Abstract
Significantly larger numbers of Toxoplasma gondii cysts were detected in the brains of RAG1−/− NOS2−/− than RAG1−/− mice following infection. In contrast, the cyst numbers markedly decreased in a same manner in both strains of mice after receiving CD8+ immune T cells. Thus, NOS2-mediated innate immunity is important for inhibiting formation of cysts in the brain but not required for the T cell-initiated cyst removal, which is associated with phagocyte accumulation. Treatment with chloroquine, an inhibitor of endolysosomal acidification, partially but significantly inhibited the T cell-mediated cyst removal, suggesting that phagosome-lysomose fusion could be involved in the T. gondii cyst elimination.
Keywords: Toxoplasma gondii, cyst, inducible nitric oxide synthase, phagolysosome, CD8+ T cells
1. Introduction
Toxoplasma gondii, an obligate intracellular protozoan parasite, is an important pathogen that can cause various diseases such as lymphadenitis and congenital infection to fetuses [9, 3]. During the acute stage of infection, tachyzoites proliferate within a variety of nucleated host cells. Whereas IFN-γ-dependent, cell-mediated immune responses control the tachyzoites proliferation [18, 19, 10], the parasite establishes a chronic infection by forming cysts in various organs, especially in the brain. The cysts reside within host cells, and one cyst can contain hundreds to thousands of bradyzoites surrounded by the cyst wall. This chronic infection is widespread in humans worldwide including developed countries, with up to one third of human population being estimated to be infected [9]. This chronic infection can reactivate and cause life-threatening toxoplasmic encephalitis in immunocompromised individuals such as those with AIDS, neoplastic diseases, and organ transplants [9, 4, 22]. Since the reactivation of the infection is initiated by rupture of cysts, followed by conversion of released bradyzoites to tachyzoites and proliferation of the tachyzoites, it is important to eradicate the tissue cysts of T. gondii to prevent the reactivation of the infection. However, there are currently no drugs effective to the cyst form of the parasite. Therefore, it is important to develop a novel method capable of removing T. gondii cysts from chronically infected individuals.
Immunological intervention appears to be an intriguing approach to combat the chronic T. gondii infection. Our recent studies uncovered that CD8+ T cells of mice genetically resistant to the chronic infection have a potent activity to eliminate T. gondii cysts when these T cells are transferred into infected immunodeficient mice that have already formed large numbers of the cysts in their brains [20]. We also discovered that the amino-terminal region of dense granule protein 6 of the parasite is a key target of CD8+ immune T cells for their recognition of cyst-containing cells to initiate the anti-cyst immune process [14]. Of interest, the anti-cyst activity of the CD8+ immune T cells does not require their production of IFN-γ, the essential mediator of the protective immunity against tachyzoites, but does require perforin [20]. Since perforin mediates the cytotoxic activity of T cells, the CD8+ T cells most likely induce the cyst removal by utilizing their cytotoxic activity.
During the T cell-mediated anti-cyst immune process, phagocytes, morphologically microglia and macrophages, accumulate around the cysts and eventually invade into the cysts [20]. Therefore, these phagocytes appear to be actual effector cells that kill bradyzoites after initiation of the anti-cyst immune process by cytotoxic activity of CD8+ T cells. Although nitric oxide (NO) production by inducible NO synthase (NOS2) has been shown to be important for inhibiting proliferation of tachyzoites within macrophages in vitro [1] and in the brains of infected mice [15], the mechanisms by which the phagocytes kill bradyzoites located within the cysts is currently unknown. In the present study, we examine the role of NOS2 in the CD8+ T cell-mediated removal of T. gondii cysts. Our study revealed that whereas NOS2-mediated activities of the innate immune cells are important for inhibiting formation of the cysts in the brain, the NOS2-mediated protective activity is not required for the immune process to eliminate the cysts. The present study also demonstrated that chloroquine, an inhibitor of phagosome-lysosome system acidification, partially inhibited the T cell-mediated cyst removal, suggesting that degradation of bradyzoites by formation of phagolysosomes is involved in the elimination process of the cysts.
