Abstract
To identify determinants of early progressive renal decline in type 2 diabetes a range of markers were studied in 1032 patients enrolled into the 2nd Joslin Kidney Study. eGFR slopes estimated from serial measurements of serum creatinine during 5–12 years of follow-up were used to define early renal decline. At enrollment, all patients had normal eGFR, 58% had normoalbuminuria and 42% had albuminuria. Early renal decline developed in 6% and in 18% patients, respectively. As determinants, we examined baseline values of clinical characteristics, circulating markers: TNFR1, KIM-1, and FGF23, and urinary markers: albumin, KIM-1, NGAL, MCP-1, EGF (all normalized to urinary creatinine) and the ratio of EGF to MCP-1. In univariate analysis, all plasma and urinary markers were significantly associated with risk of early renal decline. When analyzed together, systolic blood pressure, TNFR1, KIM-1, the albumin to creatinine ratio, and the EGF/MCP-1 ratio remained significant with the latter having the strongest effect. Integration of these markers into multi-marker prognostic test resulted in a significant improvement of discriminatory performance of risk prediction of early renal decline, compared with the albumin to creatinine ratio and systolic blood pressure alone. However, the positive predictive value was only 50% in albuminuric patients. Thus, markers in plasma and urine indicate that the early progressive renal decline in Type 2 diabetes has multiple determinants with strong evidence for involvement of tubular damage. However, new, more informative makers are needed to develop a better prognostic test for such decline that can be used in a clinical setting.
Keywords: diabetic nephropathy, diabetes, microalbuminuria
Introduction
Patients with type 2 diabetes mellitus (T2D) are at increased risk of end-stage renal disease (ESRD) and they contribute to one half of all new cases of ESRD in the US population.1, 2 This devastating outcome develops as a result of progressive renal decline.3 The rate of this decline varies greatly among patients but appears to be fairly constant over time in the same person,3–5 making it possible to search for markers that predict rate of decline and identify patients at risk many years before onset of ESRD.
There has been significant effort to understand the mechanisms and develop prognostic tests for progressive renal decline in diabetic patients with impaired renal function (late progressive renal decline).6–10 Very little is known, however, about the mechanisms, determinants and markers of early progressive renal decline in patients with normal renal function. Early progressive renal decline was recognized recently.11–13 Various definitions have been used to diagnose such decline but all rely on serially measured serum creatinine and evaluation of trajectory of creatinine based eGFR over variable follow-up period. In this study, we used a loss of >30% eGFR from baseline value during ≤5 years of follow-up as the outcome measure. Patients with normal renal function and such an eGFR loss might reach ESRD in less than 20 years.3 Decline in eGFR of >30% at any time during follow-up was recently proposed as the pre-defined endpoint of CKD progression.14
In this study we aimed to investigate determinants of early renal decline in a large cohort of T2D patients with normal renal function. We were particularly interested in evaluating the hypothesis that markers of tubular damage and kidney inflammation might be important in initiation and progression of early renal decline independently from other markers, as we have recently demonstrated in Type 1 diabetes (T1D).15,16 New, emerging risk markers of renal decline include markers of proximal and distal tubule damage (markers indicating damage: KIM-1, and NGAL, or healing: EGF) and markers of kidney inflammation (MCP-1). These proteins can indicate ongoing damage (or protection), but it is also possible that some are causally involved in initiation and progression of chronic kidney disease.17–24
For etiological consideration we examined three groups of pathogenetic factors or biomarkers at baseline: 1) clinical characteristics, 2) serum markers known to be associated with renal decline: tumor necrosis factor receptor 1 (TNFR1),6 kidney injury molecule-1 (KIM-1)15,16 and fibroblast growth factor 23 (FGF23),25 and 3) urinary markers: albumin/creatinine ratio (ACR), kidney injury molecule-1 (KIM-1cr), neutrophil gelatinase-associated lipocalin (NGALcr),26, 27 monocyte chemoattractant protein-1 (MCP-1cr),28 epidermal growth factor (EGFcr).29, 30 and EGF/MCP-1 ratio.31 We evaluated the markers individually and jointly as determinants of the development of early renal decline in T2D.
Additionally, using our findings on the determinants of early progressive renal decline, we aimed to develop a multi-marker prognostic test constructed from the strongest and statistically independent variables, and assess its prognostic performance in identifying those at high risk of early renal decline among diabetic patients with normal renal function.
Our study differs from previous publications on renal decline in T2D.6–9, 30 Those studies often focused on a single marker and they frequently did not distinguish early renal decline when patients had normal renal function from late renal decline when patients had impaired renal function. Most importantly, although the positive findings were reported and interpreted as potential prognostic tests, the authors never attempted to use their findings to construct specific tests to diagnose patients at risk of early renal decline. Obviously, they never evaluated performances of such tests among patients with low risk of early renal decline. We aimed to do both; to search in a comprehensive way for markers of etiologic pathways of early progressive renal decline and to use the findings to construct a prognostic test and evaluate its performance.
Results
Characteristics of the Study groups
One thousand three hundred sixty-eight patients with T2D attending the Joslin Clinic between 2003 and 2009 were enrolled into the 2nd Joslin Kidney Study. By design half of these patients had normoalbuminuria and half had albuminuria according to multiple ACR measurements during the 2 year interval preceding the enrollment. For the present study we selected only patients with normal eGFR at baseline (median of 98 and 1st and 3rd quartile of 85–110 mL/min) of whom 602 had normoalbuminuria and 430 had albuminuria. Table 1 shows characteristics of both groups. In both groups 80% were Caucasians and 20% were minorities. By design, patients with normoalbuminuria had very low baseline ACR (median 4 ug/mg; 1st and 3rd quartile of 2 – 7) and the majority of those with albuminuria had baseline ACR in the range of microalbuminuria (median 44 μg/mg; 1st and 3rd quartile of 20–141), only 16% of them had ACR in the proteinuria range. Otherwise both groups were similar with regard to age, duration of diabetes, proportion of treatment with insulin, and eGFR. Those with albuminuria were treated more frequently with ACE-inhibitors/ARBs than those with normoalbuminuria.
