Abstract
Rheumatoid arthritis is associated with reduced kidney function, possibly due to chronic inflammation or the use of nephrotoxic therapies. However, little is known about the effects of using the newer novel non-nephrotoxic biologic agents on the risk of incident chronic kidney disease (CKD). To study this we used a cohort of 20,757 United States veterans diagnosed with rheumatoid arthritiswith an estimated glomerular filtration rate (eGFR) of 60 mL/min/1.73m2 or more, recruited between October 2004 and September 2006, and followed through 2013. The associations of biologic use with incident CKD (eGFR under 60 with a decrease of at least 25% from baseline, and eGFR under 45 mL/min/1.73m2) and change in eGFR (<−3, −3 to <0 [reference], and ≥0 mL/min/1.73m2/year) were examined in propensity-matched patients based on their likelihood to initiate biologic treatment, using Cox models and multinomial logistic regression models, respectively. Among 20,757 patients, 4,617 started biologic therapy. In the propensity-matched cohort, patients treated (versus not treated) with biologic agents had a lower risk of incident CKD (hazard ratios 0.95, 95% confidence interval [0.82–1.10] and 0.71 [0.53–0.94] for decrease in eGFR under 60 and under 45 mL/min/1.73m2, respectively) and progressive eGFR decline (multinomial odds ratios [95% CI] for eGFR slopes <−3 and ≥0 [versus −3 to <0] mL/min/1.73m2/year, 0.67 [0.58–0.79] and 0.76 [0.69–0.83], respectively). A significant deceleration of eGFR decline was also observed after biologic administration in patients treated with biologics (−1.0 versus −0.4 [mL/min/1.73m2/year] before and after biologic use). Thus, biologic agent administration was independently associated with lower risk of incident CKD and progressive eGFR decline.
Keywords: Biologics, chronic kidney disease, estimated glomerular filtration rate, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) has been associated with a variety of kidney disorders, such as secondary amyloidosis, glomerulonephritis, and drug-induced nephropathy, principally through chronic inflammation and/or exposure to nephrotoxic agents, and the prevalence of chronic kidney disease (CKD) in patients with RA has been reported to be higher than that in the general population.1–3 A growing body of evidence has also shown the strong association between RA and higher risk of cardiovascular events.4–6 Since the systemic inflammation characteristic of RA plays a pivotal role in the development of atherosclerosis and subsequent cardiovascular disease, increasing attention has been paid to the management of cardiovascular risk factors in RA patients, with an enhanced focus on achieving remission early in the disease course with various anti-inflammatory therapeutic approaches using disease-modifying antirheumatic drugs (DMARDs) and biologic agents.7 In addition, recent meta-analyses have identified the beneficial effect of tumor necrosis factor (TNF) inhibitors on cardiovascular risk in patients with RA,8, 9 which is also reflected in the current European League Against Rheumatism (EULAR) recommendations, suggesting that disease activity should be controlled optimally to lower cardiovascular risk in RA patients.10 Considering these data and the possible biological link between systemic inflammation and CKD progression in patients with RA,11 it is plausible that reducing their inflammatory burden with biologic treatment could also have favorable effects on renal function with the potential to slow kidney disease progression and to reduce subsequent risk of incident CKD and end-stage renal disease (ESRD).
In the present study, we therefore hypothesized that patients with RA and normal kidney function who are treated with biologic agents are at a lower risk of incident kidney disease and are less likely to experience progressive decline of kidney function than those not receiving biologic treatment. To test these hypotheses, we investigated the association of biologic treatment with incident CKD and change in estimated glomerular filtration rate (eGFR) using a large nationally representative cohort of US veterans with RA and eGFR of ≥60 mL/min/1.73m2.
RESULTS
Baseline characteristics overall and in patients categorized by biologic use are shown in Supplemental Table 1. The overall mean±standard deviation (SD) age at baseline was 63.2±11.4 years; 91.7% of patients were male; 12.1% were African American; and 23.0% were diabetic. The mean baseline eGFR was 83.4±14.8 mL/min/1.73m2. During the follow-up period, 4,617 patients (22.2%) started biologic therapy. Compared with RA patients without biologic treatment, those receiving biologic treatment were younger and less likely to be male and African-American; had a higher baseline eGFR and per capita income and a lower prevalence of comorbidities except liver disease and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS); and were more likely to be service connected. They were also more likely to have RA-related articular procedures and to use non-biologic DMARDs, nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids, and less likely to use renin-angiotensin system inhibitors (RASi) and statins. Baseline characteristics were well balanced between those with and without biologic use in the propensity-matched cohort (Table 1).
Table 1.
