Abstract
Syllidae is one of the most species‐rich groups within Annelida, with a wide variety of reproductive modes and different regenerative processes. Syllids have striking ability to regenerate their body anteriorly and posteriorly, which in many species is redeployed during sexual (schizogamy) and asexual (fission) reproduction. This review summarizes the available data on regeneration in syllids, covering descriptions of regenerative mechanisms in different species as well as regeneration in relation to reproductive modes. Our survey shows that posterior regeneration is widely distributed in syllids, whereas anterior regeneration is limited in most of the species, excepting those reproducing by fission. The latter reproductive mode is well known for a few species belonging to Autolytinae, Eusyllinae, and Syllinae. Patterns of fission areas have been studied in these animals. Deviations of the regular regeneration pattern or aberrant forms such as bifurcated animals or individuals with multiple heads have been reported for several species. Some of these aberrations show a deviation of the bilateral symmetry and antero‐posterior axis, which, interestingly, can also be observed in the regular branching body pattern of some species of syllids.
Keywords: annelid, epimorphosis, fission, morphallaxis, schizogamy
1. INTRODUCTION: OVERVIEW OF ANNELID REGENERATION
Regeneration is a postembryonic morphogenesis characterized by the ability of an organism to restore lost parts of the body (Bely & Nyberg, 2010; Brockes & Kumar, 2008). Regeneration is widely distributed among animals and occurs at the level of cells, tissues, internal organs, structures, and even the whole body (Bely & Nyberg, 2010; Pfeifer, Dorresteijn, & Fröbius, 2012). Annelids, commonly referred to as “segmented worms,” have remarkable abilities of whole‐body regeneration, even from few segments (Bely, 2006, 2014; Seaver, 2003). Hence some species have been considered as excellent models to investigate regeneration, such as Alitta virens (Bakalenko, Novikova, Nesterenko, & Kulakova, 2013; Kozin, Filippova, & Kostyuchenko, 2017), Capitella teleta (Hill, Ferkowicz, & Grassle, 1988; Jong & Seaver, 2017), Cirratulus cirratus (Weidhase, Bleidorn, & Helm, 2014), Enchytraeus japonensis (Myohara, Yoshida‐Nora, Kobari, & Tochinai, 1999; Ogawa et al., 2017), Eurythoe complanata (Müller, Berenzen, & Westheide, 2003; Weidhase, Bleidorn, Beckers, & Helm, 2016), Lamellibrachia satsuma (Miyamoto, Shinozaki, & Fujiwara, 2014), Platynereis dumerilii (Prud'homme et al., 2003; Starunov, Voronezhskaya, & Nezlin, 2017), Pristina leidyi (Bely & Wray, 2001; Nyberg, Conte, Kostyun, Forde, & Bely, 2012; Özpolat, Sloane, Zattara, & Bely, 2016), Timarete punctata (Weidhase, Helm, & Bleidorn, 2015), and Typosyllis antoni (Aguado, Helm, Weidhase, & Bleidorn, 2015; Weidhase, Beckers, Bleidorn, & Aguado, 2016; Weidhase, Beckers, Bleidorn, & Aguado, 2017).
For annelids, two different processes leading to regeneration are distinguished: epimorphosis and morphallaxis (Kozin et al., 2017; Özpolat et al., 2016). Epimorphosis is characterized by the activity of somatic stem cells, dedifferentiation, and re‐differentiation processes, resulting in the appropriate re‐establishment of tissue polarity, structure, and form of the organism (Agata, Saito, & Nakajima, 2007; Alvarado & Tsonis, 2006; Bely & Nyberg, 2010; Özpolat & Bely, 2016). The dedifferentiation stage is represented by the formation of a blastema, a tissue that contains undifferentiated cells and acts as a growth zone (Agata et al., 2007; Boilly, Faulkner, Jobling, & Hondermarck, 2017). In contrast, blastema formation is absent in morphallaxis, and the remaining part of the body remodels drastically acquiring morphologies consistent with new positional identities or maintaining normal proportions of the body (Agata et al., 2007; Özpolat & Bely, 2016).
Several studies in annelids have compared juvenile development with regeneration, since both processes (may) involve the establishment of a proliferation zone, also known as a segmental addition zone (SAZ) (Balavoine, 2014; Zattara & Bely, 2016). Additionally, developmental pathways of key transcriptional factors can be triggered by regenerative processes (Bakalenko et al., 2013; Novikova, Bakalenko, Nesterenko, & Kulakova, 2013). However, during early development the body axes are established de novo, while during regeneration body axes are defined by the remaining old tissue (Boilly, Boilly‐Marer, & Bely, 2017). Thus, a different mechanism might be used for establishment of body axes in regenerating individuals (Boilly, Boilly‐Marer et al., 2017).
The annelid body consists of a prostomium (the anterior cap or head), a set of segments which are often equipped with chaetae‐bearing parapodia (body wall projections used for locomotion), and a pygidium (the posterior cap or tail) (Figure 1). In annelids, posterior regeneration is found widely distributed across taxa, whereas anterior regeneration is restricted to fewer groups (Hyman, 1940). It has been supposed that anterior regeneration is ancestral for annelids, and subsequently this ability has been lost independently across the annelid tree (Bely, Zattara, & Sikes, 2014; Zattara & Bely, 2016). The addition of segments by the action of a SAZ is clearly detectable during posterior regeneration (Balavoine, 2014). Observations of anterior regeneration show an antero‐posterior developmental gradient of newly forming segments, in which the anterior cap (prostomium) forms first, and the SAZ is not clearly detectable or transient.
Figure 1.
