Abstract
Insulin-like growth factor 1 (IGF-1) has been associated with osteoporosis, cardiovascular disease, cancer, neurodegenerative diseases, and mortality in middle and older aged adults. Cross-sectionally, IGF-1 decreases with age and levels of IGF-1 are markedly different between individuals. However, little is known about intra-individual trajectories of IGF-1. We examined baseline and serial measures of plasma total IGF-1, IGF binding protein (IGFBP)-3, and their ratio, which is a proxy for bioavailable IGF-1, among 1,618 adults, aged 50–95, enrolled in the Mayo Clinic Study of Aging. At baseline, IGF-1 and IGFBP-3 were strongly correlated (r = 0.62, p < 0.001). Total IGF-1 and IGFBP-3 decreased across age, while the ratio of IGF-1/IGFBP-3 increased across age. This pattern was consistent across ages at baseline and intra-individually over an average 2.3 years follow-up (range = 10 months-5.6 years). In age-adjusted linear regression models, baseline levels of total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 varied by participant characteristics (sex, BMI, gait speed), medical comorbidities (Charlson comorbidity index score, hypertension, diabetes, and cardiovascular disease), and hormone replacement therapy use in women. High interclass correlation coefficients (ICCs) suggest little intra-individual variability in levels of total IGF-1 (ICC=0.84), IGFBP-3 (ICC=0.88), and IGF-1/IGFBP-3 (ICC=0.81) over time. In mixed effects models that specified age as a time scale, men showed greater decreases in total IGF-1 and IGFBP-3 with age, while more comorbidities and decreasing gait speed were associated with increasing IGFBP-3. In sex-stratified models, trajectories of total IGF-1, IGFBP-3, and IGF-1/IGFBP-3, as a function of participant demographics, health characteristics, and medical conditions, differed between men and women. These results suggest that change in levels of plasma total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 are associated with demographics, health characteristics, and medical conditions, and that the trajectories of change differ by sex. Future research should consider how IGF-1 and IGFBP-3 might be useful in research or clinic, paying particular attention to how sex may impact levels as a function of demographics, health characteristics, and medical conditions.
Keywords: Insulin-like growth factor 1, Insulin-like growth factor binding protein 3, Age
1. Introduction
Insulin-like growth factor 1 (IGF-1) regulates growth hormone, and is critical during growth and development in childhood (Ghigo et al., 2000). In older adults, IGF-1 levels are associated with multiple medical conditions, including cardiovascular disease, diabetes, osteoporosis, cancer, and neurodegenerative diseases (Yang et al., 2005). Cross-sectionally, IGF-1 levels vary substantially among individuals of the same age (van Dam and Aleman, 2004), but in general decline with age (Ashpole et al., 2015). In the periphery, approximately 99% of circulating IGF-1 is bound to IGF binding proteins (IGFBPs), with more than 80% bound to IGFBP-3. The remaining 1% of circulating IGF-1 remains free, in a biologically available form (Favelyukis et al., 2001; Rajaram et al., 1997). IGF-1 bound to IGFBP-3 creates a stable complex that cannot cross the endothelium (Wacharasindhu et al., 2002). The ratio of IGF-1 to IGFBP-3 is a proxy for bioavailable IGF-1 (Rajaram et al., 1997). The majority of circulating IGF-1 is produced by the liver, but it is also generated by peripheral cells and in the brain (Favelyukis et al., 2001). Unbound IGF-1 can cross the blood brain barrier (Anlar et al., 1999; Coculescu, 1999). Within the brain, IGF-1 is associated with neuron proliferation and differentiation, and myelination.
Recently, in the Cardiovascular Health Study (CHS), it was shown that among participants aged 60–100, total plasma IGF-1 levels, measured a maximum of five times over a span of 18 years, remained stable at younger ages and started to decline slightly in the eighth decade (Newman et al., 2016). This was in contrast to related plasma biomarkers (adiponectin, interleukin-6 (IL-6), and cystatin-C), which showed much greater longitudinal intra-individual variability with age. These findings indicate that total IGF-1 levels may not be good time-varying, or state, biomarkers of health status and comorbidities among older adults, particularly when compared to inflammatory markers such as IL-6. However, there is little research on the long-term stability of IGFBP-3 or the IGF-1/IGFBP-3 ratio. In this analysis, we expanded upon the results of the CHS study and examined the intra-individual variations in total IGF-1, IGFBP-3, and the ratio of the two among 1,618 adults aged 50–95 at baseline with a maximum of five serial measures. We determined whether these markers changed with age or were affected by demographic variables, health characteristics, and medical conditions over time.
2. Methods
2.1 Participants
The Mayo Clinic Study of Aging (MCSA) is a prospective population-based study aimed at characterizing the incidence and prevalence of mild cognitive impairment (MCI) in Olmsted County, Minnesota (Roberts et al., 2008). In 2004, Olmsted County residents between the ages of 70 and 89 were identified for recruitment using an age- and sex-stratified random sampling design to ensure that men and women were equally represented in each 10-year age strata. In 2008, the study was extended to include those aged 50 and older. This study included 1,618 participants, 1,387 of whom had at least two IGF measures. Additionally, there were 37 participants who had five serial IGF measures, and a sub-analysis was performed in these individuals. The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
2.2 Participant assessment
MCSA visits included a physician examination, an interview by a study coordinator, and neuropsychological testing (Roberts et al., 2008). All MCSA participant visits are conducted approximately every 15 months. Cognitive test performance on nine tests in four domains (memory, executive function, language, and visual-spatial) and a global average of the four was compared with the age-adjusted scores of clinically normal individuals previously obtained using Mayo’s Older American Normative Studies (Ivnik et al., 1992). For the purposes of these analyses, cognitive impairment was defined as a score of < −1.0 SD below age-specific norms. Additional details on participant cognitive assessment were published by Roberts and colleagues (2008).
