Abstract
Background
Patients with gout are at an increased risk of cardiovascular disease (CVD) including myocardial infarction (MI), stroke and heart failure (HF).
Objective
We conducted a cohort study to examine comparative cardiovascular safety of the two gout treatments - probenecid and allopurinol - in patients with gout.
Methods
Among gout patients aged ≥65 years and enrolled in Medicare (2008–2013), we identified those who initiated probenecid or allopurinol. The primary outcome was a composite cardiovascular endpoint of hospitalization for MI or stroke. We assessed MI, stroke, coronary revascularization, HF and mortality separately as secondary outcomes. We estimated the incidence rate (IR) and hazard ratio (HR) of the primary and secondary outcomes in the 1:3 propensity score (PS) matched cohort of probenecid and allopurinol initiators.
Results
We included a total of 9,722 probenecid initiators PS-matched to 29,166 allopurinol initiators with mean age of 76 (±7) years, and 54% male. The IR of the primary composite endpoint of MI or stroke per 100 person-years was 2.36 in probenecid and 2.83 in allopurinol initiators with HR of 0.80 (95%CI 0.69–0.93). In the secondary analyses, probenecid was associated with a decreased risk of MI, stroke, HF exacerbation and mortality versus allopurinol. Our results were consistent in the subgroup analyses of patients without baseline CVD or those without baseline chronic kidney disease.
Conclusions
In this large cohort of 38,888 elderly gout patients, treatment with probenecid appears to be associated with a modestly decreased risk of cardiovascular events including MI, stroke and HF exacerbation compared to allopurinol.
Keywords: gout, allopurinol, probenecid, cardiovascular disease, comparative safety research
INTRODUCTION
Gout is the most common inflammatory arthritis with an increasing prevalence in many countries including the US.(1) It is caused by hyperuricemia leading to crystallization of uric acid within the joints and periarticular tissues.(2) Urate crystals then activate the NLRP3 inflammasome (i.e., cryopyrin) resulting in the production of interleukin (IL)-1β.(3) Overproduction of urate or underexcretion of urate through the kidneys leads to hyperuricemia. Allopurinol, a xanthine oxidase inhibitor, is the mainstay of treatment for gout and can be used in patients who overproduce or underexcrete urate. Probenecid is another treatment option, which has been available for gout for many decades. Probenecid inhibits organic acid reabsorption in the renal proximal tubule, causing the excretion of uric acid through the kidneys; it is not recommended in patients with overproduction of uric acid.(2,4,5)
It is well known that patients with gout are at an increased risk of cardiovascular disease (CVD) including myocardial infarction (MI), stroke and heart failure (HF).(6–8) While controversies still exists whether uric acid plays a causal role in the development of CVD, beneficial effects of allopurinol on lowering blood pressure and improving endothelial function and metabolic profile have been reported.(9–11) A randomized controlled trial in high-risk HF patients, i.e., EXACT-HF study, allopurinol did not, however, improve the composite clinical endpoint related to HF.(12) Observational cohort studies have shown conflicting results with regard to effect of xanthine oxidase inhibitors, mainly allopurinol, on reducing the risk of future CVD.(13–15) However, no data exist with regard to the effect of probenecid on CVD among gout patients. Probenecid is not only a competitive inhibitor of the organic anion transporter,(5,16) but also an inhibitor of pannexin 1 channels - an ATP release channel – involved in the activation of the inflammasome which releases IL-1β.(17) Therefore, probenecid may exhibit beneficial effects in gout by lowering serum uric acid levels and reducing systemic inflammation through the inhibition of pannexin 1 channels and reduced production of IL-1β.(17) IL-1β is also known to play a pivotal role in the pathogenesis of atherosclerosis.(18) Furthermore, probenecid may have an effect on cardiovascular risk as a potent and selective agonist of transient receptor potential vanilloid 2 (TRPV2) channels.(19,20) TRPV2 is expressed in cardiomyocytes, and several experimental studies found an inotropic effect of probenecid.(19,21–23) Therefore, it is plausible to hypothesize that probenecid may have cardioprotective effects in gout patients.
The primary objective of this study was, therefore, to compare the risk of cardiovascular events including MI or stroke in patients with gout initiating probenecid versus allopurinol in a population representative cohort. We also assessed the risk of other cardiovascular endpoints including coronary revascularization and HF, and all-cause mortality in patients with gout initiating probenecid versus allopurinol.
METHODS
Data Source
We used claims data from Medicare Parts A, B and D for the period from 2008 through 2013. Medicare is a federally funded program and provides health care coverage for nearly all legal residents of the US aged ≥65 and selected disabled populations aged <65. Medicare Part A generally covers inpatient care, Part B is for outpatient medical services including some drugs given in a physician’s office or clinic, and Part D provides outpatient prescription drug coverage.(24) Since the Medicare database does not contain laboratory results, we utilized Medicare data linked with the Brigham and Women’s Hospital’s electronic medical record (EMR) database (2007–2013) to select a subgroup of gout patients enrolled in Medicare who had laboratory test results such as serum uric acid and creatinine levels. The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital which granted a waiver of informed consent.
