Abstract
In November 2011 the National Institutes of Health convened a workshop of basic researchers, epidemiologists and clinical experts in PID in order to identify research gaps hindering advances in diagnosis, treatment and prevention. This article summarizes the presentations, discussions and conclusions of this group and highlights significant controversies that reveal aspects of PID research that would most greatly benefit from the application of newer molecular, immunological and radiological techniques. Multiple limitations exist to performing new clinical trials; however, emerging data from ongoing clinical trials will add to the current body of knowledge regarding prevention and treatment strategies. Additionally, use of established healthcare databases could serve as a valuable tool for performance of unbiased epidemiologic outcome studies.
Keywords: pelvic inflammatory disease, Chlamydia, genital mycoplasma, ectopic pregnancy, infertility
INTRODUCTION
Pelvic inflammatory disease (PID) continues to pose great risk to the reproductive health of women worldwide. In addition to costs associated with immediate treatment of acute illness, potentially devastating long-term sequelae of PID, specifically tubal factor infertility (TFI), ectopic pregnancy (EP), and chronic pelvic pain, can occur during a woman’s reproductive years. Both sexually transmitted and endogenous vaginal microorganisms may be isolated from the upper genital tract in women with symptomatic PID, suggesting that PID most likely results from ascension of microorganisms from the lower genital tract (vagina and cervix) to the endometrium and Fallopian tubes. Diagnosis of PID is based primarily on clinical signs and symptoms. Given that most women with TFI have no prior history of symptomatic PID, damage to the Fallopian tubes may be due to an inflammatory process that is clinically unapparent, a condition termed subclinical, silent, or unrecognized PID. The lack of a minimally or non-invasive diagnostic test that identifies women with upper genital tract inflammation prevents evaluation of therapies designed to improve the outcome for women with silent and clinically apparent PID.
Current guidelines instruct clinicians to treat sexually active women with lower abdominal pain and genital tract symptoms with antibiotics active against the sexually transmitted pathogens, Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC). However, these pathogens are only detected in a third of women with PID. Should broader spectrum antibiotics active against other organisms that have been isolated from the endometrium of women with PID, such as anaerobes, and Mycoplasma spp. be included in treatment protocols? It is possible that broadening the antimicrobial spectrum of drug therapy would improve outcomes. However, direct causal data for non-CT, non-GC organisms are lacking, and side effects, cost and induction of antibiotic resistance associated with increased drug therapy must be considered. Additional agents may impact the natural flora of the genital tract, and the role of the vaginal and endometrial microbiomes in inflammation and development of upper reproductive tract disease remains largely unknown.
In an effort to reduce the incidence of infection and prevent disease due to known pathogens, widespread screening and treatment programs have been introduced in developed countries. These programs target CT and GC, and they have been established long enough that analyses of their effectiveness in limiting reproductive sequelae and reducing healthcare costs have been performed. Data indicate implementation of these programs has led to improved reproductive outcomes. However, community based programs are costly and difficult to maintain and the possibility that frequent screening and early treatment could negatively impact development of adaptive immunity has spurred controversy. The absence of a test that provides for detection and tracking of upper reproductive tract inflammation and the reliance on endpoints like TFI and EP that are typically remote from the treatment intervention, makes evaluation of these programs, as well as new therapeutic or prevention strategies, extremely challenging.
In November 2011 the NIH convened a workshop of basic researchers, epidemiologists and clinical experts in PID in order to identify research gaps hindering advances in diagnosis, treatment and prevention (Table 1). Participants were charged with addressing the following questions: Does lower genital tract infection and/or colonization predict upper genital tract infection, colonization and/or development of disease? What techniques or methodologies should be evaluated as predictors of sequelae? Are current treatment regimens adequate to address PID-inducing pathogens and immune-mediated mechanisms of pathogenesis? What are the roles of CT and GC screening and treatment programs in reducing the incidence of acute disease and late complications? Could negative consequences arise from widespread screening and treatment protocols?
Table 1.
PID Research Gaps
| Basic Science |
Characterize disease pathophysiology • Determine if M. genitalium plays a causal role in PID and chronic reproductive tract disease • Determine if BV-associated organisms play a causal role in chronic reproductive tract disease • Determine whether histological endometritis correlates with subclinical PID and predicts chronic reproductive tract disease Develop biomarkers • Identify upper genital tract inflammatory responses and cellular infiltrates most closely associated with chronic reproductive tract disease • Develop a non-invasive biomarker that correlates upper genital tract infection with risk of chronic reproductive tract disease |
| Translational Research |
Develop new antibiotics • Develop well-tolerated antibiotics targeted to organisms causing PID Improve disease detection • Develop user friendly, accessible, polymicrobial, fast, reliable, inexpensive tests for STIs • Determine ability of MRI or transvaginal ultrasound to detect tubal inflammation in women with lower genital tract infection Improve mechanisms to predict reproductive sequelae • Relate presence of tubal inflammation on MRI or transvaginal ultrasound to likelihood of chronic reproductive tract disease |
| Clinical Research |
Improve PID case definition • Identify diagnostic criteria for PID that accurately correlate with upper genital tract inflammation Optimize antibiotic coverage • Determine benefit of antimicrobial coverage for anaerobes and Mycoplasma spp Investigate new treatment modalities • Determine benefit of immune modulating agents Prevent reinfection • Improve mechanisms of partner notification and treatment |
This article summarizes the presentations, discussions and conclusions of this group and highlights significant controversies that reveal aspects of PID research that would most greatly benefit from the application of newer molecular, immunological and radiological techniques. Multiple limitations exist to performing new clinical trials; however, emerging data from ongoing clinical trials will add to the current body of knowledge regarding prevention and treatment strategies. Additionally, use of established healthcare databases could serve as a valuable tool for performance of unbiased epidemiologic outcome studies.