2. Materials and methods
2.1. Mice
BALB/c, BALB/c-background RAG1−/−, NOS2−/−, and SCID mice were from the Jackson Laboratories (Bar Harbor, ME). RAG1−/−NOS2−/− mice were generated by mating the RAG1−/− with NOS2−/− mice. There were 4 female mice in each experimental group in all experiments except two groups that had 3 mice. Mouse care and experimental procedures were performed under specific pathogen-free conditions in accordance with established institutional guidance and approved protocols from the Institutional Animal Care and Use Committee.
2.2. Infection with T. gondii
RAG1−/−, RAG1−/−NOS2−/−, and SCID mice were infected with 20 cysts of the ME49 strain of T. gondii perorally by gavage and treated with sulfadiazine in the drinking water (400 mg/L) beginning at 9 or 10 days after infection for the entire period of experiment to control proliferation of tachyzoites and establish a chronic infection [20, 13]. To generate CD8+ immune T cells, BALB/c mice were infected with 10 cysts perorally. Two independent experiments were performed for each study.
2.3. Purification and transfer of CD8+ immune T cells
Spleen cells obtained from 8–11 BALB/c mice that had been infected for at least 2 months were pooled in each experiment and suspended in Hanks’ balanced salt solution (Hyclone, Logan, UT) with 2% fetal bovine serum (Sigma, St. Louis, MO). CD8+ immune T cells were purified from the spleen cells using magnetic bead-conjugated anti-mouse CD8 (53-6.7) monoclonal antibody (Miltenyi Biotech, Auburn, CA) [20, 5]. As a control, CD8+ normal T cells were purified from the spleens of uninfected BALB/c mice in the same manner. RAG1−/−, RAG1−/−NOS2−/−, and SCID mice received the purified CD8+ T cells (2.4–3.2 × 106 cells) intravenously at 3 weeks after infection [20].
2.4. Treatment of infected SCID mice with chloroquine, an inhibitor of lysosome acidification [8, 12]
Infected SCID mice were injected intraperitoneally with 0.6 mg of chloroquine (in 0.2 ml of PBS) daily [12, 11] beginning at one week before receiving CD8+ T cells for 2 weeks. Another group of infected SCID mice were injected with PBS (0.2 ml) in the same manner.
2.5. Quantifying numbers of T. gondii cysts and amounts of mRNA for bradyzoite-specific BAG1, perforin, and granzyme B in the brains of infected RAG1−/−, RAG1−/−NOS2−/−, and SCID mice
One week after a transfer of CD8+ T cells, the brains of RAG1−/−, RAG1−/−NOS2−/−, and SCID mice were removed and a half of each brain was triturated in 0.5 ml of PBS [20]. Numbers of cysts in 3–6 aliquots (20 µl each) of the brain suspensions were counted microscopically. RNA was purified from a half of each brain, and amounts of mRNA for BAG1, perforin, and granzyme B were measured by reverse transcription real-time PCR using StepOnePlus real-time PCR system with TaqMan reagents (Applied Biosystems, Branchburg, New Jersey) [20, 13]. Primers and probe for BAG1 were described previously [17]. Primers and probes for perforin, granzyme B, and β-actin were from Applied Biosystems.
2.6. Statistical Analysis
Levels of difference between experimental groups were determined by Mann-Whitney test (GraphPad Prism, La Jolla, CA). When multiple comparisons were performed within a single experiment, corrected P value (Pc) was calculated by multiplying the P value by the number of comparisons performed within the experiment. Differences which provided P<0.05 for a single comparison or Pc<0.05 for multiple comparisons were considered significant.
3. Results and discussion
RAG1−/− and RAG1−/−NOS2−/− mice were infected and treated with sulfadiazine to control tachyzoite proliferation and establish a chronic infection in their brains. Both groups of mice lack T and B cells. Only difference between these two strains is the presence (the former) or absence of NOS2 (the latter) in innate immune cells. At 3–4 weeks after infection, 2.8 times larger numbers of cysts were detected in the brains of RAG1−/−NOS2−/− than RAG1−/− mice (P<0.05, Fig. 1A). Consistently, amounts of mRNA for bradyzoite-specific BAG1 were about twice greater in the former than latter (Fig. 1B, P<0.05). Increased levels of NOS2 mRNA were detected in the brains of only infected RAG1−/− mice as expected (P<0.001, Fig. 1C). These results indicate that the innate immune responses mediated by NOS2 are important for the protective immunity to restrict formation of T. gondii cysts in the brain.