Demographic variables (e.g., education) were collected by self-report during the in-clinic exam. Participants’ height (cm) and weight (kg) were also measured during the in-clinic exam. These measures were used to calculate body mass index (BMI) (kg/m2). Self-reported medication use, including hormone (estrogen, progesterone) replacement therapy (HRT), was collected in-clinic and corroborated using information abstracted from the medical records of the record-linkage system. Medical conditions and Charlson comorbidity index (CCI) score (Charlson et al., 1987) were abstracted from the Rochester Epidemiology Project medical records linkage system. Depressive symptoms were assessed using the Beck Depression Inventory (BDI) (Beck et al., 1988); participants with a score of ≥ 13 were considered to have depression. Participants’ blood sample collected in-clinic was used to determine APOE genotype.
2.3 Laboratory analyses of IGF-1 and IGFBP-3
Participants’ blood was collected in the fasting state at the in-clinic exam, centrifuged, aliquoted, and stored at −80°C. Serum total IGF-1 and IGFBP-3 levels were measured at the Mayo Clinic Immunochemical Core Laboratory. Total IGF-1 was a solid-phase, chemiluminescent immunometric assay on the Siemens Immulite 2000 automated immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL 60015). Intra-assay coefficients of variation (CV’s) were 3.5% and 4.2% at 70 and 236 ng/mL, respectively. Inter-assay CV’s were 4.9%, 3.5% and 5.0%, at 37, 68 and 225 ng/mL respectively. IGFBP-3 was a solid-phase, chemiluminescent immunometric assay on the Siemens Immulite 2000 automated immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL 60015). Intra-assay CV’s were 4.2% and 2.5% at 1.0 and 4.4 ug/mL respectively. Inter-assay CV’s were 4.0% and 3.9% at 1.0 and 4.3 ug/mL, respectively. We calculated the ratio of total IGF-1 to IGFBP-3 for each participant as a proxy of free (bioavailable) IGF-1.
2.4 Statistical analyses
Spearman correlations were calculated between total IGF-1 and IGFBP-3 levels at baseline. Wilcoxon ranksum and Fisher’s exact tests were used to compare participant baseline variables by sex. We utilized t-tests and Wilcoxon ranksum tests to compare total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 ratio levels by participant demographics, health characteristics, and medical conditions. We used ANOVA to compare total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels by age group (50–59, 60–69, 70–79, ≥ 80). Interclass correlation coefficients (ICC) were used to assess intra-individual variability of the markers over time in all participants with longitudinal data (N=1,387), separately in those aged 50–69 and 70 and older, by the median CCI score (< 6 vs ≥ 6), and among the 37 participants with five measures. Baseline means and standard deviations plots by decade were created using the mean and standard deviation plot function.
Total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 values were skewed so we natural log-transformed them. We then fit age-adjusted linear regression models to investigate the cross-sectional association between participant demographics, health characteristics, and medical conditions (independent variables) and total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 (dependent variables). Additionally, we fit mixed effects models specifying age as a time scale to investigate the longitudinal association between demographics, health characteristics, and medical conditions and total IGF-1, IGFBP-3, and IGF-1/IGFBP-3. The models included time-varying participant demographics, health characteristics, or medical condition status (indicating average association between participant demographics, health characteristics, and medical conditions and total IGF-1, IGFBP-3, or IGF-1/IGFBP-3 over follow-up), age (indicating change in total IGF-1, IGFBP-3, or IGF-1/IGFBP-3 with age), and the interaction between the demographic/medical variable and age (indicating change in the association between participant demographics, health characteristics, and medical conditions and total IGF-1, IGFBP-3, or IGF-1/IGFBP-3 with age). We specified a random intercept, but not a random slope, and used an unstructured covariance matrix. All statistical analyses and graphing were completed using Stata version 13.0 (StataCorp LLC, College Station, TX).
3. Results
At baseline, men, as compared to women, were older, had more years of education, were more likely to have medical comorbidities, had faster gait speed, higher total IGF-1 and IGF-1/IGFBP-3 levels, and lower IGFBP-3 levels (Table 1). IGF-1 and IGFBP-3 were highly correlated (r = 0.62, p < 0.001). Cross-sectionally, total IGF-1 levels were lower in participants with vs without hypertension and diabetes, slower versus faster gait speed, and among women who used vs did not use HRT (Table 2). IGFBP-3 levels were significantly lower in those with versus without cognitive impairment and hypertension, and IGFBP-3 levels were higher among those with vs without diabetes and cardiovascular disease, and between participants with a gait speed > 1 m/s compared to those with slower gait speed. The IGF-1/IGFBP-3 ratio was higher in those with vs without cognitive impairment, hypertension, diabetes, and cardiovascular disease, and among women who did not use HRT compared to those who did. At baseline, the mean ratio of IGF-1/IGFBP-3 (F = 5.20, p < 0.001) levels were positively associated with increasing age (Fig. 1). Conversely, total IGF-1 (F = 12.63, p < 0.001) and IGFBP-3 (F = 57.65, p < 0.001) were negatively associated with increasing age. Because total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels were significantly different by decade, we included age as a covariate in linear regression and specified age as a timescale in mixed effects models.