IDENTIFICATION OF RESEARCH GAPS
Defining PID
Clinicians rely mainly on clinical signs and symptoms to diagnose PID, mainly due to the lack of a minimally or non-invasive diagnostic test that reliably identifies women with upper genital tract inflammation. Diagnosis via laparoscopy and endometrial sampling has been evaluated in the literature, but shortcomings to these approaches limit their utility.
In a landmark study by Westrom et al [1], women hospitalized at the Lund Hospital in Sweden for clinical suspicion of PID from 1960 to 1984 underwent diagnostic laparoscopy and 1,844 of 2,501 (74%) had visual evidence of salpingitis. Long-term follow-up revealed high rates of tubal factor infertility (TFI) (11.1%) and EP (9.1%) in those with salpingitis at laparoscopy but low rates in those with normal tubes at laparoscopy (TFI = 0%, EP = 1.4%). Furthermore, the rate of TFI increased as the severity of salpingitis increased. This study showed that abnormal laparoscopic findings were highly predictive of chronic sequelae. However, laparoscopy is an invasive procedure that frequently requires general anesthesia and does not contribute to improved clinical outcomes. Consequently, the use of laparoscopy as a research tool has languished in recent years. Although several gynecological experts voiced their opinion that laparoscopy is the most sensitive means to diagnose PID and determine the likelihood of reproductive sequelae, they also agreed that the cost and risks of this invasive procedure outweigh the benefits.
In the late 1980’s, Kiviat et al [2] utilized endometrial biopsy as a diagnostic tool to assess upper genital tract inflammation in women with clinically suspected PID. The authors were able to identify histologic criteria that correlated with upper genital tract infection and salpingitis diagnosed by laparoscopy, suggesting that evaluation of endometrial tissue can serve as a surrogate for Fallopian tube infection and immune pathology. The simultaneous presence of ≥ 5 neutrophils per 400× field in the endometrial surface epithelium, together with one or more plasma cells per 120× field in endometrial stroma had a 92% sensitivity and 87% specificity for predicting upper genital tract infection and salpingitis. The inclusion of stromal plasma cells enhanced specificity in menstruating women, in whom neutrophil infiltration in the surface epithelium was common.
Meeting experts raised concerns related to the capacity of endometritis determined by histological exam to predict long-term reproductive sequelae. In the PID Evaluation and Clinical Health (PEACH) trial, the presence of neutrophils or plasma cells was considered evidence of endometritis [3]. In a secondary analysis of the outcomes of participants in the PEACH study, the presence of histologic endometritis, using modified or strict Kiviat criteria, was not associated with greater reproductive morbidity when compared to participants without endometritis [4]. Since all of the patients recruited into the PEACH study met clinical diagnostic criteria for PID, the patients without histologic endometritis could have sustained prior tubal insults or may have had other causes of decreased fertility which also present with symptoms suggestive of PID (pelvic adhesions, ovarian cysts, endometriosis).
A potential advance that may improve the specificity of endometritis as a research or diagnostic tool is the use of immunohistochemistry or flow cytometry to define specific cellular infiltrates present in the endometrial biopsy specimen. A recent study determined that detection of C. trachomatis, N. gonorrhoeae, and Trichomonas vaginalis in the lower genital tract was associated with increased numbers of CD4+ T cells, B cells, plasma cells and neutrophils, but not CD8+ T cells when compared to uninfected control women [5].
General agreement was achieved amongst the workshop participants that endometrial sampling is a useful tool because it is safe and inexpensive and frequently provides sufficient endometrial tissue to allow for microbiologic, histologic, cellular, and even molecular studies to be performed on a single biopsy specimen. The decreased cost and increased availability of genetic studies for broader microbial analysis provides the opportunity to examine specimens for novel pathogens, and examine the interaction of known pathogens with novel agents. Microarray analyses can be performed to identify immunological and fibrotic pathways activated in the endometrium of women with PID that can be correlated with symptoms and long-term outcomes. The use of these newer methodologies should be considered when prospectively evaluating roles of putative PID pathogens, efficacy of PID therapies, and types of host responses that cause adverse sequelae.
Defining the risk of subclinical PID
The Centers for Disease Control and Prevention (CDC) recommends that “empiric treatment for PID should be initiated in sexually active women at risk for sexually transmitted diseases if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than PID can be identified, and if one or more of the following minimum criteria are present on pelvic examination: cervical motion tenderness, uterine tenderness, or adnexal tenderness. The requirement that all three minimum criteria be present before the initiation of empiric treatment could result in insufficient sensitivity for the diagnosis of PID”[6]. This approach is appropriate in light of data indicating that a subset of women with mild or absent symptoms remains at risk for reproductive tract sequelae.