Study Population
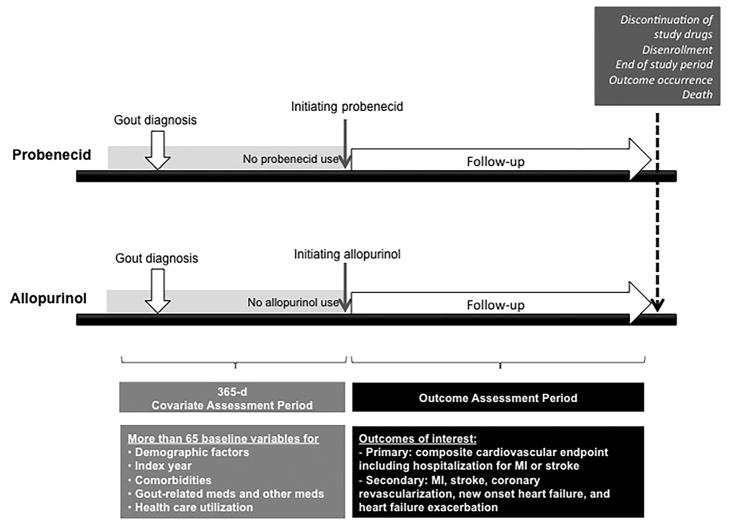
We identified adults aged 65 years older who had ≥1 International Classification of Diseases, Ninth Revision (ICD-9) code for gout (274.x). Use of probenecid or allopurinol was identified through national drug codes. Patients who were continuously enrolled in the Medicare Parts A, B, and D for ≥1 year prior to the 1st dispensing date (i.e., index date) of probenecid or allopurinol were selected as probenecid or allopurinol initiators (see Figure 1). Probenecid initiators were required to be naïve to probenecid for the 1-year baseline period prior to the index date. Similarly, allopurinol initiators were required to be naïve to allopurinol for the same baseline period. Patients who started both drugs at the same date were excluded. To assess patients’ baseline characteristics adequately, we excluded patients with no active claim in 1 year prior to the index date. We further excluded patients who used pegloticase or rasburicase, two drugs used in severe refractory gout, or had a diagnosis of end-stage renal disease or dialysis at baseline to minimize confounding by the severity of gout and renal function at baseline. For the subgroup in the linked Medicare-EMR database, we applied the same inclusion and exclusion criteria as above, and additionally required them to have ≥1 measurement for serum uric acid and serum creatinine level prior to the index date.
Figure 1.
Overview of the study design.
For the primary as-treated analysis, study subjects were followed up from the day after the index date until the earliest event of the following: 1) death; 2) outcome occurrence; 3) end of study database period; 4) insurance disenrollment; 5) nursing home admission; or 6) 30 days (grace period) after the last drug available date due to drug discontinuation or switching to the other drug. Last drug available date was defined as the last drug dispensing date plus days of supply of the exposure drug.
For the secondary intention-to-treat (ITT) analysis, patients were followed up from the day after the index date to the earliest occurrence of the following: 1) death; 2) outcome occurrence; 3) end of study database period; 4) insurance disenrollment; 5) nursing home admission; or 6) 366th day after the index date.
Outcome Definition
The primary outcome of interest was a composite cardiovascular endpoint of hospitalization for MI or stroke for any length of stay. Secondary outcomes included MI, stroke, coronary revascularization, new onset HF, and HF exacerbation all based on hospital discharge diagnosis codes, and all-cause deaths. Coronary revascularization was identified using ICD-9 procedure codes, Current Procedural Terminology codes, or diagnosis-related group codes. These claims-based algorithms for these cardiovascular outcomes had positive predictive values of ≥90%.(25–27)
Covariates
During the 1-year baseline period prior to initiation of probenecid or allopurinol (i.e., index date), we assessed more than 65 variables potentially associated with severity of gout and cardiovascular risk. These variables were demographics, the index year, regions, CVD, CKD, and other comorbidities, gout-related medications such as glucocorticoids, colchicine, or non-steroidal anti-inflammatory drugs (NSAIDs) including both non-selective NSAIDs and selective cyclooxygenase-2 inhibitors, other medications, physician orders of outpatient laboratory tests, and markers of health care utilization intensity (see the list of covariates in Table 1). History of any CVD and recent (within 60 days prior to the index date) CVD were defined as having an inpatient or outpatient diagnosis of MI, coronary artery disease, coronary revascularization, or stroke, or transient ischemic attack prior to the index date. To further assess patients’ comorbidity burden, we calculated the combined Comorbidity Score that included 47 conditions in the Charlson and Elixhauser measures.(28) We also collected data on the starting daily dose of probenecid or allopurinol in the study population. For the subgroup in the linked Medicare-EMR database, we additionally assessed their baseline serum uric acid and creatinine levels.
Table 1.
Baseline characteristics of 1:3 propensity score-matched cohort.
| Probenecid | Allopurinol | |
|---|---|---|
| N | 9,722 | 29,166 |
|
| ||
| Demographics | ||
|
| ||
| Age (mean, SD), years | 76.0 (7.4) | 76.1 (7.4) |
| Male (%) | 54.0 | 53.8 |
| Race –White (%) | 78.8 | 79.2 |
| Region | ||
| - MidWest (%) | 24.2 | 23.8 |
| - NorthEast (%) | 11.9 | 11.8 |
| - South (%) | 47.7 | 48.4 |
| - West (%) | 16.0 | 15.8 |
|
| ||
| Cardiovascular Comorbidities | ||
|
| ||
| Atrial fibrillation (%) | 22.6 | 22.2 |
| Cardiovascular disease (%) | 28.4 | 28.5 |
| Coronary artery disease (%) | 21.3 | 21.4 |
| Stroke (%) | 6.5 | 6.5 |
| Transient ischemic attach (%) | 4.6 | 4.8 |
| Heart failure (%) | 27.0 | 26.8 |
| Venous thromboembolism (%) | 7.1 | 7.1 |
| Recent coronary artery disease a (%) | 0.4 | 0.4 |
| Recent heart failure a (%) | 3.2 | 3.2 |
| Recent stroke a (%) | 0.2 | 0.2 |
| Hypertension (%) | 91.2 | 91.1 |
| Peripheral vascular disease (%) | 15.5 | 15.4 |
|
| ||
| Other Comorbidities | ||
|
| ||
| Hyperlipidemia (%) | 76.2 | 76.1 |
| Chronic kidney disease (%) | 28.4 | 28.2 |
| Chronic obstructive pulmonary disease (%) | 30.0 | 30.3 |
| Diabetes (%) | 45.7 | 45.5 |