Table 1.
Participant characteristics at baseline by sex, median (IQR) or N (%).
| All (N = 1,618) |
Men (N = 884) |
Women (N = 734) |
p | |
|---|---|---|---|---|
|
|
||||
| Age | 73.0 (64.5, 79.8) | 74.1 (65.6, 80.8) | 71.9 (63.8, 78.4) | 0.002 |
| Education (years) | 14 (12, 16) | 15 (12, 17) | 14 (12, 16) | <0.001 |
| BMI (kg/m2) | 27.7 (25.1, 31.1) | 28.0 (25.4, 30.9) | 27.4 (24.3, 31.4) | 0.054 |
| Cognitive impairment* | 88 (5) | 54 (6) | 34 (5) | 0.223 |
| CCI score | 5 (3, 8) | 6 (4, 8) | 5 (3, 7) | <0.001 |
| Hypertension | 1,002 (63) | 587 (67) | 431 (59) | 0.001 |
| Diabetes | 265 (17) | 170 (20) | 95 (13) | <0.001 |
| CABG | 90 (6) | 76 (9) | 14 (2) | <0.001 |
| Myocardial infarction | 212 (13) | 162 (19) | 50 (7) | <0.001 |
| Stroke | 57 (4) | 36 (4) | 21 (3) | 0.135 |
| Cancer | 331 (24) | 204 (27) | 127 (21) | 0.015 |
| Depression (BDI≥13) | 123 (8) | 64 (7) | 59 (8) | 0.634 |
| Gait speed (m/s) | 1.14 (0.98, 1.27) | 1.16 (1.01, 1.28) | 1.13 (0.96, 1.26) | 0.019 |
| HRT (women) | 74 (10) | – | – | – |
| Total IGF-1 (ng/ml) | 118.0 (91.0, 149.0) | 125.0 (97.0, 156.5) | 109.0 (84.0, 141.0) | <0.001 |
| IGFBP-3 (μg/ml) | 3.5 (2.9, 4.1) | 3.3 (2.7, 3.9) | 3.7 (3.1, 4.4) | <0.001 |
| IGF-1/IGFBP-3 (ng/μg/ml) | 34.4 (28.4, 41.9) | 39.1 (32.6, 46.0) | 29.6 (24.4, 35.2) | <0.001 |
Cognitive impairment defined as z global score < −1.0 SD below the age-specific norm
BMI, body mass index; CCI, Charlson comorbidity index; CABG, coronary artery bypass grafting; BDI, Beck Depression Inventory; HRT, hormone replacement therapy
Table 2.
Total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels by demographics, health characteristics, and medical conditions at baseline.
| Variable | Total IGF-1 | IGFBP-3 | IGF-1/IGFBP-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | Yes | No | p | |
| Cognitive Impairment* | |||||||||
| N | 88 | 1,530 | 88 | 1,534 | 88 | 1,530 | |||
| Median (IQR) | 106.5 (85.0. 143.5) | 118.0 (91.0, 149.0) | 0.114 | 3.1 (2.7, 3.8) | 3.5 (2.9, 4.1) | <0.001 | 36.5 (29.2, 47.7) | 34.3 (28.4, 41.7) | 0.114 |
| Hypertension | |||||||||
| N | 1,002 | 575 | 1,002 | 579 | 1,002 | 575 | |||
| Median (IQR) | 116.0 (88.0, 147.0) | 121.0 (96.0, 149.0) | 0.005 | 3.3 (2.7, 4.0) | 3.7 (3.1, 4.3) | <0.001 | 35.2 (28.7, 42.9) | 33.2 (27.8, 40.0) | 0.003 |
| Diabetes | |||||||||
| N | 265 | 1,316 | 265 | 1,320 | 265 | 1,316 | |||
| Median (IQR) | 106.0 (83.0, 145.0) | 119.0 (93.0, 149.0) | <0.001 | 3.1 (2.5, 3.7) | 3.5 (2.9, 4.2) | <0.001 | 35.7 (29.0, 44.6) | 34.2 (28.2, 41.3) | 0.036 |
| CABG | |||||||||
| N | 90 | 1,489 | 90 | 1,493 | 90 | 1,489 | |||
| Median (IQR) | 116.5 (94.5, 141.5) | 118.0 (90.0, 149.0) | 0.503 | 3.0 (2.5, 3.4) | 3.5 (2.9, 4.1) | <0.001 | 39.2 (32.2, 45.2) | 34.0 (28.1, 41.4) | <0.001 |
| Myocardial infarction | |||||||||
| N | 212 | 1,364 | 212 | 1,368 | 212 | 1,364 | |||