Baseline patient characteristics by biologic use in the propensity-matched cohort
| Characteristics | Biologic use
|
Standardized difference | |
|---|---|---|---|
| No | Yes | ||
|
| |||
| (n = 4,041) | (n = 4,041) | ||
| Mean age (SD), y | 61.6 (10.5) | 61.1 (9.8) | −0.042 |
| Mean eGFR (SD), mL/min/1.73m2 | 85.9 (14.4) | 86.6 (14.4) | 0.045 |
| Past slope of eGFR, mL/min/1.73m2 per year | −1.0 (2.4) | −1.0 (2.3) | 0.050 |
| Male, n (%) | 3,625 (89.7) | 3,597 (89.0) | −0.023 |
| African American, n (%) | 467 (11.6) | 453 (11.2) | −0.011 |
| Hypertension, n (%) | 2,326 (57.6) | 2,250 (55.7) | −0.038 |
| Diabetes mellitus, n (%) | 770 (19.1) | 751 (18.6) | −0.012 |
| Coronary heart disease, n (%) | 434 (10.7) | 414 (10.2) | −0.016 |
| Congestive heart failure, n (%) | 137 (3.4) | 124 (3.1) | −0.018 |
| Cerebrovascular disease, n (%) | 186 (4.6) | 176 (4.4) | −0.012 |
| Peripheral arterial disease, n (%) | 202 (5.0) | 197 (4.9) | −0.006 |
| Chronic lung disease, n (%) | 905 (22.4) | 875 (21.7) | −0.018 |
| Dementia, n (%) | 5 (0.001) | 7 (0.002) | 0.013 |
| Liver disease, n (%) | 46 (1.1) | 40 (1.0) | −0.015 |
| Malignancies, n (%) | 284 (7.0) | 281 (7.0) | −0.003 |
| HIV/AIDS, n (%) | 2 (0.0005) | 5 (0.001) | 0.025 |
| Depression, n (%) | 417 (10.3) | 408 (10.1) | −0.007 |
| Married, n (%) | 1,655 (41.0) | 1,650 (40.8) | −0.003 |
| Service connected, n (%) | 1,940 (48.0) | 1,997 (49.4) | 0.028 |
| Median per capita income (IQR), $ | 23,765 (12,718−33,864) | 24,708 (13,155−33,910) | 0.0004 |
| Living in area with high housing stress, n (%) | 1,341 (33.2) | 1,327 (32.8) | −0.007 |
| Living in area with low education, n (%) | 477 (11.8) | 461 (11.4) | −0.012 |
| Living in area with low employment, n (%) | 414 (10.2) | 398 (9.8) | −0.013 |
| Living in area of persistent poverty, n (%) | 225 (5.6) | 219 (5.4) | −0.013 |
| Mean BMI (SD), kg/m2 | 29.0 (5.6) | 29.1 (5.5) | 0.013 |
| Mean systolic BP (SD), mmHg | 133 (18) | 133 (18) | 0.001 |
| Mean serum albumin (SD), g/dL | 4.0 (0.4) | 4.0 (0.4) | 0.002 |
| Mean articular procedures (SD), n (per year) | 0.2 (0.5) | 0.2 (0.4) | 0.050 |
| RASi use, n (%) | 1,688 (41.8) | 1,691 (41.8) | 0.002 |
| Statin use, n (%) | 1,536 (38.0) | 1,536 (38.0) | 0.0001 |
| Methotrexate use, n (%) | 2,256 (55.8) | 2,291 (56.7) | 0.018 |
| Hydroxychloroquine use, n (%) | 1,363 (33.7) | 1,357 (33.6) | −0.003 |
| Sulfasalazine use, n (%) | 928 (23.0) | 987 (24.4) | 0.034 |
| Leflunomide use, n (%) | 350 (8.7) | 452 (11.2) | 0.085 |
| Other non-biologic DMARD use, n (%) | 197 (4.9) | 202 (5.0) | 0.085 |
| NSAID use, n (%) | 2,711 (67.1) | 2,740 (67.8) | 0.015 |
| Glucocorticoid use, n (%) | 2,325 (57.5) | 2,381 (58.9) | 0.028 |
Note: Data are presented as number (percentage), mean (standard deviation), or median (interquartile interval).
Abbreviations: AIDS = acquired immunodeficiency syndrome; BMI = body mass index; BP = blood pressure; DMARD = disease-modifying anti-rheumatic drug; eGFR = estimated glomerular filtration rate; HIV = human immunodeficiency virus; NSAID = nonsteroidal anti-inflammatory drug; RASi = renin-angiotensin system inhibitor; SD = standard deviation.
Factors associated with initial biologic treatment
Among 4,617 RA patients treated with biologics in the overall cohort, 2,779 (60.2%) were treated with etanercept, followed by adalimumab (21.4%), infliximab (9.6%), abatacept (4.1%) and others (Supplemental Table 2). The timing of biologics initiation over time was depicted in a Kaplan-Meier curve (Supplemental Figure 1). Table 2 shows the association of various baseline characteristics with initiating biologic treatment during the follow-up period in a multivariable logistic regression model. Younger age, male gender, white race, fewer comorbid conditions, married status, and higher per capita income, housing stress, eGFR, body mass index (BMI), and number of articular procedures were significantly associated with higher odds of initiating biologic treatment. Medications associated with higher odds of biologic administration included non-biologic DMARDs except hydroxychloroquine, NSAIDs, and glucocorticoids. The predictive equation for the probability of biologic use is also shown in Supplemental Table 3.
Table 2.
Baseline patient characteristics associated with biologic initiation during the follow-up period in the overall cohort (n = 4,617)
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age (1 year higher) | 0.95 | 0.94–0.95 | <0.001 |
| Sex (Female vs. Male) | 0.83 | 0.73–0.95 | 0.006 |
| Race (African American vs. white) | 0.55 | 0.48–0.62 | <0.001 |
| Hypertension | 0.89 | 0.82–0.98 | 0.013 |
| Diabetes mellitus | 0.84 | 0.76–0.92 | <0.001 |
| Coronary heart disease | 0.86 | 0.77–0.97 | 0.014 |
| Congestive heart failure | 0.58 | 0.48–0.71 | <0.001 |
| Cerebrovascular disease | 0.84 | 0.72–0.99 | 0.043 |
| Peripheral arterial disease | 0.77 | 0.66–0.89 | 0.001 |
| Chronic lung disease | 0.77 | 0.71–0.84 | <0.001 |
| Dementia | 0.33 | 0.14–0.76 | 0.009 |
| Liver disease | 0.79 | 0.56–1.11 | 0.18 |
| Malignancies | 0.68 | 0.60–0.78 | <0.001 |
| HIV/AIDS | 0.52 | 0.19–1.42 | 0.20 |
| Depression | 0.80 | 0.71–0.91 | 0.001 |
| Marital status (unmarried vs. married) | 0.92 | 0.85–1.00 | 0.048 |
| Service connected | 1.06 | 0.98–1.14 | 0.16 |
| Per capita income (1 log-unit [$] higher) | 1.05 | 1.01–1.10 | 0.013 |
| Living in area with high housing stress | 1.17 | 1.08–1.27 | <0.001 |
| Living in area with low education | 1.03 | 0.90–1.18 | 0.67 |
| Living in area with low employment | 0.91 | 0.79–1.05 | 0.21 |
| Living in area of persistent poverty | 0.92 | 0.76–1.11 | 0.37 |
| eGFR (10 mL/min/1.73m2 higher) | 1.01 | 1.00–1.01 | <0.001 |
| BMI (1 kg/m2 higher) | 1.02 | 1.01–1.02 | <0.001 |
| Systolic BP (1 mmHg higher) | 1.00 | 1.00–1.00 | 0.10 |
| Serum albumin (1 g/dL higher) | 1.01 | 0.93–1.11 | 0.80 |
| Articular procedures per year (1 count higher) | 1.29 | 1.19–1.40 | <0.001 |
| RASi use | 0.99 | 0.91–1.09 | 0.88 |
| Statin use | 0.93 | 0.86–1.02 | 0.11 |
| Methotrexate use | 1.84 | 1.70–1.98 | <0.001 |
| Hydroxychloroquine use | 0.85 | 0.78–0.92 | <0.001 |
| Sulfasalazine use | 1.40 | 1.27–1.54 | <0.001 |
| Other non-biologic DMARD use | 1.52 | 1.24–1.85 | <0.001 |
| NSAID use | 1.08 | 1.00–1.17 | 0.038 |
| Glucocorticoid use | 1.61 | 1.49–1.74 | <0.001 |
Estimates are from multivariable logistic regression model.
