knockdown of dhrs3 in Xenopus embryos also produced a phenotype characteristic of ATRA excess. Xenopus dhrs3 morphants presented with smaller heads that were reduced in diameter. In addition, the mutants also exhibit altered neuroectoderm patterning as well as defects in somitogenesis, including diminished gene expression and perturbed segmentation.141
Although DHRS3 is the primary retinaldehyde reductase known to be present in embryonic tissues, it may not be the only retinaldehyde reductase to play a role in embryogenesis. Interestingly, residual retinaldehyde reductase activity can be detected in microsomes and mouse embryonic fibroblasts (MEF) derived from Dhrs3−/− mice.139 Consistent with this observation, it is known that retinaldehyde reduction can also be carried out in vitro by several members of the aldo-keto-reductase (AKR) family. However, it remains to be determined whether AKR enzymes play such an in vivo role during embryogenesis.24,159–162 Despite this uncertainty, it is clear DHRS3 has well-defined physiological relevance based on the alterations in systemic ATRA metabolism, RAR-signaling, and developmental defects observed in Dhrs3 mutant fish, amphibian, and mouse embryos.135,139,141,148
Although members of three distinct enzyme families have been implicated in the interconversion of retinol and retinaldehyde based on enzymatic activities demonstrated in vitro, only in limited instances has a definitive in vivo role in ATRA metabolism been corroborated by loss-of-function approaches. To date, RDH10 and DHRS3 are the only retinol/retinaldehyde oxidoreductases whose ablation has been shown to result in developmental defects. The lack of an apparent developmental phenotype (except in the eye) in animals deficient in other retinoid-specific SDR, ADH, or AKR enzymes does not rule out the possibility that additional enzymes play a role in retinol/retinaldehyde interconversion. Rather the indispensable role of RDH10 and DHRS3 during embryonic development could reflect the fact that they are expressed earlier than other potential retinol/retinaldehyde oxidoreductases, or that they are expressed in a tissue or tissues where no compensatory activities exist.
Formation and Breakdown of ATRA
The second step in the metabolism of vitamin A to ATRA is the oxidation of retinal to ATRA. The conversion of retinaldehyde to ATRA is an irreversible reaction catalyzed by several retinaldehyde dehydrogenases ALDH1A1, ALDH1A2, and ALDH1A3 (formerly RALDH1, RALDH2, and RALDH3) which belong to the aldehyde dehydrogenase family.163–166 Of these, ALDH1A2 appears to be the most broadly active during early embryogenesis.164,167 ALDH1A2 plays a critical role in the conversion of retinaldehyde into ATRA in the spinal cord, developing heart, retina, lung, and inner ear olfactory epithelium.134,168–171 Aldh1a3 is expressed in sensory neuroepithelia and plays an important role in ATRA formation in the developing nasal structures and eye.172–175 Aldh1a1 is expressed in the developing neural retina, and many tissues after birth, however, a deficiency of Aldh1a1 does not result in apparent developmental defects. Rather, Aldh1a1 loss-of-function influences lipid metabolism in postnatal life in response to increased levels of retinaldehyde.167,176–181 In addition to ALDH1A1–3, several cytochrome P450 enzymes, can oxidize retinaldehyde to ATRA, and CYP1B1 in particular has been shown to contribute to the formation of ATRA in vivo.182,183
Regulation of ATRA Formation and Breakdown
The physiological levels of ATRA are tightly regulated to guard against the effects of either ATRA deficiency or excess. ATRA levels are modulated through a negative feedback mechanism, which serves to regulate ATRA-synthetic enzymes in the presence of elevated ATRA levels. Consistent with this mechanism Aldh1a1, Aldh1a2, and Aldh1a3184–186 are downregulated in response to increasing concentrations of ATRA. Furthermore, RDH10, the principal enzyme responsible for oxidation of retinol to retinal during embryogenesis was also shown to be downregulated in response to exogenous ATRA or elevated endogenous levels of ATRA as seen in Dhrs3−/− mice.29,135,148,149
A critical regulatory control point in the metabolism of vitamin A and synthesis of ATRA occurs at the first step in the reversible interconversion of retinol and all-trans-retinal. The function of the oxido-reductase enzymes, RDH10 and DHRS3, is to help alleviate excess as well as deficient levels of ATRA. DHRS3, by reducing retinaldehyde, leads to a decrease in the ATRA precursor and, therefore, protects tissues from the formation of excess ATRA. The expression of DHRS3 is responsive to ATRA levels and creates a negative feedback loop to further restrict the formation of ATRA.141,148,187,188 The oxidation of retinol to all-trans-retinal by RDH10 serves the reverse function of DHRS3 by protecting against a retinoid deficiency. This oxidative step can be modulated in the presence of excessive levels of ATRA by downregulating expression of RDH10,135 which acts as part of the negative feedback mechanism to prevent further synthesis of ATRA. Conversely, in response to a deficiency in ATRA such as in the case of Raldh2 loss-of-function, the activity of RDH10 can increase in an attempt to compensate.29
Another level of regulation in the interconversion of retinol to all-trans-retinal occurs through an interplay between RDH10 and DHRS3 which form a complex. RDH10 and DHRS3 are codependent as DHRS3 relies on RDH10 to maintain its full catalytic activity. Similarly, RDH10 requires interaction with DHRS3 for complete activation.139 This interaction has been corroborated in cell culture studies by demonstrating colocalization of the two proteins. Additionally, Rdh10 and Dhrs3 exhibit overlapping regions of activity.28,135 For example, Rdh10 and Dhrs3 have been shown to be expressed in the interdigital regions of the hindlimbs of E12.5–14.5 mouse embryos which supports the potential for their direct physical interaction.139 However, future studies aimed at thoroughly characterizing the coexpression of theses enzymes in tissues throughout embryogenesis are still critically needed.