Table 1.
Characteristics of the study groups of T2D patients according to the ascertainment criteria
| Characteristics: | Normo-albuminuria n=602 |
Albuminuria n=430 |
|---|---|---|
| 2-year pre-baseline | ||
| ACR (μg/mg) | 7.0 (4.6; 11) | 53 (26; 155) |
| Race/ethnicity | ||
| Caucasian (%) | 80% | 79% |
| African-American (%) | 13% | 11% |
| Asian (%) | 3% | 3% |
| Latino (%) | 3% | 5% |
| Native American (%) | 0% | 1% |
| Other (%) | 1% | 2% |
| At Baseline | ||
| ACR (μg/mg) | 4 (2.0; 7.0) | 44 (20; 141) |
| Gender (Male %) | 48 | 73 |
| Age (ys) | 57 (51; 62) | 56 (50; 61) |
| Duration of DM (ys) | 11 (7; 15) | 10 (5; 15) |
| Insulin Rx (%) | 58 | 51 |
| eGFR (ml/min) | 95 (84; 105) | 97 (83; 105) |
| ACE&ARB (%) | 57.4% | 79.0% |
| During Follow-up | ||
| Duration of follow-up (ys) | 7.2 (6.1; 9.7) | 7.6 (6.1; 10.6) |
| # of serum creatinine | 11 | 13 |
| eGFR slope (ml/min/y) | −1.2 (−2.4; −0.3) | −2.2 (−4.1; −0.9) |
| Loss eGFR ≥30% of baseline (n/%) within 5 ys of follow-up | 38 (6%) | 76 (18%) |
| Incidence of ESRD per 1000 p-ys | 0.53 (3/5617) | 5.32 (23/4327) |
| Mortality per 1000 p-ys due to deaths unrelated to ESRD | 0.81 (5/5615) | 1.37 (6/4391) |
Quantitative data are presented as median and 1st and 3rd quartile.
Serum creatinine-based eGFR estimated using CKD-EPI formula
Serum creatinine-based eGFR slope estimated with ordinary least squares linear regression.
All patients were followed for 5 to 12 years with median 7.4 years. Duration of follow-up and number of serum creatinine measurements to determine eGFR slopes were similar in both groups. The eGFR slopes were used to assess early renal decline in each group. The median eGFR slope was −1.2 mL/min/year (1st, 3rd quartile −2.4; −0.3) and −2.2 (−4.1; −0.9) in normoalbuminuria and albuminuria group respectively (p<0.001). The proportion of decliners defined as eGFR loss ≥30% during 5 years of follow-up was 6% in the first group and 18% in the second (p<0.001). It is important to note that in both groups we observed very fast decliners who progressed to ESRD during follow-up period. Deaths unrelated to ESRD were infrequent and occurred among non-decliners.
Comparison of Decliners vs. Non-decliners
To examine the differences in determinants of early renal decline both sub-groups of decliners (38 with normoalbuminuria and 76 with albuminuria) were combined (n=114) and compared with non-decliners (n=918). Table 2 shows comparisons of clinical characteristics, circulating and urinary markers between decliners and non-decliners. Characteristics such as sex, age, duration of T2D, insulin Rx, were not different between decliners and non-decliners. Regarding other clinical characteristics at baseline, decliners had significantly higher HbA1c, BMI, systolic and diastolic blood pressure, and significantly lower baseline eGFR than non-decliners. The concentrations of circulating markers were significantly higher in decliners than in non-decliners. Similarly the single determination of concentration at baseline of all urinary markers adjusted for urinary creatinine, were significantly higher in decliners than in non-decliners with the exception of EGFcr, which was higher in non-decliners than in decliners. Based on the previous report showing ratio of EGF over MCP-1 as a good predictor of renal outcome in IgA nephropathy,31 we analyzed such ratio in this study, as a separate variable. As shown in Table 2, the median of this ratio was very low in decliners compared with non-decliners and the difference was highly statistically significant (p <0.001).
Table 2.
Comparison of clinical characteristics, circulating and urinary markers at baseline between Decliners and Non-decliners with T2D
| Variables | Decliners (n=114) | Non-decliners (n=918) | p-value | AUC |
|---|---|---|---|---|
| A. Clinical characteristics | ||||
| Pre-baseline ACR | 62 (13.4; 654) | 12 (5.9; 32) | <0.001 | 0.71 |
| Gender (Male %) | 67% | 58% | 0.12 | 0.54 |
| Age (ys) | 57 (51; 63) | 57 (50; 61) | 0.051 | 0.56 |
| Duration of DM (ys) | 10 (7; 17) | 10 (6; 15) | 0.27 | 0.52 |
| Insulin Rx (%) | 50% | 56% | 0.33 | 0.53 |
| HbA1c (%) | 8.0 (6.8; 9.2) | 7.6 (6.9; 8.5) | 0.005 | 0.55 |
| BMI (kg/m2) | 32.4 (28.2; 38.3) | 31.1 (26.7; 35.8) | 0.04 | 0.54 |
| SysBP (mmHg) | 140 (129; 155) | 130 (120; 142) | <0.001 | 0.64 |
| DiaBP (mmHg) | 80 (72; 87) | 77 (70; 83) | 0.02 | 0.56 |
| eGFR (ml/min) | 89 (77; 99) | 97 (85; 106) | <0.001 | 0.62 |
| ACE&ARB (%) | 74% | 66% | 0.06 | 0.54 |
| B. Circulating markers at baseline | ||||
| TNFR-1 pg/ml | 1720 (1327; 2210) | 1218 (1030; 1530) | <0.001 | 0.73 |
| KIM-1 pg/ml | 18.8 (9.8; 42.3) | 9.5 (4.9; 15.0) | <0.001 | 0.72 |
| FGF-23 pg/ml | 66 (46; 85) | 55 (43; 70) | 0.002 | 0.58 |
| C. Urinary markers at baseline | ||||
| ACR ug/mg cr | 54 (11.7; 719) | 7.2 (2.9; 25) | <0.001 | 0.75 |
| KIM-1 pg/mg cr | 59 (12; 184) | 16 (3.8; 54) | <0.001 | 0.68 |
| NGAL ng/mg cr | 10.0 (4.2; 21.0) | 7.7 (4.0; 14.5) | 0.04 | 0.54 |
| MCP-1 pg/mg cr | 542 (338; 843) | 306 (162; 501) | <0.001 | 0.70 |
| EGF ng/mg cr | 10.5 (8.1; 15.0) | 13.1 (8.7; 18.6) | 0.003 | 0.58 |
| EGF ng/MCP-1 pg ratio | 19.7 (11.8; 32.4) | 43.3 (25.8; 85.2) | <0.001 | 0.75 |
Quantitative data are presented as median, 1st and 3rd quartiles.