| Alcoholism (%) | 0.5 | 0.5 |
| Malignancy (%) | 19.7 | 20.0 |
| Renal stone (%) | 3.9 | 4.0 |
| Liver disease (%) | 5.3 | 5.4 |
| Obesity (%) | 13.2 | 13.0 |
| Sleep apnea (%) | 6.6 | 6.5 |
| Smoking (%) | 6.6 | 6.7 |
| Comorbidity score (mean, SD) | 2.4 (3.0) | 2.4 (3.0) |
|
| ||
| Gout-related Medications | ||
|
| ||
| Colchicine (%) | 71.2 | 70.7 |
| NSAID (%) | 44.3 | 44.5 |
| Opioids (%) | 48.1 | 48.1 |
| Cumulative prednisone-equivalent dose, milligrams (mean, SD) | 225.9 (699.6) | 217.6 (699.2) |
| Oral steroids (%) | 35.0 | 34.6 |
| Recent oral steroid use (%) | 23.8 | 23.4 |
|
| ||
| Other Medications | ||
|
| ||
| ACEI/ARB (%) | 61.5 | 60.8 |
| Beta blockers (%) | 43.4 | 43.0 |
| Calcium channel blockers (%) | 36.7 | 36.5 |
| Diuretics (%) | 68.4 | 68.3 |
| Nitrates (%) | 14.3 | 14.2 |
| Noninsulin anti-diabetic drugs (%) | 26.4 | 25.8 |
| Insulin (%) | 9.7 | 9.6 |
| Anticoagulants (%) | 19.1 | 19.0 |
| Antiplatelets (%) | 14.5 | 14.7 |
| Phosphate binders (%) | 0.2 | 0.2 |
| Other lipid lowering drugs (%) | 13.5 | 13.5 |
| Statins (%) | 53.6 | 53.6 |
|
| ||
| Health Care Utilization | ||
|
| ||
| No. of ED visits (mean, SD) | 1.0 (1.6) | 1.0 (1.8) |
| No. of outpatient visits (mean, SD) | 13.1 (10.3) | 13.1 (10.2) |
| No. of prescription drugs (mean, SD) | 13.8 (6.8) | 13.7 (6.5) |
| Hospitalization (%) | 28.8 | 29.1 |
| No. of cardiology visits (mean, SD) | 1.2 (2.6) | 1.2 (2.5) |
| No. of rheumatology visits (mean, SD) | 0.2 (1.1) | 0.2 (0.9) |
| C-reactive protein test ordered (%) | 15.2 | 15.2 |
| Electrocardiogram ordered (%) | 57.9 | 58.1 |
| Echocardiogram ordered (%) | 1.5 | 1.4 |
| Cardiac stress test ordered (%) | 14.6 | 14.7 |
| HbA1c ordered (%) | 43.9 | 43.8 |
| Cholesterol test ordered (%) | 74.7 | 74.7 |
| Uric acid test ordered (%) | 64.5 | 63.5 |
| Serum creatinine test ordered (%) | 93.3 | 93.5 |
Recent event was defined as within 60 days from the index date.
SD=standard deviation, NSAID= non-steroidal antiinflammatory drug, ACEI=Angiotensin converting enzyme inhibitor, ARB= angiotensin II receptor blocker, ED=emergency department
Statistical Analysis
We assessed patients’ baseline characteristics by cross-tabulation. To control for over 65 potential baseline confounders simultaneously, we used propensity score (PS) matching.(29) We defined the PS as the predicted probability of a patient starting probenecid versus allopurinol given patient characteristics at baseline. The PS was estimated using multivariable logistic regression that included all the covariates listed in Table 1 and the index year (c-statistic=0.7). Probenecid and allopurinol initiators were matched with a fixed ratio of 1:3 implementing the nearest neighbor matching with a matching caliper of 0.05 on the PS scale.(30,31) The variables with the standardized differences <10% between the two groups were considered well-balanced after PS matching.(31,32)
For the primary as-treated analysis, we calculated the incidence rates (IR) with 95% confidence intervals (CI) for the primary and secondary outcomes in the PS matched cohort. For the HF exacerbation outcome, only patients with history of HF at baseline were analyzed. For the new onset HF outcome, patients with no baseline HF were examined. Cumulative incidence plots between treatment groups were compared. Cox proportional hazards regression models estimated the hazard ratio (HR) with 95% CI for the primary and secondary outcomes in the probenecid group versus the allopurinol group.
We evaluated whether the initial daily dose of probenecid or allopurinol was titrated up during followup time as recommended by the American College of Rheumatology guidelines for management of gout.(4) The daily dose for a given prescription was calculated as the number of pills or tablets prescribed multiplied by the strengths of the pills or tablets divided by the days’ supply. To estimate patients’ adherence to treatment with probenecid or allopurinol, we calculated a proportion of days covered as the number of days covered by dispensed prescriptions*100 divided by the total number of days of followup for each patient. For the secondary ITT analysis, IR and HR were calculated for the primary outcome.
Prespecified subgroup analyses stratified by the baseline CVD status were performed. In addition, because CKD is considered one of the most important confounders by indication between the two drugs, we conducted a subgroup analysis in the 1:3 PS-matched cohort of patients with no diagnosis of CKD at baseline.
To minimize potential bias due to differences in the follow-up time between the PS matched groups, we also conducted a sensitivity analysis where Cox proportional hazards models stratified on PS-matching sets (i.e., 1 probenecid initiators matched with 3 allopurinol initiators) estimated the hazard ratio (HR) of the primary or secondary outcome associated with initiation of probenecid or allopurinol.(33,34) In addition, we performed a sensitivity analysis after excluding probenecid initiators who had prior use of allopurinol and allopurinol initiators who had prior use of probenecid to make all the study patients naïve to both drugs on the index date.
Proportional hazards assumption was tested by including the interaction term between exposure and follow-up time and was not violated in any of the models for primary analysis (35). All analyses were conducted in SAS 9.4.