The interaction and subsequent activation of DHRS3 by RDH10 acts to reduce the levels of excess ATRA. In MEF derived from Dhrs3−/− mutant embryos, the production of retinol from retinaldehyde is diminished, and furthermore, cells coexpressing both Rdh10 and Dhrs3 produce less retinaldehyde and ATRA.139 Therefore, in the instance of excess ATRA, DHRS3, which is upregulated by ATRA, interacts with RDH10 enabling it to begin converting retinaldehyde back to retinol, thereby preventing its conversion by ALDH enzymes to ATRA. In the presence of RDH10, retinol may be converted back to retinaldehyde; however, the overall production of ATRA from retinaldehyde is greatly reduced in the presence of DHRS3, thereby protecting against ATRA excess. It is through this interplay between RDH10 and DHRS3, and the interconversion of retinol and retinaldehyde, that ATRA can be reduced in times of excess without stopping its production completely. This allows for the precise regulation of ATRA production, which helps guard against harmful teratogenic effects to the developing embryo.139
CONCLUSIONS
Vitamin A (retinol) and its active metabolite ATRA are essential for normal embryonic development and adult homeostasis as defects can arise in these processes in association with either retinoid excess or deficiency. Therefore, in periods of both vitamin A and ATRA excess or deficiency, compensatory mechanisms that regulate uptake, delivery and storage of retinol exist to maintain ATRA levels within a tight physiological range.
The oxidation of retinol to retinal and its reversible interconversion, which are mediated predominantly by RDH10 and DHRS3 respectively, have now been firmly established as critical control or nodal points in vitamin A metabolism and the synthesis of ATRA (Figure 1). Both RDH10 and DHRS3 are essential for embryonic development (Table 1) and together these enzymes form a codependent complex that helps to maintain appropriate levels of ATRA. It is through complex mechanisms including the precise regulation provided by RDH10 and DHRS3, that embryos and adults are protected against the potentially harmful effects of dysregulated levels of vitamin A and ATRA.
Acknowledgments
We thank all members of our respective laboratories for their continual input and discussions of this work. We are extremely grateful to Mark Miller for generating the artwork in Figure 1 and to Lisa Sandell and Naomi Tjaden for providing the images of E9.5–10.5 and E12.5 Rdh10;RARE-LacZ embryos respectively in Figure 2, as well as to Suya Wang for generously providing the images of Dhrs3−/−;RARE-LacZ embryos in Figure 2. This work was supported by the Stowers Institute for Medical Research and the National Institute for Dental and Craniofacial Research (DE016082) to PAT, a Madison and Lila Self Graduate Fellowship from the University of Kansas to SRS, and the National Institute for Child Health and Development (HD077260) to ARM and PAT.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 4.Conaway HH, Henning P, Lerner UH. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr Rev. 2013;34:766–797. doi: 10.1210/er.2012-1071. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Yanaka N, Richards JS, Shimada M. De novo-synthesized retinoic acid in ovarian antral follicles enhances FSH-mediated ovarian follicular cell differentiation and female fertility. Endocrinology. 2016;157:2160–2172. doi: 10.1210/en.2015-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinas E, Saggini A, Kritas SK, Cerulli G, Caraffa A, Antinolfi P, Pantalone A, Frydas A, Tei M, Speziali A, et al. Can vitamin a mediate immunity and inflammation? J Biol Regul Homeost Agents. 2015;29:1–6. [PubMed] [Google Scholar]
- 7.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 8.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 9.Brand N, Petkovich M, Krust A, Chambon P, de Thé H, Marchio A, Tiollais P, Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988;332:850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- 10.Krust A, Kastner P, Petkovich M, Zelent A, Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci USA. 1989;86:5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and non-genomic effects. J Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studer M, Pöpperl H, Marshall H, Kuroiwa A, Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994;265:1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Duester G. Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development. 2014;141:2972–2977. doi: 10.1242/dev.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Cunningham TJ, Duester G. Nuclear receptor corepressors Ncor1 and Ncor2 (Smrt) are required for retinoic acid-dependent repression of Fgf8 during somitogenesis. Dev Biol. 2016;418:204–215. doi: 10.1016/j.ydbio.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Lascio S, Saba E, Belperio D, Raimondi A, Lucchetti H, Fornasari D, Benfante R. PHOX2A and PHOX2B are differentially regulated during retinoic acid-driven differentiation of SK-N-BE(2)C neuroblastoma cell line. Exp Cell Res. 2016;342:62–71. doi: 10.1016/j.yexcr.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kastner P, Mark M, Ghyselinck N, Krezel W, Dupé V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 18.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 19.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Mora, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 21.Blum N, Begemann G. Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development. 2011;139:107–116. doi: 10.1242/dev.065391. [DOI] [PubMed] [Google Scholar]