The World Health Organization task force on prevention of infertility highlighted that 62% of women with TFI and serologic evidence of prior chlamydial or gonococcal infection reported no prior symptoms suggestive of PID [7]. High prevalence of antibodies to chlamydia and gonorrhea has been detected in women with TFI vs. controls [8–11]. A multitude of studies also indicate significantly higher rates of seropositivity to CT in women with EP but no history of PID compared to those with an intrauterine pregnancy [8 12–14] providing additional indirect evidence for the occurrence of subclinical PID.
A recent study by Wiesenfeld et al [15] that included women infected with CT or GC or at high risk for these STIs, and that excluded women with symptomatic PID revealed a 40% decreased incidence of pregnancy in 120 women with histologic findings of plasma cell endometritis when compared to 187 women without histologic endometritis. It should be noted that when strict Kiviat criteria, which include presence of neutrophils and plasma cells were used to establish endometritis, the association with decreased fertility was no longer observed. Meeting experts agreed that subclinical PID exists and can cause chronic sequelae. Meeting experts also agreed that endometrial biopsy is currently the most appropriate method to determine increased risk for chronic disease because it can document upper tract infection and inflammation, in both patients with symptomatic and subclinical PID.
Identified research gaps: the identification of cellular infiltrates in the endometrium that best correlate with salpingitis and risk of TFI or EP, as well as delineation of non-CT, non-GC causes of endometritis.
Non-invasive methods to detect upper genital tract disease
Identification of a blood biomarker
As previously described, establishing the diagnosis of PID in a female with pelvic pain is difficult and clinicians depend on information such as history of sexual activity and presence of cervical discharge to guide them towards a diagnosis. The imprecision of the clinical diagnosis and the possibility that subclinical PID can lead to chronic sequelae argues for a need for a simple blood test to identify women with upper genital tract inflammation and thereby at increased risk for chronic sequelae. A biomarker of upper tract inflammation could also be used as an endpoint in studies of therapeutics or preventatives. However, no such test currently exists. Studies have documented elevated levels of inflammatory proteins and cytokines in the serum of patients with clinically diagnosed PID [16–19]. However, no studies have been done to correlate specific biomarkers with upper tract infection or inflammation. Using microarray analysis to identify genes and proteins specific for upper tract inflammation combined with high-throughput genetic analyses to identify host markers of increased susceptibility may result in the development of a biomarker panel that could then be refined into a clinical test.
A major identified research gap: development of a non-invasive biomarker to identify women with upper genital tract infection who are at increased risk of chronic reproductive tract disease.
Noninvasive imaging evaluation for upper genital tract disease
Laparoscopy is invasive, costly and carries inherent surgical risks. Tukeva et al found that findings of non-contrast magnetic resonance imaging (MRI) agreed with those at laparoscopy in 17 (81%) of 21 hospitalized patients with PID [20]. Amongst 20 women hospitalized with PID, Molander et al found that Doppler transvaginal ultrasound (US) had a sensitivity of 100% and a specificity of 80% when compared to laparoscopy for confirmation of PID [21]. Additionally, transvaginal US has proved useful in a small study of ambulatory patients, where thickened fluid-filled tubes were found in 11 of 13 patients with plasma cell endometritis as revealed by endometrial biopsy and in none of those without [22].
The ability of a skilled technician, gynecologist or radiologist to perform transvaginal US at the bedside may provide logistic and cost advantages. The participants agreed that large studies are needed to examine the ability of imaging techniques to detect tubal inflammation in both acutely symptomatic and asymptomatic women with documented lower genital tract infection. Prospective investigations that combine radiological evaluation with potential serum biomarkers also are needed.
An identified research gap: determination of the ability of radiologic studies to detect tubal inflammation in women with lower genital tract infection and relate this to chronic disease risk such as TFI or EP.
Potential role of Mycoplasma genitalium in PID and development of chronic reproductive tract disease
Many women with PID or with histologic endometritis have no evidence of active chlamydial or gonococcal infection, and the microbiologic cause of inflammation in these patients remains elusive. M. genitalium has been implicated as a candidate agent. Sexual transmission of M. genitalium has been documented in infected couples [23], and can be inferred from increased prevalence rates in persons at high risk of STI [24]. Studies have demonstrated a positive association of M. genitalium with clinical PID in women from geographically diverse populations [25–30]. Nevertheless, a cohort study conducted in the United Kingdom revealed the incidence of PID over 12 months was 3.9% (3/77) in those women with M. genitalium versus 1.7% (36/2169) in those without (RR = 2.35, 0.74 to 7.46, P=0.14), indicating M. genitalium was not likely to be a major risk factor for clinical PID in this population [31]. Persistence of M. genitalium has been reported as a possible cause for continued detection of endometritis after treatment for PID [25]. This is important, because it shows the relationship to be independent of gonorrhea and chlamydia, and supports a causal relationship. However, no clear trend is observed in the majority of studies that removed co-infections with C. trachomatis or controlled for co-infections in multivariate analyses [32]. Two relatively small studies have detected a significant association between women with M. genitalium–specific serum antibodies and TFI [33] [34]. Confirmatory studies are necessary to establish whether M. genitalium infection independently causes PID and/or contributes to long-term sequelae [32].
Identified research gap: determining if M. genitalium plays a cin PID and long-term reproductive tract sequelae.