The p values are from Wilcoxon test or Pearson χ2 test.
AUC – area under the ROC curve in univariate logistic regression.
Etiological model of early renal decline: Results of Univariate and Multivariable Logistic Analyses
Many clinical characteristics, circulating and urinary markers were postulated to be involved in the early progressive renal decline on the basis of either univariate or multivariable analyses. To compare the results of our study with the previous publications we searched for determinants of initiation and progression of early progressive renal decline first by univariate and then by multivariable logistic analysis using clinical variables and markers shown in Table 2. In univariate and multivariable logistic analyses, race (p=0.13), sex (p=0.06), duration of T2D (p=0.86), insulin treatment (p=0.24), ACE-I Rx (p=0.08), and urinary NGALcr (p=0.19), were not associated with early renal decline. The others (A1c, BMI, systolic blood pressure, eGFR, TNFR1, plasma and urine KIM-1, FGF23 and EGF/MCP-1) were associated with early renal decline in univariate models and were included in multivariable logistic analysis (see Supplemental Material). The odds ratios for these variables/markers are presented in Supplementary Table 2.
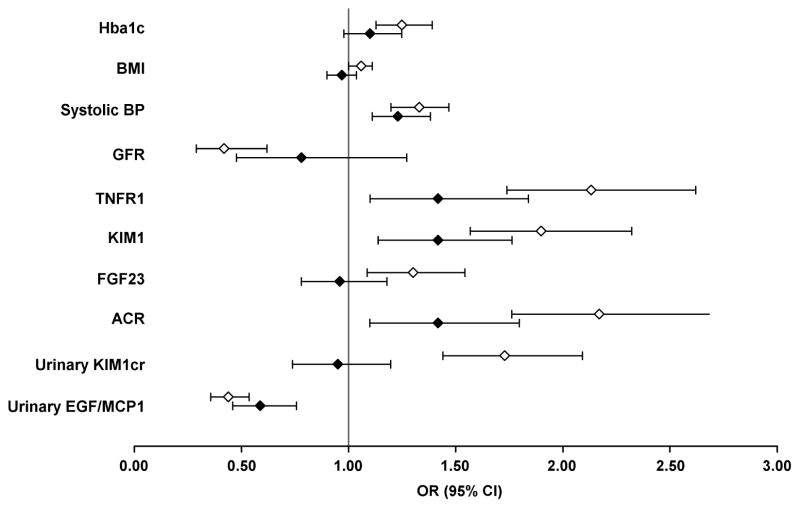
The graphical illustration of comparison of the odds ratios from univariate and multivariable analyses is shown in Figure 1. In multivariable logistic model odds ratios for HbA1c, eGFR, BMI, and urinary KIM-1cr became statistically not significant compared to univariate approach, whereas blood pressure, ACR, TNFR1, and plasma KIM-1 remained independently associated with increased risk of developing early renal decline. The magnitude of odds ratios for the latter were attenuated compared to odds ratios obtained in univariate analysis. It is important to note that in the multivariable analysis, the effect of urinary KIM-1cr disappeared whereas effect of plasma KIM-1 remained statistically significant. No difference in odds ratios between univariate and multivariable approach was observed for the EGF/MCP-1 ratio. This indicates that the high urinary concentration of EGF normalized for urinary concentration of MCP-1 had a strong protective effect against the development of early renal decline, independent from the role of the other markers and pathogenic mechanisms.
Figure 1.
Odds ratios for early renal decline from univariate and multivariable logistic analyses. For correlation matrix among the examined baseline variables see Supplementary Table 1. The multivariable model includes 10 variables, all listed in the Figure. The detailed results of logistic regression are shown in Supplementary Table 2.
Plot of odds ratios for each variable; open diamond indicates odds ratio in univariate, and closed diamond indicates odds ratio in multivariable logistic regression; 95% confidence intervals as shown as whiskers. Vertical line indicates the null effect. For easy clinical interpretability the odds ratios were calculated for 1% increase in HbA1c; 2 kg/m2 increase in BMI; 10 mmHg increase in systolic blood pressure; 20 ml/min increase in eGFR; and 1-quartile increase for the remaining covariates. The 1st quartile, median and 3rd quartile of these markers are as follows: TNFR1 - 1127, 1250 and 1602 pg/ml; FGF23 - 43, 56, and 72 pg/ml; ACR - 3, 9 and 36 ug/mg; KIM-1cr - 5, 27, 58 pg/mg; EGF/MCP-1 - 23, 41 and 79 ng/pg.
Prognostic multi-marker test to identify patients at risk of early renal decline
To select informative covariates into the multivariable prognostic logistic model, we used all the clinical characteristics and markers listed in Table 2 and a backward selection of covariates (see Supplemental material). In the final model the following markers were selected: systolic blood pressure, ACR, plasma TNFR1, plasma KIM-1 and EGF/MCP-1 ratio in urine using the significance criterion α=0.1. These were the same variables as in the etiological model described above.