- 22.Gudas LJ. Retinoids induce stem cell differentiation via epigenetic changes. Semin Cell Dev Biol. 2013;24:701–705. doi: 10.1016/j.semcdb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shmarakov IO, Jiang H, Yang KJ, Goldberg IJ, Blaner WS. Hepatic retinoid stores are required for normal liver regeneration. J Lipid Res. 2013;54:893–908. doi: 10.1194/jlr.M029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Sandell LL, Trainor PA, Koentgen F, Duester G. Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models. Biochim Biophys Acta. 2012;1821:198–205. doi: 10.1016/j.bbalip.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedishvili NY. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res. 2013;54:1744–1760. doi: 10.1194/jlr.R037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2012;1821:152–167. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta. 2012;1821:88–98. doi: 10.1016/j.bbalip.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandell LL, Lynn ML, Inman KE, McDowell W, Trainor PA. RDH10 oxidation of vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis. PLoS One. 2012;7:e30698. doi: 10.1371/journal.pone.0030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farjo KM, Moiseyev G, Nikolaeva O, Sandell LL, Trainor PA, Ma JX. RDH10 is the primary enzyme responsible for the first step of embryonic vitamin A metabolism and retinoic acid synthesis. Dev Biol. 2011;357:347–355. doi: 10.1016/j.ydbio.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosnik J, Zheng L, Rackauckas CV, Digman M, Gratton E, Nie Q, Schilling TF. Noise modulation in retinoic acid signaling sharpens segmental boundaries of gene expression in the embryonic zebrafish hindbrain. Elife. 2016;5:e14034. doi: 10.7554/eLife.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimozono S, Iimura T, Kitaguchi T, Higashijima S, Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496:363–366. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- 33.White RJ, Nie Q, Lander AD, Schilling TF. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137:283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonney S, Harrison-Uy S, Mishra S, MacPherson AM, Choe Y, Li D, Jaminet SC, Fruttiger M, Pleasure SJ, Siegenthaler JA. Diverse functions of retinoic acid in brain vascular development. J Neurosci. 2016;36:7786–7801. doi: 10.1523/JNEUROSCI.3952-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic acid oxidation. J Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 38.Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. β-carotene is an important vitamin A source for humans. J Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber D, Grune T. The contribution of β-carotene to vitamin A supply of humans. Mol Nutr Food Res. 2011;56:251–258. doi: 10.1002/mnfr.201100230. [DOI] [PubMed] [Google Scholar]
- 40.Sommer A. Vitamin a deficiency and clinical disease: an historical overview. J Nutr. 2008;138:1835–1839. doi: 10.1093/jn/138.10.1835. [DOI] [PubMed] [Google Scholar]
- 41.von Lintig J. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr. 2012;96:1234S–1244S. doi: 10.3945/ajcn.112.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloch CE. Further clinical investigations into the diseases arising in consequence of a deficiency in the fat-soluble a factor. Am J Dis Child. 1924;28:659–667. [Google Scholar]
- 43.Mason KE. Foetal death, prolonged gestation, and difficult parturition in the rat as a result of vitamin A-deficiency. Am J Anat. 1935;57:303–349. [Google Scholar]
- 44.Warkany J, Schraffenberger E. Congenital malformations of the eyes induced in rats by maternal vitamin A deficiency. Proc Soc Exp Biol Med. 1944;57:49–52. [Google Scholar]
- 45.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency—effects of restoration of vitamin-A at various times during gestation. Am J Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 46.Cohlan SQ. Excessive intake of vitamin A as a cause of congenital anomalies in the rat. Science. 1953;117:535–536. doi: 10.1126/science.117.3046.535. [DOI] [PubMed] [Google Scholar]
- 47.Cohlan SQ. Congenital anomalies in the rat produced by excessive intake of vitamin A during pregnancy. Pediatrics. 1954;13:556–567. [PubMed] [Google Scholar]
- 48.Kalter H, Warkany J. Experimental production of congenital malformations in strains of inbred mice by maternal treatment with hypervitaminosis A. Am J Pathol. 1961;38:1–21. [PMC free article] [PubMed] [Google Scholar]
- 49.Conlon RA, Rossant J. Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development. 1992;116:357–368. doi: 10.1242/dev.116.2.357. [DOI] [PubMed] [Google Scholar]
- 50.Jiang H, Gyda M, III, Harnish DC, Chandraratna RA, Soprano KJ, Kochhar DM, Soprano DR. Teratogenesis by retinoic acid analogs positively correlates with elevation of retinoic acid receptor-β 2 mRNA levels in treated embryos. Teratology. 1994;50:38–43. doi: 10.1002/tera.1420500106. [DOI] [PubMed] [Google Scholar]
- 51.Melhus H, Michaëlsson K, Kindmark A, Bergström R, Holmberg L, Mallmin H, Wolk A, Ljunghall S. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. 1998;129:770–778. doi: 10.7326/0003-4819-129-10-199811150-00003. [DOI] [PubMed] [Google Scholar]
- 52.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287:47–54. doi: 10.1001/jama.287.1.47. [DOI] [PubMed] [Google Scholar]
- 53.Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348:287–294. doi: 10.1056/NEJMoa021171. [DOI] [PubMed] [Google Scholar]
- 54.Larson RS, Tallman MS. Retinoic acid syndrome: manifestations, pathogenesis, and treatment. Best Pract Res Clin Haematol. 2003;16:453–461. doi: 10.1016/s1521-6926(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 55.Sive HL, Draper BW, Harland RM, Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990;4:932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- 56.Tickle C, Lee J, Eichele G. A quantitative analysis of the effect of all-trans-retinoic acid on the pattern of chick wing development. Dev Biol. 1985;109:82–95. doi: 10.1016/0012-1606(85)90348-3. [DOI] [PubMed] [Google Scholar]
