Abstract
Researchers can study complex developmental phenomena with all the inherent noise and complexity or simplify behaviors to hone in on the essential aspects of a phenomenon. We used the development of walking as a model system to compare the costs and benefits of simplifying a complex, noisy behavior. Traditionally, researchers simplify infant walking by recording gait measures as infants take continuous, forward steps along straight paths. Here, we compared the traditional straight-path task with spontaneous walking during 20 minutes of free play in 97 infants (10.75–19.99 months of age). We recorded infants’ footfalls on an instrumented floor to calculate gait measures in the straight-path and free-play tasks. In addition, we scored videos for other critical aspects of spontaneous walking—steps per bout, shape of walking path, and step direction. Studying infant walking during free play incurred no cost compared with the straight-path task, but considerable benefits. Straight-path gait was highly correlated with spontaneous gait and both sets of measures improved with walking age, validating use of the straight-path task as an index of development. However, a large proportion of free-play bouts were too short to permit standard gait measures, and most bouts were curved with omnidirectional steps. The high prevalence of these “non-canonical” bouts was constant over development. We propose that a focus on spontaneous walking, the phenomenon we ostensibly wish to explain, yields important insights into the problems infants solve while learning to walk. Other areas of developmental research may also benefit from retaining the complexity of complex phenomena.
Keywords: Infant walking, learning, naturalistic method, ecological validity, experimental control
One goal of developmental science is to describe and explain behavioral development. Researchers’ tasks and procedures determine how behaviors are observed and quantified. These measures, in turn, shape and constrain the descriptions of development. Consequently, theories aimed at explaining the process of development are shaped by the chosen tasks and procedures. Therefore, when the behavioral phenomenon is noisy, variable, and complex, researchers have two options. They can measure the phenomenon in all its complexity, but risk losing the ability to detect underlying processes. Alternatively, they can constrain and simplify the behavior, but risk losing focus on critical aspects of the phenomenon they wish to explain.
Research on motor development is rife with examples that illustrate the tension between ecological validity and experimental control. Although infants presumably learn to reach from a multitude of postures to objects at a multitude of locations, researchers typically study reaching to objects presented at midline while infants sit supported on a caregiver’s lap or in special chairs (Berthier & Keen, 2006; Corbetta & Thelen, 1996; Lee, Bhat, Scholz, & Galloway, 2008). Although infants presumably learn to sit on a variety of surfaces in a variety of positions, researchers typically study infants sitting with legs outstretched on a flat, rigid surface (Cignetti, Kyvelidou, Harbourne, & Stergiou, 2011; Harbourne & Stergiou, 2003). Of course, such examples are not limited to motor behaviors. Although infants’ visual exploration in real-world contexts is influenced by a complex confluence of factors (Franchak, Kretch, Soska, & Adolph, 2011; Kretch, Franchak, & Adolph, 2014; Smith, Yu, & Pereira, 2011; Yu & Smith, 2017), researchers typically study infants looking at simplified scenes on computer monitors. Indeed, most research on infant development relies on observations of constrained behaviors in structured tasks. As Bronfenbrenner (1977) and others have pointed out, this severely limits our ability to understand the noisy and variable phenomena we aim to explain (Dahl, 2017; Tamis-LeMonda, Kuchirko, Luo, & Escobar, in press).
Here, we used the development of walking as a model system to address the costs and benefits of simplifying a phenomenon to make it more tractable for study. In doing so, we show how broadening the focus to include the noisy, rich complexity of natural behavior expands researchers’ view of what and how infants learn when they learn to walk.
Traditional Straight-Path Task
Traditionally, research on learning to walk has relied on a simplified “straight-path” task in which infants are encouraged to walk repeatedly through a restricted recording area, taking forward, continuous steps in a straight line over uniform ground (e.g., Adolph, Vereijken, & Shrout, 2003; Bril, Dupuy, Dietrich, & Corbetta, 2015; Chang, Kubo, Buzzi, & Ulrich, 2006; Clark & Phillips, 1993; Hallemans, De Clercq, Otten, & Aerts, 2005; Ivanenko, Dominici, Cappellini, & Lacquaniti, 2005; McGraw & Breeze, 1941; Shirley, 1931). Of course no researcher believes that babies only walk in continuous, forward, straight lines. All of us (current authors included) have relied on the straight-path task for practical, methodological, and theoretical reasons. Specifically, a restricted recording area is necessitated by existing technologies (e.g., size of gait mat or force plate, scope of high-speed cameras, wire leads tethering infants to recording device). Steps are forward and in a straight line to ensure the symmetry of movements on both sides of the body. The sequence is continuous because at least four steps are required to calculate gait measures such as step length on both legs. And the ground is uniform because variations in surface slant, rigidity, friction, and obstacles in the path introduce issues of perceptual control and planning.
Most importantly, the straight-path task presumably captures essential aspects of walking. Indeed, a century of research based on this simple task yields robust and consistent findings on the development of infant walking (for reviews, see Adolph & Robinson, 2013; Adolph & Robinson, 2015; Ivanenko, Dominici, & Lacquaniti, 2007). Infants’ first steps are slow and choppy. Balance is precarious and infants quickly plant each moving foot to avoid falling. Infants’ legs are splayed so far apart that the side-to-side distance (step width) often exceeds the front-to-back distance (step length). Over the next three to six months, walking improves dramatically—steps become faster, longer, and narrower—so that walking more closely resembles the mature gait of adults. Explanations for the early deficits and later improvements focus on changes in muscle strength and body dimensions, control of balance and propulsion, and development of pendular mechanisms (Bril et al., 2015; Holt, Saltzman, Ho, Kubo, & Ulrich, 2006; Ivanenko et al., 2004; Thelen, 1984; Woollacott & Shumway-Cook, 1990).
Spontaneous Walking
Despite researchers’ best efforts, infants do not readily comply with the straight-path task (e.g., Bisi, Riva, & Stagni, 2014; Cole, Robinson, & Adolph, 2016; Garciaguirre, Adolph, & Shrout, 2007; Ivanenko et al., 2005; Kubo & Ulrich, 2006; Looper & Chandler, 2013; Van Dam, Hallemans, Truijen, Segers, & Aerts, 2011). Although caregivers or assistants stand at the far side of the recording area and encourage infants to walk toward them, infants stop after a few steps; they spontaneously veer out of the recording area; they turn in circles and produce curved paths; and they take steps in every direction. Researchers must remove such “irregular” segments while processing the data, and discard sequences without at least four continuous forward steps. Thus, several attempts are usually required to obtain one useable sequence, and the data analyzed are only a subset of the data collected.
When the straight-path constraints are lifted during free play, infants’ walking appears even more noisy, variable, and complex. However, to date, research on spontaneous walking has focused primarily on the overall amount and distribution of infants’ physical activity (Adolph et al., 2012; Borkhoff et al., 2015; Hnatiuk et al., 2012). Detailed video analyses of free play reveal that starting and stopping are core components of infant walking; both novice and experienced walkers produce a sizeable proportion of bouts (40–50%, on average) containing only one to three steps (Cole et al., 2016). The extent to which infants produce short bouts in the straight-path task is unknown, and the extent of curved paths and omnidirectional steps is not known in either task or over development.
Current Study
The overall goal was to examine what is lost and what is gained by examining the development of walking in a free-play task that includes all the inherent noise, variability, and complexity of spontaneous walking. Specifically, the study examined the similarities and differences between infant walking in the traditional straight-path task and in free play to assess which factors change over development and which do not.
Our first aim was to describe, for both tasks and across walking age, the extent to which infant walking comprises short versus long continuous bouts, curved versus straight paths, and omnidirectional versus forward steps. We hypothesized that, during unconstrained free play, infants would occasionally produce continuous straight-path sequences of forward steps, but they would show substantially higher rates of short bouts, curved paths, and omnidirectional steps. Moreover, we hypothesized that such “noncanonical” bouts would be more prevalent during free play than in the constrained straight-path task even when no sequences are discarded. Finally, we hypothesized that the occurrence of short bouts, curved paths, and omnidirectional steps during free play would decrease with walking age as infants become more experienced and skilled and less likely to lose balance or control.
