To the Editor
Human RAG1/2 gene mutations are associated with a spectrum of clinical and immunological phenotypes. Although null recombinase activating gene (RAG) mutations cause severe combined immunodeficiency (SCID), hypomorphic mutations may induce Omenn syndrome (OS), atypical SCID (AS), or combined immunodeficiency (CID) manifesting with autoimmunity.1 We have previously demonstrated that in patients with RAG mutations the severity of the clinical phenotype correlates with residual levels of RAG protein recombination activity and T/B-cell repertoire diversity and composition; however, whether there are molecular signatures indicative of autoimmunity remains unknown.2 Regulatory T (Treg) cells play a key role in preventing autoimmunity. Although numerical and functional Treg-cell defects have been demonstrated in patients with OS, no data are available for patients with CID/AS despite the frequent association with autoimmunity.
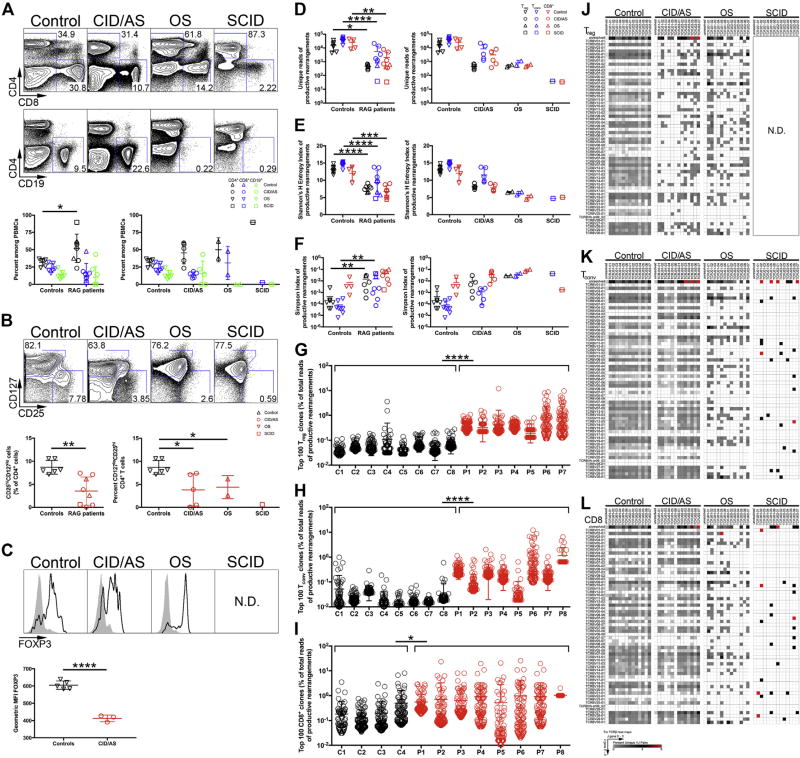
Herein, we have studied 8 patients carrying biallelic RAG mutations and presenting with SCID (n = 1), OS (n = 2), or CID/AS (n = 5) (see Table E1 and the Methods section in this article’s Online Repository at www.jacionline.org). Blood was obtained on informed consent, and the study was approved by the institutional review board of the referring institutions. Significant variability in CD4+ and CD8+ cell counts was observed in the patients, with several of them showing higher frequencies of CD4+ cells and a reduced proportion of naive CD4+ cells (Table E1; see Fig 1, A). Lower frequencies of CD4+CD25hiCD127lo cells expressing FOXP3 were observed in the patients (Fig 1, B), with significantly less FOXP3 mean fluorescent intensity in patients with CID/AS (Fig 1, C). These findings suggest that RAG deficiency disproportionately affects Treg cells compared with other T lymphocytes irrespective of the clinical phenotype.
FIG 1.