- 57.Frenz DA, Liu W, Cvekl A, Xie Q, Wassef L, Quadro L, Niederreither K, Maconochie M, Shanske A. Retinoid signaling in inner ear development: a "Goldilocks" phenomenon. Am J Med Genet A. 2010;152a:2947–2961. doi: 10.1002/ajmg.a.33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee LM, Leung CY, Tang WW, Choi HL, Leung YC, McCaffery PJ, Wang CC, Woolf AS, Shum AS. A paradoxical teratogenic mechanism for retinoic acid. Proc Natl Acad Sci USA. 2012;109:13668–13673. doi: 10.1073/pnas.1200872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Aniello E, Rydeen AB, Anderson JL, Mandal A, Waxman JS. Depletion of retinoic acid receptors initiates a novel positive feedback mechanism that promotes teratogenic increases in retinoic acid. PLoS Genet. 2013;9:e1003689. doi: 10.1371/journal.pgen.1003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. β-Carotene and its cleavage enzyme β-carotene-15,15’-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. 2011;25:1641–1652. doi: 10.1096/fj.10-175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashique AM, May SR, Kane MA, Folias AE, Phamluong K, Choe Y, Napoli JL, Peterson AS. Morphological defects in a novel Rdh10 mutant that has reduced retinoic acid biosynthesis and signaling. Genesis. 2012;50:415–423. doi: 10.1002/dvg.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costabile BK, Kim Y-K, Iqbal J, Zuccaro MV, Wassef L, Narayanasamy S, Curley RW, Jr, Harrison EH, Hussain MM, Quadro L. β-Apo-10’-carotenoids modulate placental microsomal triglyceride transfer protein expression and function to optimize transport of intact β-carotene to the embryo. J Biol Chem. 2016;291:18525–18535. doi: 10.1074/jbc.M116.738336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.During A, Harrison EH. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J Lipid Res. 2007;48:2283–2294. doi: 10.1194/jlr.M700263-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W. Cloning and expression of β,β-carotene 15,15’-dioxygenase. Biochem Biophys Res Commun. 2000;271:334–336. doi: 10.1006/bbrc.2000.2619. [DOI] [PubMed] [Google Scholar]
- 65.von Lintig J, Vogt K. Filling the gap in vitamin A research molecular identification of an enzyme cleaving β-carotene to retinal. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 66.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, et al. CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 67.Mora O, Kuri-Melo L, González-Gallardo A, Meléndez E, Morales A, Shimada A, Varela-Echavarría A. A potential role for β-carotene in avian embryonic development. Int J Vitam Nutr Res. 2004;74:116–122. doi: 10.1024/0300-9831.74.2.116. [DOI] [PubMed] [Google Scholar]
- 68.Lindqvist A, He YG, Andersson S. Cell type-specific expression of β-carotene 9’,10’-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 69.Shmarakov I, Fleshman MK, D'Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, et al. Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid. Arch Biochem Biophys. 2010;504:3–10. doi: 10.1016/j.abb.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray PA, Shivdasani RA. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–4129. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- 71.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, et al. ISX participates in the maintenance of vitamin A metabolism by regulation of β-carotene 15,15’-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–4911. doi: 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- 72.Bondurand N, Kuhlbrodt K, Pingault V, Enderich J, Sajus M, Tommerup N, Warburg M, Hennekam RC, Read AP, Wegner M, et al. A molecular analysis of the yemenite deaf-blind hypopigmentation syndrome: SOX10 dysfunction causes different neurocristopathies. Hum Mol Genet. 1999;8:1785–1789. doi: 10.1093/hmg/8.9.1785. [DOI] [PubMed] [Google Scholar]
- 73.Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem. 2013;288:9017–9027. doi: 10.1074/jbc.M112.444240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta. 2012;1821:70–77. doi: 10.1016/j.bbalip.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients. 2013;5:3563–3581. doi: 10.3390/nu5093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ong DE, Kakkad B, MacDonald PN. Acyl-CoA-independent esterification of retinol bound to cellular retinol-binding protein (type II) by microsomes from rat small intestine. J Biol Chem. 1987;262:2729–2736. [PubMed] [Google Scholar]
- 78.E X, Zhang L, Lu J, Tso P, Blaner WS, Levin MS, Li E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem. 2002;277:36617–36623. doi: 10.1074/jbc.M205519200. [DOI] [PubMed] [Google Scholar]
- 79.Kane MA, Bright FV, Napoli JL. Binding affinities of CRBPI and CRBPII for 9-cis-retinoids. Biochim Biophys Acta. 2011;1810:514–518. doi: 10.1016/j.bbagen.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- 82.Wassef L, Quadro L. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem. 2011;286:32198–32207. doi: 10.1074/jbc.M111.253070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dueker SR, Lin Y, Buchholz BA, Schneider PD, Lamé MW, Segall HJ, Vogel JS, Clifford AJ. Long-term kinetic study of β-carotene, using accelerator mass spectrometry in an adult volunteer. J Lipid Res. 2000;41:1790–1800. [PubMed] [Google Scholar]