In order to determine the incremental prognostic value of each of the selected markers in the complete study cohort we sequentially added systolic blood pressure, plasma TNFR1, plasma KIM-1 and EGF/MCP-1 ratio in urine to an initial logistic model containing ACR. The sequence was based on how strongly each covariate is already established as a clinical predictor of kidney injury. The results are shown in Table 3. For easy clinical interpretability we present odds ratio per one quartile increase in concentration of each marker. C-statistic is provided for each model and we calculated Integrated Discrimination Improvement (IDI) for pairwise comparisons between subsequent models. The IDI quantify improved discriminatory performance with addition of a subsequent marker. The base model #1 with ACR only had c-statistic of 0.71 (95% CI, 0.66; 0.76) and model #2 with ACR and systolic BP had c-statistic of 0.73 (95% CI, 0.68, 0.79; p for the difference=0.03). The C-statistic improved significantly with subsequent inclusion of TNFR1 (Model #3) to 0.77 (95% CI, 0.72, 0.81; p for difference p=0.03), then it increased to 0.78 (95% CI, 0.74; 0.83; p for difference p=0.08) with addition of plasma KIM-1 (Model #4) and to 0.81 (95% CI, 0.77; 0.85; p=0.03) with EGF/MCP-1 added (Model #5). The IDI values were significant for comparisons of all subsequent models. The detailed performance of the models was also assessed with likelihood ratio tests and other reclassification metrics (Supplementary Table 3).
Table 3.
Comparison of odds ratios, C-statistics and Integrated Discrimination Improvement in the sequence of nested logistic models for risk of early renal decline in patients with Type 2 diabetes.
| Logistic Models for early renal decline | |||||
|---|---|---|---|---|---|
|
| |||||
| Model | 1 | 2 | 3 | 4 | 5 |
| OR (95% CI) | |||||
| ACR | 2.15 (1.74; 2.6) | 1.92 (1.64; 2.50) | 1.73 (1.39; 2.14) | 1.58 (1.26; 1.96) | 1.44 (1.15; 1.81) |
| Systolic BP | 1.24 (1.11; 1.38) | 1.22 (1.09; 1.37) | 1.23 (1.10; 1.37) | 1.21 (1.08; 1.35) | |
| TNFR1 | 1.76 (1.42; 2.18) | 1.62 (1.31; 2.00) | 1.44 (1.16; 1.81) | ||
| KIM-1 | 1.53 (1.23; 1.87) | 1.45 (1.16; 1.82) | |||
| EGF/MCP-1 | 0.58 (0.46; 0.74) | ||||
| C-statistic | 0.711 | 0.734 | 0.767 | 0.784 | 0.808 |
| Δ C-statistic, 95% CI | n.a. | 0.023 (0.002; 0.045) | 0.033 (0.004; 0.062) | 0.017 (−0.002; 0.035) | 0.024 (0.002; 0.047) |
| IDI, 95% CI | n.a. | 0.026 (0.012; 0.041) | 0.039 (0.025; 0.055) | 0.020 (0.010; 0.031) | 0.026 (0.013; 0.039) |
ACR – urine albumin to urine creatinine ratio, systolic BP – Systolic Blood pressure, IDI - Integrated Discrimination Improvement. Effects for individual markers are presented as odds ratios per 1 quartile increase (or for 10 mmHg increase for systolic BP) from the nested multivariable logistic regression models
As a sensitivity analysis, the same approach was applied to patients with T2D and normoalbuminuria (low risk of early renal decline, see Table 1), and it showed a similar pattern of associations between the same predictors and early renal decline, albeit somewhat weaker and not always statistically significant (see Supplementary Table 4).
In order to develop a prognostic test to identify patients at high risk of early progressive renal decline, we developed a multi-marker prognostic score using the whole study group of patients with T2D.54–56 The model for score derivation included variables in the Model #5: ACR, systolic blood pressure (SBP), TNFR1, KIM-1 and EGF/MCP-1 ratio, all log-transformed. The score is calculated with the following formula:
where urinary ACR is expressed in mg/g, SBP in mmHg, serum TNFR1 and serum KIM-1 in ng/ml, and urinary MCP-1 and EGF in ng/ml. For details of an underlying logistic model see Supplementary Table 5.
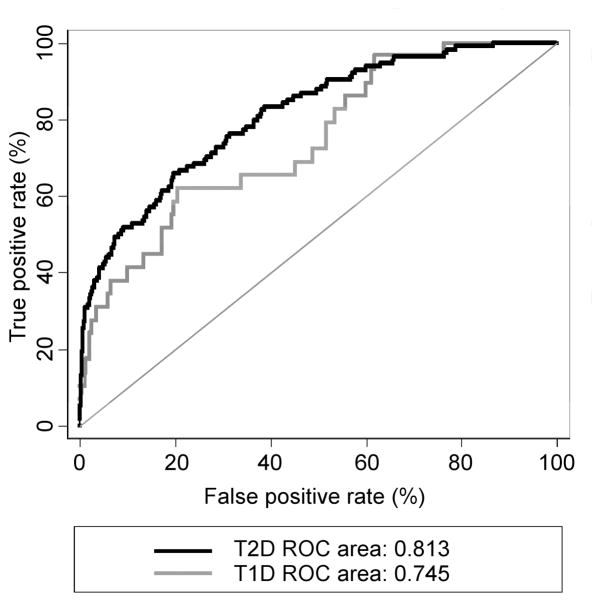
The area under the ROC curve was 0.81 (95% CI 0.77, 0.85). We performed a 10-fold cross-validation, which yielded an AUC 0.80, reflecting a 0.015 optimism. The score was further validated by applying it in our previously described cohort of patients with T1D.13 The ROC analysis resulted in AUC 0.74 (95% CI, 0.65; 0.85) in T1D. The results are plotted in Figure 2.
Figure 2.
ROC curves for the multi-marker risk score of early renal decline in type 2 diabetes (T2D, training cohort, solid black line) and type 1 diabetes (T1D, validation cohort dotted grey line). The T1D cohort characteristics are provided in the Supplemental Material.