Flow cytometric and repertoire analysis of peripheral blood lymphocyte populations in patients with RAG mutations. A, Gating strategy for circulating CD4+, CD8+, and CD19+ lymphocytes (top) and relative proportions (bottom) in controls and RAG-mutated patients. B, Identification (top) and frequency (bottom) of Treg cells among CD4+ lymphocytes by CD25 and CD127 expression. C, FOXP3 expression in CD4+CD25hiCD127lo Treg cells (black line) and CD4+CD25−CD127+ Tconv cells (gray fill) from controls or patients (top). Geometric mean fluorescent intensity of FOXP3 protein expression in controls and patients with CID/AS (bottom). D, Unique TRB productive rearrangements for each subject’s T-cell subpopulation. Shannon’s H Entropy (E) and Simpson (F) index of diversity for productive TRB rearrangements. Frequency of 100 most abundant productive TRB clonotypes identified in Treg (G), CD4+ Tconv (H), and CD8+ (I) cells for patients (P1–P8) and controls (C1–C8). Representative heat maps depicting TRBV (rows) and TRBJ (columns) gene pairing in unique productive TRB rearrangements in Treg (J), Tconv (K), orCD8+ T cells (L) (5′to 3′ orientation of V and J genes). ND, Not done. Student t test (Gaussian distribution) or Mann-Whitney test (non-Gaussian distribution) was used for flow cytometric statistical analysis and 2-way ANOVA was used for repertoire analysis with *P < .05, **P < .01, ***P < .001, and ****P < .0001. Bars represent mean ± SD.
We have previously shown that thymic Treg-cell generation is compromised in RAG-mutated patients.3 However, whether Treg TCR repertoire composition and diversity and Treg-cell function are also compromised remains unknown. To investigate this, we used high throughput sequencing to analyze the diversity and composition of T-cell receptor β (TRB) repertoire in conventional CD4+ (Tconv), Treg, and CD8+ cells from RAG-deficient patients and healthy controls. Purified cell populations of CD25hiCD127low (Treg), CD25lowCD127hi (CD4+ Tconv), and CD4− CD8+ cells were isolated for genomic DNA collection (see Fig E1, A, in this article’s Online Repository at www.jacionline.org), and rearranged TRB products were sequenced (Fig E1, B). Interestingly, the number of unique TRB reads obtained from Treg cells was reduced 100-fold in patients with OS and CID/AS compared with controls (Fig 1, D), and a 10-fold difference was observed for Tconv and CD8+ cells (Fig 1, D), further indicating that RAG mutations disproportionately affect Treg-cell development and repertoire.
Ecology parameters are used to measure diversity and composition of the T- and B-cell repertoires. In particular, Shannon’s entropy (H) and Simpson’s indices measure repertoire diversity, taking into account total sequences and clonal distribution within overall repertoire.4 The TRB repertoire of RAG-mutated patients had markedly reduced Shannon’s entropy and increased Simpson’s indices compared with control T-cell subpopulations (Fig 1, E and F), indicating significant restriction of repertoire diversity. To assess for individual T-cell clonotypic expansion, the 100 most abundant TRB clonotypes were identified, and their relative frequency among total reads was assessed. Significantly higher Treg-cell and Tconv-cell clone frequencies were present in patients compared with controls irrespective of disease phenotype (Fig 1, G and H). A similar pattern was also observed among CD8+ cells, although with significant interindividual variability (Fig 1, I).
Given the striking reduction in patients’ unique TRB reads, we determined whether this reflected skewed usage and pairing of V and J genes. The Treg, Tconv, and CD8+ T-cell populations in RAG-mutated patients demonstrated severe VJ pairing restriction (Fig 1, J–L; see Fig E2 in this article’s Online Repository at www.jacionline.org). This skewing in VJ gene pairing was driven predominantly by usage bias of individual TRBV genes (see Table E2 in this article’s Online Repository at www.jacionline.org).