- 84.Shirakami Y, Lee SA, Clugston RD, Blaner WS. Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta. 2012;1821:124–136. doi: 10.1016/j.bbalip.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schreiber R, et al. Retinyl ester hydrolases and their roles in vitamin A homeostasis. Biochim Biophys Acta. 2012;1821:113–123. doi: 10.1016/j.bbalip.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blaner WS, Obunike JC, Kurlandsky SB, al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester: possible implications for retinoid uptake by cells. J Biol Chem. 1994;269:16559–16565. [PubMed] [Google Scholar]
- 87.Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein and transthyretin mRNA levels in visceral yolk sac and liver during fetal development in the rat. Proc Natl Acad Sci USA. 1986;83:7330–7334. doi: 10.1073/pnas.83.19.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bavik C, Ward SJ, Chambon P. Developmental abnormalities in cultured mouse embryos deprived of retinoic by inhibition of yolk-sac retinol binding protein synthesis. Proc Natl Acad Sci USA. 1996;93:3110–3114. doi: 10.1073/pnas.93.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen J, Shi D, Suzuki T, Xia Z, Zhang H, Araki K, Wakana S, Takeda N, Yamamura K, Jin S, et al. Severe ocular phenotypes in Rbp4-deficient mice in the C57BL/6 genetic background. Lab Invest. 2016;96:680–691. doi: 10.1038/labinvest.2016.39. [DOI] [PubMed] [Google Scholar]
- 90.Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- 91.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Décimo D, Dollé P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 92.Sapin V, Bouillet P, Oulad-Abdelghani M, Dastugue B, Chambon P, Dollé P. Differential expression of retinoic acid-inducible (Stra) genes during mouse placentation. Mech Dev. 2000;92:295–299. doi: 10.1016/s0925-4773(00)00241-0. [DOI] [PubMed] [Google Scholar]
- 93.Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, Von Lintig J. STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum Mol Genet. 2014;23:5402–5417. doi: 10.1093/hmg/ddu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skazik C, Amann PM, Heise R, Marquardt Y, Czaja K, Kim A, Rühl R, Kurschat P, Merk HF, Bickers DR, et al. Downregulation of STRA6 expression in epidermal keratinocytes leads to hyperproliferation-associated differentiation in both in vitro and in vivo skin models. J Invest Dermatol. 2014;134:1579–1588. doi: 10.1038/jid.2013.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci. 2012;53:3027–3039. doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O'Byrne SM, DeSantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, et al. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem. 2013;288:24528–24539. doi: 10.1074/jbc.M113.484014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terra R, Wang X, Hu Y, Charpentier T, Lamarre A, Zhong M, Sun H, Mao J, Qi S, Luo H, et al. To investigate the necessity of STRA6 upregulation in T cells during T cell immune responses. PLoS One. 2013;8:e82808. doi: 10.1371/journal.pone.0082808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Redondo C, Vouropoulou M, Evans J, Findlay JB. Identification of the retinol-binding protein (RBP) interaction site and functional state of RBPs for the membrane receptor. FASEB J. 2008;22:1043–1054. doi: 10.1096/fj.07-8939com. [DOI] [PubMed] [Google Scholar]
- 99.Kawaguchi R, Zhong M, Kassai M, Ter-Stepanian M, Sun H. STRA6-catalyzed vitamin A influx, efflux, and exchange. J Membr Biol. 2012;245:731–745. doi: 10.1007/s00232-012-9463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y, Clarke OB, Kim J, Stowe S, Kim YK, Assur Z, Cavalier M, Godoy-Ruiz R, von Alpen DC, Manzini C, et al. Structure of the STRA6 receptor for retinol uptake. Science. 2016;353 doi: 10.1126/science.aad8266. pii:aad8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muenzner M, Tuvia N, Deutschmann C, Witte N, Tolkachov A, Valai A, Henze A, Sander LE, Raila J, Schupp M. Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor alpha activity. Mol Cell Biol. 2013;33:4068–4082. doi: 10.1128/MCB.00221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alapatt P, Guo F, Komanetsky SM, Wang S, Cai J, Sargsyan A, Rodríguez Díaz E, Bacon BT, Aryal P, Graham TE. Liver retinol transporter and receptor for serum retinol-binding protein (RBP4) J Biol Chem. 2013;288:1250–1265. doi: 10.1074/jbc.M112.369132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am J Physiol Endocrinol Metab. 2004;286:E844–E851. doi: 10.1152/ajpendo.00556.2003. [DOI] [PubMed] [Google Scholar]
- 105.Rask L, Peterson PA. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey’s small intestine. J Biol Chem. 1976;251:6360–6366. [PubMed] [Google Scholar]
- 106.Sivaprasadarao A, Findlay JB. The mechanism of uptake of retinol by plasma-membrane vesicles. Biochem J. 1988;255:571–579. [PMC free article] [PubMed] [Google Scholar]
- 107.Smeland S, Bjerknes T, Malaba L, Eskild W, Norum KR, Blomhoff R. Tissue distribution of the receptor for plasma retinol-binding protein. Biochem J. 1995;305(pt 2):419–424. doi: 10.1042/bj3050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 109.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 110.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, Ogawa I. Toxicological pathology in the rat placenta. J Toxicol Pathol. 2011;24:95–111. doi: 10.1293/tox.24.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dancis J, Levitz M, Katz J, Wilson D, Blaner WS, Piantedosi R, Goodman DS. Transfer and metabolism of retinol by the perfused human placenta. Pediatr Res. 1992;32:195–199. doi: 10.1203/00006450-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 112.Sapin V, Chaïb S, Blanchon L, Alexandre-Gouabau MC, Lémery D, Charbonne F, Gallot D, Jacquetin B, Dastugue B, Azais-Braesco V. Esterification of vitamin A by the human placenta involves villous mesenchymal fibroblasts. Pediatr Res. 2000;48:565–572. doi: 10.1203/00006450-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 113.Amengual J, Golczak M, Palczewski K, von Lintig J. Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J Biol Chem. 2012;287:24216–24227. doi: 10.1074/jbc.M112.353979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yost RW, Harrison EH, Ross AC. Esterification by rat liver microsomes of retinol bound to cellular retinol-binding protein. J Biol Chem. 1988;263:18693–18701. [PubMed] [Google Scholar]