Our second aim was to collect gait measures in both tasks and to compare the trajectories across walking age. We used a large instrumented mat that allowed us to record footfall measures of walking speed, step length, and step width during free play. We expected gait measures across tasks to differ in their average magnitude because the straight-path task is designed to optimize infants’ average speed, step length, and step width. But such a difference would provide only limited insight into the development of walking. The critical questions were whether infants would show similar trajectories of improvement (faster, longer, narrower steps) with increase in walking age in both tasks, and whether the individual differences in walking skill indexed by these measures would be stable and consistent across the two tasks. If trajectories differed or if individual differences were not stable across tasks, this would suggest that the straight-path task indexes a different developmental process from that involved in everyday real-world walking.
Method
Participants
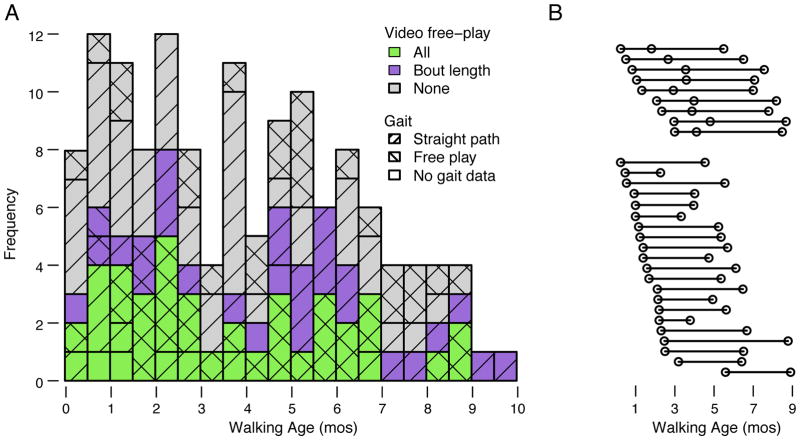
We tested 97 infants (48 girls, 49 boys) between 10.75 and 19.99 months of age (M = 16.54 months) from the New York City area; 67 infants were observed one time and 30 were observed two or three times for a total of 136 sessions. All were born at term and typically developing; 40% were white, 3% Asian, 2% black, 16% multi-race, 22% other, and 17% did not state their race (97% of those reporting other or not reporting a race identified as Hispanic). Parents reported infants’ walking onset age in a structured interview (Adolph et al., 2003) based on the first day they saw their infants walk 3 m across a room without stopping or falling. Onset dates were verified against cell phone videos, photographs, and calendars when available. Figure 1A shows the distribution of all 136 test sessions by walking age (elapsed time between test day and onset day, M = 3.93 months, range = 2 days to 10.26 months) and the type of data each infant contributed at each session; Figure 1B shows the timing of sessions by walking age for the subset of infants observed two or three times.
Figure 1.
Distribution of infants’ walking age for (A) all 136 test sessions and (B) the subset of 30 infants in A who were tested repeatedly (69 sessions). The diagonals denote sessions with gait measures collected on the instrumented floor and verified with video. Bars with left diagonals denote sessions (N = 130) in which infants contributed useable gait data in the traditional straight-path task. Bars with right diagonals denote sessions (N = 57) in which infants contributed gait data during spontaneous walking in free play. Bars with both right and left diagonals denote sessions (N = 56) in which infants contributed gait data to both the straight-path and free-play tasks. Bars with no diagonals denote sessions (N = 5) in which infants did not contribute useable gait data on the instrumented floor in either task. Bar color denotes measures scored only from video during free play: Green bars denote sessions (N = 40) for which we scored bout length (steps/bout), path shape (straight/curved), and step direction (forward/not forward). Purple bars denote sessions (N = 29) for which we scored only bout length. Gray bars denote sessions (N = 67) for which we did not score bout length, path, or step direction during free play. Note, bar color refers only to the free-play task because coders scored bout length, path shape, and step direction for all 135 sessions in which we had useable data in the straight-path task.
Instrumented Floor and Procedure
Infants were tested in a large laboratory playroom (5.97 m × 9.42 m) filled with toys, a couch, and elevations (slide with stairs, risers, pedestal, and platform). For the first 71 sessions, we instrumented the floor with a (0.92 m × 5.73 m) pressure-sensitive mat (gaitrite.com) that recorded the timing and placement of steps during the straight-path task (120 Hz, 4 sensors/in2). For the remaining 65 sessions, we used a larger mat (1.21 m × 4.88 m, 120 Hz, 4 sensors/in2, protokinetics.com) that allowed us to collect gait data during both free play (if they happened to step on the mat) and the straight-path task. A fixed camera near the ceiling recorded the entire playroom with a fish-eye lens. Two additional fixed cameras recorded activity on the instrumented mat. An assistant focused on infants’ activities using a hand-held camera. The four camera views and output from the mat were synced and mixed onto a single frame for ease of later video coding and data processing.
We observed each infant’s spontaneous walking during free play and straight-path tasks, with task order counterbalanced across infants. In the free-play task, parents were instructed to interact normally with their infants and to mind their safety for 20 minutes. Sessions always began with the toys in designated locations. Infants and parents could move freely throughout the room, stepping on the instrumented portion of the floor or not (Figure 2A). In the straight-path task, an experimenter stood infants at one end of the instrumented mat and encouraged them to walk straight to their parent who cheered them from the other end and offered toys and snacks as incentives (Figure 2B). We aimed to collect six sequences of continuous, straight-path walking. However, as is typical, infants frequently refused to comply and sequences were often repeated multiple times. Infants contributed M = 10.44 sequences in total (SD = 5.24) and M = 5.28 were useable for gait analyses (SD = 3.31). On average, we could use only 57.81% of the data each infant produced.
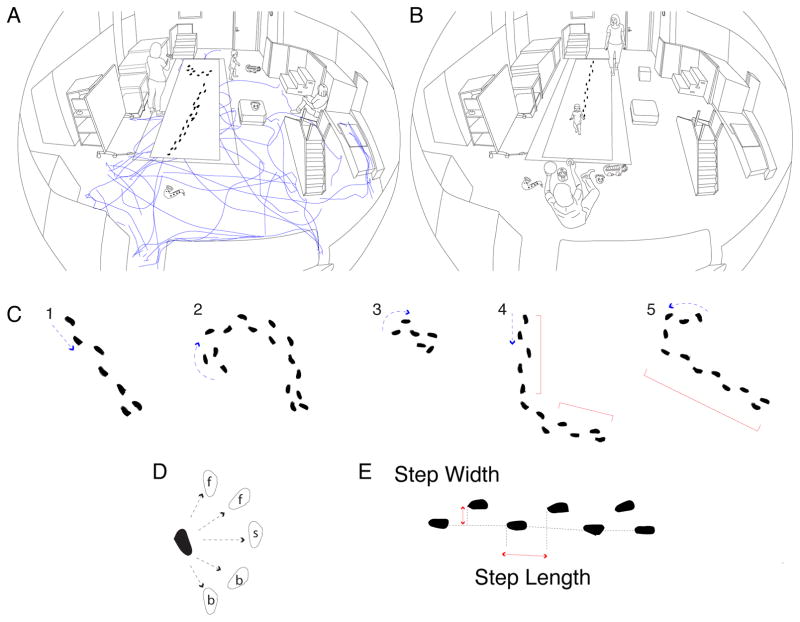
Figure 2.