To illustrate a possible clinical application of such a score we examined its value in the normoalbuminuria and albuminuria study groups separately. The results are shown in Table 4. We chose cut-points, which yielded 60% sensitivity. In normoalbuminuric patients, a prognostic test based on multi-marker score would have a very low positive predictive value (PPV) of 16%. In patients with albuminuria this test would have a reasonable PPV of 50%. For comparison, Table 4 shows performance of prognostic tests based on baseline ACR concentration only. PPV of test based on ACR was impossible to derive in normoalbuminurics and in albuminurics PPV was only 32%.
Table 4.
Multi-marker score positive predictive value in total T2D cohort, patients with normoalbuminuria, and albuminuria. Positive predictive value of ACR in albuminuria patients provided for comparison. Cut-off values were selected to be able to identify 60% (sensitivity) of the decliners in the study group.
| Cut off | Sensitivity (%) | Positive predictive value (%) | |
|---|---|---|---|
| Multi-marker score in total cohort in points | 16 | 60 | 30 |
| Multi-marker score in normo-albuminuria in points | 8.0 | 60 | 16 |
| Multi-marker score in Albuminuria in points | 40 | 60 | 50 |
| ACR (μg/mg) in albuminuria | 89 | 60 | 32 |
Discussion
By following a large cohort of mainly Caucasian patients with T2D and normal eGFR at baseline, we found that 6% of patients with normoalbuminuria and 18% of those with albuminuria developed early renal decline. Many patients with such decline developed ESRD during the current observation. The other decliners will most likely develop ESRD within the next 20 years of follow-up.3–5
In this study we found that, in addition to high systolic blood pressure and ACR, the risk of early renal decline was strongly associated with high circulating levels of TNFR1, KIM-1 and with decreased urinary EGF/MCP-1 ratio. We summed up all of these markers to differentiate the etiology and to screen for patients with incipient progressive renal decline. Taken together the results indicate that multiple determinants may contribute to the development of early renal decline.
In our study, the effect of systolic blood pressure was strong and significant in univariate analysis, and decreased only moderately when other variables were considered in multivariable logistic regression. These findings indicate that elevated systolic blood pressure exerts its impact on early renal decline through a unique and independent mechanism. The effect of elevated blood pressure on renal decline in diabetes has long been recognized.32 Our study simply extends the previous observations to early renal decline.
Circulating levels of TNFR1, KIM-1 and urinary ACR had very strong effects on risk of early renal decline in univariate analysis. In multivariable analyses, when all of them were included, those effects were still strong, however, attenuated. This suggests that the effects and, therefore mechanisms of action of these three determinants overlapped to some degree. At this time it is difficult to define this common overlapping mechanism. The existing literature mainly considered mechanisms that could account for effects of the markers separately.17, 21,33
Our findings regarding the stronger effect of plasma KIM-1 vs. urinary KIM-1 are of interest. The strong effect of urinary KIM-1 on risk of early renal decline vanished in multivariable logistic analyses specifically after accounting for effects of ACR and plasma KIM-1. This observation might be explained by two mechanisms. First, plasma KIM-1 most likely reflects longer term changes in tubular damage whereas urinary KIM-1 reflects short term variation of this damage. Second, as some authors have postulated elevated ACR reflects both glomerular and tubular damage and therefore might account for the effect of urinary KIM-1.
In agreement with recent publications, we found increased risk of early renal decline associated with decreased urinary levels of EGF.29, 30 Relevant to this observation is our finding that the effect of EGF became much stronger when urinary EGF excretion was standardized by urinary MCP-1 levels and expressed as EGF/MCP-1 ratio. It is important to emphasize that the effect of this ratio was as strong in univariate as in multivariable logistic analysis, i.e. that its effect was not attenuated by other determinants. This indicates that this ratio might either be causally related or it measured an intensity of a disease process which contributed to early renal decline through an independent causal process. Previously the urinary EGF1/MCP-1 ratio was found to be a prognostic marker in IgA nephropathy.31, 34 It was also suggested that downregulation of EGF with simultaneous upregulation of MCP-1 and increased apoptosis in the proximal tubules might be involved in tubulointerstitial damage in reflux nephropathy.35
EGF is a potent trophic factor produced mainly in kidney tubules, and plays a role in kidney development and in tissue repair.36 Experimental studies have shown the importance of EGF and EGFR signaling in maintaining tubular epithelial cell integrity.19, 20, 37,38 In the recent study by Ju et al., low urinary EGF was found to predict loss of renal function in three cohorts of patients with advanced CKD. The authors hypothesized that urinary EGF excretion might be a marker of regenerative tubular functional reserve.30 Low urinary excretion of EGF was also found to be associated with increased risk of renal function loss in normoalbuminuric patients with T2D in the Edinburgh study.30 However, its prognostic value was not statistically significant when other markers and clinical characteristics were included.
MCP-1 is a chemokine with a critical role in recruiting activated macrophages/monocytes to the kidney in response to damage and MCP-1 is upregulated in the diabetic kidney predominantly in response to tubular cell damage.18–20,39 Blocking the action of MCP-1 or chemokine receptor-2 (CCR2) in animal models of progressive renal injury resulted in reduction of the extent of tubulointerstitial inflammation and kidney fibrosis.40–44 Therefore, it is possible that the urinary EGF/MCP-1 ratio becomes such a significant determinant of early renal decline because it represents either better measure of tubulointerstitial damage or interplay between availability of EGF as a protective factor in the face of elevated MCP-1.