Figure 1.
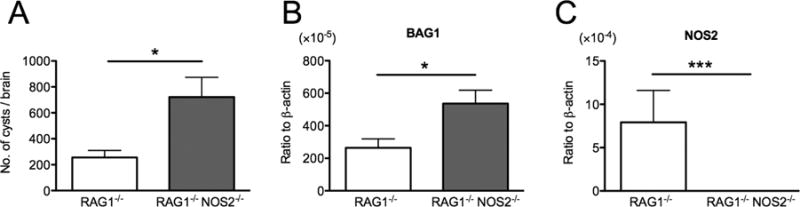
NOS2-mediated innate immunity is important for inhibiting formation of T. gondii cysts in the brain. RAG1−/− and RAG1−/−NOS2−/− mice were infected with 20 cysts of the ME49 strain perorally and treated with sulfadiazine beginning at 9 days after infection to establish a chronic infection in their brains. At 3 or 4 weeks after infection, numbers of T. gondii cysts (A) and the amounts of bradyzoite-specific BAG1 mRNA (B) and NOS2 (C) in their brains were measured. All data represent mean ± SEM. The data from two independent studies were combined. In total, there were 12 mice in each experimental group for cyst counting and 10 mice in each experimental group for quantification of mRNA for BAG1 and NOS2. *P<0.05 and ***P<0.001.
We next examined whether NOS2 is important for CD8+ T cell-mediated immune process to remove T. gondii cysts, in which phagocytes accumulate and penetrate into the cysts [20]. Infected, sulfadiazine-treated RAG1−/− and RAG1−/−NOS2−/− mice received a systemic transfer of CD8+ immune T cells at 3 weeks after infection. Seven days after the T cell transfer (Day 7), numbers of cysts in the brains of animals that had received the T cells were markedly fewer than those that had received no T cells in both RAG1−/− and RAG1−/−NOS2−/− mice (Pc<0.05 for both strains, Fig. 2A). In addition, the ratios of cyst numbers in the mice with the T cell transfer versus those in the animals without the cell transfer did not differ between RAG1−/− and RAG1−/−NOS2−/− mice (the mean ± SEM: 3.99 ± 1.72 % [n=8] and 6.36 ± 2.32 % [n=8], respectively), although the latter had larger numbers of cysts before the cell transfer (Fig. 2A). In agreement with the results in cyst numbers, amounts of bradyzoite-specific BAG1 mRNA were markedly less in the mice with the immune T cell transfer than in those without the cell transfer in both RAG1−/− and RAG1−/−NOS2−/− strains (Pc<0.05, Fig. 2B), and the ratios of BAG1 mRNA levels in the mice with the T cell transfer versus those in the animals without the cell transfer did not differ between these two strains (the mean ± SEM: 3.19 ± 1.12 % [n=8] and 3.78 ± 0.98 % [n=7], respectively). In contrast to the transfer of the immune T cells, numbers of cysts and amounts of BAG1 mRNA in the brains of mice that had received CD8+ normal T cells from uninfected BALB/c mice did not differ from those of the control mice that had received no T cells in both RAG1−/− and RAG1−/−NOS2−/− mice (Figs. 2A and 2B). These results indicate that the immune process initiated by CD8+ immune T cells is able to remove T. gondii cysts in the absence of NOS2 in innate immune cells including phagocytes.
Figure 2.