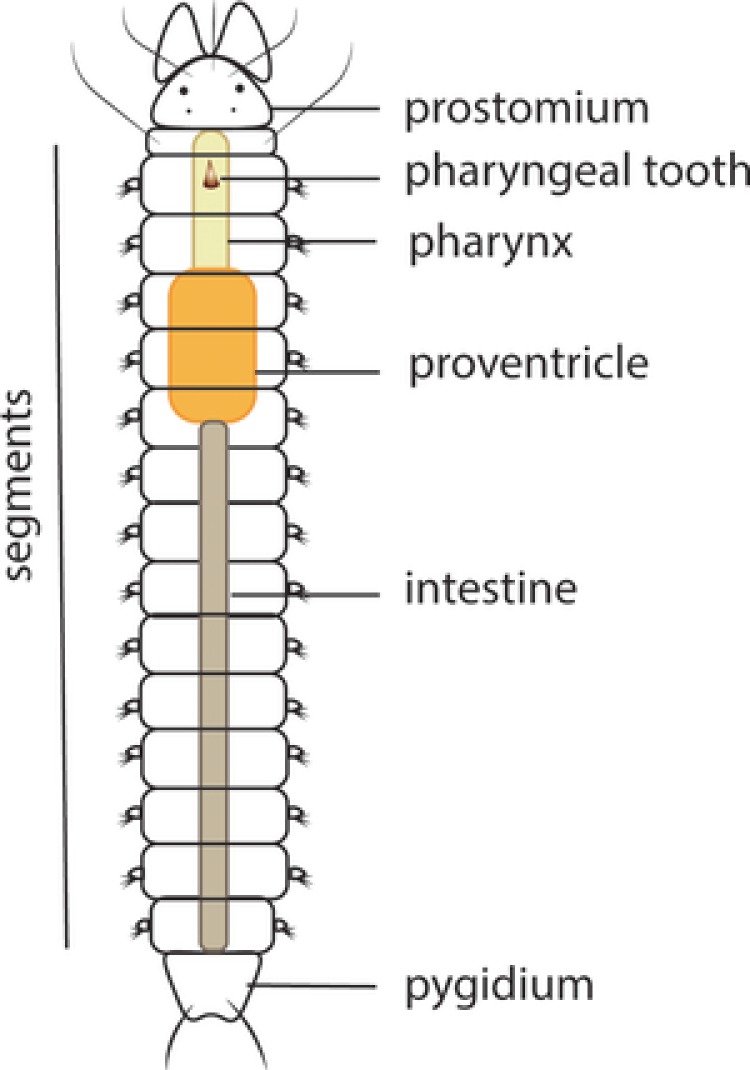
Schematic drawing of the syllid body plan. Illustration of the main features in the morphology of a syllid: prostomium, segments, pygidium and digestive tube components, pharynx, pharyngeal tooth, proventricle, and intestine
Additionally, the regenerative processes in invertebrates are closely related to agametic reproduction (Brockes & Kumar, 2008; Zattara & Bely, 2016). Several species, such as Pristina leidyi (Bely & Wray, 2001; Özpolat & Bely, 2015; Özpolat et al., 2016; Zattara & Bely, 2011) and E. japonensis (Myohara, Niva, & Lee, 2006; Myohara et al., 1999; Yoshida‐Noro, Myohara, Kobari, & Tochinai, 2000), reproduce asexually in a process that involves regeneration. Furthermore, groups such as Eunicida and Syllidae have reproductive modes (schizogamy or stolonization) that include stages of segment regeneration in the posterior body (Aguado, Glasby, Schroeder, Weigert, & Bleidorn, 2015; Aguado, Helm et al., 2015; Franke, 1999; Rouse & Pleijel, 2006) (Figure 2, Table 1).
Figure 2.
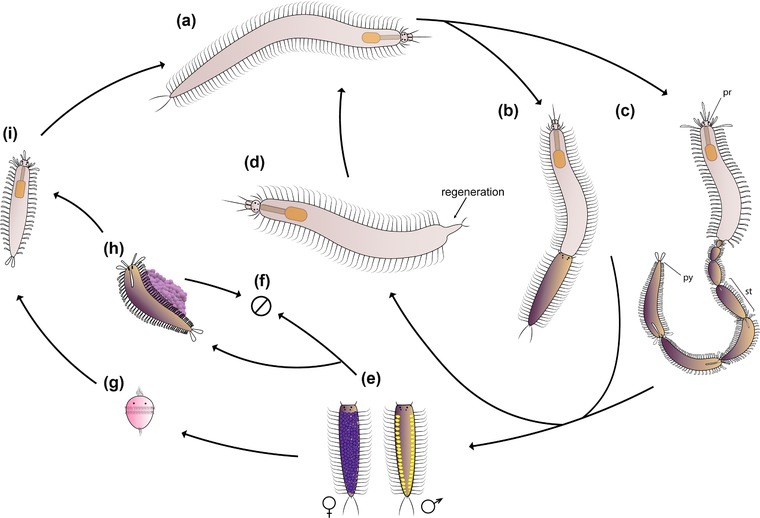
Schematic representation of schizogamy in syllids. (A) Adult individual of a schizogamous species. Schizogamy can occur by scissiparity or gemmiparity. (B) Scissiparous individual during stolonization, based on Syllis spp. (Franke, 1999). (C) Gemmiparous individual during sequential gemmiparity based on Myrianida pachycera (Okada, 1933b). When the stolons are completely developed, they are detached for spawning. The stock animal regenerates the posterior body after the detachment of stolons. (D) Individual during posterior regeneration. (E) Male and female stolons perform spawning for exchange of gametes; scheme based on stolons of Syllis spp. (F) After spawning, stolons die. (G) Larval development in non‐brooding species; larvae disperse in ocean. (H) Alternatively, autolytine female stolons live during external brooding and development of the embryos; mature female carries an egg sack until the release of juveniles; ventral brooding; based on Myrianida pachycera (Okada, 1933b). (I) Growth of juveniles until mature stage to start a new stolonization process. pr, prostomium; py, pygidium; st, stolon
Table 1.