Potential role for anaerobes and bacterial vaginosis (BV) in PID and development of chronic reproductive tract disease
The role of anaerobic bacteria and BV in contributing to PID remains unclear. A retrospective cross-sectional study compared patients with acute PID to women with subclinical PID as defined by presence of histologic endometritis on biopsy; a control group of women included those who were enrolled because of predicted risk factors for PID but were found to be negative for endometritis on biopsy. The frequency of BV was higher among patients with acute and subclinical PID when compared to controls [35]. Although examination of the endometrium for BV organisms was not performed in this study, the detection of BV in 229/356 or 64% of women without evidence of histologic endometritis clearly indicates that BV can be present in a high percentage of women without coincident endometrial inflammation.
Bacterial vaginosis-associated bacteria have been isolated from Fallopian tubes of women with symptomatic salpingitis and anaerobic gram-negative rods have been isolated from the endometrium of subjects with endometritis but without CT or GC [36 37]. In the study conducted by Hillier, et al, the detection of obligate anaerobic gram-negative rods from the endometrium was independently associated with histologic endometritis. The CDC states that “antimicrobial regimens that provide coverage of anaerobes should be considered” for the treatment of PID [6]. A formal recommendation for anti-anaerobic coverage awaits more definitive data.
Exploiting molecular techniques to characterize the endometrial microbiome using biopsy samples from women with symptomatic and subclinical PID for correlation with evidence of endometritis and long-term outcomes provides a viable approach to more definitively link these organisms with PID [38]. In addition, anaerobic culture methods by microbiological experts combined with deep sequencing methods should allow for identification of specific organisms within BV-associated bacteria that have more invasive potential and are more likely to persist and cause inflammatory changes in the upper genital tract. Lastly, prospective treatment trials that examine the effect of anti-anaerobic therapy on resolution of clinical symptoms and histologic endometritis may also provide an indication of the role of these organisms in disease pathogenesis.
Identified research gap: determining if anaerobes and BV play a causal role in PID and long-term reproductive tract sequelae.
Treatment of PID
Meeting experts agreed that the minimum requirement of any PID antibiotic treatment regimen is efficacy in treating N. gonorrhoeae and C. trachomatis. Therapy should be initiated as soon as a presumptive diagnosis is made. The standard regimen consists of ceftriaxone 250 mg IM and oral doxycycline 100 mg twice daily for 2 weeks. Inpatient and outpatient therapy have similar short- and long-term outcomes in women with mild or moderate clinical severity [3]. Although doxycycline continues to be recommended as the first-line agent for empiric coverage of C. trachomatis, a Brazilian study revealed equivalent efficacy when oral azithromycin 1 g per week for 2 weeks was substituted for oral doxycycline 100 mg twice daily for 2 weeks; both treatment regimens included standard IM treatment with ceftriaxone 250 mg [39].
The effect of antimicrobial treatment in women with non-CT, non-GC endometritis has been examined in a small study. A prospective antimicrobial treatment trial compared the clinical evaluation and endometrial biopsy results before and after antimicrobial therapy in women at high risk for STI who had histologic evidence of endometritis but no clinical indications of PID [40]. Of the 37 patients with endometritis, 16 (43%) tested positive for either chlamydia or gonorrhea. The remaining endometritis cases had no clear etiology despite thorough microbiologic assessment including culture for Mycoplasma hominis, Ureaplasma urealyticum, and aerobic and anaerobic bacteria. Bacterial vaginosis was highly prevalent in both women with endometritis (22 of 37, 59%) and without (85 of 170, 50%). Subjects were treated with oral doses of cefixime 400 mg, azithromycin 1 g, and metronidazole 500 mg twice daily for 7 days. Of the 48 women with endometrial biopsy both before and after therapy, histologic endometritis was present in 18 subjects before therapy and in only 2 subjects after antimicrobial therapy (P<0.001). The long-term effect of resolution of endometritis on tubal patency is not known.
Antibiotic coverage for gonococcal or chlamydial infection, mycoplasmas, and anaerobes will require combination regimens associated with increased drug cost and side effects compared to currently recommended treatments. Support was expressed for prospective longitudinal treatment trials to establish the risk- and cost-benefit ratios of providing broad-spectrum coverage that is effective against multiple organisms.
Identified research gap: determination if adding drugs to the treatment recommendations for PID that would provide coverage for anaerobes and for Mycoplasma spp. provide benefit.
A global look at prevention – Chlamydia as a model
In many developed countries, screening programs for chlamydia were established to reduce transmission and reproductive tract morbidity [41–43]. These public programs were initiated in the U.S. in the late 1980s, in British Columbia in the late 1990s, and in the United Kingdom in 2003. Recent studies have assessed the impact of these control programs.
Reported chlamydial infection rates among women in the U.S. have been increasing annually since programs for screening and treatment were first established [44]. Similar increases have been observed in British Columbia, where the relative risk of reinfection has been increasing at 4.6% per year since 1996, coinciding with the widespread institution of azithromycin treatment for PCR positive individuals [45]. The observation that prolonged infection seems to be required to induce an effective immune response to chlamydiae [46] has led Dr. Robert Brunham to propose that these rising rates of infection are due to arrested immunity during this era of early case identification and treatment [45]. However, the increase in chlamydia case reports may not represent a true increase in chlamydial burden, but rather the result of expanded screening for this frequently asymptomatic infection, development and use of more sensitive tests, and more complete national reporting [47].