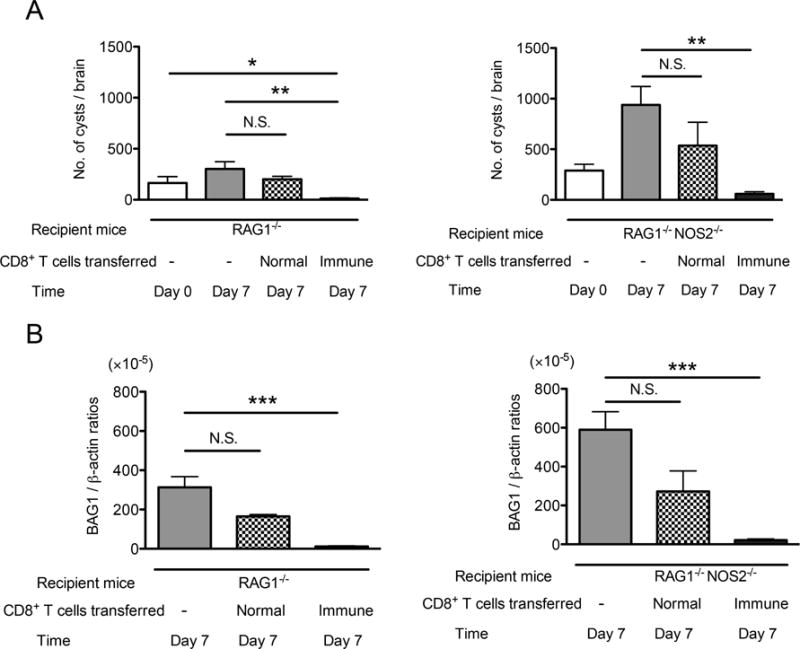
NOS2 is not required for the CD8+ T cell-initiated immune process to remove T. gondii cysts from the brain. RAG1−/− and RAG1−/−NOS2−/− mice were infected with 20 cysts of the ME49 strain perorally and treated with sulfadiazine beginning at 9 days after infection to establish a chronic infection in their brains. At 3 weeks after infection, the mice received a systemic transfer of CD8+ immune T cells (3.2 ×106 cells) from chronically infected BALB/c mice (Day 0). As a control, a group of infected RAG1−/− and RAG1−/−NOS2−/− mice received CD8+ normal T cells from uninfected BALB/c mice in the same manner. Seven days after the T cell transfer (Day 7), numbers of T. gondii cysts (A) and the amounts of bradyzoite-specific BAG1 mRNA (B) in their brains were measured. All data represent mean ± SEM. The data from two independent studies were combined. There were 3 or 4 mice in each experimental group in each experiment. *Pc<0.05, **Pc<0.01, and ***Pc<0.001. N.S.: not significant.
After the initiation of anti-cyst immune process by CD8+ T cells, it is possible that phagocytes, which invade into the cysts, phagocytose bradyzoites and destroy them by phagolysosome formation. To address this possibility, infected, sulfadiazine-treated SCID mice lacking T and B cells were injected with chloroquine, the inhibitor of phagosome-lysosome system acidification, daily beginning at 7 days before receiving CD8+ immune T cells. As a control, another group of infected SCID mice were injected with PBS in the same manner. At 7 days after the T cell transfer, numbers of cysts in the brains of both chloroquine- and PBS-treated mice that had received the T cells were significantly fewer than those of a negative control mice that had not received the T cells (Pc<0.01 for chloroquine-treated mice and Pc<0.001 for PBS-treated animals, Fig. 3A). However, when compared between the chloroquine- and PBS-treated mice with the T cell transfer, numbers of cysts were significantly greater in the former than the latter (Pc<0.05, Fig. 3A). Amounts of bradyzoite-specific BAG1 mRNA were also greater in the brains of the chloroquine- than PBS-treated mice with the T cell transfer (Fig. 3B, P<0.05).
Figure 3.