Features of regeneration in Syllidae: sexual reproduction, resegmentation and literature review
| Anterior resegmentation | Posterior resegmentation | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Reproduction | Prt | Time | N seg | Pyg | Time | N seg | References |
| Amblyosyllis formosa | Epigamy | NA | NA | NA | 4 | − | 7 | Abeloos, 1952 |
| Epigamia alexandri | Epigamy | − | − | − | − | − | − | Malaquin, 1893; Saint‐Joseph, 1886 |
| Epigamia macrophtalma | Epigamy | NA | NA | NA | − | 42 | 10 | Allen, 1927a |
| Myrianida edwardsi | Schizogamy | − | − | 2 | 5 | − | 13 | Okada, 1929; Saint‐Joseph, 1886 |
| Myrianida pinnigera | Schizogamy | − | − | − | − | − | >20 | Dehorne, 1917; Okada, 1935 |
| Proceraea picta | Schizogamy | − | − | 8 | − | − | 11 | Allen, 1927a, 1927b; Okada, 1929, 1934 |
| Proceraea scapularis | Schizogamy | − | − | − | − | − | − | Okada, 1933a |
| Procerastea halleziana | Schizogamy | 4 | 39 | 29 | 3 | 39 | 35 | Allen, 1923, 1927a; Caullery, 1925 |
| Eusyllis assimilis | Epigamy | − | 9 | − | − | − | Malaquin, 1893 | |
| Eusyllis blomstrandi | Epigamy | − | 53 | 4 | − | − | − | Boilly, 1961 |
| Odontosyllis fulgurans | Epigamy | − | − | 6 | − | − | − | Okada, 1929; Saint‐Joseph, 1886 |
| Odontosyllis ctenostoma | Epigamy | − | − | − | − | − | − | Michel, 1909b; Schroeder & Hermans, 1975 |
| Synmerosyllis lamelligera | Epigamy | − | − | − | − | − | − | Allen, 1927b; Okada, 1929 |
| Exogone naidina | Epigamy | − | − | − | − | − | − | Pagenstecher, 1862; Viguier, 1884 |
| Alcyonosyllis aidae | Schizogamy | − | − | − | − | − | − | Álvarez‐Campos et al., 2013 |
| Branchiosyllis cirropunctata | Schizogamy | − | − | − | − | − | − | Michel, 1909b, 1909c |
| Haplosyllis spongicola | Schizogamy | − | − | 2 | − | − | 18 | Albert, 1887; Okada, 1929; Saint‐Joseph, 1886; Wissocq, 1966 |
| Opisthosyllis brunnea | Schizogamy | − | − | 2 | − | − | 3 | Okada, 1929 |
| Parahaplosyllis brevicirra | Schizogamy | − | − | − | − | − | − | San Martín, Hutchings, & Aguado, 2010 |
| Parahaplosyllis kumpol | Schizogamy | − | − | − | − | − | − | Álvarez‐Campos et al., 2013 |
| Ramisyllis multicaudata | Schizogamy | − | − | − | − | − | − | Aguado, Glasby et al., 2015; Glasby et al., 2012; Schroeder et al., 2017 |
| Syllis amica | Schizogamy | 5 | 70 | 4 | 9 | 70 | 20 | Boilly, 1962a, 1962b, 1965a, 1965b, 1967a, 1967b, 1967c, 1967d; Durchon, 1951; Verguer‐Bocquet, 1979 |
| Syllis armillaris | Schizogamy | − | − | 4 | − | − | − | Malaquin, 1893; Saint‐Joseph, 1886 |
| Syllis gracilis | Schizogamy | 8 | − | 18 | − | − | 8 | Boilly & Thibaut, 1974; Mesnil, 1901; Mesnil & Caullery, 1919 |
| Syllis hyalina | Schizogamy | − | − | 2 | − | − | − | Malaquin, 1893; Durchon, 1959 |
| Syllis prolifera | Schizogamy | 35 | 40 | 0 | 7? | − | 18 | Franke, 1980, 1983, 1986; Franke & Pfannenstiel, 1984 |
| Syllis rosea | Schizogamy | − | − | 2 | − | − | − | Langerhans, 1879 |
| Syllis variegata | Schizogamy | − | − | 2 | − | − | − | Andrews, 1892; Langerhans, 1879; Malaquin, 1893; Saint‐Joseph, 1886 |
| Syllis vittata | Schizogamy | − | − | − | − | − | − | Durchon, 1959; Michel, 1909b |
| Trypanosyllis krohnii | Schizogamy | − | − | − | − | − | − | Marion & Bobretzky, 1875; Michel, 1909a |
| Trypanosyllis zebra | Schizogamy | − | 4 | 5 | − | − | 24 | Delye, 1962; Junqua, 1957; Michel, 1909b |
| Typosyllis antoni | Schizogamy | 4 | 14 | 6 | 4 | 18 | 16 | Aguado, Helm et al., 2015; Weidhase, Beckers et al., 2016; Weidhase et al. 2017 |
| Typosyllis pigmentata | Schizogamy | − | 60 | 3 | − | − | 10 | Heacox & Schroeder, 1982 |
Prt,: time for prostomium appearing, in days; Time, maximum time of resegmentation, in days; N seg, maximum number of regenerated segments; Pyg, time for pygidium appearing, in days. NA, not applicable. Dashes represent lack of data.
Syllidae is an interesting group for studying regeneration. It is one of the largest groups within Annelida in terms of biodiversity, with a wide variety of reproductive modes and different regenerative processes (Aguado, Nygren, & Siddall, 2007; Franke, 1999; Okada, 1929). Syllids occur in all marine benthic habitats and about 700 species are currently known, classified in five families, Anoplosyllinae, Autolytinae, Eusyllinae, Exogoninae, and Syllinae, and some independent groups, such as Amblyosyllis and Perkinsyllis (Aguado & San Martín, 2009; Aguado, San Martín, & Siddall, 2012; Aguado et al., 2007). They are easily identified to the family level by the presence of the proventricle, a differentiated element of the digestive tube, which can be considered as a synapomorphy of the group (Figure 1) (Aguado et al., 2007, 2012; Fauchald & Rouse, 1997; Franke, 1999; Glasby, 1993; Okada, 1929).