Estimating prevalence of infection from national case reporting data can be biased by healthcare seeking behavior and is not representative of disease burden in the United States. Trends in chlamydial prevalence may best be estimated using population-based surveys, such as studies reported by Datta et al [48]. Among a nationally representative sample of men and women aged 14 to 39 years in the United States, the overall prevalence of chlamydia decreased significantly from 1999-2008, but the prevalence did not decrease among females aged 14 to 25 years, the population targeted for routine annual screening [48].
An important question is whether STI screening and treatment leads to decreased incidence of STI-induced adverse outcomes. Studies indicate rates of PID have decreased in the U.S., British Columbia, and the United Kingdom since control programs were initiated. The percentage of women in the U.S. who report ever being treated for PID has decreased over the past 3 decades to a low of 5% in 2008 (Figure 1) [49]. Additionally, using National Center for Health Statistics Datasets, Sutton, et al [50] found that rates of hospitalization for PID declined 68% overall from 1985 through 2001 (P <0.0001), and ambulatory visits to physicians’ offices for PID decreased 47%. Insurance claims data reveal that the rates of PID diagnoses among privately insured women declined 25.5% from 2001 to 2005 among all age groups examined and within all geographic regions [51]. In British Columbia, rates of PID have fallen significantly in the 20 years since implementation of control measures, and continue to fall [52]. In the United Kingdom, the rate of definite/probable PID decreased by 10.4% per year over the past decade with control efforts [53].
Figure 1.
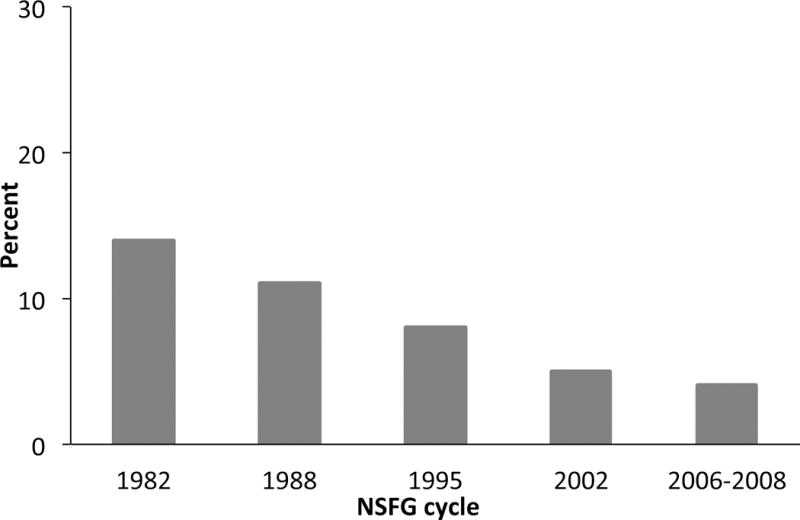
U.S. women aged 15-44 years who reported ever being treated for PID, National Survey of Family Growth, 1982-2008 (With permission of Anjani Chandra, National Center for Health Statistics, Centers for Disease Control and Prevention).
Dr. Pippa Oakeshott and her colleagues in the United Kingdom screened over 2500 young women in the Prevention of Pelvic Infection (POPI) trial, and 94% were followed up after 12 months[54]. Screening and treatment resulted in a 35% reduction in the risk of clinical PID (P = 0.07). A high prevalence of chlamydial infection amongst this group may have skewed the results towards the null. Importantly, most episodes of PID occurred in women who tested negative for chlamydia at baseline but developed infection later (79%, 30/38). This high rate of incident infection indicates the importance of targeting those at higher risk, such as women with a new sexual partner or a past history of chlamydial infection. The authors concluded that inexpensive, rapid screening methods for STIs and optimized methods of partner notification are needed to reduce PID rates further.
Since chlamydial infection is directly linked to Fallopian tube scarring, it is important to assess how chlamydial control programs have impacted rates of EP. Time trends in the diagnosis and treatment of EP among commercially insured women in the U.S. from 2002 to 2007 were estimated by Hoover et al using a large claims database with information for several million women aged 15–44 years (Figure 2) [55]. The overall rate of EP was 0.6%, and did not change over the 6-year time period, but was lower than that described in a study of a managed care population from 1997 to 2000 that found a rate of 2.1% [56]. Interestingly, EP rates determined from 2002 to 2007 increased with age; 0.3% among girls and women aged 15–19 years and 1.0% among women aged 35– 44 years. Women aged 35 years or older make up more than half of those using assisted reproductive technologies (ART) [57]., The increased rate of ectopic pregnancies observed in older women may reflect increased numbers of women seeking therapy who sustained oviduct damage during teen-age/young adulthood, during a time when chlamydial infection was more prevalent (prior to initiation of intensive screening and treatment). It is also possible that the increased rate amongst older women reflects changes in the structure and function of the Fallopian tube that occur with age, or other unknown mechanisms resulting from ART. In British Columbia, the rate of EP‐related physician billings and hospital discharges declined during the 1990’s, and remained relatively stable after 2001 except for increases in 2007 and 2008 [52]. An increase in chlamydial reinfection rates reported in recent years in British Columbia were proposed by Dr. Brunham as a potential cause for this rise in EP rates[58]. However, continued monitoring and additional data are needed before conclusions are possible.
Figure 2.