Abbreviations: AIDS = acquired immunodeficiency syndrome; BMI = body mass index; BP = blood pressure; DMARD = disease-modifying anti-rheumatic drug; eGFR = estimated glomerular filtration rate; HIV = human immunodeficiency virus; NSAID = nonsteroidal anti-inflammatory drug; RASi = renin-angiotensin system inhibitor.
Incident CKD
In the propensity-matched cohort, 700 (8.7%) and 199 (1.5%) patients experienced decrease in eGFR reaching <60 (with a decrease of at least 25% from baseline) and <45 mL/min/1.73m2, respectively (corresponding crude incidence rate [95% confidence interval (CI)], 24.4 [22.6–26.2] and 6.6 [5.7–7.6] per 1000 patient-years), during a median follow-up of 4.5 years. Patients treated with (vs. without) biologics had a higher CKD-event free survival, with more evident separation for decrease in eGFR <45 mL/min/1.73m2 (97.7% vs. 96.7% at 4 years, log rank P = 0.018; Figure 1B) than for decrease in eGFR <60 mL/min/1.73m2 (90.2% vs. 89.9% at 4 years, log rank P = 0.50; Figure 1A). Compared to patients without biologic treatment, those with biologic treatment showed lower risk of incident CKD, with significantly lower risk seen only for decrease in eGFR <45 mL/min/1.73m2 (hazard ratios [HRs] [95% CI], 0.95 [0.82–1.10] and 0.71 [0.53–0.94] for decrease in eGFR <60 and <45 mL/min/1.73m2, respectively).
Figure 1. Kaplan-Meier curve for incident CKD (decrease in eGFR [A] <60 and [B] <45 mL/min/1.73m2) in the propensity-matched cohort.
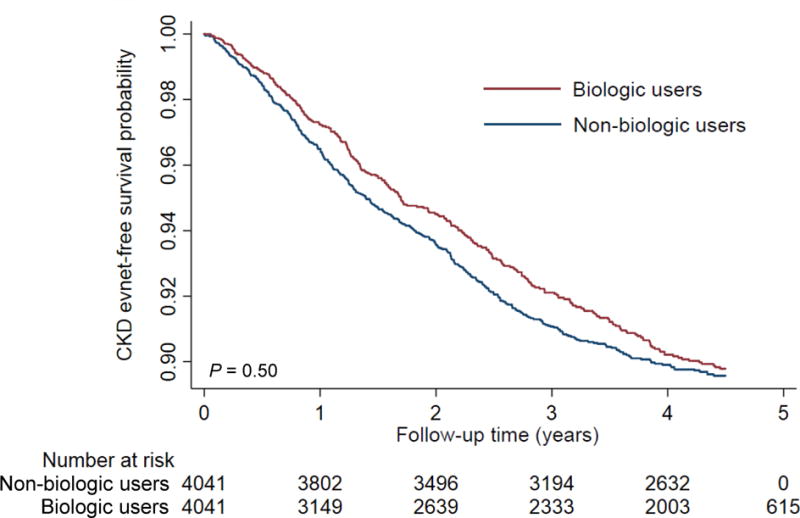
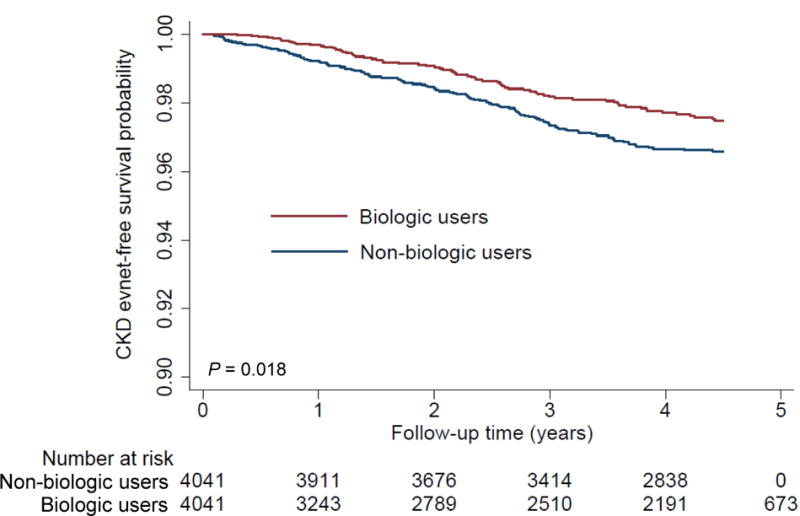
Abbreviations: CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
In subgroup analyses, the trend of association was qualitatively similar across multiple subgroups (Figures 2A and 2B), with the exception of the decrease in eGFR to <45 mL/min/1.73m2 in subgroups categorized by coronary heart disease (CHD) and articular procedures (Figure 2B). The association between biologic treatment and incident CKD was also robust in the overall analysis using time-dependent Cox models, with significantly lower risks seen in patients treated with (vs. without) biologics (adjusted HRs [95% CI], 0.83 [0.72–0.96] and 0.42 [0.32–0.56] for decrease in eGFR <60 and <45 mL/min/1.73m2, respectively; Supplemental Table 4).