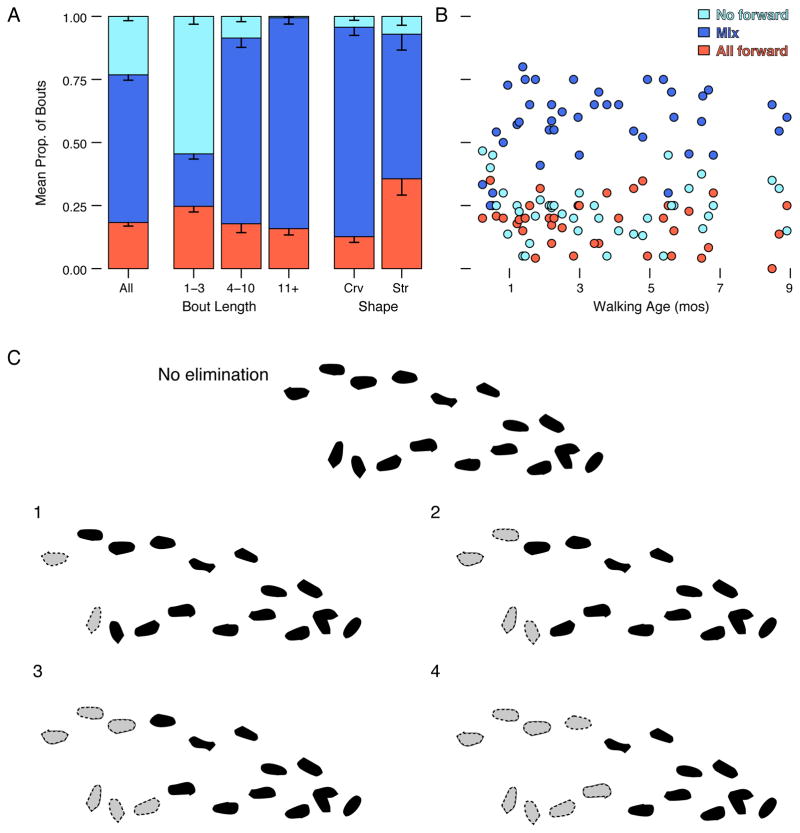
Procedure and measures of walking. (A) Free-play task. Illustration of one infant’s spontaneous walking in the laboratory playroom filled with toys, couch, and elevations. Part of the floor (shown by rectangle) was instrumented. Infants were free to move or not as they wished; parents (shown sitting) were told to interact normally and mind infants’ safety. An experimenter (shown standing) followed discretely from a distance to record infants’ movements with a handheld camera. Footprints denote two sequences of the infant’s steps on the instrumented floor and smoothed lines show the infant’s path on the remaining floor area during free play. Play on elevations is not shown. (B) Straight-path task. Illustration of procedure for testing infants in the straight-path task on the instrumented floor. The experimenter placed infants at one end of the mat while parents at the other end urged infants to walk straight over using toys and snacks as incentives. The task was repeated as many times as necessary to obtain several useable sequences of continuous, forward steps along a straight path. (C) Path shape. Example of (#1) path coded as straight. Examples of curved paths due to: (#2) a twisting, serpentine change in direction, (#3) turning and stepping in place, (#4) angular, zigzag change in direction, and (#5) an otherwise straight path that began with a hook. To be coded as “curved”, infants had to change direction over at least two consecutive steps or step in place. Red brackets show curved paths containing straight segments with at least four steps. Note, for illustrative purposes, we built examplars in the figure from actual data from infants stepping on the instrumented mat. However, all path shapes were scored only from video because many bouts were not on the mat or were only partially on the mat. (D) Step direction. Examples of forward, backward, and sideways steps. Sequences could contain any combination of steps. Note, for illustrative purposes, we built exemplars in the figure from actual data from infants stepping on the instrumented mat. However, all step direction data were scored only from video because many bouts were not on the mat or were only partially on the mat. (E) Calculation of gait measures for sequences with at least four continuous steps. Step length is the front-to-back distance between steps; step width is the side-to-side distance between steps; and speed (not shown) is the time from the first to last steps. For the straight-path task, only forward steps were selected for calculating gait measures. For the free-play task, steps could be in any direction so distances were calculated as the absolute value.
Data Coding and Processing
Data were processed in three ways as described in detail below. First, to compare falls, bout length, path shape, and step direction in the free-play versus straight-path tasks, we analyzed all bouts produced on the floor in both tasks (i.e., walking on elevations was not included). Second, to measure gait during free play, we analyzed all bouts occurring on the instrumented floor, regardless of path shape and step direction. Third, to measure gait in the straight-path task, we analyzed only straight, continuous, forward steps on the instrumented floor.
Measures of falls, bout length, path shape, and step direction
Because of the labor-intensive nature of video coding, we scored only a subset of free play sessions for bout length (N = 69), path shape (N = 40), and step direction (N = 40) distributed across the range of walking age (colored bars in Figure 1), but all straight-path data because infants contributed fewer bouts. Due to technical problems, straight-path data were missing for one session, resulting in N = 135 sessions coded.
A primary coder used Datavyu (datavyu.org), a computerized video coding software to score falls and to count the number of steps per bout (a single step or series of steps flanked by stationary periods). We defined walking bouts as the time between the first step, when a foot lifted from the floor, and the last step when a foot rested on the floor for at least 0.5 s and infants were not shifting weight to another upright step (Cole et al., 2016). The total number of floor bouts in free play was 6121; infants contributed 15–150 bouts (M = 87.44). The total number of bouts in the straight-path task was 1345; infants contributed 1–33 bouts (M = 9.96).
Coders used the overhead camera view to score path shape for bouts with at least four steps (N = 1465 bouts for free play and 1267 bouts for straight path). Straight bouts maintained the body’s direction of heading over the entire bout (Figure 2C #1). Most “non-straight” bouts were curved (serpentine, zigzag, began or ended in a “hook,” turning in place, see Figure 2C #2–5); the remainder were steps in place. For simplicity, we refer to all “non-straight” bouts as curved. Coders also scored every fifth free-play bout for step direction (N = 890 bouts) and all bouts for the straight-path task—whether each step was forward or not forward (sideways, backward, or stepping in place; Figure 2D).
A second coder independently scored 25% of each infant’s falls, number of steps per bout, and step direction, and 100% of each infant’s path shape data. Inter-rater agreement was high: steps per bout, r(1082) = .95, p < .00; falls (kappa = .74), path shape (kappa = .78), and step direction (kappa = .85), ps < .001).
Gait measures
During free play, infants sometimes stepped on the instrumented floor. We used all complete bouts or sections of bouts that occurred on the instrumented floor in which infants took at least four continuous steps (to include a complete gait cycle on each leg), regardless of path shape or step direction; no steps were removed from these bouts during processing. Infants contributed 1–69 bouts (M = 17.42) for analyses of spontaneous gait (993 bouts in total). We calculated three standard gait measures: speed (distance from first to last step divided by time), step length (front-to-back distance between steps), and step width (side-to-side distance between steps); see Figure 2E. However, because infants did not always take forward steps in straight lines, values for step lengths and step widths were sometimes negative; thus, we calculated absolute values for each step.
As is customary in the straight-path task, the experimenter removed 1–3 steps from the beginning and end of each sequence to eliminate steps when infants were ramping up and slowing down, and eliminated segments that were not forward, continuous, and straight (Bisi et al., 2014; Bisi & Stagni, 2015; Ivanenko et al., 2005; Van de Walle et al., 2010; Yaguramaki & Kimura, 2002). Of the remaining segments, only those with at least four steps were used for analyses. Infants contributed 1–21 sequences (M = 5.28, N = 682 sequences in total) to calculations of gait measures.
Results
We used generalized estimating equations (GEEs) rather than ANOVAs for all analyses to account for the subset of infants who had repeated sessions (Hardin & Hilbe, 2003). For free play, we tested effects of walking age on bout length and then tested effects of walking age and bout length on path shape and step direction. Statistical comparisons of task effects necessarily included only the subset of infants with data in both tasks. We did not include walking age or bout length in tests of task effects on these measures because walking age was confounded with compliance (younger infants were less likely to “follow instructions” by walking on the mat) and bout length (most infants did not produce short bouts in the straight-path task). Analyses of gait measures tested the effects of walking age, task, and sequence selection.
Prevalence of Continuous, Straight-Path, Forward Steps
To preview the results below, findings confirmed our hypothesis that non-canonical bouts would be over-represented in free play. Although infants occasionally produced long, straight, forward bouts during free play comparable to those selected for analyses in the straight-path task, short bouts, curved paths, and omnidirectional steps were more prevalent during free play. However, contrary to our hypothesis, the high rates of these behaviors during free play did not decrease with walking age.
Bout length
As shown by the distribution of the blue bars in Figure 3A, short bouts of 1–3 steps were prevalent during free play. Every infant produced short bouts (range = 10%–69% of bouts, M = 34.18%). However, as shown in the figure, infants also produced long bouts during free play. Every infant spontaneously produced bouts with at least 12 steps, and 84% of infants occasionally produced very long bouts of 31–121 steps—longer than the maximum bout length allowed by the length of the mat in the straight-path task.