RESULTS
Cohort Selection
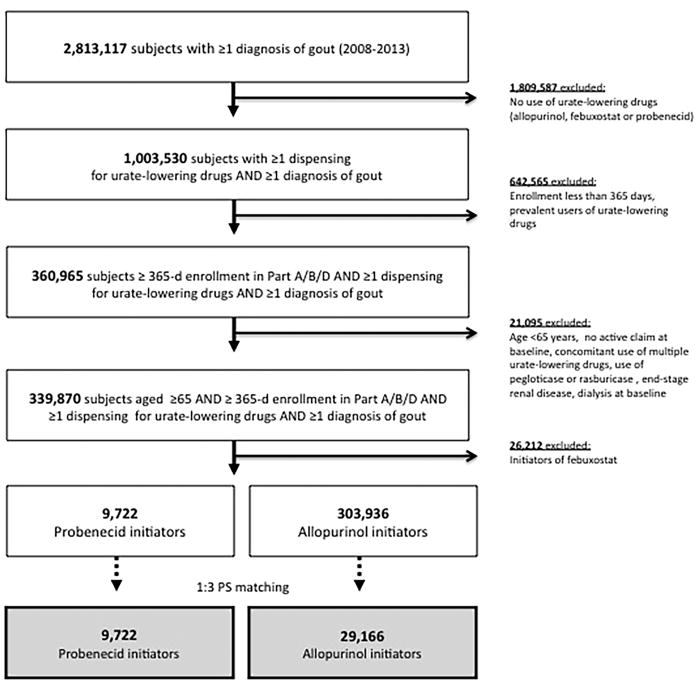
We identified more than 2.8 million patients with ≥1 diagnosis of gout enrolled in Medicare Parts A, B, and D during the study period. After applying inclusion and exclusion criteria (Figure 2), we identified 339,870 patients aged ≥65 years with ≥1 diagnosis of gout who initiated a urate-lowering drug (i.e., allopurinol, febuxostat or probenecid). Of those, 9,722 initiated probenecid and 303,936 started allopurinol. After PS matching with a 1:3 fixed ratio, 100% of probenecid initiators and 9.6% of all allopurinol initiators were included for the study cohort.
Figure 2. Study cohort selection flow.
Among over 2.8 million Medicare enrollees with ≥1 diagnosis of gout between 2008 and 2013, we selected 9,722 probenecid and 303,936 allopurinol initiators. The final study cohort included 9,722 probenecid initiators propensity score-matched on 29,166 allopurinol initiators with a fixed ratio of 1:3.
Patient Characteristics
Before PS matching, probenecid initiators were less likely to have CKD (28.4% vs. 39.0%) and recent HF (3.2% vs. 5.6%) and have their serum uric acid level tested (64.5% vs. 74.8%) compared to allopurinol initiators (Supplemental Table 1).
Table 1 shows baseline characteristics of the two groups matched on PS. The mean (SD) age of the PS-matched groups was 76 (7) years. 54% were male and 79% were white. At baseline, 28% in both groups had CVD, 27% HF, 28% CKD and 46% diabetes. Use of gout-related medications including colchicine (71%), oral steroids (35%), NSAID (44%), and opioids (48%) was common in both groups. All the baseline covariates were well-balanced between the PS-matched groups with a standardized difference <10%.(31) Prior to the index date, 1% of the allopurinol group used probenecid and 14% of the probenecid group took allopurinol. The majority of colchicine use was noted at the time of treatment initiation with probenecid or allopurinol as 63.9% of probenecid initiators and 42.1% of allopurinol initiators had a dispensing for colchicine at the index date.
Patterns of Probenecid or Allopurinol Treatment
In the PS matched cohort, the median (IQR) initial daily dose was 1,000 mg (500–1,000 mg) for the probenecid group and 176 mg (100–300 mg) for the allopurinol group. 44.0% of probenecid initiators were started on a daily dose lower than 1000 mg and 62.6% of allopurinol initiators were started on a daily dose lower than 300 mg. During the followup, 9.2% of probenecid initiators and 22.3% of allopurinol initiators had the daily dose increased. There was a large difference in the adherence between the two groups. The median (IQR) proportion of days covered for 180 days was 39.8% (17.1–88.4%) with probenecid and 87.3% (50.3–100%) with allopurinol. The median (IQR) proportion of days covered for 365 days was 26.1% (8.5–80.9%) with probenecid and 82.2% (34.1–99.5%) with allopurinol. Nearly a third of patients who discontinued probenecid were switched to allopurinol or febuxostat after their followup time ended.
The median (IQR) followup time for the primary as-treated analysis was 118 days (61–469 days) among probenecid initiators and 358 days (103–854 days) among allopurinol initiators. However, given the large size of the study cohort, 2,890 (29.7%) probenecid and 14,468 (49.6%) allopurinol initiators had a followup time over 1 year and 1,534 (15.8%) probenecid and 8,817 (30.2%) allopurinol initiators were followed up for >2 years.