| Median (IQR) | 118.0 (95.0, 152.0) | 117.5 (90.0, 148.0) | 0.800 | 3.1 (2.5, 3.7) | 3.5 (2.9, 4.2) | <0.001 | 38.5 (32.2, 47.2) | 33.6 (27.9, 41.1) | <0.001 |
| Stroke | |||||||||
| N | 57 | 1,521 | 57 | 1,525 | 57 | 1,521 | |||
| Median (IQR) | 129.5 (93.0, 149.0) | 118.0 (91.0, 149.0) | 0.617 | 3.5 (2.9, 3.9) | 3.5 (2.8, 4.1) | 0.281 | 35.8 (29.4, 46.3) | 34.3 (28.2, 41.7) | 0.152 |
| Cancer (any) | |||||||||
| N | 331 | 1,026 | 331 | 1,029 | 331 | 1,026 | |||
| Median (IQR) | 115.0 (90.0, 146.0) | 116.0 (90.0, 146.0) | 0.934 | 3.4 (2.8, 3.9) | 3.4 (2.8, 4.1) | 0.587 | 34.7 (28.7, 43.4) | 34.5 (28.3, 41.7) | 0.517 |
| Depression (BDI ≥ 13) | |||||||||
| N | 123 | 1,477 | 123 | 1,481 | 123 | 1,477 | |||
| Median (IQR) | 121.0 (89.0, 152.0) | 118.0 (91.0, 149.0) | 0.809 | 3.4 (2.8, 4.0) | 3.5 (2.9, 4.1) | 0.456 | 33.3 (28.4, 43.3) | 34.5 (28.4, 41.7) | 0.881 |
| Gait speed < 1 m/s | |||||||||
| N | 413 | 1,085 | 413 | 1,089 | 413 | 1,085 | |||
| Median (IQR) | 110.0 (85.0, 143.0) | 121.0 (95.0, 151.0) | <0.001 | 3.2 (2.6, 3.9) | 3.6 (3.0, 4.2) | <0.001 | 34.3 (27.8, 42.8) | 34.5 (28.6, 41.7) | 0.920 |
| Hormone replacement therapy (women) | |||||||||
| N | 74 | 660 | 75 | 661 | 74 | 660 | |||
| Median (IQR) | 98.0 (70.0, 138.0) | 109.5 (85.0, 141.0) | 0.058 | 3.8 (3.0, 4.4) | 3.7 (3.1, 4.4) | 0.507 | 26.8 (22.0, 33.9) | 29.9 (24.6, 35.2) | 0.014 |
Cognitive impairment defined as z global score < −1.0 SD below the age-specific norm
BMI, body mass index; CCI, Charlson comorbidity index; CABG, coronary artery bypass grafting; BDI, Beck Depression Inventory; HRT, hormone replacement therapy
Fig. 1.
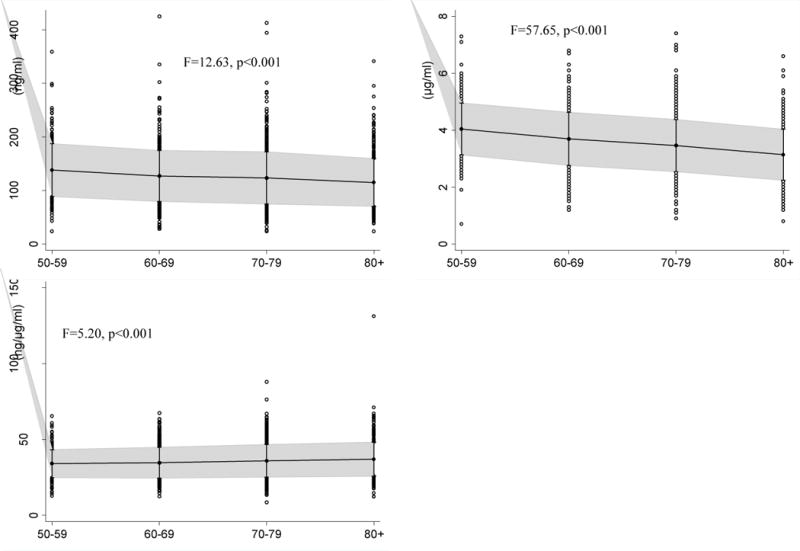
Mean, 95% CI, and data points above and below the CI of plasma levels of total IGF-1 (ng/ml), IGFBP-3 (μg/ml), and IGF-1/IGFBP-3 (ng/μg/ml) ratio by decade at baseline (N=1,618).
In age-adjusted linear regression analyses, being a man and faster gait speed were associated with higher levels of log total IGF-1, while diabetes and HRT were associated with lower levels (Table 3). Being a man and greater BMI, cognitive impairment, higher CCI score, hypertension, diabetes, cardiovascular disease, and slower gait speed were associated with lower levels of log IGFBP-3. Finally, being a man, greater CCI score, and cardiovascular disease were associated with higher log IGF-1/IGFBP-3 levels, while HRT use in women was associated with lower levels. Because plasma levels of log total IGF-1, IGFBP-3, and their ratio differed between men and women, we additionally investigated whether sex was an effect modifier by including an interaction term between sex and the participant demographic, health characteristic, or medical condition variable in each model. We did not find any evidence that sex was an effect modifier in cross-sectional models (results not shown).
Table 3.