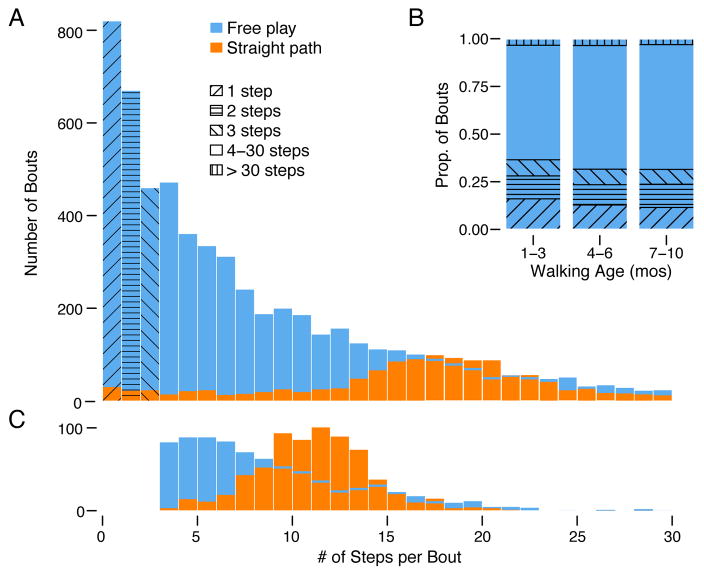
Figure 3.
Number of steps per bout. (A) Frequency distribution of bout length in free-play and straight-path tasks. Height of the blue bars denotes the number of walking bouts during free play, N = 69 sessions. Note, the x-axis excludes bouts between 31 and 105 steps. Height of the orange bars denotes walking bouts during the straight-path task (including short bouts not useable for gait measures); N = 135 sessions. (B) Average proportion of bouts of various lengths in free play task. Data are represented for novice (1–3 months), intermediate (4–6 months), and expert groups (7–10 months) by walking age; N = 69 sessions. (C) Frequency distribution of bout length (after processing) used for analyses of gait measures in free play and straight-path tasks. The y-axis shows the number of bouts. Height of the blue bars denotes bouts used for calculating gait measures during the free-play task. The left side of the distribution begins abruptly at 4 steps due to data processing constraints. The height of the orange bars denotes bouts used for calculating gait measures during the straight-path task. The distribution begins at 4 steps due to processing constraints and ends at 22 steps due to the length of the instrumented mat. Note, the distribution for free-play gait (N = 57 sessions) is partially hidden behind the distribution for straight-path gait (N = 130 sessions); the height of the hidden blue bars is denoted by the horizontal blue lines.
Bout length during free play did not change over development (Figure 3B). During free play, the GEEs showed no change with walking age in the proportion of short bouts, mean bout length, or longest bout, χ2 < 2.82, ps > .09. On average, bout length was shorter in free play (8.87 steps) than in the straight-path task (17.91 steps), χ2 = 545.15, p < .001. As shown by the distribution of orange bars in Figure 3A, short bouts were more prevalent during free play (M = 34.18% of bouts, SD = 11.51) compared with the straight-path task (M = 4.37%, SD = 8.65), χ2 = 483.49, p < .001, but 28% of infants produced short bouts in the straight-path task, even when encouraged to walk continuously.
Falls
Infants fell in both tasks, Ms = 15.80 falls/hour in free play (SD = 16.26) and 8.08 falls/hour in the straight-path task (SD = 19.50), χ2 = 6.45, p < .05. However, falls were rare relative to the total number of steps in both tasks (Ms = 0.009 falls/step and 0.003 falls/step for free play and straight path, respectively), and falls were spread evenly across bout lengths. Thus, the prevalence of short bouts was not due to falling. Most bouts ended because infants stopped walking, not because they fell.
Path shape
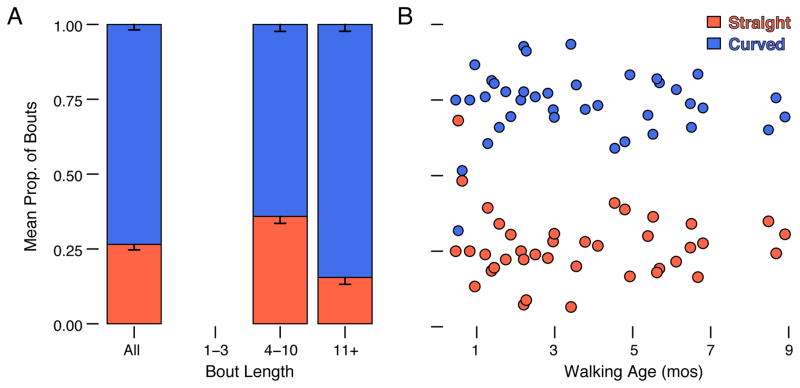
As shown by the left-most bar in Figure 4A, curved paths, not straight ones, characterized spontaneous walking during free play. Every infant produced bouts with curved paths (range = 32%–93% of bouts, M = 73.49%). As shown by the two right bars in Figure 4A, curved paths were more likely in longer bouts (M = 84.51%, SD = 14.38) than shorter ones (M = 64.13, SD = 14.50), likely because infants had more opportunities to turn in bouts with more steps, χ2 = 5.18, p < .05. Despite the high prevalence of curved paths during free play, 14.8% of curved paths contained straight segments with at least four continuous steps (Figure 2C). Figure 4B shows that the prevalence of curved paths did not change with walking age during free play, χ2 = 0.29, p = .59. Curved paths were more prevalent during free play than in the straight-path task (M = 35.50% bouts, SD = 23.95), χ2 = 124.41, p < .001, but 87% of infants produced curved paths in the straight-path task, even when encouraged to walk in a straight line.
Figure 4.
Mean proportion of curved and straight bouts during free play (N = 40 sessions). (A) Left-most bar shows all bouts and two right bars show bouts with 4–10 steps and 11+ steps, respectively. Note, bouts with 1–3 steps were too short to be scored for path shape as represented by the empty spot along the x-axis. Error bars denote standard errors. Blue bars denote curved paths and orange bars denote straight paths. (B) Scatterplot showing proportion of curved (blue symbols) and straight bouts (orange symbols) by walking age. Each symbol represents the proportion of curved or straight bouts in that session.
Step direction
As shown by the left-most bar in Figure 5A, most bouts in free play were not composed entirely of forward steps. Every infant produced bouts with omnidirectional steps (range = 65%–100% of bouts, M = 81.69%). As shown by the three middle bars in Figure 5A, the proportion of bouts containing only forward steps did not vary with bout length, χ2 = 4.86, p = .09. However, as shown by the two right-most bars in Figure 5A, the proportion of bouts containing only forward steps was greater for straight paths than for curved ones. For the straight bouts, the large proportion of “mixed” steps resulted from single sideways or backward steps while maintaining the same direction of heading. The small proportion of straight bouts with no forward steps occurred when infants took a series of sideways or backward steps. As shown in Figure 5B, the prevalence of bouts with all forward steps did not change with walking age in free play, χ2 = 0.46, p = .50. Bouts with mixed or no forward steps were more prevalent during free play than in the straight-path task (M = 17.27% bouts, SD = 17.45), χ2 = 482.97, p < .001, but 67% of infants produced omnidirectional steps even when encouraged to walk forward.
Figure 5.
Mean proportion of bouts in the free-play task containing all forward steps, a mixture of forward and non-forward steps, and no forward steps (N = 40 sessions). (A) Left-most bar shows all bouts. Middle three bars show bouts with 1–3 steps, 4–10 steps and 11+ steps, respectively. Two right-most bars show step direction for free-play bouts scored as curved paths and straight paths, respectively. Error bars denote standard errors. (B) Scatterplot shows proportion of free-play bouts with all forward, mixed, and no forward steps by walking age. (C) Procedure and effects of progressively eliminating steps at the beginning and end of each free-play bout on proportion of omnidirectional steps.
When in a bout did infants take omnidirectional steps? For the free-play data, we progressively tested the effect of eliminating the first and last steps in a bout, the first and last two steps in a bout, the first and last three steps in a bout, and so on (Figure 5C). The percentage of omnidirectional steps remaining decreased from 28% omnidirectional steps with all steps included to 17% omnidirectional steps with five steps removed from the beginning and end of the bouts. Removing more than five steps at each end of the bout did not change the percentage of omnidirectional steps. The decrease means that omnidirectional steps were more prevalent at the beginning and end of bouts. But the fact that bouts contained at least 17% non-forward steps regardless of whether steps were removed from the beginning and end of the bout means that omnidirectional steps pervade the entire bout regardless of bout length.