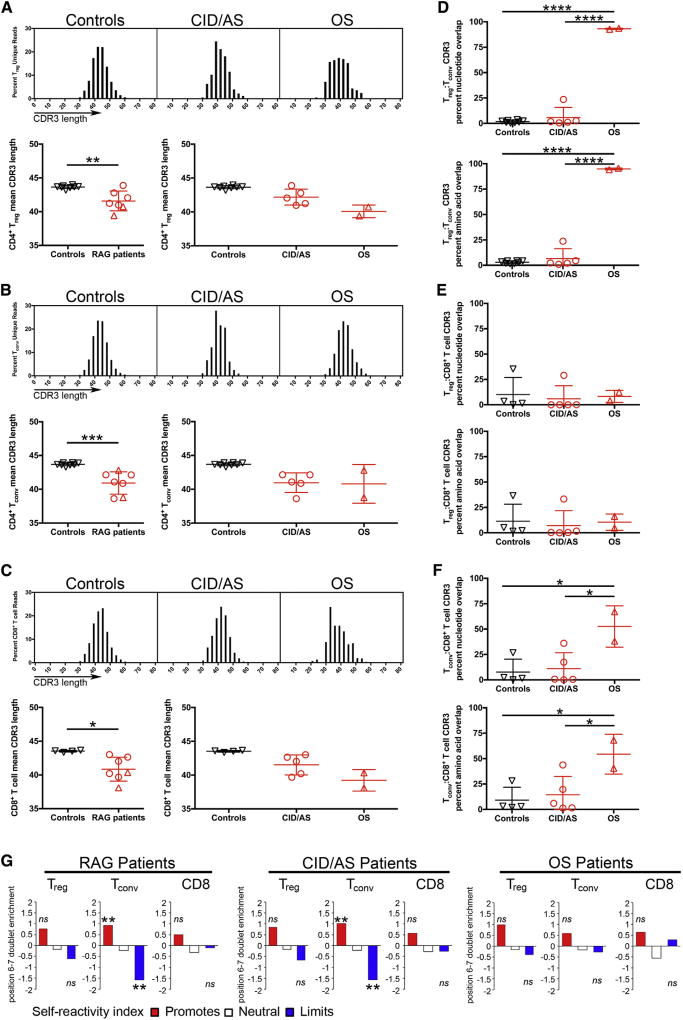
Given the risk for RAG-mutated patients to develop autoimmunity, we characterized their TRB repertoire for complementary determining region 3 (CDR3) properties that may associate with self-reactivity. The CDR3 length from all T-cell subpopulations in patients with CID/AS and OS was significantly shorter than in equivalent control populations (Fig 2, A–C). Although Treg and Tconv cells from controls have largely nonoverlapping repertoires,5 a high degree of overlap (>90%) was detected both at the nucleotide level and at the amino acid level in patients with OS (Fig 2, D). Furthermore, although no repertoire overlap was detected between Treg and CD8+ cells (Fig 2, E), some overlap was also observed for Tconv and CD8+ T-cell repertoire in patients with OS (Fig 2, F). The high degree of repertoire overlap between Treg and Tconv cells from patients with OS may suggest that circulating CD25hiCD127low cells from these patients do not represent bona fide Treg cells, but rather peripherally activated cells. Indeed, in humans, FOXP3 expression is not restricted to Treg cells, and FOXP3+ effector T cells do not display suppressive activity.6 In this regard, although circulating CD4+CD25hiFOXP3+ cells may be present in normal number in patients with OS, they express markers of effector/memory T cells (CCR7−CD45RO+) and have impaired suppressive function.7
FIG 2.
Molecular characteristics of CDR3 of productive TRB rearrangements. Virtual spectratyping of CDR3 nucleotide length (top) and mean CDR3 length (bottom) of unique rearranged products in Treg (A), CD4+ Tconv (B), andCD8+ T (C) cells from controls and patients. TRB-CDR3 sequence homology at the nucleotide (top) and amino acid (bottom) level in different T-cell subsets (D–F). Self-reactivity index of Treg, Tconv, and CD8+ cells from all RAG-mutated patients (left), patients with CID/AS (middle), or patients with OS (right) compared with equivalent control subpopulations (G). ns, Not significant. Student t test was used for statistical analysis with *P < .05, **P < .01, ***P < .001, and ****P < .0001. In panels A–F, bars represent mean ± SD. **P < 10−10.