Cross-sectional association between participant characteristics and log total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels adjusted for age (N=1,618)
| Log Total IGF-1 | Log IGFBP-3 | Log IGF-1/IGFBP-3 | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | |
| Men | 0.15 (0.11, 0.19) | <0.001 | −0.13 (−0.16, −0.10) | <0.001 | 0.28 (0.25, 0.31) | <0.001 |
| BMI | −0.003 (−0.007, 0.001) | 0.146 | −0.005 (−0.007, −0.002) | 0.001 | 0.002 (−0.001, 0.005) | 0.237 |
| Cognitive impairment* | −0.07 (−0.25, 0.03) | 0.115 | −0.12 (−0.18, −0.06) | <0.001 | 0.05 (−0.01, 0.11) | 0.124 |
| CCI score | 0.0008 (−0.006, 0.008) | 0.821 | −0.009 (−0.01, −0.004) | 0.001 | 0.01 (0.004, 0.01) | <0.001 |
| Hypertension | −0.02 (−0.06, 0.02) | 0.398 | −0.04 (−0.07, −0.01) | 0.006 | 0.02 (−0.008, 0.06) | 0.134 |
| Diabetes | −0.07 (−0.12, −0.02) | 0.007 | −0.10 (−0.14, −0.06) | <0.001 | 0.03 (−0.009, 0.07) | 0.136 |
| CABG | 0.01 (−0.07, 0.09) | 0.810 | −0.09 (−0.15, −0.03) | 0.002 | 0.10 (0.04, 0.17) | 0.001 |
| Myocardial infarction | 0.04 (−0.02, 0.10) | 0.179 | −0.09 (−0.13, −0.05) | <0.001 | 0.13 (0.09, 0.17) | <0.001 |
| Stroke | 0.09 (−0.02, 0.19) | 0.108 | 0.04 (−0.04, 0.11) | 0.356 | 0.05 (−0.03, 0.13) | 0.217 |
| Cancer | 0.03 (−0.02, 0.08) | 0.273 | 0.02 (−0.01, 0.06) | 0.196 | 0.005 (−0.03, 0.04) | 0.814 |
| Depression (BDI ≥ 13) | −0.01 (−0.08, 0.06) | 0.730 | −0.02 (−0.07, 0.03) | 0.403 | 0.01 (−0.05, 0.06) | 0.733 |
| Gait speed | 0.20 (0.10, 0.29) | <0.001 | 0.12 (0.05, 0.19) | 0.001 | 0.07 (−0.002, 0.15) | 0.058 |
| HRT (women) | −0.16 (−0.26, −0.06) | 0.001 | −0.06 (−0.12, 0.003) | 0.060 | −0.09 (−0.16, −0.03) | 0.007 |
Cognitive impairment defined as z global score < −1.0 SD below the age-specific norm
BMI, body mass index; CCI, Charlson comorbidity index; CABG, coronary artery bypass grafting; BDI, Beck Depression Inventory; HRT, hormone replacement therapy
Among participants with serial measures (N=1,387), ICCs showed little intra-individual variation over time for total IGF-1 (ICC=0.84), IGFBP-3 (ICC=0.88), and the IGF-1/IGFBP-3 ratio (ICC=0.81). Intra-individual variation was similar when comparing older (70–95) and younger (50–69) participants and those with fewer (CCI < 6) versus more (CCI ≥ 6) medical comorbidities (results not shown). Additionally, we modeled total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 ratio levels among 37 participants who had five measures (12 women and 25 men). Compared to the rest of the participants, these 37 were significantly (p < 0.05) older (median (interquartile range) [IQR]) = 78.1 (75.0, 82.0)), had lower BMI (median (IQR) =26.5 kg/m2 (24.3, 29.2)), higher CCI score (median (IQR) = 7 (6, 9)), and were more likely to have hypertension (89%) and myocardial infarction (MI) (22%). Trajectories of intra-individual total IGF-1 (ICC=0.80), IGFBP-3 (ICC=0.87), and IGF-1/IGFBP-3 (ICC=0.79) among these 37 participants also did not change substantially over time (Supplementary Figure 1).
In mixed effects models, men had greater intra-individual decreases in log total IGF-1 (B = −0.004, 95% CI −0.007, −0.0004) and log IGFBP-3 (B = −0.004, 95% CI −0.007, −0.002) with age. Additionally, higher CCI score (B = 0.0004, 95% CI 0.00004, 0.0007) and hypertension (B = 0.003, 95% CI 0.0005, 0.006) were associated with increasing log IGFBP-3 levels, while increasing gait speed was associated with decreasing log IGFBP-3 levels (B = −0.005, 95% CI −0.009, −0.0002) (Table 4). In sensitivity analyses, we additionally fit linear regression and mixed effects models with an age-squared term, but found this did not improve model fit.
Table 4.