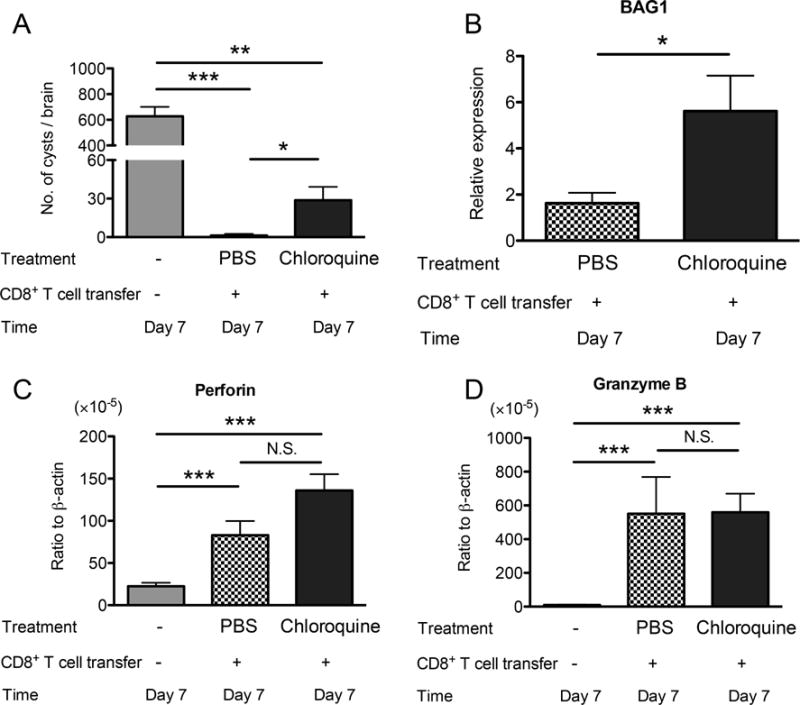
Chloroquine, the inhibitor of phagosome-lysosome system acidification, partially inhibits the immune process to remove T. gondii cysts from the brain. SCID mice were infected with 20 cysts of the ME49 perorally and treated with sulfadiazine beginning at 10 days after infection to establish a chronic infection in their brains. At 3 weeks after infection, two groups of the mice received a systemic transfer of CD8+ immune T cells (2.4 or 2.5 ×106 cells) from chronically infected BALB/c mice. One group was injected intraperitoneally with 0.6 mg of chloroquine (in 0.2 ml of PBS) daily beginning at one week before receiving CD8+ T cells for 2 weeks. Another group of infected SCID mice were injected with PBS (0.2 ml) in the same manner. Numbers of cysts (A) and amounts of BAG1 mRNA (B) were measured at 7 days after the T cell transfer (Day 7). Amounts of mRNA for perforin (C) and granzyme B (D) were also measured at Day 7. All data represent mean ± SEM. The data from two independent studies were combined. There were 6–8 mice in total in each experimental group. *Pc<0.05, **Pc<0.01, and ***Pc<0.001. N.S.: not significant.
As mentioned earlier, anti-cyst activity of CD8+ immune T cells requires their perforin (9), and therefore, the T cells most likely initiate the anti-cyst immune process by utilizing their perforin-mediated cytotoxic activity against cyst-containing cells. Granzyme B also plays an important role in the cytotoxic activity of CD8+ T cells. Amounts of mRNA for both perforin and granzyme B were markedly greater in the brains of both chloroquine- and PBS-treated mice that had received the CD8+ immune T cells, when compared to animals that had received no T cells (Pc<0.001, Fig. 3C). Furthermore, perforin and granzyme B mRNA levels did not differ between the chloroquine- and PBS-treated mice (Fig. 3C), strongly suggesting that cytotoxic activity of CD8+ immune T cells was not affected by the chloroquine treatment. These results suggest that phagosome-lysosome system acidification in innate immune cells, most likely phagocytes, is involved, at least in part, in the T cell-mediated immune process for destruction of cysts and/or the elimination of the bradyzoites located within the cysts.
The present study uncovered an important difference in the roles of NOS2 in the protective immune responses to inhibit formation of T. gondii cysts and those to remove the cysts that have already been developed. The comparison between RAG1−/− and RAG1−/−NOS2−/− mice revealed that innate immune system is able to upregulate cerebral expression of NOS2 following T. gondii infection, and that the NOS2 induced by the innate immunity plays an important role in restricting T. gondii cyst formation in the brain. Previous study by others comparing NOS2−/− and wild-type mice demonstrated that NOS2 is not required for controlling tachyzoite proliferation in the peritoneal cavity during the acute stage of infection following an intraperitoneal injection of the parasite but required for preventing tachyzoite growth in the brain during the later stage of the infection [15]. Mortality in infected NOS2−/− mice during the later stage of infection [15] depicts the importance of the NOS2-mediated control of cerebral tachyzoite growth in host resistance. In the present study, it is possible that the innate immunity-induced NOS2 inhibited cerebral tachyzoite growth in RAG1−/− mice before beginning of sulfadiazine treatment and thereby contributed to formation of fewer cysts in their brains when compared to RAG1−/−NOS2−/−mice.