Developmental Changes and Individual Differences in Gait
Figure 3C shows the distribution of bouts used for analyzing gait measures in the free-play and straight-path tasks. To preview the results below, our expectation regarding task differences in gait measures was largely supported. In terms of the magnitude of differences, infants walked faster and took longer steps in the straight-path task compared with free play, but step widths were similar across tasks. The more important questions concerned improvements with walking age and the stability of individual differences. Fortunately (for ourselves and other researchers who have invested in the straight-path task), step length and step width showed the same developmental trajectory across tasks. Although speed showed a steeper trajectory for the straight-path task compared with free play, infants showed considerable overlap in speed in both tasks. Moreover, the stability of individual differences across tasks was strongly supported: Infants with more mature gait patterns in the straight-path task were also “better walkers” in free play.
Developmental changes in gait
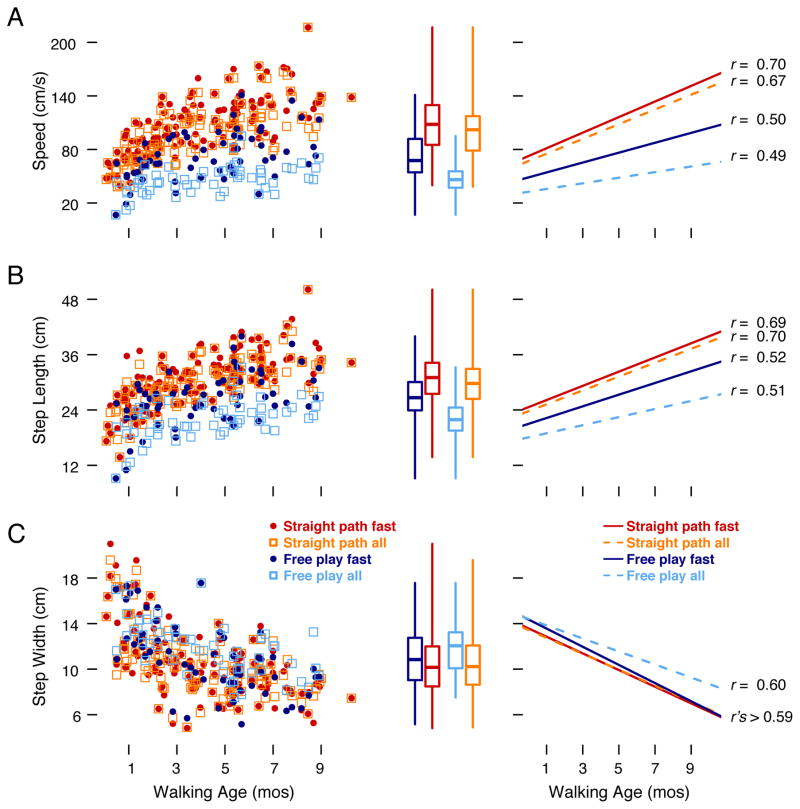
Because infants cannot follow instructions, they walk at self-produced speeds that are likely to vary widely across sequences. Speed is strongly correlated with step length—imagine the front-to-back distance of your steps while walking slowly and quickly (Carty & Bennett, 2009; Murray, 1967; Murray, Mollinger, Gardner, & Sepic, 1984). In infants, faster, longer steps are indicative of more mature gait (Bril & Breniere, 1992; Hallemans, De Clercq, & Aerts, 2006; Van Dam, Hallemans, Truijen, & Aerts, 2010). Thus, some researchers select infants’ best sequences for analyses (e.g., Cole, Lingeman, & Adolph, 2012; Looper & Chandler, 2013) and some analyze all useable sequences (e.g., Holt, Saltzman, Ho, & Ulrich, 2007; Looper, Wu, Barroso, Ulrich, & Ulrich, 2006). Here, we analyzed the data both ways—including all useable sequences (at least four forward steps) and selecting the two “best” (fastest, long) sequences. We also used the two best sequences to assess test-retest reliability in each task (i.e., whether infants’ gait measures were reproducible). For free play, rs were .94, .89, and .66 for speed, step length, and step width, respectively, all ps < .01; for the straight-path task, rs were .97, .95, and .84 for speed, step length, and step width, respectively, all ps < .01
Figure 6 illustrates effects of task (free play versus straight-path), sequence selection (all sequences versus two fastest sequences), and walking age. Table 1 shows main effects for task, sequence selection, and walking age. Infants walked faster and took longer steps in the straight-path task compared with free play, but showed no differences for step width; accordingly, the GEEs confirmed a main effect for task for speed and step length, but not for step width (row 1 in the table). Infants also took longer steps in the two fastest sequences compared with all sequences, but sequence selection did not affect step width; the GEEs confirmed a main effect for sequence selection for speed and step length, but not for step width (row 2 in the table). Importantly, the fastest sequences in free play showed considerable overlap with the data for the straight-path task (see symbols in the scatterplots and the whiskers in the box plots), meaning that infants spontaneously produced similar gait sequences during free play as in the straight-path task. As shown by the slope of the scatterplots and curves in Figure 6A–C (rs ranged from .49–.70, all ps < .001) and by row 3 in the table, all three measures improved with walking age—infants took faster, longer, narrower steps.
Figure 6.
Gait measures for (A) speed, (B) step length, and (C) step width. Scatterplots in left panel show improvements with walking age in the free-play task (blue symbols, N = 57 sessions) and straight-path task (red symbols, N = 130 sessions) for all bouts (open symbols) and the two fastest (closed symbols) bouts in each task of spontaneous walking and the straight-line paradigm; each symbol represents the average for an individual infant’s session. The scatterplots highlight the wide range in individual differences across walking age. Box plots in the middle panel show the 25th to 75th percentiles (box size), median (horizontal line), and ranges (whiskers) for each measure. Line graphs in the right panel show linear curve fits and correlation coefficients for the data in the scatterplots; blue lines denote free play, red lines denote the straight-path task, solid lines represent all sequences, and dashed lines represent the two fastest sequences.
Table 1.
Results of GEEs for main effects of task (free play versus straight-path), sequence selection (all sequences versus two fastest sequences), and walking age for speed, step length, and step width
| Speed | Step length | Step width | ||||
|---|---|---|---|---|---|---|
| χ2 | p | χ2 | p | χ2 | p | |
| Task | 41.86 | <.001 | 22.94 | <.001 | 2.49 | .12 |
| Sequence | 43.86 | <.001 | 34.21 | <.001 | .040 | .85 |
| Walking age | 66.95 | <.001 | 86.38 | <.001 | 75.95 | <.001 |
The analyses also revealed several interactions. The GEEs showed a task × walking age interaction for speed, χ2 = 18.06, p < .001, meaning that infants with more days of walking walked faster in the straight-path task than in free play. The GEEs also showed a sequence selection × walking age interaction for all three measures: speed (χ2 = 11.29, p < .01), step length (χ2 = 6.96, p < .01), and step width (χ2 = 8.48, p < .01). This means that infants’ gait looked more mature with walking age when the two fastest sequences were selected.
Individual differences and intraindividual variability in gait
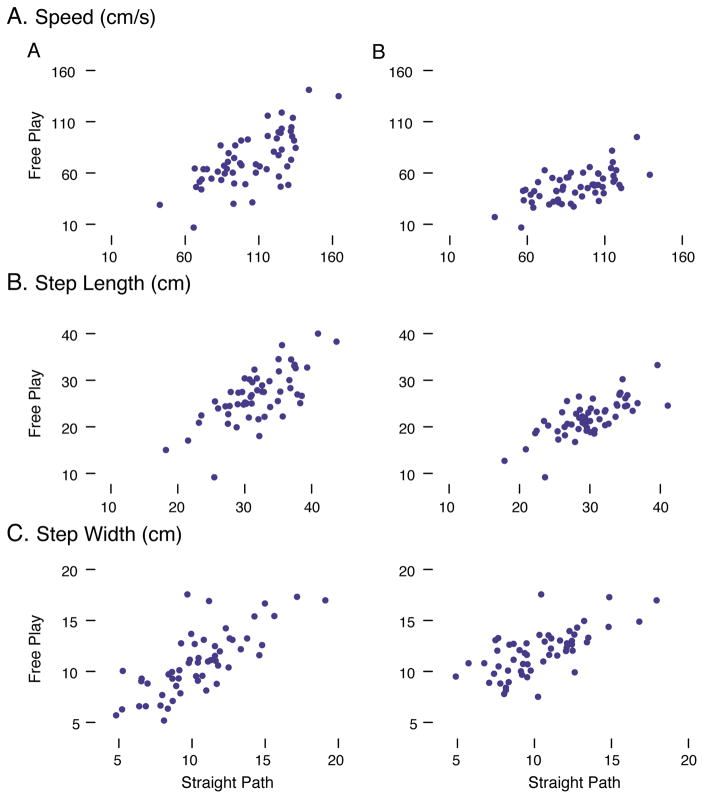
Individual differences were stable across the two tasks, regardless of sequence selection (Figure 7). Infants with more mature gait patterns in the straight-path task (faster, longer, narrower steps) also displayed more mature gait patterns during spontaneous walking in free play, regardless of whether we compared all sequences produced by each child or only the two fastest sequences. Moreover, as expected, gait measures were intercorrelated within tasks and processing method, for free play r(56)s = .61 to .92, ps < .001 and for straight path r(129)s = .51 to .88, ps < .001.