Longitudinal association between participant characteristics and total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels (N=1,387)
| Demographics, health characteristics, and medical conditions (predictor variable) | Log Total IGF-1 | Log IGFBP-3 | Log IGF-1IGFBP-3 | |||
|---|---|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | |
| Men | 0.44 (0.18, 0.69) | 0.001 | 0.18 (0.004, 0.37) | 0.045 | 0.24 (0.07, 0.42) | 0.007 |
| Time | −0.004 (−0.007, −0.001) | 0.003 | −0.004 (−0.006, −0.003) | <0.001 | 0.0007 (−0.001, 0.002) | 0.448 |
| Men* Time | −0.004 (−0.007, −0.0004) | 0.028 | −0.004 (−0.007, −0.002) | 0.001 | 0.0006 (−0.002, 0.003) | 0.655 |
| BMI | −0.02 (−0.05, 0.002) | 0.070 | −0.009 (−0.03, 0.009) | 0.318 | −0.01 (−0.03, 0.005) | 0.138 |
| Time | −0.01 (−0.02, −0.003) | 0.008 | −0.01 (−0.02, −0.003) | 0.005 | −0.003 (−0.01, 0.004) | 0.366 |
| BMI* Time | 0.0003 (−0.0008, 0.0006) | 0.128 | 0.00008 (−0.0002, 0.0003) | 0.504 | 0.0002 (−0.00006, 0.0005) | 0.140 |
| Cognitive impairment* | 0.34 (−0.25, 0.93) | 0.264 | 0.04 (−0.26, 0.44) | 0.843 | 0.29 (−0.18, 0.77) | 0.221 |
| Time | −0.005 (−0.007, −0.004) | <0.001 | −0.007 (−0.008, −0.006) | <0.001 | 0.002 (0.0006, 0.003) | 0.005 |
| Cognition* Time | −0.004 (−0.01, 0.003) | 0.262 | −0.0007 (−0.005, 0.004) | 0.789 | −0.003 (−0.009, 0.002) | 0.248 |
| CCI | −0.006 (−0.04, 0.03) | 0.773 | −0.03 (−0.06, −0.008) | 0.011 | 0.02 (−0.004, 0.05) | 0.091 |
| Time | −0.007 (−0.01, −0.004) | <0.001 | −0.008 (−0.01, −0.005) | <0.001 | 0.0002 (−0.002, 0.003) | 0.849 |
| CCI* Time | 0.0001 (−0.0003, 0.0006) | 0.568 | 0.0004 (0.00004, 0.0007) | 0.029 | −0.0002 (−0.0005, 0.0002) | 0.340 |
| Hypertension | −0.25 (−0.53, 0.02) | 0.074 | −0.28 (−0.48, −0.08) | 0.005 | 0.009 (−0.20, 0.22) | 0.937 |
| Time | −0.007 (−0.01, −0.004) | <0.001 | −0.008 (−0.01, −0.006) | <0.001 | 0.002 (−0.0008, 0.004) | 0.198 |
| Hypertension* Time | 0.003 (−0.0007, 0.007) | 0.112 | 0.003 (0.0005, 0.006) | 0.021 | 0.0002 (−0.003, 0.003) | 0.910 |
| Diabetes | −0.33 (−0.72, 0.05) | 0.090 | −0.20 (−0.48, 0.07) | 0.148 | −0.14 (−0.44, 0.16) | 0.363 |
| Time | −0.006 (−0.008, −0.004) | <0.001 | −0.007 (−0.008, −0.006) | <0.001 | 0.002 (0.00004, 0.003) | 0.044 |
| Diabetes* Time | 0.004 (−0.001, 0.009) | 0.158 | 0.002 (−0.002, 0.005) | 0.398 | 0.002 (−0.002, 0.006) | 0.265 |
| CABG | −0.28 (−1.08, 0.51) | 0.486 | −0.60 (−1.16, −0.04) | 0.035 | 0.35 (−0.26, 0.96) | 0.266 |
| Time | −0.006 (−0.008, −0.004) | <0.001 | −0.007 (−0.008, −0.006) | <0.001 | 0.002 (0.0002, 0.003) | 0.024 |
| CABG* Time | 0.004 (−0.006, 0.01) | 0.451 | 0.006 (−0.0007, 0.01) | 0.076 | −0.003 (−0.01, 0.005) | 0.468 |
| Myocardial infarction | 0.20 (−0.28, 0.68) | 0.419 | 0.23 (−0.11, 0.57) | 0.183 | −0.04 (−0.40, 0.32) | 0.830 |
| Time | −0.006 (−0.007, −0.004) | <0.001 | −0.006 (−0.008, −0.005) | <0.001 | 0.0009 (−0.0005, 0.002) | 0.209 |
| MI* Time | −0.002 (−0.008, 0.004) | 0.477 | −0.004 (−0.009, 0.00004) | 0.052 | 0.002 (−0.003, 0.007) | 0.367 |
| Stroke | −0.23 (−1.20, 0.73) | 0.632 | −0.32 (−1.00, 0.35) | 0.348 | 0.08 (−0.66, 0.82) | 0.834 |
| Time | −0.006 (−0.008, −0.004) | <0.001 | −0.007 (−0.009, −0.006) | <0.001 | 0.002 (0.0004, 0.003) | 0.011 |
| Stroke* Time | 0.004 (−0.008, 0.02) | 0.522 | 0.004 (−0.004, 0.01) | 0.316 | −0.0003 (−0.009, 0.009) | 0.957 |
| Cancer | 0.19 (−0.20, 0.57) | 0.336 | 0.11 (−0.16, 0.38) | 0.417 | 0.07 (−0.24, 0.37) | 0.670 |
| Time | −0.005 (−0.008, −0.003) | <0.001 | −0.007 (−0.009, −0.005) | <0.001 | 0.002 (0.0003, 0.004) | 0.025 |
| Cancer* Time | −0.002 (−0.007, 0.003) | 0.482 | −0.0009 (−0.004, 0.003) | 0.600 | −0.0007 (−0.005, 0.003) | 0.736 |
| Depression | 0.009 (−0.34, 0.36) | 0.962 | 0.01 (−0.23, 0.26) | 0.913 | 0.005 (−0.27, 0.28) | 0.974 |
| Time | −0.006 (−0.007, −0.004) | <0.001 | −0.007 (−0.008, −0.006) | <0.001 | 0.002 (0.0006, 0.003) | 0.005 |
| Depression* Time | 0.0002 (−0.004, 0.005) | 0.931 | −0.0002 (−0.003, 0.003) | 0.883 | 0.0003 (−0.003, 0.004) | 0.870 |
| Gait speed | 0.46 (−0.04, 0.96) | 0.073 | 0.42 (0.07, 0.76) | 0.018 | 0.03 (−0.37, 0.42) | 0.897 |
| Time | 0.001 (−0.006, 0.009) | 0.723 | −0.0009 (−0.006, 0.004) | 0.734 | 0.002 (−0.004, 0.008) | 0.432 |
| Gait speed* Time | −0.004 (−0.01, 0.002) | 0.180 | −0.005 (−0.009, −0.0002) | 0.039 | 0.0003 (−0.005, 0.005) | 0.898 |
| HRT | −0.08 (−0.60, 0.44) | 0.771 | −0.06 (−0.40, 0.28) | 0.729 | 0.03 (−0.36, 0.41) | 0.888 |
| Time | −0.004 (−0.007, −0.001) | 0.004 | −0.005 (−0.006, −0.003) | <0.001 | 0.0005 (−0.001, 0.003) | 0.601 |
| HRT* Time | −0.001 (−0.008, 0.006) | 0.791 | 0.0001 (−0.005, 0.005) | 0.960 | −0.002 (−0.007, 0.004) | 0.551 |
Cognitive impairment defined as z global score<−1.0 SD below age specific norm
BMI, body mass index; CCI, Charlson comorbidity index; CABG, coronary artery bypass grafting; HRT, hormone replacement therapy