Figure 2. Association of biologic treatment with incident CKD (decrease in eGFR [A] <60 and [B] <45 mL/min/1.73m2) in predefined subgroups in the propensity-matched cohort.
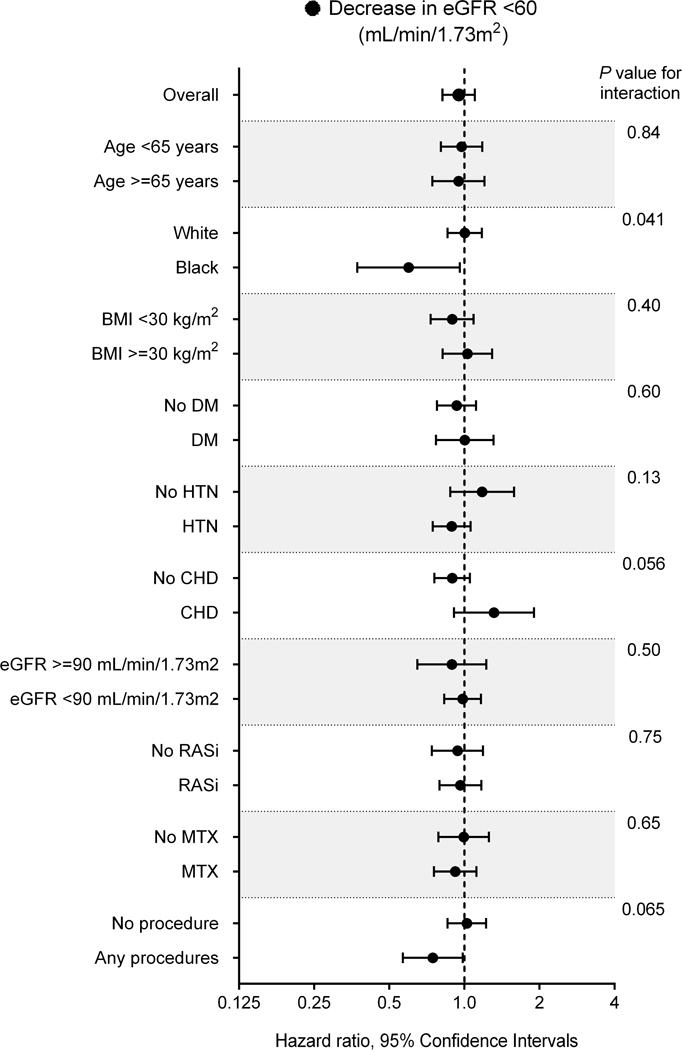
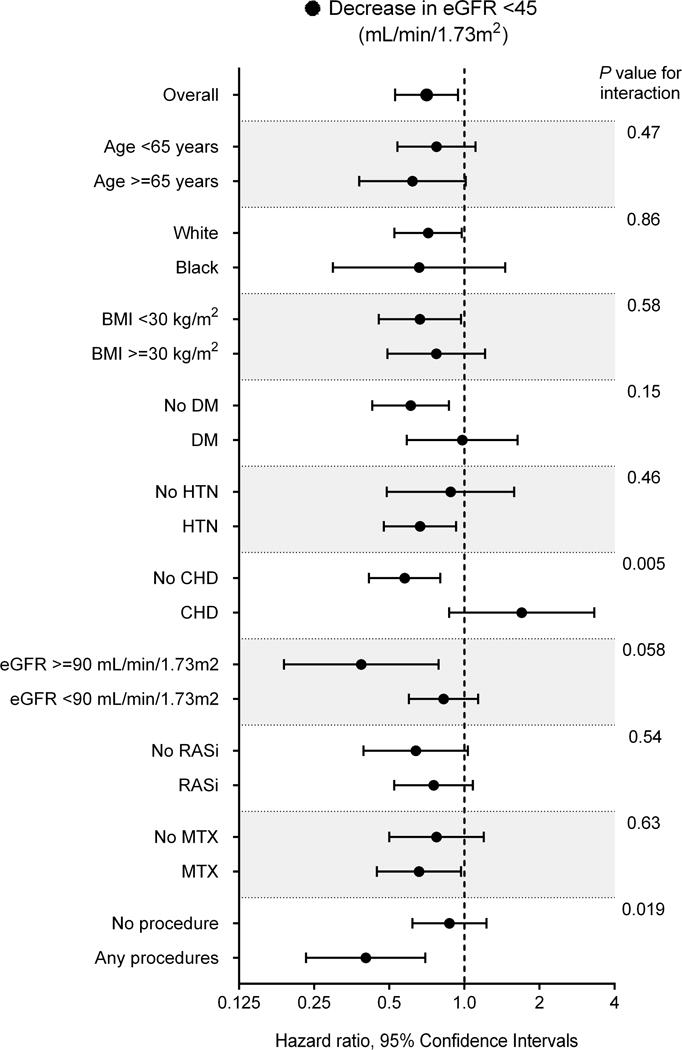
Abbreviations: BMI = body mass index; CHD = coronary heart disease; DM = diabetes mellitus; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HTN = hypertension.
Change in eGFR
Among 8,082 patients in the propensity-matched cohort, 729 (9.0%) and 4,356 (53.9%) experienced a decline in eGFR of <−3 and −3 to <0 mL/min/1.73m2/year, respectively; whereas 2,997 patients (37.1%) had a stable or increasing eGFR (≥0 mL/min/1.73m2/year). Patients treated with (vs. without) biologics were at a lower risk of experiencing faster eGFR decline, and a similar association was also present for increasing eGFR (multinomial odds ratios [95% CI] of eGFR slope <−3 and ≥0 [vs. −3 to <0] mL/min/1.73m2/year, 0.67 [0.58–0.79] and 0.76 [0.69–0.83], respectively).