Risk of Cardiovascular Events
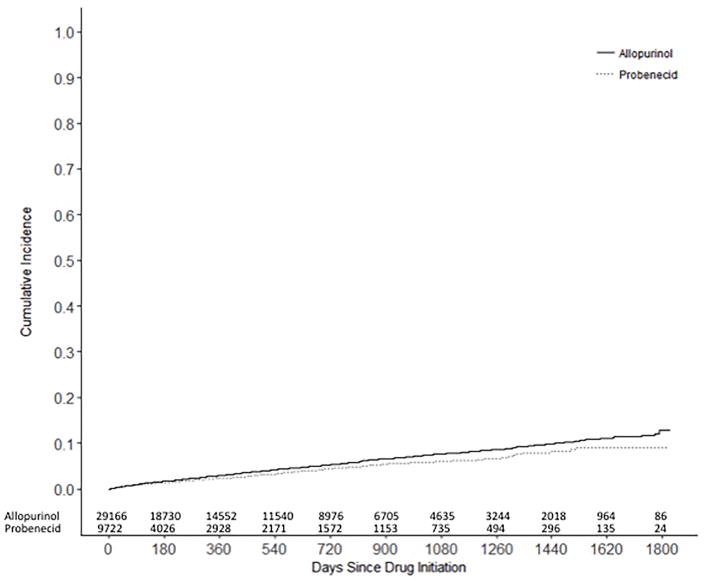
During a total of 50,427 person-years of followup in the PS-matched cohort for the as-treated analysis, 1,385 patients – 203 probenecid and 1,182 allopurinol initiators - developed the primary composite endpoint of hospitalization for MI or stroke (Table 2). The IR of the composite endpoint of MI or stroke per 100 person-years was 2.36 (95% CI 2.05–2.71) among probenecid initiators and 2.83 (95% CI 2.67–2.99) among allopurinol initiators. The HR of the primary outcome was 0.80 (95% CI 0.69–0.93) in the probenecid group compared with the allopurinol group. Cumulative incidence plots showed consistent results with the log rank test p-value of 0.003 (Figure 3).
Table 2.
Risk of cardiovascular events in probenecid initiators versus allopurinol: 1:3 propensity score-matched analysis
| Probenecid (n=9,722) | Allopurinol (N=29,166) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Event (n) | Person-years | IR * (95% CI) | HR (95% CI) | Event (n) | Person-years | IR * (95% CI) | HR (95% CI) | |
| As-treated analysis | ||||||||
|
| ||||||||
| Primary outcome | ||||||||
| MI or stroke | 203 | 8,611 | 2.36 (2.05–2.71) | 0.80 (0.69–0.93) | 1,182 | 41,816 | 2.83 (2.67–2.99) | Ref |
| Secondary outcomes | ||||||||
| MI | 121 | 8,670 | 1.40 (1.17–1.67) | 0.81 (0.67–0.99) | 693 | 42,142 | 1.64 (1.53–1.77) | Ref |
| Stroke | 83 | 8,695 | 0.96 (0.77–1.18) | 0.72 (0.57–0.90) | 539 | 42,367 | 1.27 (1.17–1.38) | Ref |
| Coronary revascularization | 213 | 8,550 | 2.49 (2.18–2.85) | 0.94 (0.81–1.09) | 1,033 | 41,564 | 2.49 (2.34–2.64) | Ref |
| All-cause deaths | 255 | 8,753 | 2.91 (2.58–3.29) | 0.87 (0.76–1.00) | 1,387 | 42,719 | 3.25 (3.08–3.42) | Ref |
|
| ||||||||
| ITT365-d analysis | ||||||||
|
| ||||||||
| Primary outcome | ||||||||
| MI or stroke | 224 | 7,905 | 2.83 (2.49–3.23) | 0.86 (0.74–1.00) | 767 | 23,333 | 3.29 (3.06–3.53) | Ref |
| Secondary outcomes | ||||||||
| MI | 126 | 7,932 | 1.59 (1.33–1.89) | 0.85 (0.70–1.04) | 438 | 23,413 | 1.87 (1.70–2.06) | Ref |
| Stroke | 104 | 7,944 | 1.31 (1.08–1.59) | 0.86 (0.69–1.07) | 358 | 23,475 | 1.53 (1.38–1.69) | Ref |
| Coronary revascularization | 235 | 7,881 | 2.98 (2.62–3.39) | 1.00 (0.86–1.15) | 698 | 23,291 | 3.00 (2.78–3.23) | Ref |
| All-cause deaths | 314 | 7,971 | 3.94 (3.53–4.40) | 0.92 (0.81–1.04) | 1,014 | 23,561 | 4.30 (4.05–4.58) | Ref |
IR is per 100 person-years
IR= incidence rate, HR=hazard ratio, CI=confidence interval, MI= myocardial infarction, ITT 365-d=intention-to-treat analysis up to the 365th day of followup
Central Illustration – Figure 3. Cumulative incidence curves of the composite endpoint of MI or stroke.
Among the propensity score matched cohort, the cumulative incidences of the composite endpoint of MI or stroke were compared between probenecid and allopurinol initiators with the log-rank test (p=0.003).
In the as-treated analysis, the IRs of the secondary outcomes were also lower in the probenecid group versus the allopurinol group with the HR of 0.81 for MI (95% CI 0.67–0.99) and the HR of 0.72 (95% CI 0.57–0.90) for stroke (Table 2). There was no difference in the IR of coronary revascularization between the two groups (HR 0.94, 95% CI 0.81–1.09). Among the patients with no baseline HF (Table 3), the IR of hospitalization for new onset HF was similar between the two groups (HR 0.95, 95% CI 0.84–1.08). However, the IR of hospitalization for HF exacerbation in patients with baseline HF was lower in the probenecid group than the allopurinol group (HR 0.91, 95% CI 0.83–0.997). The rate of all-cause deaths (Table 2) was also lower among the probenecid group versus the allopurinol group with the HR of 0.87 (95% CI 0.76–0.997).
Table 3.