We investigated whether there was an interaction between sex and participant demographics, health characteristics, or medical conditions longitudinally by including interaction terms in the mixed effects models. There was evidence of an interaction (p < 0.10), and thus, we conducted sex-stratified analyses. In women, but not men, increasing BMI (p = 0.024), hypertension (p = 0.001), and MI (p = 0.047) were associated with increasing log total IGF-1 levels with age, while increasing gait speed was associated with decreasing levels (p = 0.001). Similarly, in women but not men, increasing BMI (p = 0.024) and hypertension (p = 0.030) were associated with increasing log IGFBP-3 levels with age, while increasing gait speed was associated with decreasing levels (p = 0.026). In men, but not women, increasing scores on the CCI were associated with decreasing log IGF-1/IGFBP-3 levels (p = 0.001) while increasing gait speed was associated with increasing levels (p < 0.001) with age. In women, but not men, hypertension (p = 0.008), diabetes (p = 0.018), and MI (p = 0.003) were associated with increasing log IGF-1/IGFBP-3 ratio levels, while increasing gait speed was associated with decreasing levels (p = 0.015) with age.
4. Discussion
Cross-sectionally, plasma levels of total IGF-1 and IGFBP-3 decreased with advancing age, while the ratio of IGF-1/IGFBP-3, a proxy for bioavailable IGF-1, increased with age. Levels of all three markers remained relatively stable within individuals over time. However, longitudinally, participant demographics, health characteristics, and medical conditions impacted levels of total IGF-1, IGFBP-3, and their ratio. Notably, both baseline levels and trajectories of total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 as a function of participant demographics, health characteristics, and medical conditions differed between men and women.
Our finding that the ratio of IGF-1/IGFBP-3 increases with age, while total IGF-1 and IGFBP-3 levels decrease with age, which is consistent with past studies (Ashpole et al., 2015), suggests that either older adults have more free IGF-1 or IGFBP-3 is binding other proteins with different affinities at differing ages. Few studies have examined intra-individual trajectories of total IGF-1 and IGFBP-3 and their ratio. The one study that longitudinally examined measures of IGF-1 in older adults has shown that intra-individual levels do not change substantially over time (Newman et al., 2016), which is consistent with the findings presented here.
Additionally, we found that baseline levels of total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 varied by participant demographics (sex), health characteristics (BMI, gait speed), medical comorbidities (CCI score, hypertension, diabetes, and cardiovascular disease), and HRT use in women. Moreover, in sex-stratified longitudinal analyses, participant demographics, health characteristics, and medical conditions affected trajectories of IGF-1, IGFBP-3, and IGF-1/IGFBP-3 differently in men and women. Additionally, men, who had more comorbidities at baseline, had steeper declines in IGF-1 and IGFBP-3 with age. Because the etiological processes associated with these diseases and health characteristics (e.g., weight gain, decline leading to decreased gait speed) begin years or decades prior to onset or diagnosis, it may be that these etiological processes affect total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels. Therefore, once the disease or health marker has become established in the individual, trajectories are determined. Indeed, evidence from transgenic mouse models suggests that IGF-1 levels early in the lifespan are “drivers of disease,” while levels later in life do not affect health outcomes (e.g., functionality, cancer, and mortality), and that these associations differ by sex (Ashpole et al., 2017). This is further supported by evidence in humans showing that trajectories of total IGF-1 are similar in healthy men and women, but that unhealthy men show steeper decline in total IGF-1 as compared to unhealthy women (Newman et al., 2016).
In middle- and older-aged adults, IGF-1 has been associated with diabetes, cardiovascular disease, osteoporosis, cancer, and neurodegenerative diseases (Yang et al., 2005). However, much of the research surrounding IGF-1 levels and these diseases has been inconclusive. For example, studies have shown that Alzheimer’s disease (AD) patients show both higher and lower levels of IGF-1 compared to controls (Ostrowski et al., 2016). Though, these contrary findings may be attributable to age differences between patients and controls (Hu et al., 2016). Similarly, IGFBP-3 has shown both proliferative and apoptotic effects. Stimulation of cell proliferation can occur either by enhancing IGF-stimulated proliferation or in the absence of IGF-1 (Baxter, 2000; Hollowood et al., 2000). However, IGFBP-3 inhibits cell function and promotes apoptosis both by blocking availability of IGF-1 and via independent pathways (Baxter, 2000; Hollowood et al., 2000). IGFBP-3 has also been associated with diseases common in the elderly, including AD (Watanabe et al., 2015) and type II diabetes (Drogan et al., 2016). The mixed evidence regarding the association between IGF-1 and IGFBP-3 and disease may be attributable to age-dependent changes between IGF-1 and disease and differential sex associations.