The amino acid composition of the CDR3 TRB (CDR3β) may distinguish normal versus autoimmune-prone T-cell repertoires. In particular, hydrophobic amino acids at CDR3β positions 6 and 7 promote TCR self-reactivity.8 A self-reactivity index has been proposed to measure how differential usage of amino acids at positions 6 and 7 of the CDR3β may promote self-reactivity contributing to autoimmunity.8 When applied to patients with hypomorphic RAG mutations, enrichment for amino acids associated with self-reactivity and decreases in those limiting autoimmunity were identified in CDR3β from Tconv cells of patients with CID/AS (Fig 2, G). No significant difference in the self-reactivity index was observed for Treg and CD8+ cells from these patients, nor for any subset from patients with OS (Fig 2, G). The increased hydrophobicity demonstrated at the CDR3β position 6–7 doublet is indicative of higher self-reactivity of Tconv cells in patients with CID/AS. This may reflect impaired negative selection of self-reactive T cells in this group of patients, as also indicated by impaired expression of autoimmune regulator (AIRE) in the thymus.9
Finally, to determine whether abnormalities of TRB repertoire in patients with CID/AS could be magnified by Treg-cell functional defects, PBMCs from 2 patients with CID/AS (P1 and P4) and controls were sort-purified into CD25hi CD127low Treg and CD25− CD127hi Tconv cells (see Fig E3, A, in this article’s Online Repository at www.jacionline.org). The Treg-cell activity was measured via suppression of control Tconv-cell division to CD2/CD3/CD28 stimulation. In both patients, Treg cells displayed reduced suppressive activity compared with controls (Fig E3, B). Because genetic defects affecting Treg-cell development and/or function in humans are associated with autoimmunity,10 these findings suggest that functional Treg-cell defects in patients with CID/AS may contribute to autoimmunity.
In summary, our findings suggest a model in which hypomorphic RAG mutations drive disproportionate loss of Treg cells, and Treg-cell and TCR repertoire restriction is compounded by increased Tconv-cell self-reactivity with diminished Treg-cell function in patients with CID/AS. These findings also provide evidence for distinctive cellular and molecular signatures of autoimmunity in these patients.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant nos. 2R01AI100887 and 2U54AI082973) and by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (protocol 16-IN139).
J. H. Rowe is employed by Dana Farber Cancer Institute. B. D. Stadinski has received grants from the National Institutes of Health (grant nos. U19 AI109858-01, R01 DK095077-05, and R01 AR071269-02) and is employed by the University of Massachusetts Medical School. Y. N. Lee has received travel support from the Jeffrey Modell Foundation. M. T. de la Morena has received a grant from the National Institutes of Health (grant no. 5R01AI114523). A. Hayward is employed by Brown University and has received grants from the National Institutes of Health. C. H. Huang is employed by the National University of Singapore. J. E. Walter has consultant arrangements with and has received payment for lectures from Shire and CSL-Behring, has received a grant from the National Institute of Allergy and Infectious Diseases (grant no. K08AI103035), and a grant from the National Institutes of Health (grant no. 5R01AI100887). E. S. Huseby has received a grant from the National Institutes of Health. L. D. Notarangelo has received a grant from the National Institute of Allergy and Infectious Disease; has board memberships with the Journal of Clinical Immunology, Clinical Immunology, and Frontiers in Immunology; and has received royalties from UpToDate.
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen TG, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood. 2001;97:81–8. doi: 10.1182/blood.v97.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol. 2014;133:1099–108. doi: 10.1016/j.jaci.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poliani PL, Facchetti F, Ravanini M, Gennery AR, Villa A, Roifman CM, et al. Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood. 2009;114:105–8. doi: 10.1182/blood-2009-03-211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magurran AE. Biological diversity. Curr Biol. 2005;15:R116–8. doi: 10.1016/j.cub.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Golding A, Darko S, Wylie WH, Douek DC, Shevach EM. Deep sequencing of the TCR-beta repertoire of human forkhead box protein 3 (FoxP3)+ and FoxP3− T cells suggests that they are completely distinct and non-overlapping. Clin Exp Immunol. 2017;188:12–21. doi: 10.1111/cei.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, et al. Activation- induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 7.Cassani B, Poliani PL, Moratto D, Sobacchi C, Marrella V, Imperatori L, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol. 2010;125:209–16. doi: 10.1016/j.jaci.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Stadinski BD, Shekhar K, Gomez-Tourino I, Jung J, Sasaki K, Sewell AK, et al. Hydrophobic CDR3 residues promote the development of self-reactive T cells. Nat Immunol. 2016;17:946–55. doi: 10.1038/ni.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–71. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.