A few comments must be made regarding factors identified previously but not confirmed in our study. First, the lack of association between HbA1c, BMI and circulating FGF23 and early renal decline in our multivariable logistic analysis can be the result of their weak effects. Perhaps, a much larger study would demonstrate their significant independent effects. Second, the strong effects of baseline eGFR and urinary KIM-1 in univariate analysis did not sustain in multivariable analysis due to likely common causal processes shared with other determinants, such as circulating serum TNFR1, KIM-1, and urinary ACR. Finally, lack of positive finding with urinary NGAL in our study most likely reflects the role of that marker in tubular damage in late but not early renal decline. 45
Independent determinants found in our study could be used to develop a prognostic test to identify patients at risk of early renal decline in T2D. Integration of these determinants into a multi-marker score resulted in significant improvement of the accuracy of prediction of early renal decline, compared with a model that included only clinical variables. However, this improvement is less impressive when considering its application in the care of patients with low risk of renal decline, as those with normoalbuminuria. First, the higher values of AUC of our prognostic test are due to improvement in classification of not only decliners but also non-decliners, with the latter being predominant in T2D population with normal renal function. Second, the sensitivity and positive predictive value (PPV) of any prognostic test are profoundly influenced by the prevalence of patients at risk of early renal decline.
For example, in our study our multi-marker prognostic test with sensitivity selected at 60% (desirable for patient care and for recruitment of patients for clinical trials) had PPV of only 15% in patients with normoalbuminuria, in whom risk of early renal decline was low (6%). The same multi-marker test had PPV of 50%, if used in patients with microalbuminuria in whom risk of early renal function was higher (18%). Clearly, our multi-marker prognostic test does not have much value in identifying patients with early renal decline in T2D patients with normoalbuminuria, and its value in albuminurics is only moderate. Therefore, there is still a great need to find new informative markers to develop a better prognostic test to identify early renal decline in patients with T2D and normal renal function.
Finally, limitations of our study should be acknowledged. A potential concern in our study is the determination of early renal decline based on serum creatinine measurements. Compared with directly measured GFR, eGFR might underestimate “hyperfiltration” in T2D. This might reduce the steepness of eGFR slopes and underestimate the frequency of early progressive renal decline. However, it is unlikely that the major conclusions from our study were affected. With regard to the etiological model of early renal decline, our study cannot provide evidence about causality. It generates a hypothesis about the importance of tubular damage for early renal decline but this hypothesis needs to be further validated in a setting of animal study or clinical trial. Our study was conducted in T2D patients of mainly European ancestry. The findings are similar to our previous observations in similar patients with T1D, however, it is not certain whether our findings can be generalized to a broad population of T2D patients from different countries and different ethnic origins. Our multi-marker prognostic test represents a new approach to utilize the results of etiological study to develop a prognostic test. However, the test has limited prognostic value and, at this moment, with too few informative markers known, it cannot be recommended for clinical application.
Methods
Study group
Participants for the 2nd Joslin Kidney Study (JKS) were recruited from among patients attending the Adult Endocrinology Unit at Joslin Clinic between 2003 and 2009. Residents of New England with T2D diagnosed after age 30 years and age 35 – 64 at study enrollment were eligible for the study. We excluded patients, who were on dialysis, had renal transplant, or had a history of HIV or hepatitis C infection.
For 4500 eligible patients the archived clinical laboratory results were searched for measurements of albumin-to-creatinine ratio (ACR) in urine specimens performed during a 2 year period preceding the clinic visits at which patients were considered for enrollment into the 2nd JKS. Patients with median value of ACR <14 and <24 μg albumin/1 mg of urinary creatinine accordingly in men and women were considered normoalbuminurics. Those with median ACR values above were considered albuminurics.
This 2nd JKS aimed to enroll eligible patients with albuminuria and a similar number of eligible patients taken randomly from the much larger pool of patients with normoalbuminuria. Between 2003 and 2009, 1476 patients were enrolled, 743 patients with albuminuria and 733 patients with normoalbuminuria. They were examined during routine visits to the Clinic as baseline examination and biannually afterwards with specimens of blood and urine taken for laboratory determinations and storage in −80 C. Patients with less frequent clinic visits or those who stopped coming to the clinic were examined at their homes.
All patients in the 2nd JKS were queried against rosters of the United States Renal Data System (USRDS) and the National Death Index (NDI) covering all events up to the end of 2013. USRDS maintains a roster of U.S. patients receiving renal replacement therapy, which includes dates of dialysis and transplantation. The NDI is a comprehensive roster of deaths in the U.S., which includes date and cause of death.
For the current study only patients with normal baseline eGFR and at least 5 year follow-up were included, this yielded 430 and 602 patients accordingly with albuminuria and normoalbuminuria.
Laboratory Determinations
Assessment of abnormalities in urinary albumin excretion
In the Joslin Clinic albumin and creatinine concentrations in spot urines are measured at least once a year for all patients. The laboratory methods used to determine albumin and creatinine concentrations in urine were reported previously46.
Definition of albuminuria
The 2-year median pre-baseline ACR was used for patient ascertainment; the definitions of normoalbuminuria, microalbuminuria and proteinuria were reported previously.46
Definition of baseline ACR
For the purpose of prediction and comparison with other determinants of renal decline, we used a single ACR measurement performed in urine specimen obtained at enrollment into the study, on the same occasion as the other markers were measured.
Assessment of renal function
In 2013–2015, serum specimens obtained at baseline and during follow-up in patients participating in the study were retrieved and used to measure creatinine concentrations. The measurements were performed in the Advanced Research and Diagnostic Laboratory at the University of Minnesota using the Roche enzymatic assay (Prod No. 11775685) on a Roche/Hitachi Mod P analyzer. This method was previously calibrated to be traceable to an isotope dilution mass spectrometry (IDMS) reference assay and was verified by measuring National Institutes of Standards and Technology Standard Reference Material (NIST SRM) No. 967. The CKD-EPI formula was used to estimate eGFR.47
Definition of early renal decline
In this study only patients with normal renal function at baseline (eGFR above 60 mL/min, median 98 mL/min, with 1st and 3rd quartile of 85–110 mL/min) were included. Early renal decline was defined as eGFR loss ≥30% from baseline during the first 5 years of follow-up. Using a linear regression on the longitudinal subject-specific values of eGFR, we estimated baseline eGFR (intercept) and eGFR slope (rate of renal function loss in mL/min/year) for each patient. Using these two indices we identified patients who lost at least 30% of baseline eGFR during 5 year follow-up. These patients were referred to as decliners. To exclude acute kidney injury as a cause of the early progressive renal decline, we manually reviewed eGFR trajectories in patients classified as decliners.