5. Conclusion
Findings suggest that total IGF-1, IGFBP-3, and their ratio do not vary significantly in individuals over time, but do vary as a function of demographics, health characteristics, and medical conditions separately in men and women. We hypothesize that the etiological processes of the diseases and health markers associated with differences in total IGF-1, IGFBP-3, and ratio levels occur years or decades prior to onset of the condition and that these differences are affected by sex. Some studies have suggested IGF-1 as a therapeutic target (Sadaba et al., 2016). However, more research, taking care to examine sex differences, is needed to understand the associations between levels of IGF-1 and IGFBP-3 and disease, and how they might be useful as clinical or research markers.
Supplementary Material
Highlights.
Cross-sectional and longitudinal patterns of IGF-1, IGFBP-3, and IGF-1/IGFBP-3 are explored
IGF-1, IGFBP-3, and IGF-1/IGFBP-3 levels are stable within individuals over time
Trajectories vary by demographics, health characteristics, and medical conditions
Health characteristic- and medical condition-dependent trajectories differ by sex
Acknowledgments
Funding
This study was supported by the National Institutes of Health/National Institute on Aging grants U01 AG006786, P50 AG44170, and R01 AG049704; and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Role of the Funding Source:
The funding sources had no role in the design of and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations
- AD
Alzheimer’s disease
- BDI
Beck Depression Inventory
- BMI
body mass index
- CCI
Charlson comorbidity index
- CV’s
coefficients of variation
- HRT
hormone replacement therapy
- IGF-1
Insulin-like growth factor 1
- IGFBP-3
Insulin-like growth factor 3
- ICC
interclass correlation coefficients
- MCSA
Mayo Clinic Study of Aging
- MCI
mild cognitive impairment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
A. Wennberg and C. Hagen have no disclosures to report. R. Petersen is a consultant for Roche, Inc, Merck, Inc., Biogen, Inc. and Eli Lilly and Company Genentech, Inc; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the National Institutes of Health. M. Mielke served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc. She receives research support from the National Institute on Aging, National Institutes of Health and unrestricted research grants from Biogen, Lundbeck, and Roche.
References
- Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm Metab Res. 1999;31:120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Coculescu M. Blood-brain barrier for human growth hormone and insulin-like growth factor-I. J Pediatr Endocrinol Metab. 1999;12:113–124. doi: 10.1515/jpem.1999.12.2.113. [DOI] [PubMed] [Google Scholar]
- Drogan D, Schulze MB, Boeing H, Pischon T. Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor-Binding Protein 3 in Relation to the Risk of Type 2 Diabetes Mellitus: Results From the EPIC-Potsdam Study. Am J Epidemiol. 2016;183:553–560. doi: 10.1093/aje/kwv188. [DOI] [PubMed] [Google Scholar]
- Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–1063. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Arvat E, Gianotti L, Lanfranco F, Broglio F, Aimaretti G, Maccario M, Camanni F. Hypothalamic growth hormone-insulin-like growth factor-I axis across the human life span. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1493–1502. doi: 10.1515/jpem-2000-s624. [DOI] [PubMed] [Google Scholar]
- Hollowood AD, Lai T, Perks CM, Newcomb PV, Alderson D, Holly JM. IGFBP-3 prolongs the p53 response and enhances apoptosis following UV irradiation. Int J Cancer. 2000;88:336–341. [PubMed] [Google Scholar]
- Hu X, Yang Y, Gong D. Circulating insulin-like growth factor 1 and insulin-like growth factor binding protein-3 level in Alzheimer’s disease: a meta-analysis. Neurol Sci. 2016;37:1671–1677. doi: 10.1007/s10072-016-2655-1. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s older americans normative studies: WAIS-R norms for ages 56 to 97. Clinical Neuropsychologist. 1992;6:1–30. [Google Scholar]
- Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, Arnold AM. Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol. 2016;45:1135–1145. doi: 10.1093/ije/dyw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski PP, Barszczyk A, Forstenpointner J, Zheng W, Feng ZP. Meta-Analysis of Serum Insulin-Like Growth Factor 1 in Alzheimer’s Disease. PLoS One. 2016;11:e0155733. doi: 10.1371/journal.pone.0155733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaba MC, Martin-Estal I, Puche JE, Castilla-Cortazar I. Insulin-like growth factor 1 (IGF-1) therapy: Mitochondrial dysfunction and diseases. Biochim Biophys Acta. 2016;1862:1267–1278. doi: 10.1016/j.bbadis.2016.03.010. [DOI] [PubMed] [Google Scholar]
- van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur J Pharmacol. 2004;490:87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Wacharasindhu S, Aroonparkmongkol S, Srivuthana S. Measurement of IGF-1, IGFBP-3 and free IGF-1 levels by ELISA in growth hormone (GH) deficient children before and after GH replacement. Asian Pac J Allergy Immunol. 2002;20:155–160. [PubMed] [Google Scholar]
- Watanabe K, Uemura K, Asada M, Maesako M, Akiyama H, Shimohama S, Takahashi R, Kinoshita A. The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer’s disease. Mol Brain. 2015;8:82. doi: 10.1186/s13041-015-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Anzo M, Cohen P. Control of aging and longevity by IGF-I signaling. Exp Gerontol. 2005;40:867–872. doi: 10.1016/j.exger.2005.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