Studies about syllid regeneration are limited and inhomogeneous in terms of investigated species, processes, and applied methods. Nevertheless, the available information represents a treasure trove for future investigations. The purpose of this study is to summarize available data on regeneration in this group of animals. We focus on the regenerative abilities and reproductive modes of syllids, differences between anterior and posterior regeneration, cells and tissues involved, and aberrant forms as a result of anomalous regenerative processes.
2. SYLLID REGENERATION
Most studies dealing with regeneration in syllids were published at the end of the 19th century (Langerhans, 1879; Malaquin, 1893; Marion & Bobretzky, 1875) and during the 20th century (Allen, 1923; Boilly & Thibaut, 1974; Delye, 1962; Durchon, 1959; Franke, 1980; Heacox & Schroeder, 1982; Junqua, 1957; Michel, 1909b; Okada, 1929; Wissocq, 1966). The first experiment under laboratory conditions was conducted with the species Syllis gracilis by Mesnil (1901). Following studies were focused mainly on the relationship between regeneration and sexual reproduction based on experiments of removal of the pharynx and/or proventricle, which might play a role during stolonization (Durchon, 1957, 1959; Junqua, 1957; Okada, 1938; Wissocq, 1966, 1970a). Some of the most recent studies were those published by Franke (1980, 1983), Heacox and Schroeder (1982), and Franke and Pfannenstiel (1984). After three decades, this topic re‐emerged with a study about the role of the proventricle during the stolonization of T. antoni (Weidhase, Bleidorn et al., 2016). This species was also used for investigating muscular and nervous system regeneration (Weidhase, Beckers et al., 2016; Weidhase et al., 2017).
Currently, syllids known to be able to regenerate belong to Autolytinae, Syllinae, Eusyllinae, Exogoninae, and Amblyosyllis (Figures 3,4A−D and Table 1). No information is available for Anoplosyllinae or Perkinsyllis species. Despite that, all schizogamous species are supposed to regenerate at least posteriorly. In general, syllids regenerate from fragments containing the pygidium or prostomium, or from isolated midbody segments. For instance, a fragment comprising the prostomium and two more chaetigers in Trypanosyllis zebra is able to regenerate up to 24 segments (Okada, 1929). From an isolated segment, Amblyosyllis formosa was observed regenerating posteriorly the pygidium and three segments (Abeloos, 1952). Autolytines are able to regenerate anteriorly and posteriorly including short fragments with only two, three, and four segments (Allen, 1923, 1927a, 1927b). Fragments with two segments (chaetiger 12−13) of Procerastea halleziana can regenerate anteriorly up to seven segments and prostomium, and posteriorly the pygidium plus four segments (Allen, 1923). In contrast, most of the sylline species, such as Syllis amica (Boilly, 1967d), T. antoni (Weidhase, Beckers et al., 2016) and Odontosyllis fulgurans (Okada, 1929), revealed a limited anterior regeneration demonstrated, for example, by the lack of a new proventricle, pharynx, or pharyngeal tooth (Figures 3, 4A, Table 1).
Figure 3.
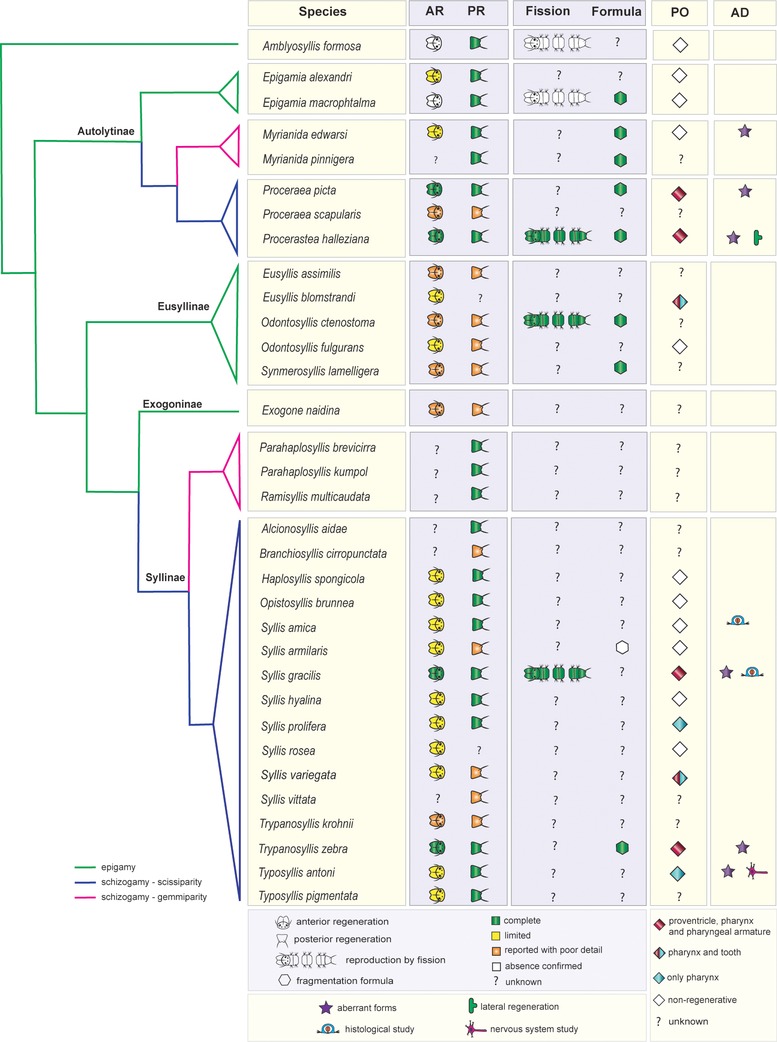
Distribution of some regenerative abilities and fragmentation in Syllidae. Regeneration data are based on reports of regenerating species and experimental studies (see Table 2). Presence and absence of regeneration was considered following, in general, categories by Zattara and Bely (2016), with some modifications: (1) axial regeneration absent (not even small portions of terminal tissue regenerate), (2) type I axial regeneration (caps can be regenerated after partial removal of these), (3) type II axial regeneration (caps, one or more segments and part of digestive tube can be regenerated), and (4) type III axial regeneration (like type II, but all digestive tube can be regenerated). Limited regeneration comprises inability to restore complete organs and extent comparable with the lost body before bisection, which includes type I and II axial categories. Type III was considered as presence of complete anterior or posterior regeneration. As suggested by Aguado, Glasby et al. (2015) and Aguado, Helm et al., (2015), Syllis and Typosyllis names remain as they were originally described. See Table 1 for data covering the information in this figure. AD, additional information; AR, anterior regeneration; PO, pharyngeal organ regeneration; PR, posterior regeneration. Phylogeny and distribution of reproductive modes following the most recent hypotheses (Aguado et al., 2012; Aguado, Glasby et al., 2015; Aguado, Helm et al., 2015)
Figure 4.