Laboratory procedures to measure plasma and urinary markers
A detailed description of procedures to measure plasma TNFR1, FGF23, urinary and plasma KIM-1, urine MCP-1 and EGF is provided in the supplemental material
Statistical analyses
To make the results of the current study easily comparable with our previous publications,6,8,24 continuous variables are presented as medians and 1st and 3rd quartile values, and qualitative variables are presented as frequencies and percentages. Incidence rates of ESRD and deaths were calculated using SAS macro provided by the Mayo Clinic.48, 49 Differences among the two outcome groups were tested using χ2 test for categorical variables, and Wilcoxon Rank-Sum test for continuous variables. The effects of baseline clinical characteristics and examined markers on early renal decline were estimated using logistic regression model and were expressed in terms of odds ratios (OR) and their confidence intervals. Interactions between markers were tested by the addition of interaction terms into the final multivariable logistic model. The multicollinearity was quantified using the variance inflation factor.
The detailed information about the development and assessment of the etiological and prognostic logistic models for the early renal decline are described in Supplemental Material. The strategy applied for deriving prognostic index was described previously.50–52 We used a multivariable logistic regression model with natural log-transformed values of markers of early progressive renal decline as covariates. The regression coefficients used to build the score are shown in Supplementary Table 5.
Statistical analyses were performed using SAS version 9.4. Two-sided P < .05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was supported by the JDRF grant “Biomarkers of Diabetic Nephropathy Collaborative Research Initiative (DN-BIO)” No.3-SRA-2015-106-Q-R to ASK and JVB; by the National Institutes of Health grant DK-041526 to ASK; by the NIH Diabetes Research Center (DRC) grant P30 DK036836 and by grants from the Lilly Inc., and Pfizer Inc. to ASK to study biomarkers of early renal decline in T2D.
The authors thank the technical staff of the Krolewski laboratory Stephanie Croall, Johann Roebelen, and Kevin McDonnell for their help with biological measurements.
Footnotes
Disclosures:
ASK and MAN are co-inventors of the TNFR1 and TNFR2 patent for predicting risk of ESRD. This patent was licensed by the Joslin Diabetes Center to EKF Diagnostics.
Author Contributions: NN contributed to the study design, performed laboratory measurements and data analysis, interpreted the results and wrote manuscript; JS contributed to study design, worked together with NN on data analysis, interpreted the results and edited manuscript; AS supervised data collection, and reviewed manuscript; MY reviewed manuscript; MAN contributed to research data collection, reviewed manuscript; AG, KLD, MDB, NP, and JVB all contributed intellectually to the final plans of data analysis, final interpretation of the results and writing/editing the manuscript; ASK was responsible for design the study, supervised data collection and data analysis, contributed to writing and editing manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A. US renal data system 2010 annual data report. Am J Kidney Dis. 2011;57(Suppl 1):A8-e1–526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nature Reviews Nephrology. 2016;12:73–81. doi: 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 3.Krolewski AS, Skupien J, Rossing P, Warram JH. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int. 2017 Jun;91(6):1300–1311. doi: 10.1016/j.kint.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skupien J, Warram JH, Smiles AM, Niewczas MA, Gohda T, Pezzolesi MG, Cantarovich D, Stanton R, Krolewski AS. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int. 2012;82:589–97. doi: 10.1038/ki.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skupien J, Warram JH, Smiles AM, Stanton RC, Krolewski AS. Patterns of Estimated Glomerular Filtration Rate Decline Leading to End-Stage Renal Disease in Type 1 Diabetes. Diabetes Care. 2016;39:2262–2269. doi: 10.2337/dc16-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, Karoly ED, Kensicki EM, Berry GT, Bonventre JV, Pennathur S, Meyer TW, Krolewski AS. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int. 2014;85:1214–24. doi: 10.1038/ki.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamanouchi M, Skupien J, Niewczas MA, Smiles AM, Doria A, Stanton RC, Galecki AT, Duffin KL, Pullen N, Breyer MD, Bonventre JV, Warram JH, Krolewski AS. Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int. 2017;92(1):258–266. doi: 10.1016/j.kint.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, Wong MC, Turner C, Palmer CN, Nogoceke E, Groop L, Salomaa V, Dunger DB, Agakov, McKeigue PM, Colhoun HM SUMMIT Investigators. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015;88:888–96. doi: 10.1038/ki.2015.199. [DOI] [PubMed] [Google Scholar]
- 10.Saulnier PJ, Gand E, Velho G, Mohammedi K, Zaoui P, Fraty M, Halimi JM, Roussel R, Ragot S, Hadjadj S SURDIAGENE Study Group. Association of Circulating Biomarkers (Adrenomedullin, TNFR1, and NT-proBNP) With Renal Function Decline in Patients With Type 2 Diabetes: A French Prospective Cohort. Diabetes Care. 2017;40:367–374. doi: 10.2337/dc16-1571. [DOI] [PubMed] [Google Scholar]
- 11.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–61. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 12.Krolewski AS, Niewczas MA, Skupien J, Gohda T, Smiles A, Eckfeldt J, Doria A, Warram JH. Early Progressive Renal Decline Precedes the Onset of Microalbuminuria and Its Progression to Macroalbuminuria. Diabetes Care. 2014;37:226–234. doi: 10.2337/dc13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2015;38:954–62. doi: 10.2337/dc15-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS CKD Prognosis Consortium. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–311. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang Ch, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. Kidney Injury Molecule-1 is a Biomarker of Acute and Chronic Kidney Injury and Predicts Progression to ESRD. J Am Soc Nephrol. 2014;25:2177–86. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, Smiles A, Warram JH, Bonventre JV, Krolewski AS. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016;89:459–67. doi: 10.1038/ki.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–68. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol. 2002;13:1534–47. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]