In subgroup analyses, the pattern of association between biologic treatment and change in eGFR was qualitatively similar across subgroups, with a greater contribution of biologic treatment to progressive eGFR decline among patients who had received articular procedures at baseline (Figure 3). The association between biologic treatment and faster eGFR decline was similar in the overall cohort (Supplemental Table 5). When comparing the eGFR slopes estimated by unadjusted mixed-effects models between pre- and post-biologic administration among patients treated with biologics in the overall cohort, a significant deceleration of eGFR decline was seen after the biologic administration, with a mean improvement in crude eGFR slope of 0.6±3.0 (−1.0±1.9 vs. −0.4±2.2 [mL/min/1.73m2/year] before and after biologic administration, respectively, P <0.001; Supplemental Figure 2). The improvement in eGFR slope was similarly observed even after using eGFR slopes estimated by multivariable-adjusted mixed-effects models (Supplemental Figure 2). The changes in eGFR slope were similar between patients treated with TNF inhibitors and with non-TNF biologics (Supplemental Figure 3).
Figure 3. Association of biologic treatment with change in eGFR in predefined subgroups in the propensity-matched cohort.
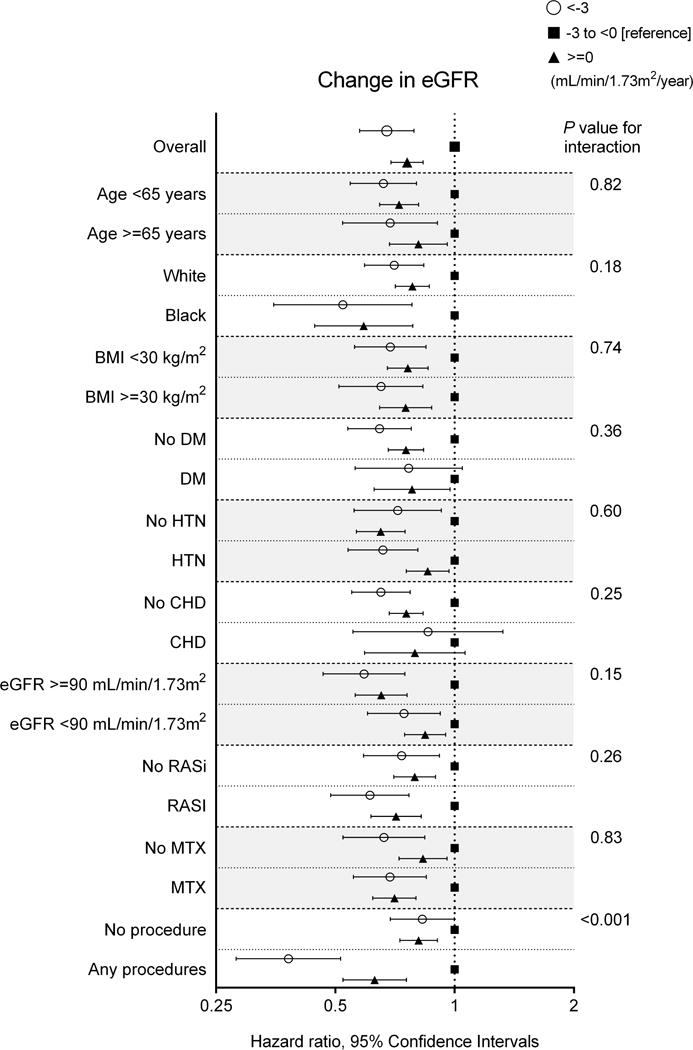
Abbreviations: BMI = body mass index; CHD = coronary heart disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HTN = hypertension.
DISCUSSION
In this large cohort of US veterans with RA and eGFR ≥60 mL/min/1.73m2, we found that administration of biologic agents was associated with lower risk of incident CKD, particularly for advanced stages of CKD (i.e., eGFR <45 mL/min/1.73m2), and progressive eGFR decline, after accounting for confounding by indication in the propensity-matched cohort. Furthermore, we also found a significant deceleration of eGFR decline after biologic administration in patients treated with biologics, irrespective of the type of agent used (TNF inhibitor or other). Findings were similarly observed in selected subgroups and were largely robust after accounting for time-dependent confounders in the overall cohort.
These results are similar to some aspects of a few previous studies that reported a beneficial association of the use of TNF inhibitors with CKD progression.12–14 In a retrospective cohort of 70 patients with RA and CKD, Kim et al.14 demonstrated that administration of anti-TNF-α drugs stabilized renal function, showing a significant difference of change in eGFR slope between patients treated with and without anti-TNF-α drugs during a mean follow-up of 2.9 years. In these studies, however, the change in eGFR was calculated using a single first and single last measurement during a relatively short follow-up period, which may be subject to potentially substantial intra-individual variability of eGFR over time,15, 16 and thus may not be precise. Most importantly, these previous studies included only RA patients with CKD, and hence, they are unable to determine whether administration of anti-TNF-α is associated with the development of de novo kidney disease in RA patients with preserved kidney function. We therefore estimated eGFR slopes using a more sophisticated modeling technique and extended the observation to a large nationwide cohort of US veterans with RA and normal kidney function, and for the first time demonstrated the independent association of biologic treatment with incident CKD and change in eGFR.