- 116.Ghyselinck NB, Båvik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Håkansson H, Sauvant P, et al. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saari JC, Nawrot M, Garwin GG, Kennedy MJ, Hurley JB, Ghyselinck NB, Chambon P. Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest Ophthalmol Vis Sci. 2002;43:1730–1735. [PubMed] [Google Scholar]
- 118.Wei S, Lai K, Patel S, Piantedosi R, Shen H, Colantuoni V, Kraemer FB, Blaner WS. Retinyl ester hydrolysis and retinol efflux from BFC-1β adipocytes. J Biol Chem. 1997;272:14159–14165. doi: 10.1074/jbc.272.22.14159. [DOI] [PubMed] [Google Scholar]
- 119.Strom K, Gundersen TE, Hansson O, Lucas S, Fernandez C, Blomhoff R, Holm C. Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. FASEB J. 2009;23:2307–2316. doi: 10.1096/fj.08-120923. [DOI] [PubMed] [Google Scholar]
- 120.Pirazzi C, Valenti L, Motta BM, Pingitore P, Hedfalk K, Mancina RM, Burza MA, Indiveri C, Ferro Y, Montalcini T, et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum Mol Genet. 2014;23:4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taneja R, Bouillet P, Boylan JF, Gaub MP, Roy B, Gudas LJ, Chambon P. Reexpression of retinoic acid receptor (RAR) gamma or overexpression of RAR alpha or RAR beta in RAR gamma-null F9 cells reveals a partial functional redundancy between the three RAR types. Proc Natl Acad Sci USA. 1995;92:7854–7858. doi: 10.1073/pnas.92.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier JM, Schuhbaur B, Dollé P, Chambon P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2) Dev Biol. 1995;170:420–433. doi: 10.1006/dbio.1995.1226. [DOI] [PubMed] [Google Scholar]
- 123.Kashyap V, Laursen KB, Brenet F, Viale AJ, Scandura JM, Gudas LJ. RARγ is essential for retinoic acid induced chromatin remodeling and transcriptional activation in embryonic stem cells. J Cell Sci. 2013;126:999–1008. doi: 10.1242/jcs.119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mangelsdorf DJ, Umesono K, Kliewer SA, Borgmeyer U, Ong ES, Evans RM. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991;66:555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- 125.Smith WC, Nakshatri H, Leroy P, Rees J, Chambon P. A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J. 1991;10:2223–2230. doi: 10.1002/j.1460-2075.1991.tb07758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rajan N, Blaner WS, Soprano DR, Suhara A, Goodman DS. Cellular retinol-binding protein messenger RNA levels in normal and retinoid-deficient rats. J Lipid Res. 1990;31:821–829. [PubMed] [Google Scholar]
- 127.Wu L, Ross AC. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res. 2009;51:378–387. doi: 10.1194/jlr.M001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zolfaghari R, Ross AC. An essential set of basic DNA response elements is required for receptor-dependent transcription of the lecithin:retinol acyltransferase (Lrat) gene. Arch Biochem Biophys. 2009;489:1–9. doi: 10.1016/j.abb.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dixon JL, Kim YK, Brinker A, Quadro L. Loss of β-carotene 15,15’-oxygenase in developing mouse tissues alters esterification of retinol, cholesterol and diacylglycerols. Biochim Biophys Acta. 2014;1841:34–43. doi: 10.1016/j.bbalip.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ang HL, Deltour L, Zgombic-Knight M, Wagner MA, Duester G. Expression patterns of class I and class IV alcohol dehydrogenase genes in developing epithelia suggest a role for alcohol dehydrogenase in local retinoic acid synthesis. Alcohol Clin Exp Res. 1996;20:1050–1064. doi: 10.1111/j.1530-0277.1996.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 131.Molotkov A, Deltour L, Foglio MH, Cuenca AE, Duester G. Distinct retinoid metabolic functions for alcohol dehydrogenase genes Adh1 and Adh4 in protection against vitamin A toxicity or deficiency revealed in double null mutant mice. J Biol Chem. 2002;277:13804–13811. doi: 10.1074/jbc.M112039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farrés J, Parés X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously expressed alcohol dehydrogenase Adh3. Proc Natl Acad Sci USA. 2002;99:5337–5342. doi: 10.1073/pnas.082093299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deltour L, Foglio MH, Duester G. Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice: overlapping roles of Adh1 and Adh4 in ethanol clearance and metabolism of retinol to retinoic acid. J Biol Chem. 1999;274:16796–16801. doi: 10.1074/jbc.274.24.16796. [DOI] [PubMed] [Google Scholar]
- 134.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 135.Billings SE, Pierzchalski K, Butler Tjaden NE, Pang XY, Trainor PA, Kane MA, Moise AR. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 2013;27:4877–4889. doi: 10.1096/fj.13-227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cunningham TJ, Chatzi C, Sandell LL, Trainor PA, Duester G. Rdh10 mutants deficient in limb field retinoic acid signaling exhibit normal limb patterning but display interdigital webbing. Dev Dyn. 2011;240:1142–1150. doi: 10.1002/dvdy.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cunningham TJ, Zhao X, Sandell LL, Evans SM, Trainor PA, Duester G. Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Rep. 2013;3:1503–1511. doi: 10.1016/j.celrep.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Farjo KM, Moiseyev G, Takahashi Y, Crouch RK, Ma JX. The 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle proteins. Invest Ophthalmol Vis Sci. 2009;50:5089–5097. doi: 10.1167/iovs.09-3797. [DOI] [PubMed] [Google Scholar]