- 19.Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 21.Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015;87:281–96. doi: 10.1038/ki.2014.285. [DOI] [PubMed] [Google Scholar]
- 22.Lucarelli G, Mancini V, Galleggiante V. Emerging Urinary Markers of Renal Injury in Obstructive Nephropathy. BioMed Research International. 2014 doi: 10.1155/2014/303298. Article ID 303298, 7 pages, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackle I, Gunther H, Von Gise H, Alt JM, Bohle A, Stolte H. Kidney function and protein excretion in relation to pathomorphology of glomerular diseases. Contrib Nephrol. 1988;68:128–135. doi: 10.1159/000416503. [DOI] [PubMed] [Google Scholar]
- 24.Remuzzi Giuseppe, Ruggenenti Piero, Benigni Ariela. Understanding the nature of renal disease progression. Kidney International. 1997;51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 25.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P MMKD Study Group. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 26.Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scandinavian journal of clinical and laboratory investigation Supplementum. 2008;241:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolignano D, Coppolino G, Lacquaniti A, et al. Pathological and prognostic value of urinary neutrophil gelatinase-associated lipocalin in macroproteinuric patients with worsening renal function. Kidney Blood Press Res. 2008;31:274–9. doi: 10.1159/000151665. [DOI] [PubMed] [Google Scholar]
- 28.Tam FW, Riser BL, Meeran K. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine. 2009;47:37–42. doi: 10.1016/j.cyto.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song P, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7:316–25. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betz BB, Jenks SJ, Cronshaw AD, Lamont DJ, Cairns C, Manning JR, Goddard J, Webb DJ, Mullins JJ, Hughes J, McLachlan S, Strachan MW, Price JF, Conway BR. Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int. 2016;89:1125–35. doi: 10.1016/j.kint.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Torres DD, Rossini M, Manno C, Mattace-Raso F, D’Altri C, Ranieri E, Pontrelli P, Grandaliano G, Gesualdo L, Schena FP. The ratio of epidermal growth factor to monocyte chemotactic peptide- 1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int. 2008;73:327–333. doi: 10.1038/sj.ki.5002621. [DOI] [PubMed] [Google Scholar]
- 32.Parving HH, Mauer M, Ritz E. Diabetic nephropathy, chapter 38. In: Brenner BM, editor. Brenner and Rector’s the Kidney. 7. Philadelphia: WB Saunders; 2004. pp. 1777–1818. [Google Scholar]
- 33.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Movahedi, Naini S, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–35. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranieri E, Gesualdo L, Petrarulo F, Schena FP. Urinary IL-6/EGF ratio: a useful prognostic marker for the progression of renal damage in IgA nephropathy. Kidney Int. 1996;50:1990–2001. doi: 10.1038/ki.1996.521. [DOI] [PubMed] [Google Scholar]
- 35.Chertin B, Farkas A, Puri P. Epidermal growth factor and monocyte chemotactic peptide-1 expression in reflux nephropathy. Eur Urol. 2003;44:144–149. doi: 10.1016/s0302-2838(03)00190-8. [DOI] [PubMed] [Google Scholar]
- 36.Mullin JM, McGinn MT. Epidermal growth factor-induced mitogenesis in kidney epithelial cells (LLC-PK1) Cancer Res. 1988;48:4886–4891. [PubMed] [Google Scholar]
- 37.Zhuang S, Dang Y, Schnellmann RG. Requirement of the epidermal 422 growth factor receptor in renal epithelial cell proliferation and migration. Am J Physiol Renal Physiol. 2004;287:F365–372. doi: 10.1152/ajprenal.00035.2004. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Chen J-K, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney International. 2012;82:45–52. doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 40.Mizu H, Maruyama S, Yuzawa Y, Kato T, Miki Y, Suzuki S, Sato W, Morita Y, Maruyama H, Egashira K, Matsuo S. Anti-monocyte chemoattractant protein-1 gene therapy attenuates renal injury induced by protein-overload proteinuria. J Am Soc Nephrol. 2007;14:1496–1505. doi: 10.1097/01.asn.0000069223.98703.8e. [DOI] [PubMed] [Google Scholar]
- 41.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol. 2003;14:2503–15. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 42.Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N, Tomosugi N, Matsushima K, Egashira K, Yokoyama H. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol. 2004;15:940–8. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- 43.Ramos MV, Auvynet C, Poupel L, Rodero M, Mejias MP, Panek CA, Vanzulli S, Combadiere C, Palermo M. Chemokine receptor CCR1 disruption limits renal damage in a murine model of hemolytic uremic syndrome. Am J Pathol. 2012;180:1040–8. doi: 10.1016/j.ajpath.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Sayyed SG, Ryu M, Kulkarni OP, Schmid H, Lichtnekert J, Grüner S, Green L, Mattei P, Hartmann G, Anders HJ. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80:68–78. doi: 10.1038/ki.2011.102. [DOI] [PubMed] [Google Scholar]
- 45.Nickolas Thomas L, Forster Catherine S, Sise Meghan E, Barasch Nicholas, Valle David Solá-Del, Viltard Melanie, Buchen Charles, Kupferman Shlomo, Carnevali Maria Luisa, Bennett Michael, Mattei Silvia, Bovino Achiropita, Argentiero Lucia, Magnano Andrea, Devarajan Prasad, Mori Kiyoshi, Erdjument-Bromage Hediye, Tempst Paul, Allegri Landino, Barasch Jonathan. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82(6):718–722. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–7. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 47.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 49.Bergstralh E. [Accessed 2010 Feb 3];SAS Macro That Performs Cumulative Incidence in Presence of Completing Risks. Available: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/comprisk.sas.
- 50.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 51.Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22–28. doi: 10.1016/j.jclinepi.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 52.Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA. Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients With Stable Coronary Heart Disease. JAMA. 2016;315:2532–41. doi: 10.1001/jama.2016.5951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.