IFN-γ is crucial for cerebral NOS2 expression during T. gondii infection [3]. Our recent studies demonstrated that in addition to T cells [21], innate immune cells including brain-resident cells produce IFN-γ to inhibit tachyzoite growth in the brains of infected mice [5, 14]. The results of the present study strongly suggest that IFN-γ produced by innate immune cells is able to efficiently upregulate cerebral NOS2 expression to suppress tachyzoite proliferation. In regard to the effector cell population that becomes activated by IFN-γ and suppresses tachyzoite growth through NOS2-dependent manner in the brain, a previous study using bone marrow chimeric mice showed that NOS2 expression only by hematopoietic cell populations is sufficient to inhibit tachyzoite growth during the chronic stage of infection [23]. Since in vitro studies showed that macrophages activated by IFN-γ utilize NOS2 as a part of the mechanisms to inhibit intracellular proliferation of tachyzoites [1], and since inflammatory monocytes that migrate into the brain play an important role in preventing cerebral tachyzoite growth in mice [2], NOS2-mediated activity of the blood-derived macrophages and inflammatory monocytes most likely contributes to restricting cerebral tachyzoite growth and inhibiting cyst formation. However, these results do not exclude a possibility that NOS2 contributes to inhibiting the stage conversion from the tachyzoites to the bradyzoites and thereby suppressing cyst formation. Further studies are required to determine the role of NOS2 in macrophages and inflammatory monocytes to control cerebral tachyzoite proliferation and the role of NOS2 in inhibiting the stage conversion from tachyzoites to bradyzoites in the brain.
In contrast to the protective effects of NOS2-mediated innate immunity in inhibiting cerebral tachyzoite proliferation and restricting formation of T. gondii cysts in the brain, the present study revealed that the absence of NOS2 in innate immune cells does not affect the capability of the immune system to remove the cysts from the brain. In contrast, chloroquine treatment partially inhibited the immune process to remove T. gondii cysts. Since the cyst elimination is associated with penetration of phagocytes into the cysts [20], it is most likely that the phagocytes employ, at least in part, endolysosomal acidification during the process to remove the cysts. Thus, the innate immune cells employ two distinct mechanisms, NOS2-mediated and endosomal acidification-mediated, against tachyzoites and cysts, respectively. We previously demonstrated that T cells utilize two distinct mechanisms to control T. gondii; IFN-γ for preventing tachyzoite proliferation [18, 17], and perforin for initiating the immune process to eliminate the cysts [20]. Thus, both innate and T cell-mediated immune system employ different strategies depending on two different differentiation stages of T. gondii, the tachyzoite and the cyst, to combat this intracellular microorganism.
The reason for the only partial inhibition of the cyst removal by chloroquine in the present study is unclear. Since the brain is isolated from the periphery by the blood-brain barrier that blocks migration of small compounds into the brain, the amount of chloroquine that had reached to the brain in the present study may not have been sufficient to display a complete inhibition of the cyst removal. Another possibility would be that phagocytes utilize multiple mechanisms to eliminate T. gondii cysts, and that the presence of other mechanisms may have allowed phagocytes to remove the cysts in the presence of chloroquine, although the efficiency of their anti-cyst activity partially decreased in this condition.