Although our observational study cannot conclude a causal relationship, there are several plausible explanations for the association of biologic use with lower risk of renal events. The chronic inflammatory state in RA plays a contributory role in the development and progression of atherosclerosis and adverse atherosclerotic cardiac events.4–6, 17 In line with these findings, a recent observational study has revealed an independent association between persistently high C-reactive protein level and higher risk of incident CKD in patients with RA.11 Given the multiple beneficial properties of biologic agents on endothelial function,18, 19 insulin resistance,20 and lipid metabolism,21 as well as their anti-inflammatory effects, it seems plausible that biologic treatment can directly (e.g., through attenuation of renal inflammation and endothelial dysfunction) or indirectly (e.g., through modified metabolic profiles and/or increased physical activity accompanied by improved joint function) contribute to attenuate the heightened risk of adverse renal outcomes in RA patients. Indeed, there are a few reports describing that TNF-α inhibitors were not only effective in the treatment of active arthritis, but also stabilized or improved renal function by reducing both acute-phase reactant and proteinuria in RA patients with renal dysfunction caused by secondary amyloidosis.12, 13, 22 In this context, it may be worthy of note that, despite the fact that patients with more severe RA (i.e., higher number of articular procedures and more use of anti-inflammatory drugs) at baseline had significantly higher odds of initiating biologic treatment, the biologic use remained associated with lower risk of renal events. Although still speculative, the lower risk of increasing eGFR seen in patients treated with biologics in the propensity-matched cohort might reflect loss of muscle mass associated with chronic inflammatory state among those without biologic treatment, which might be supported by the lack of such association in the overall cohort analysis which accounted for BMI as a time-dependent covariate. As another potential explanation, improved pain management associated with biologic treatment may help to reduce the need for potentially nephrotoxic anti-inflammatory agents such as NSAIDs and certain types of non-biologic DMARDs like D-penicillamine and cyclosporine,23–25 which could consequently reduce the risk of drug-induced nephrotoxicity and thereby contribute to the lower risk of renal events. Unlike these potentially nephrotoxic non-biologic agents, the unique pharmacokinetic features of biologic agents that are primarily metabolized by the reticuloendothelial system without apparent nephrotoxicity,26–28 along with the fact that some biologic agents have been used to treat established kidney diseases,29 may also support this hypothesis. In our study, however, the association of biologic use with favorable renal outcomes still remained statistically significant even after accounting for various medications that were modeled as time-dependent covariates in our overall cohort, suggesting potential renoprotective benefits of biologic agents in patients with RA. At the same time, it may also be important for clinicians to acknowledge that case reports have suggested a possible pathogenic role of biologic agents in the development of glomerulonephritis.30–32 Therefore, the effects of therapeutic interventions and optimal control of inflammation using biologic agents toward improving renal outcomes in RA patients, as well as the variability of the effects between individual agents, may deserve further investigation, including clinical trials with a renal endpoint.
Our study results must be interpreted in light of several limitations. Most of our patients were male US veterans; hence, the results may not apply to women or patients from other geographical areas. Information about RA disease activity measures such as the Clinical Disease Activity Index and Disease Activity Score, as well as erythrocyte sedimentation rate and C-reactive protein values, were not available in this cohort. Instead, we included the number of various RA-related articular procedures as a measure of RA severity as reported previously.33–36 Finally, as with all observational studies, we cannot eliminate the possibility of unmeasured confounders. Specifically, albuminuria data were not sufficiently available to be accounted for in our analyses, which might affect the risk estimates for CKD progression.
In conclusion, in this large nationwide cohort of 20,757 patients with RA, biologic administration was associated with lower risk of incident CKD and progressive eGFR decline, independent of established kidney disease risk factors. Further studies are warranted to test whether active interventions with biologic agents can attenuate the heightened risk of development and progression of CKD associated with RA.
METHODS
Cohort Definition
Our study used data from a retrospective cohort study examining risk factors in patients with incident CKD (Racial and Cardiovascular Risk Anomalies in CKD [RCAV] study).37–39 We extracted all serum creatinine measurements obtained in clinical settings in all US Department of Veteran Affairs (VA) health care facilities between October 1, 2004 and September 30, 2006 (baseline period) from the national VA Corporate Data Warehouse LabChem data files.40 Overall, 4,447,691 veterans had at least 1 available serum creatinine measurement, representing ~94% of all veterans who received VA health care during this time period.41 The RCAV cohort included 3,582,478 patients with eGFR ≥60 mL/min/1.73m2. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.42 We defined RA as either having at least 2 diagnoses for RA, as identified by the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) (Supplemental Table 6), that were recorded ≥30 days apart; or having a single RA diagnosis code plus ≥3-month supply of non-biologic DMARDs approved for use in RA during the two-year baseline period,43, 44 based on information obtained from VA Pharmacy dispensation records.45 Among the 3,582,478 patients, 30,130 (0.8%) were identified as having RA. We defined de novo exposure to biologics as initiation of biologics in RA patients who had not been exposed to any biologics for at least 6 months after the cohort entry, which was the date of the first eGFR ≥60 mL/min/1.73m2 during the baseline period, but were subsequently exposed to any biologics until the end of follow-up. Therefore, RA patients exposed to any biologics within 6 months after the cohort entry were excluded (n = 992) due to the possibility that they may have received treatment prior to cohort entry. After further exclusion of patients with missing covariates (n = 8,377) and erroneous data (n = 4), 20,757 RA patients were included in our final cohort (Supplemental Figure 4). Compared with patients in the final cohort, those who were excluded from the source cohort were older (66.2 vs. 63.2 years) and were less likely to be African-American (8.0% vs. 12.1%). To minimize confounding by indication, we generated from the final cohort a propensity score-matched cohort of 8,082 patients (4,041 exposed and 4,041 unexposed to biologics) for our primary analyses (Supplemental Figure 4).
Data collection
Exposures and covariates
The primary exposure of interest was an exposure to any types of biologics during the follow-up period. Biologics included etanercept, adalimumab, infliximab, abatacept, certolizumab, golimumab, rituximab, tocilizumab, and anakinra. The administration of biologics was modeled using an intention-to-treat approach, in which patients with de novo biologic exposure were considered part of the treated group until the end of follow-up irrespective of subsequent discontinuation of biologics.
Sociodemographic characteristics, comorbid conditions, laboratory characteristics, and medication use were obtained as previously described.46, 47 Briefly, data on patients’ age, sex, race, marital status (married, single, divorced or widowed), BMI, blood pressure, comorbid conditions, medication use, mean per capita income, and service connectedness (a measure indicating whether one or more of a patient’s comorbidities were caused by military service, resulting in certain privileges such as preferential access to care and lower copayments) were collected from various national VA research data files.48 Medication use was defined as at least one dispensation of ≥30-day supply each. Prevalent comorbidities were defined as the presence of relevant ICD-9-CM and Current Procedural Terminology (CPT) codes recorded from October 1, 2004 to September 30, 2006 (Supplemental Table 6).37 Data on the number of RA-related articular procedures was obtained from relevant CPT codes (Supplemental Table 6). In addition to the information derived from VA sources, we included select socioeconomic indicators using 2004 county topology codes (housing stress, low education, low employment, and persistent poverty; Supplemental Table 7).