Figure 7.
Scatterplots showing correlations between straight-path and free-play tasks for (A) speed, step length, and step width with all sequences selected, and (B) speed, step length, and step width with the two fastest sequences selected. N = 56 sessions where infants contributed gait data to both tasks.
Intraindividual variability was higher in the free play task for speed, step length, and step width compared with the straight-path task; the coefficient of variation for all three measures was significantly higher during free play compared with the straight-path task, χ2s > 5.36, ps < .05.
Discussion
This study addressed the tension between experimental control and behavioral complexity endemic in developmental research on motor skills, language, attention, visual exploration, and so on (Bronfenbrenner, 1977; Dahl, 2017; Tamis-LeMonda et al., in press). Our reliance as a field on standard lab tasks over more natural, everyday behaviors reflects our comfort with traditional, tried-and-true tasks, limitations of recording technologies, and, in some cases, a failure of imagination.
We used the development of infant walking as a model system to assess the costs and benefits of simplifying a complex and variable behavior versus characterizing the natural behavior with all its inherent messy complexity. Traditionally, infant walking has been studied in the context of a simplified “straight-path” task in which infants are encouraged to produce long sequences of forward steps along a straight path and any “non-canonical” bouts are eliminated during data processing. However, previous work has not assessed the extent to which walking in the straight-path task is representative of the development of natural walking, presumably the phenomenon researchers wish to explain. Thus, we compared infant walking in the traditional straight-path task, which eliminates much of the variability and complexity, with spontaneous walking during free-play, which retains all of the natural richness.
The Cost and Benefits of Naturalistic versus Simplified Tasks
The potential cost of simplifying a complex behavior is to risk losing sight of the critical aspects of the phenomenon to be explained. For example, a 5-minute traditional structured play task fails to capture the normal pattern of infants’ everyday language experiences, which includes far less talk, long periods of silence, and less diversity of words (Tamis-LeMonda et al., in press). Traditional structured tasks of infants’ visual exploration of scenes on a computer monitor fail to capture the influence of posture on what infants see in everyday contexts (Frank, Simmons, Yurovsky, & Pusiol, 2013; Jayaraman, Fausey, & Smith, 2015; Kretch et al., 2014).
Similarly, simplifying walking with the straight-path task comes at a tremendous cost. As we hypothesized, infants’ spontaneous walking in free play was characterized by frequent short bouts with omnidirectional steps along curved paths. Although infants also produced such non-canonical bouts in the straight-path task, they did so less frequently, and of course such bouts are eliminated during standard data processing. Put another way, essential characteristics of infant walking are lost from the straight-path task. And these aspects of walking are precisely the characteristics that make walking functional, that allow infants to flexibly adapt their walking patterns to steer through a cluttered environment. These findings will be no surprise to researchers in motor development. Nonetheless, researchers rely on the straight-path task because they expect spontaneous walking in free play to be too noisy to yield useful measures of walking skill and because their recording technologies are impractical for capturing spontaneous walking as infants roam freely around a large room. But is there really an empirical cost to using the free-play task instead of the straight-path task?
The potential cost of analyzing a complex behavior in all its complexity is to risk losing signal in noise. In alignment with previous work, the current study considered developmental improvements in gait measures to be an important “signal” (for reviews, see Adolph & Robinson, 2013, 2015; Ivanenko et al., 2007). We found that observing infants’ gait during free play came at no cost in terms of empirical findings and theory. And this is likely to be a surprise to researchers in motor development. The free-play task yielded largely the same developmental story regarding improvements in gait as in the straight-path task. As shown in Figure 6, infants took faster, longer, narrower steps with increase in walking age. These findings held for free play despite the fact that infants mostly walked curved paths with omnidirectional steps. Indeed, an important contribution of the current study is our demonstration that with the appropriate recording equipment (here, a large instrumented floor), researchers can obtain robust measures of infants’ gait during spontaneous walking in free play.
Moreover, the developmental trajectories shown in Figure 6 coupled with the stability of individual differences shown in Figure 7 provides a strong demonstration of the ecological validity of gait measures collected in the straight-path task. Similarly, individual differences among infants’ language experiences are stable across a 5-minute traditional structured play task and 45 minutes of natural activity in the home (Tamis-LeMonda et al., in press). Indeed, infants’ fast sequences during free play overlapped with their performance in the straight-path task (see Figure 6 left column), and each infant spontaneously produced straight sequences with a comparable number of forward steps during free play as they did in the straight-path task (see Figure 4A). In fact, we replicated the same developmental improvements for gait measures in free play when we analyzed only the first six gait sequences (what we aimed to collect in the straight-path task).
Put another way, the straight-path task tells us only what infants can do (their best performance), whereas the free-play task tells us both what they can do and what they actually do. Similarly, in the language domain, peak language input during natural home activity is comparable to the intensity of language input during structured play (Tamis-LeMonda et al., in press). Thus, the free-play task confers considerable benefits for understanding improvements in walking during the more complex behaviors involved in controlling balance and forces while taking omnidirectional steps along curved paths. Moreover, understanding the development of gait during spontaneous, natural walking has important implications for studying the development of walking in infants with impairments.
A final consideration of “cost” includes practical and financial costs. One practical consideration is that infants do not want to walk in straight, forward, continuous paths; they want to play! Given that it is easier and less intrusive to collect spatial-temporal footfall measures of gait during free play, there is little reason for researchers to rely on the straight-path task for these measures. In other words, for footfall measures of gait, the free-play task with all its inherent messiness yields all the benefits of the simplified straight-path task with no additional costs. Moreover, practical costs in terms of labor and time of processing gait data from the instrumented floor and video coding other measures (bout length, path shape, step direction) are equivalent across the two tasks. However, for other types of measures (muscle actions, joint angles, forces, etc.), current technologies do not readily allow recording during unconstrained free play. As in other areas of science (e.g., mobile brain imaging), consumer demand for increased ecological validity can push advances in technologies (e.g., wireless EMG and motion tracking, larger recording areas) and reduction in financial cost.
What Changes and What Is Constant Across Development
We observed infants across the spectrum of walking age, including novice walkers with only a few weeks of practice and experienced walkers with several months of practice. Results indicate that spatiotemporal footfall measures of infants’ gait are robust indices of how well infants walk and how quickly they improve, regardless of task. However, the two fastest sequences were a more sensitive measure of development as shown by the interactions between sequence selection and walking age for speed and step length, but not for step width. Why not step width?
In real time, young adults generally maintain a constant step width regardless of how fast they walk (Murray, 1967; Murray et al., 1984). But adults increase their speed in part by increasing step length until they are running (Diedrich & Warren, 1995; Jordan, Challis, & Newell, 2007; Murray, 1967; Murray et al., 1984). Like adults, real-time changes in infants’ speed do not affect their step width; but real-time increases in speed accompany increases in infants’ step length (Bril & Breniere, 1992; Hallemans et al., 2006). In addition to these real-time relations, measures of speed, step length, and step width are related over development (Adolph et al., 2003; Bril & Breniere, 1992; Hallemans et al., 2006). But the developmental relations among these measures reflect concurrent improvements in walking skill. Thus, in terms of development, infants’ “best” performance (their fastest sequences) is a stronger index of improvements in speed and step length than their average performance (all sequences). Step width is robust to changes in speed and yields the same developmental outcome regardless of which sequences are selected.