Risk of heart failure (HF) in probenecid initiators versus allopurinol: 1:3 propensity score-matched analysis
| Probenecid | Allopurinol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Outcome | N | Event (n) | Person-years | IR * (95% CI) | HR (95% CI) | N | Event (n) | Person-years | IR * (95% CI) | HR (95% CI) |
| As-treated analysis | ||||||||||
|
| ||||||||||
| New-onset HF a | 7,101 | 289 | 6,665 | 4.34 (3.86–4.87) | 0.95 (0.84–1.08) | 21,303 | 1,421 | 32,197 | 4.41 (4.19–4.65) | Ref |
| HF exacerbation b | 2,621 | 590 | 1,600 | 36.88 (34.02–39.98) | 0.91 (0.83–0.997) | 7,863 | 2,627 | 7,090 | 37.05 (35.66–38.50) | Ref |
|
| ||||||||||
| ITT365-d analysis | ||||||||||
|
| ||||||||||
| New-onset HF a | 7,101 | 279 | 5,962 | 4.68 (4.16–5.26) | 0.93 (0.82–1.07) | 21,303 | 892 | 17,779 | 5.02 (4.70–5.36) | Ref |
| HF exacerbation b | 2,621 | 709 | 1,678 | 42.26 (39.26–45.49) | 0.91 (0.84–0.995) | 7,863 | 2,210 | 4,746 | 46.57 (44.67–48.55) | Ref |
IR is per 100 person-years
among the subgroup of patients with no baseline history of HF
among the subgroup of patients with baseline history of HF, only counting the 1st exacerbation after the index date
IR= incidence rate, HR=hazard ratio, CI=confidence interval, MI= myocardial infarction, ITT 365-d=intention-to-treat analysis up to the 365th day of followup
The secondary ITT analysis up to 365 days of followup for the primary and secondary outcomes also yielded similar results (Tables 2 and 3).
Subgroup and Sensitivity Analyses
For the subgroup in the linked Medicare-EMR database, we identified 5,973 patients aged ≥65 years with ≥1 diagnosis of gout who initiated a urate-lowering drug. Of those, 1,969 (33%) had ≥1 measurement for serum uric acid and creatinine level prior to the index date. After applying other exclusion criteria, there were only 34 probenecid and 1,847 allopurinol initiators. In this subgroup, the mean (SD) serum uric acid level was 7.2 (2.1) mg/dl in the probenecid and 7.8 (2.3) mg/dl in the allopurinol group. The mean (SD) serum creatinine level was 1.2 (0.5) mg/dl in the probenecid and 1.3 (0.5) mg/dl in the allopurinol group. Due to the small number of probenecid initiators, none developed MI or stroke during followup.
In the analysis stratified by the baseline CVD status (Table 4), the HR for the composite endpoint of MI or stroke was 0.82 (95% CI 0.67–0.99) among probenecid initiators with baseline CVD (n=2,758) versus allopurinol initiators with baseline CVD (n=8,274) matched on PS. Among patients with no baseline CVD, the HR for the composite endpoint of MI or stroke was 0.77 (95% CI 0.61–0.98) associated with probenecid (n=6,964) versus allopurinol (n=20,892). In the subgroup of patients with no baseline CKD (Table 4), the HR for the composite endpoint of MI or stroke was 0.84 (95% CI 0.70–1.01) associated with probenecid (n=6,962) versus allopurinol (n=20,886).
Table 4.
Subgroup analyses for risk of the composite endpoint of MI or stroke: 1:3 propensity score-matched analysis
| Probenecid | Allopurinol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Outcome | N | Event (n) | Person-years | IR * (95% CI) | HR (95% CI) | N | Event (n) | Person-years | IR * (95% CI) | HR (95% CI) |
| As treated analysis | ||||||||||
|
| ||||||||||
| With baseline CVD | 2,758 | 82 | 2,084 | 3.94 (3.17–4.89) | 0.82 (0.67–0.99) | 8,274 | 493 | 10,412 | 4.74 (4.34–5.17) | Ref |
| Without baseline CVD | 6,964 | 121 | 6,527 | 1.85 (1.55–2.22) | 0.77 (0.61–0.98) | 20,982 | 691 | 31,356 | 2.20 (2.05–2.38) | Ref |
| Without baseline CKD | 6,962 | 140 | 6,578 | 2.13 (1.80–2.51) | 0.84 (0.70–1.01) | 20,886 | 767 | 31,475 | 2.44 (2.27–2.62) | Ref |
IR is per 100 person-years
In the sensitivity analysis where we used Cox proportional hazards models stratified on PS-matching sets, the HR of the primary outcome was similar to the main result with a slightly wider confidence interval (0.81, 95% CI 0.68–0.97) in the probenecid group compared with the allopurinol group. The sensitivity analyses for the secondary outcomes also showed consistent results (data not shown). In addition, we found similar results from the sensitivity analysis where patients were required to be naïve to both probenecid and allopurinol at baseline with the HR of 0.78 (95% CI 0.67–0.92) for the primary outcome in the probenecid group (n=8,351) versus the allopurinol group (n=25,053).
DISCUSSION
This large observational study including Medicare-enrolled elderly patients with gout found an association between probenecid use and a lower risk of CV events compared with allopurinol. While both probenecid and allopurinol have been available for a long time for the management of gout, to the best of our knowledge this is the first study that has evaluated the CV effect of probenecid directly compared with allopurinol in a population-representative cohort of gout patients. Inflammation plays a critical role in the pathogenesis of both gout and CVD. IL-1β, a proinflammatory cytokine primarily produced by monocytes and macrophages, is the main cytokine involved in gout.(3) Monocytes and macrophages are also important cells in the development of atherosclerotic plaque, and accumulating data support the role of IL-1β in atherothrombosis.(18,36) Probenecid lowers serum uric acid by blocking reuptake of uric acid in the kidneys and may exhibit anti-inflammatory effect through its inhibition of pannexin 1 channels, thereby reducing IL-1β. Prior studies have hypothesized potential beneficial effects of IL-1β inhibition on CVD,(37) and canakinumab, an IL-1β inhibitor, reduced the risk of major adverse CV events in patients with prior heart attack and inflammatory atherosclerosis in the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) trial. Nevertheless, further investigation on the pathophysiologic mechanisms by which probenecid may modulate the risk of CV events is needed. Probenecid is also a potent and selective agonist of TRPV2 channels;(19,20) several studies support its inotropic effect.(19,21–23) In our study, probenecid was associated with a 20% lower risk of hospitalization for MI or stroke compared to allopurinol, whereas the magnitude of the association between probenecid and hospitalization for HF exacerbation was smaller (9%). We also did not observe any link between probenecid and new onset HF. Probenecid is known to increase the concentration of some drugs such as antibiotics and NSAIDs when used concomitantly, but drug interactions between probenecid and statins or other CV drugs are not reported.