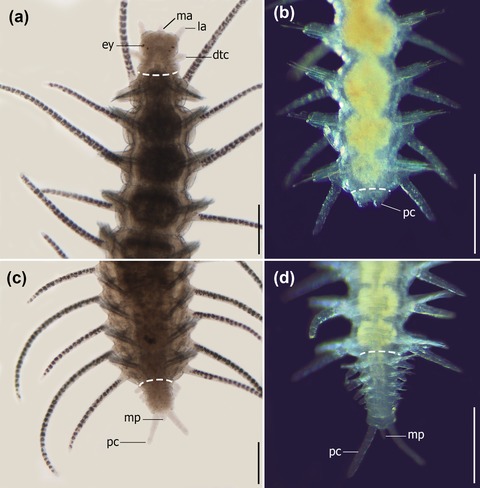
Light microscope pictures of two regenerating species of syllids, Typosyllis antoni (A and C) (pictures by Michael Weidhase reproduced by permission) (Aguado, Glasby et al., 2015; Aguado, Helm et al., 2015), and Syllis sp. (B and D). (A) Anterior regeneration, 6 days after dissection (dad). (B) Early stages of posterior regeneration, pygidium and cirri present. (C) Posterior regeneration in 6 dad. (D) Growth stage of posterior regeneration. Dashed line represents the dissection region. All pictures are dorsal view. dtc, dorsal tentacular cirrus; ey, eye; ma, median antenna; mp, median papila; pc, pygidial cirrus; la, lateral antenna. Scale bar 250 μm
Regeneration in syllids occurs by an epimorphic process, but morphallaxis is also observed (see Section 6) (Boilly, 1969; Delye, 1962; Özpolat & Bely, 2016; Weidhase et al., 2017; Zattara & Bely, 2016). After amputation, at first an invagination is formed at the edge of the wound; after about 1–3 days the wound heals, and then dedifferentiation or blastema formation is initiated (Boilly, 1962b, 1967a, 1967c; Weidhase, Beckers et al., 2016; Weidhase et al. 2017). The next steps are blastema growth, re‐differentiation, tissue remodeling, and resegmentation (Boilly, 1968a, 1968b; Wissocq, 1970a, 1970b) (see Section 5). The total regeneration time is variable among species, and there is a lack of detailed information about how long the process takes (Table 1). In comparison with other species the development of the prostomium and brain of T. antoni is relatively fast (Weidhase et al., 2017) (Table 1). Similarly, P. halleziana and S. amica also show an early appearance of the prostomium 4 and 5 days after amputation, respectively (Allen, 1923; Boilly, 1967a).
3. REGENERATION AND SEXUAL REPRODUCTION IN SYLLIDAE
Syllids can reproduce sexually by epitoky, a process in which adult individuals undergo a metamorphosis before spawning (Aguado et al., 2012; Franke, 1999). Epitoky can be subdivided into two different modes: epigamy and schizogamy (Aguado et al., 2012; Franke, 1999; Nygren & Sundberg, 2003). In epigamy, the sexually mature organisms modify the body by developing a hypertrophied cephalic sensory apparatus and swimming notochaeta in the midbody‐posterior segments. After spawning the adult organisms usually die (Aguado et al., 2012; Schroeder & Hermans, 1975). In schizogamy, the modified segments additionally develop a head and become sexual units called stolons that can be detached from the adult body (Figure 2) (Aguado, Glasby et al., 2015; Aguado et al., 2012). Eventually, the stolons die after spawning and/or release of juveniles (for species in which brooding occurs), while the adult body regenerates new posterior segments and is able to reproduce again (Figure 2) (Aguado, Helm et al., 2015; Franke, 1999). Sometimes regeneration of a new posterior end precedes the release of stolons (Álvarez‐Campos, Martín, & Aguado, 2013; San Martín, Hutchings, & Aguado, 2008). Therefore, in the case of schizogamous syllids regeneration represents part of the normal reproductive process (Figure 2D).
Additionally, there are two types of schizogamic reproduction named gemmiparity and scissiparity (Figures 2 and 5). In gemmiparity several successive stolons are produced simultaneously, while in scissiparity only one stolon is produced at a time (Nygren & Sundberg, 2003). In scissiparity, the stolons result from the modification of existing segments, whereas in gemmiparity the stolons are developed de novo, i.e., new segments are generated to form the stolons (Franke, 1999; Schroeder & Hermans, 1975). Scissiparity is also found, although with some differences, in other groups of annelids such as Eunicidae (Rouse & Pleijel, 2006); in contrast, gemmiparity is unique to syllids (Aguado, Glasby et al., 2015).
Figure 5.