Outcomes
The co-primary outcomes of interest were incident CKD and change in eGFR. Incident CKD was defined as two eGFR levels <60 mL/min/1.73m2 separated by ≥90 days, and a >25% decrease from baseline eGFR.49 We also examined more advanced stages of CKD (i.e., two eGFR levels <45 mL/min/1.73m2 separated by ≥90 days) as the outcome of incident CKD. Change in eGFR (slope) was calculated in each patient from linear mixed-effects models using all outpatient eGFR measurements available from the index date (vide infra) to October 13, 2012 (the last date of available serum creatinine measurement), and stratified into 3 categories as follows: <−3, −3 to <0 (reference), and ≥0 mL/min/1.73m2/year. Information about all-cause mortality was obtained from the VA Vital Status Files.50
Statistical analysis
Baseline patient characteristics were presented as number (percent) for categorical variables and mean±SD for continuous variables with normal distribution or median (interquartile interval) for those with skewed distribution. The timing of biologics initiation over time was depicted in a Kaplan-Meier curve, and factors independently associated with biologic initiation were identified by multivariable logistic regression. Based on a-priori knowledge and their availability in this study, the following explanatory variables were included: age, sex, race, prevalent comorbidities (diabetes mellitus, hypertension, CHD, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic lung disease, liver disease, dementia, malignancy, depression, and HIV/AIDS), socioeconomic parameters (mean per capita income, marital status, service connectedness, housing stress, low education, low employment, and persistent poverty), eGFR, BMI, systolic blood pressure, serum albumin, number of articular procedures per year as a measure of RA severity,33–36 and medications (statins, RASi, methotrexate, hydroxychloroquine, sulfasalazine, other non-biologic DMARDs, NSAIDs, and glucocorticoids). In the overall cohort, the start of follow-up was the date of cohort entry. Patients were followed up until the earliest date of incident CKD, death or the last date of VA contact for incident CKD analyses, and until death or the last date of VA contact for analyses of change in eGFR. In order to account for the confounding arising from the differences in clinical characteristics of patients treated with (vs. without) biologics, we performed a propensity score-matched analysis as our primary analysis. The characteristics at the time of biologic initiation among biologic users were matched to those at a corresponding time point among non-biologic users by redefining their index date as the date of cohort entry plus the median number of days from cohort entry to biologic initiation in biologic users (i.e., 3.0 years). Propensity scores for the likelihood of presence vs. absence of biologic treatment were calculated by logistic regression using all of the above-mentioned variables at the new baseline (i.e., the date of biologic initiation and the index date for biologic users and non-users, respectively). We then matched patients with biologic treatment to comparable patients without treatment using a 1:1 nearest-neighbor matching without replacement. Differences between variables were examined by calculating standardized differences, and values <0.1 were considered acceptable for the matching. The associations of biologic treatment with renal outcomes were assessed with the Kaplan-Meier method and using Cox proportional hazards models (for incident CKD) and multinomial logistic regression models (for change in eGFR). For the Cox models, the proportionality assumption was tested by scaled Schoenfeld residuals, which showed no violations.
We performed several sensitivity analyses to evaluate the robustness of our main findings. The associations of biologic treatment with outcomes were examined in relevant subgroups of patients. Potential interactions were formally tested by including interaction terms. Analyses were repeated in the overall cohort using the multivariable-adjusted Cox models (for incident CKD) by modeling biologic treatment as a time-dependent exposure, with the time between cohort entry and the start of biologic therapy allocated to the unexposed group,51 in order to avoid immortal time bias arising from the relevant follow-up period among patients treated with biologics. Along with biologic exposure, eGFR, BMI, SBP, serum albumin, number of articular procedures per year, and medications were also treated as time-dependent covariates in the Cox models. Additionally, among patients treated with biologics in the overall cohort, we calculated eGFR slopes by both unadjusted and multivariable-adjusted mixed-effects models separately for the pre- and post-biologic administration periods in each individual, and compared the changes in eGFR slopes for these two periods using paired t-tests in overall biologic users and in those stratified by two different types of biologic agents (i.e., TNF inhibitors vs. other biologics). The multivariable mixed-effects model was adjusted for the same covariates as above. The reported P values are two-sided and reported as significant at <0.05 for all analyses. All of the analyses were conducted using Stata/MP version 14 (Stata Corporation, College Station, TX). The study was approved by the institutional review boards at the Memphis and Long Beach VA medical centers, with exemption from informed consent.
Supplementary Material
Acknowledgments
This study was supported by grant R01DK096920 to Drs. Kovesdy and Kalantar-Zadeh and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (project numbers SDR 02-237 and 98-004).
The sponsors had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Drs. Kovesdy and Kalantar-Zadeh are employees of the US Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs.
None of the authors have relevant conflicts of interest.