While our study generated important and interesting findings, our results should be interpreted with caution. First, as noted in any non-randomized observational studies, this study is subject to residual or unmeasured confounding and cannot prove the causality. In other words, probenecid initiators might be different from allopurinol initiators with regard to severity of gout or CKD, serum uric acid levels, body mass index, smoking, or severity of underlying comorbidities such as CVD, hypertension, diabetes, or HF as such clinical or laboratory data were not available in the Medicare data. However, we included several proxies of gout severity such as use of baseline steroids, NSAIDs, and colchicine as well as proxies of CVD severity such as use of diuretics, nitrates and statins and physician orders of electrocardiogram, echocardiogram, and cardiac stress test in the PS model. We also conducted subgroup analyses by CKD and CVD at baseline and found similar results. While the number of probenecid initiators was small in the subgroup of patients in the linked Medicare-EMR database, we confirmed that there was no substantial difference in the mean serum creatinine level between probenecid (1.2 mg/dL) and allopurinol (1.3 mg/dL) initiators. Nonetheless, in order to completely avoid confounding, we would need a randomized controlled trial to determine the potential benefit of probenecid. Second, potential misclassification of outcomes is possible as we mainly relied on billing diagnosis and procedure codes. Furthermore, because we defined new onset HF as well as HF exacerbation based on hospital discharge diagnosis, this study did not capture patients who were managed in an outpatient setting for mild HF in the secondary outcome analysis. Third, this study may not be able to answer the long-term CV effect of probenecid or allopurinol as the median duration of active treatment with either drug was less than 1 year. However, over 10,000 patients remained in the study for longer than 2 years. Fourth, while our study likely reflects current clinical practice for management of gout in the elderly, the doses for probenecid and allopurinol were generally lower with the starting daily dose for allopurinol in over 60% of allopurinol initiators lower than 300 mg. Furthermore, <25% of allopurinol initiators had the daily dose increased during followup. Fifth, there was a difference in the adherence to probenecid versus allopurinol. Although it is possible that the observed difference in the medication adherence between the two groups may lead to a biased estimate, we found consistent results in the ITT analysis up to 365 days of followup as well as the sensitivity analysis with Cox regression stratifying on PS matching sets. This analysis specifically compared each probenecid initiator to the three PS-matched allopurinol initiators for the same duration of followup.(33,34)
Given the limitations of observational studies of drugs including confounding and suboptimal adherence to treatment, future studies are needed to further determine the effect of probenecid on cardiovascular risk. Prospective interventional studies that evaluate the effect of probenecid on intermediate endpoints of CVD may be helpful to delineate the potential mechanism by which probenecid modulate CV risks. In our study cohort with the mean age of 76 years, the IR of hospitalization for MI or stroke was greater than 2 per 100 person years in both drug groups and the rate difference in the PS-matched cohort was 0.47 per 100 person-years. Based on the absolute risk difference that we observed, the number of gout patients needed to treat (NNT) with probenecid versus allopurinol to prevent one additional hospitalization for MI or stroke would be 213. If a similar magnitude of the effect noted in our study is confirmed in a trial, the NNT will not be very large given the high prevalence of CV risk factors in the typical older gout population.
CONCLUSIONS
In this large cohort study of 38,888 elderly gout patients enrolled in Medicare, use of probenecid appears to be associated with a modestly decreased risk of CV events including MI, stroke and HF exacerbation compared to allopurinol. Given the high CV morbidity and mortality in gout patients, potential positive effects of probenecid should be further examined.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
Gout is the most common inflammatory arthritis and associated with an increased risk of cardiovascular disease. Urate-lowering drugs, probenecid and allopurinol, may affect the risk of cardiovascular disease. In this study, use of probenecid appears to be associated with a modestly decreased risk of cardiovascular events including myocardial infarction, stroke and heart failure exacerbation compared to allopurinol.
Translational Outlook
Future research should study the pathophysiologic mechanisms by which probenecid may modulate the risk of cardiovascular events. In addition, the effect of probenecid on cardiovascular risk needs to be further determined, ideally in a randomized controlled trial setting.
ABBREVIATIONS
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- HF
heart failure
- HR
hazard ratio
- IR
incidence rate
- ITT
intention-to-treat
- MI
myocardial infarction
- NSAID
nonsteroidal anti-inflammatory drug
- PS
propensity score
- TRPV2
transient receptor potential vanilloid 2
Footnotes
Potential Conflicts of Interest: Dr. Kim has received research grants to the Brigham and Women’s Hospital from Roche/Genentech, Pfizer, Bristol-Myers Squibb, Merck, and AstraZeneca for unrelated studies. Dr. Desai has received research grants to the Brigham and Women’s Hospital from Merck. Drs. Neogi, Kang, Liu and Zhang have nothing to disclose. Dr. Solomon has received research grants to the Brigham and Women’s Hospital from Lilly, Pfizer, AstraZeneca, Genentech, Amgen and CORRONA. Dr. Solomon serves in an unpaid role on a trial sponsored by Pfizer unrelated to the current study.
Contributors: All authors made substantial contributions to the concept and design, or analysis and interpretation of data, and to the drafting of the manuscript or revising it critically for important intellectual content. SC Kim drafted the paper and all authors provided final approval of the manuscript.
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: Dr. Kim has received research grants to the Brigham and Women’s Hospital from Roche/Genentech, Pfizer, Bristol-Myers Squibb, Merck, and AstraZeneca for unrelated studies. Dr. Desai has received research grants to the Brigham and Women’s Hospital from Merck. Drs. Neogi, Kang, Liu and Zhang have nothing to disclose. Dr. Solomon has received research grants to the Brigham and Women’s Hospital from Lilly, Pfizer, AstraZeneca, Genentech, Amgen and CORRONA. Dr. Solomon serves in an unpaid role on a trial sponsored by Pfizer unrelated to the current study.