The developmental story about the prevalence of non-canonical walking (short bouts, curved paths, omnidirectional steps) during free play was surprising. We hypothesized that non-canonical walking would decrease with walking age, but it did not. Moreover, omnidirectional steps occurred at all locations in the bout. Together, the findings about gait and non-canonical walking indicate that short bouts, curved paths, and omnidirectional steps are not a by-product of poor balance control in novice walkers. One- to three-step bouts and backward steps, for example, are not merely the result of novice walkers’ attempts to recapture balance; curved paths do not merely reflect novices’ inability to control the path of progression. Rather, short bouts with frequent starting and stopping, changes in speed and path direction, and stepping in all directions are core characteristics of infant walking at all stages of development—even when infants have become competent walkers. Moreover, the prevalence of non-canonical walking across all periods of development indicates that infants need not wait for sufficient skill to perform these more complex maneuvers.
Implications for What and How Infants Learn
If short bouts, curved paths, and omnidirectional steps are not signs of poor or immature walking, how should these aspects of infant walking be interpreted? We offer three suggestions. First, walking is not the rote repetition of forward alternating steps. Walking—like talking and thinking—is a fundamentally creative act (Bernstein, 1996; Thelen, 1996). Infants do not learn to step the same way over and over, just as they do not learn to produce the same utterances or think the same thoughts over and over. A second, related suggestion is that infants are learning to produce those aspects of walking that make upright locomotion functional. That is, infants are learning—from their first upright steps—how to generate the rich and infinitely varied combination of movements that allows them to explore and navigate the larger environment (Adolph, Cole, & Vereijken, 2015). Indeed, robots trained to walk using algorithms similar to infants’ twisting omnidirectional paths are far more adaptive and functional than robots trained to walk in straight lines (MacAlpine, Barrett, Urieli, Vu, & Stone, 2012).
Third, if we are correct in our first two suggestions, then learning to walk is more difficult than previously imagined. Decerebrate cats, anencephalic infants, and newborns can produce alternating forward steps using only spinal pattern generators (Adolph & Berger, 2006; Duysens & Van de Crommert, 1998). But only an intimate coupling between perception and action would allow infants to control the two sides of their body differently to produce curved paths, to cope with gravitational and inertial forces to initiate and terminate bouts every few steps, and to plan which direction to plant their feet to steer around obstacles or step in place. From their very first steps, infants are acquiring strength, balance control, and control of pendular mechanisms in the context of highly complex and varied movements. They do not first learn to walk in a straight line and then add on the complexity.
A final point: This study suggests that gait measures such as step length and speed, which can be collected in either free play or the straight-path task are a robust index of the maturity of infants’ gait. In contrast, measures such as bout length, path shape, and step direction are not. But these messy, complex, and variable walking patterns produced during free play comprise the training set for the dramatic improvements that researchers have documented over the last century of work. What’s needed now is a characterization of infants’ natural everyday experiences walking over different surfaces, through different environmental layouts, while involved in different tasks with different goals.
Conclusions
When weighing the costs and benefits of simplifying a phenomenon to make it tractable for rigorous study, sometimes complexity and noise do not incur empirical and theoretical cost and may enjoy considerable benefits. In the case of infant walking—as in the case of infant language and visual exploration—new technologies allow us to validate individual differences and developmental trajectories in simplified laboratory tasks and to study important aspects of the natural phenomenon that were previously unavailable. Perhaps Gibson (1979) said it best: “It is not true that the laboratory can never be like life. The laboratory must be like life!” (p. 109).
Research Highlights.
Decisions whether to simplify a developmental phenomenon or study the behavior in all its complexity influence the data obtained and conclusions drawn about development.
Studying infant walking during free play in all its complexity incurs no empirical or conceptual costs compared with the traditional simplified “straight-path” task, and yields considerable benefits.
Walking during free play is an equally robust indicator of development as in the straight-path task, but reveals essential characteristics of infant walking (short bouts, curved paths, omnidirectional steps) that are eliminated in the straight-path task.
A focus on spontaneous walking—ostensibly, the phenomenon we wish to explain—yields important insights into the problems infants solve while learning to walk.
Acknowledgments
The project described was supported by Award Number R37HD033486 from NICHD to KEA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD.
We thank Orit Herzberg-Keller, Carli Heiman, and Joshua Schneider for video-coding and Daniella Corbetta for her discussions of the data. Do Kyeong Lee, Whitney Cole, and Laura Golenia agree to share first authorship.
References
- Adolph KE, Berger SE. Motor development. In: Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2 Cognition, perception, and language (6th ed., Vol. 2 Cognitive Processes. New York: Wiley; 2006. pp. 161–213. [Google Scholar]
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, Lingeman JM, … Sotsky RB. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science. 2012;23:1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, Vereijken B. Intra-individual variability in the development of motor skills in childhood. In: Diehl M, Hooker K, Sliwinski M, editors. Handbook of intra-individual variability across the lifespan. New York: Routledge/Taylor & Francis Group; 2015. pp. 59–83. [Google Scholar]
- Adolph KE, Robinson SR. The road to walking: What learning to walk tells us about development. In: Zelazo P, editor. Oxford handbook of developmental psychology. New York: Oxford University Press; 2013. pp. 403–443. [Google Scholar]
- Adolph KE, Robinson SR. Motor development. In: Liben L, Muller U, editors. Handbook of child psychology and developmental science (7th ed., Vol. 2 Cognitive Processes. New York: Wiley; 2015. pp. 114–157. [Google Scholar]
- Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Development. 2003;74:474–497. doi: 10.1111/1467-8624.7402011. [DOI] [PubMed] [Google Scholar]
- Bernstein NA. On dexterity and its development. In: Latash ML, Turvey MT, editors. Dexterity and its development. Mahwah, NJ: Erlbaum; 1996. pp. 3–244. [Google Scholar]
- Berthier NE, Keen RE. Development of reaching in infancy. Experimental Brain Research. 2006;169:507–518. doi: 10.1007/s00221-005-0169-9. [DOI] [PubMed] [Google Scholar]
- Bisi MC, Riva F, Stagni R. Measures of gait stability: Performance on adults and toddlers at the beginning of independent walking. Journal of NeuroEngineering and Rehabilitation. 2014;11:131–140. doi: 10.1186/1743-0003-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisi MC, Stagni R. Evaluation of toddler different strategies during the first six-months of independent walking: A longitudinal study. Gait and Posture. 2015;41:574–579. doi: 10.1016/j.gaitpost.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Borkhoff CM, Heale LD, Anderson LN, Tremblay MS, Maguire JL, Parkin PC, Birken CS. Objectively measured physical activity of young Canadian children using accelerometry. Applied Physiology, Nutrition, and Metabolism. 2015;40:1302–1308. doi: 10.1139/apnm-2015-0164. [DOI] [PubMed] [Google Scholar]
- Bril B, Breniere Y. Postural requirements and progression velocity in young walkers. Journal of Motor Behavior. 1992;24:105–116. doi: 10.1080/00222895.1992.9941606. [DOI] [PubMed] [Google Scholar]
- Bril B, Dupuy L, Dietrich G, Corbetta D. Learning to tune the antero-posterior propulsive forces during walking: A necessary skill for mastering upright locomotion in toddlers. Experimental Brain Research. 2015;233:2903–2912. doi: 10.1007/s00221-015-4378-6. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Toward an experimental ecology of human development. American Psychologist. 1977;32:513–531. [Google Scholar]