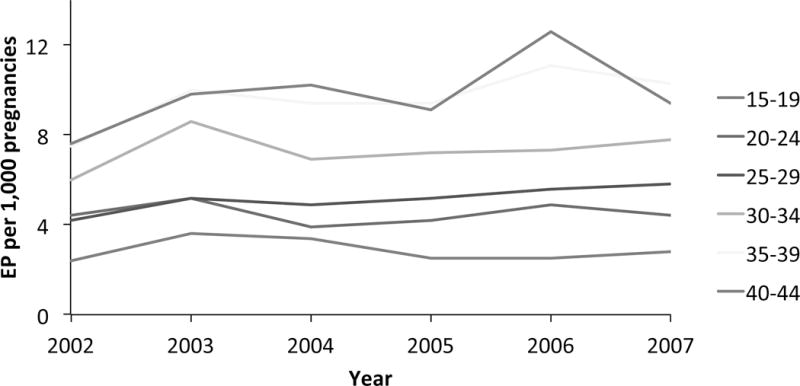
Ectopic pregnancies in commercially insured U.S. women by age group, MarketScan, 2002-2007 (Adapted from Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstetrics and gynecology 2010;115:495-502.)
Although it is difficult to estimate the proportion of TFI attributable to chlamydial infection, the National ART Surveillance System observed a steady decline from 1999 to 2009 in the rate of ART cycles for TFI among women in the U.S. Over this same time frame, the rate of ART cycles for decreased ovarian reserve have increased, in agreement with women of older age seeking pregnancy in recent years. Additionally, married women in the U.S. aged 15-44 years who reported 12-month infertility decreased significantly from 1982 to 2002 and again from 2002 to 2008 [59]. Thus, although incidence rates of chlamydial infection continue to rise in several countries, population-based surveys indicate overall infection prevalence has decreased in the United States over the past decade. In addition, considerable evidence suggests that chlamydial screening and treatment programs are leading to decreased rates of PID, EP and TFI.
Identified research gaps: development of inexpensive, rapid screening methods, and determination of the time required for development of an effective adaptive immune response to chlamydial infection…
CONCLUSION
The most substantial advance in PID treatment made in the last twenty years has been the move to outpatient-based therapy. Although this provided a major cost savings in the acute phase, and enabled proactive treatment of women with minimal criteria of PID, these women remain at risk for long-term sequelae. The failure of current antimicrobial treatment to prevent these long-term outcomes exposes outstanding research gaps, including causes of non-GC, non-CT PID, the contributory role of host commensals, and host responses that lead to chronic tissue damage. Endometrial biopsy via catheter suction device provides a safe means to gather microbiological, histological and immunological information from women with sexually transmitted infection to inform a more specific diagnosis, treatment and prognosis. Imaging methods need to be examined for their ability to determine presence of oviduct inflammation and disease in asymptomatic and symptomatic women with sexually transmitted infections. Oviduct imaging should be correlated with results of endometrial biopsy to determine if endometrial inflammation predicts oviduct disease. Bacterial genomic studies should accelerate the identification of novel pathogens that cause PID. Human transcriptomic and genomic studies should allow for the determination of biomarkers of disease risk which will enable more cost effective screening and treatment programs. Use of these novel tools should enhance provision of more specific therapy and guidance to sexually active women and thereby promote their reproductive health.
SUMMARY.
Proceedings of a 2011 National Institutes of Health-sponsored workshop on pelvic inflammatory disease where past and recent data were reviewed and goals were defined for future research.
Acknowledgments
The authors would like to acknowledge the support and contribution of Marcia Hobbs, M. Elizabeth Rogers, the NIH-funded STI CRC investigators and other participants of the NIH-sponsored PID workshop. Toni Darville is supported by United States National Institutes of Health grants (AI054624 and U19 AI084024).
Dr. Toni Darville is supported by United States National Institutes of Health grants (AI054624 and U19 AI084024).
Footnotes
Dr. Darville has no conflict of interest.