Disclosures: This study received no specific funding. Dr. Kim is partially supported by NIH R21 AR069271. Dr. Neogi’s effort was supported by NIH K24 AR070892. Dr. Solomon’s effort was supported by NIH K24 AR055989.
Competing interests: No specific funding was available for this study. SC Kim is partially supported by NIH R21-AR069271. Dr. Neogi’s effort was supported by NIH K24 AR070892. Dr. Solomon’s effort was supported by NIH K24 AR055989.
References
- 1.Roddy E, Choi HK. Epidemiology of gout. Rheumatic diseases clinics of North America. 2014;40:155–75. doi: 10.1016/j.rdc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neogi T. Clinical practice. Gout The New England journal of medicine. 2011;364:443–52. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 4.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis care & research. 2012;64:1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moller JV. The tubular site of urate transport in the rabbit kidney, and the effect of probenecid on urate secretion. Acta pharmacologica et toxicologica. 1965;23:329–36. [PubMed] [Google Scholar]
- 6.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Annals of the rheumatic diseases. 2010;69:1162–4. doi: 10.1136/ard.2009.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seminog OO, Goldacre MJ. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology (Oxford, England) 2013;52:2251–9. doi: 10.1093/rheumatology/ket293. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. The American journal of medicine. 2012;125:679–687e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA : the journal of the American Medical Association. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez-Ramirez G, Perez-Mendez O, Lopez-Osorio C, Kuri-Alfaro J, Espinola-Zavaleta N. Effect of the treatment with allopurinol on the endothelial function in patients with hyperuricemia. Endocrine research. 2012;37:1–6. doi: 10.3109/07435800.2011.566235. [DOI] [PubMed] [Google Scholar]
- 11.Roncal CA, Reungjui S, Sanchez-Lozada LG, et al. Combination of captopril and allopurinol retards fructose-induced metabolic syndrome. American journal of nephrology. 2009;30:399–404. doi: 10.1159/000235731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of Xanthine Oxidase Inhibition in Hyperuricemic Heart Failure Patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) Study. Circulation. 2015;131:1763–71. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SC, Schneeweiss S, Choudhry N, Liu J, Glynn RJ, Solomon DH. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. The American journal of medicine. 2015;128:653e7–653e16. doi: 10.1016/j.amjmed.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen KS, Pottegard A, Lindegaard HM, Hallas J. Effect of Allopurinol on Cardiovascular Outcomes in Hyperuricemic Patients: A Cohort Study. The American journal of medicine. 2016;129:299–306. e2. doi: 10.1016/j.amjmed.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Singh JA, Ramachandaran R, Yu S, Curtis JR. Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC cardiovascular disorders. 2017;17:76. doi: 10.1186/s12872-017-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roch-Ramel F, Guisan B. Renal Transport of Urate in Humans. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 1999;14:80–84. doi: 10.1152/physiologyonline.1999.14.2.80. [DOI] [PubMed] [Google Scholar]
- 17.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. American journal of physiology Cell physiology. 2008;295:C761–7. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–9. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 19.Koch SE, Tranter M, Robbins N, et al. Probenecid as a noninjurious positive inotrope in an ischemic heart disease murine model. Journal of cardiovascular pharmacology and therapeutics. 2013;18:280–9. doi: 10.1177/1074248412469299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neuroscience letters. 2007;425:120–5. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert M, Robbins N, Conway G, Effat M, Rubinstein J. Probenecid Improves Cardiac Function in Patients With Heart Failure. Interim Analysis of the Re-Prosper HF Trial. Journal of Cardiac Failure. 21:S47. [Google Scholar]
- 22.Robbins N, Koch SE, Rubinstein J. Targeting TRPV1 and TRPV2 for potential therapeutic interventions in cardiovascular disease. Translational research : the journal of laboratory and clinical medicine. 2013;161:469–76. doi: 10.1016/j.trsl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein J, Lasko VM, Koch SE, et al. Novel role of transient receptor potential vanilloid 2 in the regulation of cardiac performance. American journal of physiology Heart and circulatory physiology. 2014;306:H574–84. doi: 10.1152/ajpheart.00854.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennessy S, Freeman C, Cunningham F. US Government Claims Databses. In: Strom B, Kimmel S, Hennessy S, editors. Pharmacoepidemiology. 5. Philadelphia PA: Wiley-Blackwell; 2012. pp. 209–23. [Google Scholar]
- 25.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiology and drug safety. 2012;21(Suppl 1):100–28. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Kumamaru H, Judd SE, Curtis JR, et al. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circulation Cardiovascular quality and outcomes. 2014;7:611–9. doi: 10.1161/CIRCOUTCOMES.113.000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin D. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 30.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiology and drug safety. 2012;21(Suppl 2):69–80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical statistics. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Statistics in medicine. 2014;33:1685–99. doi: 10.1002/sim.6058. [DOI] [PubMed] [Google Scholar]
- 33.Cummings P, McKnight B, Greenland S. Matched cohort methods for injury research. Epidemiologic reviews. 2003;25:43–50. doi: 10.1093/epirev/mxg002. [DOI] [PubMed] [Google Scholar]
- 34.Walker AM, Jick H, Hunter JR, et al. Vasectomy and non-fatal myocardial infarction. Lancet. 1981;1:13–5. doi: 10.1016/s0140-6736(81)90116-1. [DOI] [PubMed] [Google Scholar]
- 35.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. 3. New York, NY: Springer New York; 2012. Evaluating the Proportional Hazards Assumption; pp. 161–200. [Google Scholar]
- 36.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) American heart journal. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.