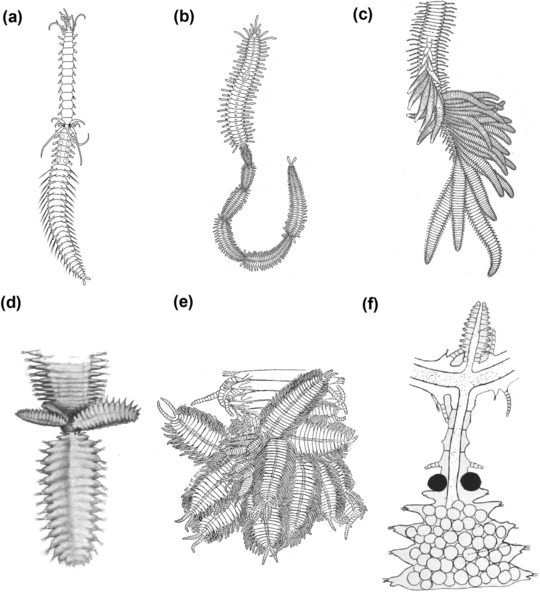
Different types of schizogamy: (A) scissiparity and (B)–(F) gemmiparity. (A) Scissiparous species Proceraea cornuta modified after Okada (1933a); a single stolon is produced per stolonization event. (B) Sequential gemmiparity in Myrianida sp., modified after Okada (1933b). (C). Successive budding in Trypanobia asterobia, modified after Okada (1933b). (D) Collateral budding in Trypanosyllis crosslandi Potts, 1911, modified after Potts (1911). (E) Collateral budding in Trypanedenta gemmipara (Johnson, 1901), modified after Johnson (1902). (F) Branching gemmiparity: branches and stolon in Syllis ramosa, modified after McIntosh (1885). All modified figures are in the public domain
The phylogenetic relationships within Syllidae are well resolved (Aguado et al., 2007, 2012; Aguado, Glasby et al., 2015; Aguado, Helm et al., 2015) and thus the evolution of certain features and processes, such as reproduction, can be traced. Epigamy (in Amblyosyllis, Eusyllinae, Exogoninae, Epigamia, and Perkinsyllis) is hypothesized to be the plesiomorphic condition in Syllidae, and schizogamy has evolved separately in Syllinae and Autolytinae (Figure 3) (Aguado et al., 2007, 2012; Nygren & Sundberg 2003). Regarding the schizogamous modes, gemmiparity is derived from scissiparity in two independent clades: Myrianida (Autolytinae) and in some genera of the so‐called “ribbon” clade (Aguado, Glasby et al., 2015) (within Syllinae), such as Parahaplosyllis, Ramisyllis, Trypanedenta, and Trypanobia (Aguado et al., 2012; Aguado, Glasby et al., 2015).
Schizogamous species seem to show higher anterior regenerative potential (Weidhase et al., 2017) (Figure 3, Table 1). On the other hand, the regeneration abilities in epigamous species are frequently limited, such as in Eusyllinae (Boilly, 1961; Okada, 1929), autolytines of genus Epigamia (Allen, 1927a), and A. formosa (Abeloos, 1952). Only few details regarding the regeneration in Exogoninae are available (Figure 3, Table 1).
Interestingly, regeneration studies of stolonizing individuals of Proceraea picta revealed the regression of stolon and different types of regenerated heads (Durchon & Wissocq, 1962, 1964; Okada, 1929). The experiments consisted of bisecting the animals in segments 13 or 14, where usually the stolon head is formed (Durchon & Wissocq, 1964). When this bisection is made in early stages of stolonization, in which a differentiation of a brain, eyes and appendages occurs, the posterior piece regenerates an adult head at the level of the stolon head (Okada, 1929) while the anterior piece regenerates a new tail, and the previous stolon head is completely regressed (Durchon & Wissocq, 1962, 1964). This regression depends on the section region. Dissections closer to the stolon head promote the regression, whereas dissections closer to the posterior end do not interfere in stolon formation (Durchon & Wissocq, 1962). These results point out that regeneration inhibits stolonization depending on the distance of the bisection from the stolon head (Durchon & Wissocq, 1962, 1964). Similar findings were also described in P. leidyi, a clitellate annelid that reproduces by paratomic fission, where amputation after the fission zone could provoke its resorption (Zattara & Bely, 2013).
Multiple SAZs are observed during stolonization of gemmiparous species, such as those of autolytine Myrianida and the syllines Trypanobia, Parahaplosyllis, Trypanosyllis, and Trypanedenta (Figure 5B–F) (Delye, 1962; Okada, 1933b; Potts, 1913). Myrianida stolonizes by sequential gemmiparity, in which stolons are generated in the same antero‐posterior axis of the body (Figure 5B) (Aguado, Glasby et al., 2015; Okada, 1933b). Trypanobia asterobia, for example, stolonizes by successive gemmiparity (Figure 5C) (one stolon per segment, projected from the ventral side) (Aguado, Glasby et al., 2015; Okada, 1933b). Species of Parahaplosyllis, Trypanedenta and Trypanosyllis can stolonize by collateral budding (Figure 5D–E) (stolons are projected in different directions from a specific segment). Additionally, a branching gemmiparity was reported for Syllis ramosa (McIntosh, 1879) and Ramisyllis multicaudata (Glasby, Schroeder, & Aguado, 2012) (Figure 5F). The body of these species is clearly ramified consisting of several branches and each terminal branch has a pygidium (Aguado, Glasby et al., 2015; Glasby et al., 2012; Schroeder, Aguado, Malpartida, & Glasby, 2017). These species develop stolons in the posterior end of different terminal branches (Aguado, Glasby et al., 2015; Glasby et al., 2012). Parahaplosyllis, Trypanosyllis, and Trypanobia are phylogenetically closely related with R. multicaudata, within the “ribbon clade” (Aguado, Glasby et al., 2015). Therefore, ribbon‐clade species share the ability to develop multiple SAZs and stolons with antero‐posterior axes different from the original body. This process apparently violates the bilateral symmetry in annelids, maintained by the activity of only one SAZ (Balavoine, 2014; Bely, 2006). Formation of multiple SAZs during stolonization appears to be restricted to gemmiparous species.