- 139.Adams MK, Belyaeva OV, Wu L, Kedishvili NY. The retinaldehyde reductase activity of DHRS3 is reciprocally activated by Retinol Dehydrogenase 10 to control retinoid homeostasis. J Biol Chem. 2014;289:14868–14880. doi: 10.1074/jbc.M114.552257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kam RK, Shi W, Chan SO, Chen Y, Xu G, Lau CB, Fung KP, Chan WY, Zhao H. Dhrs3 protein attenuates retinoic acid signaling and is required for early embryonic patterning. J Biol Chem. 2013;288:31477–31487. doi: 10.1074/jbc.M113.514984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rhinn M, Schuhbaur B, Niederreither K, Dolle P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci USA. 2011;108:16687–16692. doi: 10.1073/pnas.1103877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chatzi C, Cunningham TJ, Duester G. Investigation of retinoic acid function during embryonic brain development using retinaldehyde-rescued Rdh10 knockout mice. Dev Dyn. 2013;242:1056–1065. doi: 10.1002/dvdy.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.D’Aniello E, Ravisankar P, Waxman JS. Rdh10a provides a conserved critical step in the synthesis of retinoic acid during zebrafish embryogenesis. PLoS One. 2015;10:e0138588. doi: 10.1371/journal.pone.0138588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sahu B, Sun W, Perusek L, Parmar V, Le Y-Z, Griswold MD, Palczewski K, Maeda A. Conditional ablation of retinol dehydrogenase 10 in the retinal pigmented epithelium causes delayed dark adaption in mice. J Biol Chem. 2015;290:27239–27247. doi: 10.1074/jbc.M115.682096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wright DM, Buenger DE, Abashev TM, Lindeman RP, Ding J, Sandell LL. Retinoic acid regulates embryonic development of mammalian submandibular salivary glands. Dev Biol. 2015;407:57–67. doi: 10.1016/j.ydbio.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tong MH, Yang QE, Davis JC, Griswold MD. Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc Natl Acad Sci USA. 2013;110:543–548. doi: 10.1073/pnas.1214883110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feng L, Hernandez RE, Waxman JS, Yelon D, Moens CB. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol. 2010;338:1–14. doi: 10.1016/j.ydbio.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Strate I, Min TH, Iliev D, Pera EM. Retinol dehydrogenase 10 is a feedback regulator of retinoic acid signalling during axis formation and patterning of the central nervous system. Development. 2009;136:461–472. doi: 10.1242/dev.024901. [DOI] [PubMed] [Google Scholar]
- 150.Zhang M, Hu P, Krois CR, Kane MA, Napoli JL. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J. 2007;21:2886–2896. doi: 10.1096/fj.06-7964com. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Kane MA, Napoli JL. Multiple retinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes. J Biol Chem. 2011;286:6542–6553. doi: 10.1074/jbc.M110.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Deltour L, Foglio MH, Duester G. Impaired retinol utilization in Adh4 alcohol dehydrogenase mutant mice. Dev Genet. 1999;25:1–10. doi: 10.1002/(SICI)1520-6408(1999)25:1<1::AID-DVG1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 153.Molotkov A, Fan X, Duester G. Excessive vitamin A toxicity in mice genetically deficient in either alcohol dehydrogenase Adh1 or Adh3. Eur J Biochem. 2002;269:2607–2612. doi: 10.1046/j.1432-1033.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 154.Molotkov A, Ghyselinck NB, Chambon P, Duester G. Opposing actions of cellular retinol-binding protein and alcohol dehydrogenase control the balance between retinol storage and retinoic acid synthesis. Biochem J. 2004;383:295–302. doi: 10.1042/BJ20040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem Rev. 2014;114:194–232. doi: 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lundova T, Zemanová L, Malčeková B, Skarka A, Štambergová H, Havránková J, Šafr M, Wsól V. Molecular and biochemical characterisation of human short-chain dehydrogenase/reductase member 3 (DHRS3) Chem Biol Interact. 2015;234:178–187. doi: 10.1016/j.cbi.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 158.Kam RK, Chen Y, Chan SO, Chan WY, Dawid IB, Zhao H. Developmental expression of Xenopus short-chain dehydrogenase/reductase 3. Int J Dev Biol. 2010;54:1355–1360. doi: 10.1387/ijdb.092984rk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gallego O, Ruiz FX, Ardèvol A, Domínguez M, Alvarez R, de Lera AR, Rovira C, Farrés J, Fita I, Parés X. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci USA. 2007;104:20764–20769. doi: 10.1073/pnas.0705659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ruiz FX, Gallego O, Ardèvol A, Moro A, Domínguez M, Alvarez S, Alvarez R, de Lera AR, Rovira C, Fita I, et al. Aldo-keto reductases from the AKR1B subfamily: retinoid specificity and control of cellular retinoic acid levels. Chem Biol Interact. 2009;178:171–177. doi: 10.1016/j.cbi.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 161.Ruiz FX, Porté S, Gallego O, Moro A, Ardèvol A, Del Río-Espínola A, Rovira C, Farrés J, Parés X. Retinaldehyde is a substrate for human aldo-keto reductases of the 1C subfamily. Biochem J. 2011;440:335–344. doi: 10.1042/BJ20111286. [DOI] [PubMed] [Google Scholar]