Macrophages activated by IFN-γ in vitro control tachyzoites not only by NOS2 [1] but also by inducing a destruction of parasitophorous vacuoles by accumulation of the immunity-related GTPases and guanylate-binding proteins [16, 24, 7]. Both of these processes do not employ lysosomal acidification, and therefore differ from the observations in the present study. This difference could reflect, at least in part, the aggressive capability of tachyzoites to penetrate into host cells. When tachyzoites egress from host cells after intracellular proliferation, they quickly invade into adjacent host cells by secreting molecules from specialized organelles, such as micronames and rhoptories, and form parasitophorous vacuoles in which the invaded parasite proliferates. In contrast, when phagocytes penetrate into the T. gondii cysts after perforin-mediated attack of cyst-containing cells by CD8+ immune T cells, bradyzoites located within the cysts are probably not ready to invade into these phagocytes. Therefore, the invaded phagocytes readily phagocytose the bradyzoites and destroy them by formation of phagolysosomes. Another possibility may be that phagocytes activated in vivo with T. gondii infection kill the parasite by utilizing a different mechanism(s) from those employed by the in vitro activated macrophages, since a previous study showed that macrophages from mice infected with an attenuated strain of T. gondii kill tachyzoites through a mechanism involving lysosomal fusion [6]. The inhibitory effects of chloroquine observed in the present study was only partial as mentioned earlier, and therefore further studies are needed to elucidate the detailed mechanisms of the T cell-initiated immune process to remove T. gondii cysts. However, the results from the present study depicted intriguing variations in the protective immune mechanisms, including NOS2, depending on the stages, the tachyzoite and the cyst, of T. gondii within the same host.
Acknowledgments
The studies are supported in part by NIH grants (AI095032, and AI078756).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that there is no conflict of interest.
References
- 1.Adams LB, Hibbs JB, Jr, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990;144:2725–9. [PubMed] [Google Scholar]
- 2.Biswas A, Bruder D, Wolf SA, Jeron A, Mack M, Heimesaat MM, et al. Ly6C(high) monocytes control cerebral toxoplasmosis. J Immunol. 2015;194:3223–35. doi: 10.4049/jimmunol.1402037. [DOI] [PubMed] [Google Scholar]
- 3.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–81. [PubMed] [Google Scholar]
- 4.Israelski DM, Remington JS. Toxoplasmosis in the non-AIDS immunocompromised host. In: Remington JS, Swrltz M, editors. Curr Clin Top Infect Dis. Blackwell Scientific Publications; London: 1993. pp. 322–56. [PubMed] [Google Scholar]
- 5.Kang H, Suzuki Y. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect Immun. 2001;69:2920–7. doi: 10.1128/IAI.69.5.2920-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–71. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, et al. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misinzo G, Delputte PL, Nauwynck HJ. Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells. J Virol. 2008;82:1128–35. doi: 10.1128/JVI.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 10.Munoz M, Liesenfeld O, Heimesaat MM. Immunology of Toxoplasma gondii. Immunol Rev. 2011;240:269–85. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 11.Nalbandian A, Llewellyn KJ, Nguyen C, Yazdi PG, Kimonis VE. Rapamycin and chloroquine: the in vitro and in vivo effects of autophagy-modifying drugs show promising results in valosin containing protein multisystem proteinopathy. PloS One. 2015;10:e0122888. doi: 10.1371/journal.pone.0122888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndolo RA, Forrest ML, Krise JP. The role of lysosomes in limiting drug toxicity in mice. J Pharmacol Exp Ther. 2010;333:120–8. doi: 10.1124/jpet.109.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochiai E, Sa Q, Brogli M, Kudo T, Wang X, Dubey JP, et al. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. Am J Pathol. 2015;185:314–24. doi: 10.1016/j.ajpath.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sa Q, Ochiai E, Tiwari A, Perkins S, Mullins J, Gehman M, et al. Cutting Edge: IFN-gamma produced by brain-resident cells is crucial to control cerebral infection with Toxoplasma gondii. J Immunol. 2015;195:796–800. doi: 10.4049/jimmunol.1500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HWT, et al. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Patog. 2013;9:e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki Y, Conley FK, Remington JS. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–50. [PubMed] [Google Scholar]
- 18.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Sa Q, Gehman M, Ochiai E. Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert Rev Mol Med. 2011;13:e31. doi: 10.1017/S1462399411002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol. 2010;176:1607–13. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Kang H, Kikuchi T, Suzuki Y. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect Immun. 2004;72:4432–8. doi: 10.1128/IAI.72.8.4432-4438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong SY, Remington JS. Toxoplasmosis in the setting of AIDS. In: Broder S, Mergan TC Jr, Bolognesi D, editors. Text Book of AIDS Medicine. Williams & Wikins; Baltimore: 1994. pp. 223–57. [Google Scholar]
- 23.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–92. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]