4. REGENERATION AND ASEXUAL REPRODUCTION IN SYLLIDAE
In annelids, asexual reproduction is always accomplished by subdivision of the body and regeneration of missing parts (Schroeder & Hermans, 1975). This process is widely known as fission and can occur in two different ways: fission or architomy, in which physical separation precedes the development of new tissues; and paratomy, in which new tissues are developed prior to physical separation (Zattara & Bely, 2016). Phylogenetic analyses in annelids suggest that fission has repeatedly evolved by co‐option of regenerative abilities and the presence of anterior regeneration is necessary to evolve fission (Zattara & Bely, 2016).
True asexual reproduction seems to be extremely rare in syllids (Franke, 1999). Some few species with high regenerative abilities may reproduce asexually by fission, such as P. halleziana (Figure 6) (Allen, 1923, 1927a, see below) and S. gracilis (Mesnil & Caullery, 1919) (Figure 3). This reproductive mode implies the development of new individuals from fragments of the stock organism that are capable of regenerating new anterior and/or posterior ends (Allen, 1923, 1927a, 1927b; Boilly & Thibaut, 1974; Okada, 1929) (Figures 6, 7).
Figure 6.
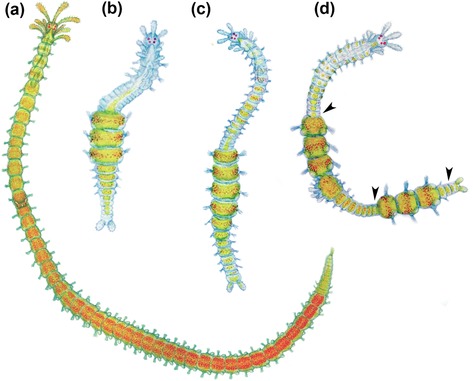
Regeneration of Procerastea halleziana. (A) Adult specimen starting stolonization. (B) Specimen regenerating from three original segments of midbody. (C) Specimen regenerating from four original segments of midbody after defective fragmentation. (D) Aberrant specimen regenerating after fragmentation, there is a regenerate between two fragments; arrows point at putative SAZs. Modified from Allen (1923). All modified figures are in the public domain
Figure 7.
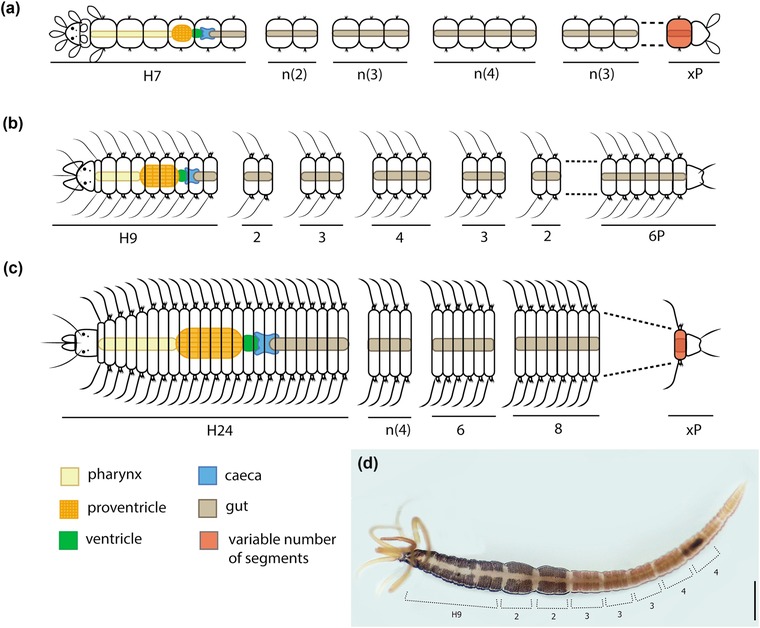
Fragmentation series in species of three subfamilies of Syllidae. (A) Autolytinae, Procerastea halleziana, after Allen (1923, 1927a, 1927b). (B) Eusyllinae, Synmerosyllis lamelligera (Allen, 1927b). (C) Syllinae, Trypanosyllis zebra, after Okada (1929). (D) Autolytinae, Procerea picta, collected from Ferrol, Galicia, Spain; the method of intercalated addition of distilled water and seawater was performed with this specimen, following Allen (1923). Regions of constrictions are marked indicating their number of segments. The series obtained with the constriction regions is comparable to the fragmentation series described for this species, following the color pattern (Allen, 1927a; Okada, 1929). Formula characters: H, head; n, variable number of fragments; x, variable number of segments; P, pygidium. Scale bar 500 μm
During the first decades of the 20th century, several interesting investigations were carried out regarding a possible established program of fission and regeneration in certain species of syllids. Allen (1923) found that P. halleziana revealed some exact fission areas in the body, after experimental induction of fission by wetting the animals alternately with distilled water and seawater (Figures 6, 7, Table 2). As a result, the pattern of fission areas in P. halleziana could be described mathematically by a “fragmentation formula”: H7 + 2 + 2 + 2 + 3 + 3 + 3 + 4 + 4 + 4 + 4(+4) + 3 + 3 + 3 + 3 + n(+3) + xP, where the numbers indicate the segments of each fragmented piece (H, head; P, pygidium) (Allen, 1923) (Figure 7A, Table 2). The possible existence of specific fragmentation formulae stimulated a series of experiments in different groups of syllids, especially in Autolytinae (Allen, 1927a, 1927b; Okada, 1929). Okada (1929) found that the addition of a KCl solution induced this fragmentation as well. The contact with the solution provoked an unusually strong contraction of the longitudinal muscles of specific segments (Okada, 1929). Both methods (distilled/salted water; KCL solution) revealed that the animals fragmented in specific areas of the body (Figure 7, Table 2). However, the fragmentation formula of each species may vary depending on the method used, either with distilled water or KCl solution (Okada, 1929).