References
- 1.Helin HJ, Korpela MM, Mustonen JT, et al. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. 1995;38:242–247. doi: 10.1002/art.1780380213. [DOI] [PubMed] [Google Scholar]
- 2.Karie S, Gandjbakhch F, Janus N, et al. Kidney disease in RA patients: prevalence and implication on RA-related drugs management: the MATRIX study. Rheumatology (Oxford) 2008;47:350–354. doi: 10.1093/rheumatology/kem370. [DOI] [PubMed] [Google Scholar]
- 3.Hickson LJ, Crowson CS, Gabriel SE, et al. Development of reduced kidney function in rheumatoid arthritis. Am J Kidney Dis. 2014;63:206–213. doi: 10.1053/j.ajkd.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 5.Meune C, Touze E, Trinquart L, et al. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–1313. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 6.Levy L, Fautrel B, Barnetche T, et al. Incidence and risk of fatal myocardial infarction and stroke events in rheumatoid arthritis patients. A systematic review of the literature. Clin Exp Rheumatol. 2008;26:673–679. [PubMed] [Google Scholar]
- 7.Roubille F, Kritikou EA, Roubille C, et al. Emerging anti-inflammatory therapies for atherosclerosis. Curr Pharm Des. 2013;19:5840–5849. doi: 10.2174/13816128113199990351. [DOI] [PubMed] [Google Scholar]
- 8.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:522–529. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 9.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 11.Kochi M, Kohagura K, Shiohira Y, et al. Inflammation as a Risk of Developing Chronic Kidney Disease in Rheumatoid Arthritis. PLoS One. 2016;11:e0160225. doi: 10.1371/journal.pone.0160225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Nebro A, Tomero E, Ortiz-Santamaria V, et al. Treatment of rheumatic inflammatory disease in 25 patients with secondary amyloidosis using tumor necrosis factor alpha antagonists. Am J Med. 2005;118:552–556. doi: 10.1016/j.amjmed.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Higashi S, Tomoda K, et al. Effectiveness of etanercept vs cyclophosphamide as treatment for patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford) 2012;51:2064–2069. doi: 10.1093/rheumatology/kes190. [DOI] [PubMed] [Google Scholar]
- 14.Kim HW, Lee CK, Cha HS, et al. Effect of anti-tumor necrosis factor alpha treatment of rheumatoid arthritis and chronic kidney disease. Rheumatol Int. 2015;35:727–734. doi: 10.1007/s00296-014-3146-4. [DOI] [PubMed] [Google Scholar]
- 15.Turin TC, Coresh J, Tonelli M, et al. Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant. 2012;27:3835–3843. doi: 10.1093/ndt/gfs263. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59:504–512. doi: 10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Juanatey C, Testa A, Garcia-Castelo A, et al. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor alpha antibody. Arthritis Rheum. 2004;51:447–450. doi: 10.1002/art.20407. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Juanatey C, Llorca J, Vazquez-Rodriguez TR, et al. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor alpha blocker therapy. Arthritis Rheum. 2008;59:1821–1824. doi: 10.1002/art.24308. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–86. [PubMed] [Google Scholar]
- 21.Popa C, Netea MG, Radstake T, et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottenberg JE, Merle-Vincent F, Bentaberry F, et al. Anti-tumor necrosis factor alpha therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a followup report of tolerability and efficacy. Arthritis Rheum. 2003;48:2019–2024. doi: 10.1002/art.11163. [DOI] [PubMed] [Google Scholar]
- 23.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 24.Hall CL, Jawad S, Harrison PR, et al. Natural course of penicillamine nephropathy: a long term study of 33 patients. Br Med J (Clin Res Ed) 1988;296:1083–1086. doi: 10.1136/bmj.296.6629.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dijkmans BA, van Rijthoven AW, Goei The HS, et al. Cyclosporine in rheumatoid arthritis. Semin Arthritis Rheum. 1992;22:30–36. doi: 10.1016/0049-0172(92)90046-g. [DOI] [PubMed] [Google Scholar]
- 26.Don BR, Spin G, Nestorov I, et al. The pharmacokinetics of etanercept in patients with end-stage renal disease on haemodialysis. J Pharm Pharmacol. 2005;57:1407–1413. doi: 10.1211/jpp.57.11.0005. [DOI] [PubMed] [Google Scholar]
- 27.Hammoudeh M. Infliximab treatment in a patient with rheumatoid arthritis on haemodialysis. Rheumatology (Oxford) 2006;45:357–359. doi: 10.1093/rheumatology/kei264. [DOI] [PubMed] [Google Scholar]
- 28.Sumida K, Ubara Y, Suwabe T, et al. Adalimumab treatment in patients with rheumatoid arthritis with renal insufficiency. Arthritis Care Res (Hoboken) 2013;65:471–475. doi: 10.1002/acr.21800. [DOI] [PubMed] [Google Scholar]
- 29.Jayne D, Tesar V. Biologic treatment in glomerular disease. Nephron Clin Pract. 2014;128:203–204. doi: 10.1159/000369646. [DOI] [PubMed] [Google Scholar]
- 30.Stokes MB, Foster K, Markowitz GS, et al. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant. 2005;20:1400–1406. doi: 10.1093/ndt/gfh832. [DOI] [PubMed] [Google Scholar]
- 31.Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11–14. doi: 10.1053/j.ajkd.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Piga M, Chessa E, Ibba V, et al. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohort. Autoimmun Rev. 2014;13:873–879. doi: 10.1016/j.autrev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Askling J, Fored CM, Brandt L, et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis. 2007;66:1339–1344. doi: 10.1136/ard.2006.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754–1764. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 35.Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125–1133. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 36.Al-Aly Z, Pan H, Zeringue A, et al. Tumor necrosis factor-alpha blockade, cardiovascular outcomes, and survival in rheumatoid arthritis. Transl Res. 2011;157:10–18. doi: 10.1016/j.trsl.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132:1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. J Am Coll Cardiol. 2016;68:1375–1386. doi: 10.1016/j.jacc.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumida K, Molnar MZ, Potukuchi PK, et al. Constipation and Incident CKD. J Am Soc Nephrol. 28:1248–1258. doi: 10.1681/ASN.2016060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gosmanova EO, Lu JL, Streja E, et al. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64:951–957. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Department of Veterans Affairs. Veteran population. http://www.va.gov/vetdata/Veteran_Population.asp. Accessed December 15, 2014.
- 42.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51:952–957. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 44.Curtis JR, Yang S, Patkar NM, et al. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:990–997. doi: 10.1002/acr.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.VA Information Resource Center. VIReC Research User Guide: VHA Pharmacy Prescription Data. 2nd. Hines, IL: US Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2008. [Google Scholar]
- 46.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159:233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovesdy CP, Lu JL, Molnar MZ, et al. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med. 2014;174:1442–1449. doi: 10.1001/jamainternmed.2014.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US Department of Veterans Affairs. VA Information Resource Center. http://www.virec.research.va.gov/Resources/Info-About-VA-Data.asp. Accessed December 15. 2014.
- 49.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 50.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molnar MZ, Kalantar-Zadeh K, Lott EH, et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650–658. doi: 10.1016/j.jacc.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