- Carty CP, Bennett MB. The use of dimensionless scaling strategies in gait analysis. Human Movement Science. 2009;28:218–225. doi: 10.1016/j.humov.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Chang CL, Kubo M, Buzzi U, Ulrich B. Early changes in muscle activation patterns of toddlers during walking. Infant Behavior and Development. 2006;29:175–188. doi: 10.1016/j.infbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignetti F, Kyvelidou A, Harbourne RT, Stergiou N. Anterior-posterior and medial-lateral control of sway in infants during sitting acquisition does not become adult-like. Gait and Posture. 2011;33:88–92. doi: 10.1016/j.gaitpost.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JE, Phillips SJ. A longitudinal study of intralimb coordination in the first year of independent walking: A dynamical systems analysis. Child Development. 1993;64(4):1143–1157. doi: 10.1111/j.1467-8624.1993.tb04192.x. [DOI] [PubMed] [Google Scholar]
- Cole WG, Lingeman JM, Adolph KE. Go naked: Diapers affect infant walking. Developmental Science. 2012;15:783–790. doi: 10.1111/j.1467-7687.2012.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WG, Robinson SR, Adolph KE. Bouts of steps: The organization of infant exploration. Developmental Psychobiology. 2016;58:341–354. doi: 10.1002/dev.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta D, Thelen E. The developmental origins of bimanual coordination: A dynamic perspective. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:502–522. doi: 10.1037//0096-1523.22.2.502. [DOI] [PubMed] [Google Scholar]
- Dahl A. Ecological commitments: Why developmental science needs naturalistic methods. Child Development Perspectives. 2017;11:79–84. doi: 10.1111/cdep.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich FJ, Warren WH. Why change gaits? Dynamics of the walk-run transition. Journal of Experimental Psychology. 1995;21:183–202. doi: 10.1037//0096-1523.21.1.183. [DOI] [PubMed] [Google Scholar]
- Duysens J, Van de Crommert HW. Neural control of locomotion; Part 1: The central pattern generator from cats to humans. Gait and Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Franchak JM, Kretch KS, Soska KC, Adolph KE. Head-mounted eye tracking: A new method to describe infant looking. Child Development. 2011;82:1738–1750. doi: 10.1111/j.1467-8624.2011.01670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Simmons K, Yurovsky D, Pusiol G. Developmental and postural changes in children’s visual access to faces. In: Knauff M, Pauen M, Sebanz N, Wachsmuth I, editors. Proceedings of the 35th Annual Meeting of the Cognitive Science Society; Austin, TX: Cognitive Science Society; 2013. pp. 454–459. [Google Scholar]
- Garciaguirre JS, Adolph KE, Shrout PE. Baby carriage: Infants walking with loads. Child Development. 2007;78:664–680. doi: 10.1111/j.1467-8624.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The ecological approach to visual perception. Boston, MA: Houghton Mifflin Company; 1979. [Google Scholar]
- Hallemans A, De Clercq D, Aerts P. Changes in 3D joint dynamics during the first 5 months after the onset of independent walking: A longitudinal follow-up study. Gait and Posture. 2006;24:270–279. doi: 10.1016/j.gaitpost.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hallemans A, De Clercq D, Otten B, Aerts P. 3D joint dynamics of walking in toddlers: A cross-sectional study spanning the first rapid development phase of walking. Gait and Posture. 2005;22:107–118. doi: 10.1016/j.gaitpost.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Developmental Psychobiology. 2003;42:368–377. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman & Hall CRC; 2003. [Google Scholar]
- Hnatiuk J, Ridgers ND, Salmon J, Campbell K, McCallum Z, Hesketh K. Physical activity levels and patterns of 19-month-old children. Medical Science and Sports Exercise. 2012;44:1715–1720. doi: 10.1249/MSS.0b013e31825825c4. [DOI] [PubMed] [Google Scholar]
- Holt KG, Saltzman E, Ho CL, Kubo M, Ulrich BD. Discovery of the pendulum and spring dynamics in the early stages of walking. Journal of Motor Behavior. 2006;38:206–218. doi: 10.3200/JMBR.38.3.206-218. [DOI] [PubMed] [Google Scholar]
- Holt KG, Saltzman E, Ho CL, Ulrich BD. Scaling of dynamics in the earliest stages of walking. Physical Therapy. 2007;87(11):1458–1467. doi: 10.2522/ptj.20060299. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Dan B, Cheron G, Lacquaniti F. Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. The Journal of Experimental Biology. 2004;207:3797–3810. doi: 10.1242/jeb.01214. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Cappellini G, Lacquaniti F. Kinematics in newly walking toddlers does not depend upon postural stability. Journal of Neurophysiology. 2005;94:754–763. doi: 10.1152/jn.00088.2004. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Lacquaniti F. Development of independent walking in toddlers. Exercise and Sport Sciences Reviews. 2007;35:67–73. doi: 10.1249/JES.0b013e31803eafa8. [DOI] [PubMed] [Google Scholar]
- Jayaraman W, Fausey CM, Smith LB. The faces in infant-perspective scenes change over the first year of life. PLoS ONE. 2015 doi: 10.1371/journal.pone.0123780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait and Posture. 2007;26:128–134. doi: 10.1016/j.gaitpost.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Kretch KS, Franchak JM, Adolph KE. Crawling and walking infants see the world differently. Child Development. 2014;85:1503–1518. doi: 10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Ulrich B. A biomechanical analysis of the ‘high guard’ position of arms during walking in toddlers. Infant Behavior and Development. 2006;29:509–517. doi: 10.1016/j.infbeh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Lee HM, Bhat A, Scholz JP, Galloway JC. Toy-oriented changes during early arm movements IV: Shoulder-elbow coordination. Infant Behavior and Development. 2008;31:447–469. doi: 10.1016/j.infbeh.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Looper J, Chandler LS. How do toddlers increase their gait velocity? Gait and Posture. 2013;37:631–633. doi: 10.1016/j.gaitpost.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Looper J, Wu J, Barroso RA, Ulrich D, Ulrich BD. Changes in step variability of new walkers with typical development and with down syndrome. Journal of Motor Behavior. 2006;38:367–372. doi: 10.3200/JMBR.38.5.367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlpine P, Barrett S, Urieli D, Vu V, Stone P. Design and optimization of an omnidirectional humanoid walk: A winning approach at the RoboCup 2011 3D simulation competition. Vol. 1. Palo Alto, CA: Association for the Advancement of Artificial Intelligence; 2012. [Google Scholar]
- McGraw MB, Breeze KW. Quantitative studies in the development of erect locomotion. Child Development. 1941;12:267–303. [Google Scholar]
- Murray MP. Gait as a total pattern of movement. American Journal of Physical Medicine. 1967;46:290–333. [PubMed] [Google Scholar]
- Murray MP, Mollinger LA, Gardner GM, Sepic SB. Kinematic and EMG patterns during slow, free, and fast walking. Journal of Orthopaedic Research. 1984;2:272–280. doi: 10.1002/jor.1100020309. [DOI] [PubMed] [Google Scholar]
- Shirley MM. The first two years: A study of twenty-five babies. Postural and locomotor development. Vol. 1. Minneapolis, MN: University of Minnesota Press; 1931. [Google Scholar]
- Smith LB, Yu C, Pereira AF. Not your mother’s view: The dynamics of toddler visual experience. Developmental Science. 2011;14:9–17. doi: 10.1111/j.1467-7687.2009.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Kuchirko Y, Luo R, Escobar K. Power in methods: Language to infants in structured and naturalistic contexts. Developmental Science. doi: 10.1111/desc.12456. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Learning to walk: Ecological demands and phylogenetic constraints. Advances in Infancy Research. 1984;3:213–260. [Google Scholar]
- Thelen E. The improvising infant: Learning about learning to move. In: Merrens MR, Brannigan GG, editors. The developmental psychologists: Research adventures across the lifespan. New York: McGraw-Hill; 1996. pp. 21–36. [Google Scholar]
- Van Dam M, Hallemans A, Truijen S, Aerts P. A cross-sectional study about the relationship between morphology and step-time parameters in children between 15 and 36 months. Gait and Posture. 2010;32:400–404. doi: 10.1016/j.gaitpost.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Van Dam M, Hallemans A, Truijen S, Segers V, Aerts P. A cross-sectional study about the relationship between morphology and kinematic parametersin children between 15 and 36 months. Gait and Posture. 2011;34:159–163. doi: 10.1016/j.gaitpost.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Van de Walle P, Desloovere K, Truijen S, Gosselink R, Aerts P, Hallemans A. Age-related changes in mechanical and metabolic energy during typical gait. Gait and Posture. 2010;31:495–501. doi: 10.1016/j.gaitpost.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A. Changes in postural control across the lifespan—A systems approach. Physical Therapy. 1990;70:799–807. doi: 10.1093/ptj/70.12.799. [DOI] [PubMed] [Google Scholar]
- Yaguramaki N, Kimura T. Acquirement of stability and mobility in infant gait. Gait and Posture. 2002;16:69–77. doi: 10.1016/s0966-6362(01)00205-3. [DOI] [PubMed] [Google Scholar]
- Yu C, Smith LB. Multiple sensory-motor pathways lead to coordinated visual attention. Cognitive Science. 2017;41:5–31. doi: 10.1111/cogs.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]