- 162.Porté S, Xavier Ruiz F, Giménez J, Molist I, Alvarez S, Domínguez M, Alvarez R, de Lera AR, Parés X, Farrés J. Aldo-keto reductases in retinoid metabolism: search for substrate specificity and inhibitor selectivity. Chem Biol Interact. 2012;202:186–194. doi: 10.1016/j.cbi.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 163.Bhat PV, Labrecque J, Boutin JM, Lacroix A, Yoshida A. Cloning of a cDNA encoding rat aldehyde dehydrogenase with high activity for retinal oxidation. Gene. 1995;166:303–306. doi: 10.1016/0378-1119(96)81752-5. [DOI] [PubMed] [Google Scholar]
- 164.Wang X, Penzes P, Napoli JL. Cloning of a cDNA encoding an aldehyde dehydrogenase and its expression in Escherichia coli: recognition of retinal as substrate. J Biol Chem. 1996;271:16288–16293. doi: 10.1074/jbc.271.27.16288. [DOI] [PubMed] [Google Scholar]
- 165.Zhao D, McCaffery P, Ivins KJ, Neve RL, Hogan P, Chin WW, Dräger UC. Molecular identification of a major retinoic-acid-synthesizing enzyme, a retinaldehyde-specific dehydrogenase. Eur J Biochem. 1996;240:15–22. doi: 10.1111/j.1432-1033.1996.0015h.x. [DOI] [PubMed] [Google Scholar]
- 166.Penzes P, Wang X, Sperkova Z, Napoli JL. Cloning of a rat cDNA encoding retinal dehydrogenase isozyme type I and its expression in E. coli. Gene. 1997;191:167–172. doi: 10.1016/s0378-1119(97)00054-1. [DOI] [PubMed] [Google Scholar]
- 167.McCaffery P, Tempst P, Lara G, Drager UC. Aldehyde dehydrogenase is a positional marker in the retina. Development. 1991;112:693–702. doi: 10.1242/dev.112.3.693. [DOI] [PubMed] [Google Scholar]
- 168.Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CY, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- 169.Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- 170.Chen F, Marquez H, Kim YK, Qian J, Shao F, Fine A, Cruikshank WW, Quadro L, Cardoso WV. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest. 2014;124:801–811. doi: 10.1172/JCI70291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romand R, Albuisson E, Niederreither K, Fraulob V, Chambon P, Dollé P. Specific expression of the retinoic acid-synthesizing enzyme RALDH2 during mouse inner ear development. Mech Dev. 2001;106:185–189. doi: 10.1016/s0925-4773(01)00447-6. [DOI] [PubMed] [Google Scholar]
- 172.Grun F, Hirose Y, Kawauchi S, Ogura T, Umesono K. Aldehyde dehydrogenase 6, a cytosolic retinaldehyde dehydrogenase prominently expressed in sensory neuroepithelia during development. J Biol Chem. 2000;275:41210–41218. doi: 10.1074/jbc.M007376200. [DOI] [PubMed] [Google Scholar]
- 173.Mic FA, Molotkov A, Fan X, Cuenca AE, Duester G. RALDH3, a retinaldehyde dehydrogenase that generates retinoic acid, is expressed in the ventral retina, otic vesicle and olfactory pit during mouse development. Mech Dev. 2000;97:227–230. doi: 10.1016/s0925-4773(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 174.Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yahyavi M, Abouzeid H, Gawdat G, de Preux AS, Xiao T, Bardakjian T, Schneider A, Choi A, Jorgenson E, Baier H, et al. ALDH1A3 loss of function causes bilateral anophthalmia/microphthalmia and hypoplasia of the optic nerve and optic chiasm. Hum Mol Genet. 2013;22:3250–3258. doi: 10.1093/hmg/ddt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kiefer FW, Orasanu G, Nallamshetty S, Brown JD, Wang H, Luger P, Qi NR, Burant CF, Duester G, Plutzky J. Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology. 2012;153:3089–3099. doi: 10.1210/en.2011-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kiefer FW, Vernochet C, O'Brien P, Spoerl S, Brown JD, Nallamshetty S, Zeyda M, Stulnig TM, Cohen DE, Kahn CR, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012;18:918–925. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nallamshetty S, Wang H, Rhee EJ, Kiefer FW, Brown JD, Lotinun S, Le P, Baron R, Rosen CJ, Plutzky J. Deficiency of retinaldehyde dehydrogenase 1 induces BMP2 and increases bone mass in vivo. PLoS One. 2013;8:e71307. doi: 10.1371/journal.pone.0071307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yasmeen R, Reichert B, Deiuliis J, Yang F, Lynch A, Meyers J, Sharlach M, Shin S, Volz KS, Green KB, et al. Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62:124–136. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang QY, Dunbar D, Kaminsky L. Human cytochrome P-450 metabolism of retinals to retinoic acids. Drug Metab Dispos. 2000;28:292–297. [PubMed] [Google Scholar]
- 183.Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development. 2007;134:1369–1383. doi: 10.1242/dev.02815. [DOI] [PubMed] [Google Scholar]
- 184.Elizondo G, Corchero J, Sterneck E, Gonzalez FJ. Feedback inhibition of the retinaldehyde dehydrogenase gene ALDH1 by retinoic acid through retinoic acid receptor α and CCAAT/enhancer-binding protein β. J Biol Chem. 2000;275:39747–39753. doi: 10.1074/jbc.M004987200. [DOI] [PubMed] [Google Scholar]
- 185.Elizondo G, Medina-Diaz IM, Cruz R, Gonzalez FJ, Vega L. Retinoic acid modulates retinaldehyde dehydrogenase 1 gene expression through the induction of GADD153-C/EBPβ interaction. Biochem Pharmacol. 2009;77:248–257. doi: 10.1016/j.bcp.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Niederreither K, McCaffery P, Drager UC, Chambon P, Dolle P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 187.Cerignoli F, Guo X, Cardinali B, Rinaldi C, Casaletto J, Frati L, Screpanti I, Gudas LJ, Gulino A, Thiele CJ, et al. retSDR1, a short-chain retinol dehydrogenase/reductase, is retinoic acid-inducible and frequently deleted in human neuroblastoma cell lines. Cancer Res. 2002;62:1196–1204. [PubMed] [Google Scholar]
- 188.Zolfaghari R, Chen Q, Ross AC. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am J Physiol Gastrointest Liver Physiol. 2012;303:G578–G588. doi: 10.1152/ajpgi.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]