References
- 1.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–92. [PubMed] [Google Scholar]
- 2.Kiviat NB, Wolner-Hanssen P, Eschenbach DA, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990;14(2):167–75. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Amer J of Obstet and Gynecol. 2002;186(5):929–37. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 4.Haggerty CL, Ness RB, Amortegui A, et al. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Amer J of Obstet and Gynecol. 2003;188(1):141–8. doi: 10.1067/mob.2003.87. [DOI] [PubMed] [Google Scholar]
- 5.Reighard SD, Sweet RL, Vicetti Miguel C, et al. Endometrial leukocyte subpopulations associated with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis genital tract infection. Amer J of Obstet and Gynecol. 2011;205(4):324 e1–7. doi: 10.1016/j.ajog.2011.05.031. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Workowski KA, Berman SM. Centers for Disease Control and Prevention Sexually Transmitted Disease Treatment Guidelines. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(Suppl 3):S59–63. doi: 10.1093/cid/cir694. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 7.Tubal infertility: serologic relationship to past chlamydial and gonococcal infection. World Health Organization Task Force on the Prevention and Management of Infertility. Sexually transmitted diseases. 1995;22(2):71–7. [PubMed] [Google Scholar]
- 8.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(Suppl 2):S134–55. doi: 10.1086/652395. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 9.Tiitinen A, Surcel HM, Halttunen M, et al. Chlamydia trachomatis and chlamydial heat shock protein 60-specific antibody and cell-mediated responses predict tubal factor infertility. Human Reproduction. 2006;21(6):1533–8. doi: 10.1093/humrep/del014. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 10.Sellors JW, Mahony JB, Chernesky MA, Rath DJ. Tubal factor infertility: an association with prior chlamydial infection and asymptomatic salpingitis. Fertility and Sterility. 1988;49:451–57. doi: 10.1016/s0015-0282(16)59772-6. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen A, Heinonen PK, Teisala K, Hakkarainen K, Punnonen R. Serologic evidence for the role of Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma hominis in the etiology of tubal factor infertility and ectopic pregnancy. Sex Transm Dis. 1990;17:10–14. [PubMed] [Google Scholar]
- 12.Svensson L, Mardh PA, Ahlgren M, Nordenskjold F. Ectopic pregnancy and antibodies to Chlamydia trachomatis. Fertility and Sterility. 1985;44:313–17. [PubMed] [Google Scholar]
- 13.Walters MD, Eddy CA, Gibbs RS, Schachter J, Holden AEC, Pauerstein CJ. Antibodies to Chlamydia trachomatis and risk for tubal pregnancy. American Journal of Obstetrics and Gynecology. 1988;159:942–46. doi: 10.1016/s0002-9378(88)80177-7. [DOI] [PubMed] [Google Scholar]
- 14.Chow JM, Yonekura ML, Richwald GA, Greenland S, Sweet RL, Schachter J. The association between Chlamydia trachomatis and ectopic pregnancy. A matched-pair, case-control study. JAMA. 1990;263:3164–67. [PubMed] [Google Scholar]
- 15.Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL. Subclinical Pelvic Inflammatory Disease and Infertility. Obstetrics & Gynecology. 2012 doi: 10.1097/AOG.0b013e31825a6bc9. Publish Ahead of Print. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 16.Lee SA, Wang PH, Chiou HL, Chou MC, Tsai HT, Yang SF. Markedly elevated plasma myeloperoxidase protein in patients with pelvic inflammatory disease who have A allele myeloperoxidase gene polymorphism. Fertility and Sterility. 2010;93(4):1260–6. doi: 10.1016/j.fertnstert.2008.11.024. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao PC, Wang PH, Tee YT, et al. Significantly elevated concentration of plasma monocyte chemotactic protein 1 of patients with pelvic inflammatory disease. Reprod Sci. 2010;17(6):549–55. doi: 10.1177/1933719110362593. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 18.Wang PH, Liu YF, Tsai HT, et al. Elevated plasma osteopontin level is associated with pelvic inflammatory disease. Reprod Sci. 2010;17(11):1052–8. doi: 10.1177/1933719110379270. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 19.Lee SA, Tsai HT, Ou HC, et al. Plasma interleukin-1beta, -6, -8 and tumor necrosis factor-alpha as highly informative markers of pelvic inflammatory disease. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2008;46(7):997–1003. doi: 10.1515/CCLM.2008.196. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 20.Tukeva TA, Aronen HJ, Karjalainen PT, Molander P, Paavonen T, Paavonen J. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209–16. doi: 10.1148/radiology.210.1.r99ja04209. [DOI] [PubMed] [Google Scholar]
- 21.Molander P, Sjoberg J, Paavonen J, Cacciatore B. Transvaginal power Doppler findings in laparoscopically proven acute pelvic inflammatory disease. Ultrasound Obstet Gynecol. 2001;17(3):233–8. doi: 10.1046/j.1469-0705.2001.00353.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 22.Cacciatore B, Leminen A, Ingman-Friberg S, Ylostalo P, Paavonen J. Transvaginal sonographic findings in ambulatory patients with suspected pelvic inflammatory disease. Obstetrics and gynecology. 1992;80(6):912–6. [PubMed] [Google Scholar]
- 23.Hjorth SV, Bjornelius E, Lidbrink P, et al. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. Journal of clinical microbiology. 2006;44(6):2078–83. doi: 10.1128/JCM.00003-06. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen B, Sokolowski I, Ostergaard L, Kjolseth Moller J, Olesen F, Jensen JS. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sexually transmitted infections. 2007;83(3):237–41. doi: 10.1136/sti.2006.022970. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haggerty CL, Totten PA, Astete SG, et al. Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sexually transmitted infections. 2008;84(5):338–42. doi: 10.1136/sti.2008.030486. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haggerty CL, Totten PA, Astete SG, Ness RB. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infectious diseases in obstetrics and gynecology. 2006;2006:30184. doi: 10.1155/IDOG/2006/30184. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. Bjog. 2010;117(3):361–4. doi: 10.1111/j.1471-0528.2009.02455.x. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 28.Bjartling C, Osser S, Persson K. Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. American journal of obstetrics and gynecology. 2012 doi: 10.1016/j.ajog.2012.02.036. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 29.Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet. 2002;359(9308):765–6. doi: 10.1016/S0140-6736(02)07848-0. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 30.Simms I, Eastick K, Mallinson H, et al. Associations between Mycoplasma genitalium, Chlamydia trachomatis and pelvic inflammatory disease. Journal of clinical pathology. 2003;56(8):616–8. doi: 10.1136/jcp.56.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “New Chlamydia?” A community-based prospective cohort study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51(10):1160–6. doi: 10.1086/656739. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 32.McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS pathogens. 2011;7(5):e1001324. doi: 10.1371/journal.ppat.1001324. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility–a prospective study. Fertility and sterility. 2008;90(3):513–20. doi: 10.1016/j.fertnstert.2006.12.056. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 34.Clausen HF, Fedder J, Drasbek M, et al. Serological investigation of Mycoplasma genitalium in infertile women. Human reproduction. 2001;16(9):1866–74. doi: 10.1093/humrep/16.9.1866. [DOI] [PubMed] [Google Scholar]
- 35.Wiesenfeld HC, Sweet RL, Ness RB, Krohn MA, Amortegui AJ, Hillier SL. Comparison of acute and subclinical pelvic inflammatory disease. Sexually transmitted diseases. 2005;32(7):400–5. doi: 10.1097/01.olq.0000154508.26532.6a. [DOI] [PubMed] [Google Scholar]
- 36.Hillier SL, Kiviat NB, Hawes SE, et al. Role of bacterial vaginosis-associated microorganisms in endometritis. American journal of obstetrics and gynecology. 1996;175(2):435–41. doi: 10.1016/s0002-9378(96)70158-8. [DOI] [PubMed] [Google Scholar]
- 37.Paavonen J, Teisala K, Heinonen PK, et al. Microbiological and histopathological findings in acute pelvic inflammatory disease. Br J Obstet Gynaecol. 1987;94(5):454–60. doi: 10.1111/j.1471-0528.1987.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 38.Marrazzo JM, Martin DH, Watts DH, et al. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sexually Transmitted Diseases. 2010;37(12):732–44. doi: 10.1097/OLQ.0b013e3181fbbc95. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstetrics and gynecology. 2007;110(1):53–60. doi: 10.1097/01.AOG.0000268801.90261.27. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 40.Eckert LO, Thwin SS, Hillier SL, Kiviat NB, Eschenbach DA. The antimicrobial treatment of subacute endometritis: a proof of concept study. American Journal of Obstetrics and Gynecology. 2004;190(2):305–13. doi: 10.1016/j.ajog.2003.09.024. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 41.Control ECfDPa. Chlamydia control in Europe. European Center for Disease Control; 2009. [Google Scholar]
- 42.Bell TA, Grayston JT. Centers for Disease Control guidelines for prevention and control of Chlamydia trachomatis infections. Summary and Commentary. Annals of Internal Medicine. 1986;104(4):524–26. doi: 10.7326/0003-4819-104-4-524. [DOI] [PubMed] [Google Scholar]
- 43.Division of STD/AIDS Control BCCfDC. STD/AIDS control annual report 2003. Vancouver: British Columbia Centre for Disease Control; 2004. [Google Scholar]
- 44.Prevention CfDCa. Sexually Transmitted Disease Surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 45.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The Unexpected Impact of a Chlamydia trachomatis Infection Control Program on Susceptibility to Reinfection. J Infect Dis. 2005;192(10):1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 46.Molano M, Meijer CJ, Weiderpass E, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis. 2005;191(6):907–16. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 47.Vickers DM, Osgood ND. Current crisis or artifact of surveillance: insights into rebound chlamydia rates from dynamic modelling. BMC infectious diseases. 2010;10:70. doi: 10.1186/1471-2334-10-70. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999-2008. Sexually transmitted diseases. 2012;39(2):92–6. doi: 10.1097/OLQ.0b013e31823e2ff7. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 49.Chandra AMGM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital Health Stat. 2005 [PubMed] [Google Scholar]
- 50.Sutton MY, Sternberg M, Zaidi A, St Louis ME, Markowitz LE. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985-2001. Sexually transmitted diseases. 2005;32(12):778–84. doi: 10.1097/01.olq.0000175375.60973.cb. [DOI] [PubMed] [Google Scholar]
- 51.Bohm MK, Newman L, Satterwhite CL, Tao G, Weinstock HS. Pelvic inflammatory disease among privately insured women, United States, 2001-2005. Sexually transmitted diseases. 2010;37(3):131–6. doi: 10.1097/OLQ.0b013e3181bf576f. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 52.Control BCCfD. STI/HIV Prevention and Control Surveillance: Annual Surveillance Report, 2008. 2008 [Google Scholar]
- 53.French CE, Hughes G, Nicholson A, et al. Estimation of the rate of pelvic inflammatory disease diagnoses: trends in England, 2000-2008. Sexually Transmitted Diseases. 2011;38(3):158–62. doi: 10.1097/OLQ.0b013e3181f22f3e. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 54.Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340:c1642. doi: 10.1136/bmj.c1642. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstetrics and gynecology. 2010;115(3):495–502. doi: 10.1097/AOG.0b013e3181d0c328. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 56.Van Den Eeden SK, Shan J, Bruce C, Glasser M. Ectopic pregnancy rate and treatment utilization in a large managed care organization. Obstetrics and gynecology. 2005;105(5 Pt 1):1052–7. doi: 10.1097/01.AOG.0000158860.26939.2d. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 57.Sunderam S, Chang J, Flowers L, et al. Assisted reproductive technology surveillance–United States, 2006. MMWR Surveill Summ. 2009;58(5):1–25. [PubMed] [Google Scholar]
- 58.Rekart ML, Gilbert M, Meza R, et al. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis. 2013;207(1):30–8. doi: 10.1093/infdis/jis644. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]
- 59.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982-2002. Fertility and sterility. 2006;86(3):516–23. doi: 10.1016/j.fertnstert.2006.02.129. published Online First: Epub Date. [DOI] [PubMed] [Google Scholar]