Table 2.
Fragmentation series of syllids
| Species | Formula | Reference |
|---|---|---|
| Procerastea halleziana | H7+2+2+2+3+3+3+4+4+4+4(+4)+3+3+3+3+x(+3)+nP | Allen, 1923 |
| Myrianida prolifera | H7+2+2+2+3+3+3+4+4+4+4+4+3+3+4+4+4+25P | |
| H13+3+3+3+4+4+x(+3)+12P | Allen, 1927a | |
| Myrianida inermis | H9+2+2+3+3+3+4+nS | Allen, 1927a |
| H9+2+2+3+3+3+4+4+4+14P | ||
| H11+2+3+3+3+4+4+4+4+4+3+3+7P | ||
| Myrianida edwarsi | H7+2+2+2+3+3+3+4+4+nS | Allen, 1927a |
| H11+2+3 + 3+3+4+4+4+3+nS | ||
| Myrianida pinnigera | H13 +3 +3+3+4 +4 +4 +3 +3 +4 +4 +4 +4 +4 +4 +3 +3+3+(…)+nP | Okada, 1929 |
| Proceraea picta | H9+2+2+3+3+3+4+4+4+4+4+3+3+4+4 +4+4+4+4+1+3+5P | Allen, 1927a |
| Epigamia macrophtalma | H7+2+2+2+3+3+3+4+4+4+4+4+3+3+4+4+4+4+4+4+4+4+4+11P | Allen, 1927a |
| H7+2+2+2+3+3+3+4+4+4+4+4+3+3+4+4+4+4+4+4+4+4+4+6P | ||
| Synmerosyllis lamelligera | H9+2+2+3+3+3+4+3+4+2+2+6P | Allen, 1927b |
| Nudisyllis divaricata | H9+4+3+3+3+4+5+4+3+4+4+4+5P | Okada, 1929 |
| Trypanosyllis zebra | H24+4+4+4+6+8+4+6+4+18+6+6+12+3+4+39P | Okada, 1929 |
| H24+4+4+4+6+8+4+6+4+18+6+6+12+3+4+nS |
The fragment with the prostomium is represented by H and the number of segments, and the last fragment, with the pygidium is represented by the number of segments and P, adapted after Allen (1923). H, head; P, pygidium; nS, number of segments corresponding to stolon; nP, variable number of segments plus pygidium.
According to these studies, the first fragment (with prostomium) usually breaks in an odd number of segments and it is usually longer than the rest (Figure 7A–C, Table 2). In autolytines and eusyllines, the number of segments in the anterior fragments is usually 7, 9, 11 or 13 (Allen, 1927a, 1927b; Okada, 1929). Generally, posterior fragments vary in number of segments and in a stolonizing individual the break occurs at the level where the stolon head is developed (Allen, 1927a; Okada, 1929). Furthermore, some species exhibited high variation in the formula, depending on the presence or absence of stolon (Allen, 1927a). Interestingly, the fragmentation formulae of Proceraea picta and Epigamia macrophtalma coincide with the color pattern found in the body surface of these species (Allen, 1927a). We have repeated the experiment of fragmentation induction with distilled water in a specimen of P. picta. Although the animal did not break, constrictions appeared in the same fragmenting areas previously described coinciding with its color pattern (Figure 7D).
Ten species are known to show a fragmentation formula, including those that do not reproduce by fission, i.e., species in which the fragments are unable to regenerate completely (Table 2). Fission can be an asexual reproductive mode easily confounded with non‐reproductive autotomy and post‐injury regeneration, especially from field observations (Zattara & Bely, 2016). The fragmentation formulae observed experimentally in syllids (Allen, 1923, 1927a; Okada, 1929) might indeed be the result of a stress induction and not a truly asexual reproductive process (Franke, 1999; Langhammer, 1928). The addition of distilled water could be the factor that provokes autotomy as a defensive strategy based on discard of part of the body.
5. ANTERIOR VERSUS POSTERIOR REGENERATION
Most of the studied species regenerate anterior as well as posterior ends of the body, with the exception of A. formosa and E. macrophtalma that are not able to regenerate anteriorly (Figure 3, Table 1). However, fragments of both these species can survive for some weeks without a regenerated head (Abeloos, 1952; Allen, 1927a). Interestingly, in A. formosa, a species with a fixed number of segments, the regeneration of the posterior end never exceeds the original number of segments before amputation (Abeloos, 1952). All remaining investigated syllids are able to redevelop a new anterior end, although this is limited to a regenerated prostomium and few segments with missing organs in many species, i.e., Eusyllis blomstrandi (Boilly, 1961), O. fulgurans (Saint‐Joseph, 1886), Syllis prolifera (Abeloos, 1950), and T. antoni (Aguado, Helm et al., 2015; Weidhase, Beckers et al., 2016; Weidhase et al., 2017) (Figure 3).
Anterior resegmentation and posterior resegmentation involve different processes that vary among species (Figure 8). Most authors observed that posterior regeneration involves the establishment of a SAZ, immediately before the pygidium (Allen, 1923; Weidhase, Beckers et al., 2016) (Figure 8B, C). Concerning anterior resegmentation, two patterns were observed in different species of syllids. In most syllids the blastema grows and differentiates into a prostomium (at first) and regenerated segments, and a SAZ is transient or not detectable (Figure 8B) (Aguado, Helm et al., 2015; Boilly, 1961, 1965a; Weidhase, Beckers et al., 2016, Weidhase et al., 2017). However, in some cases the anterior regeneration seems to bear a SAZ, because segments are regenerated by sequential addition from the original segment of the stock (Figure 8C) (Aguado, Helm et al., 2015; Allen, 1923). Whereas the first pattern is widely recognized in annelid regeneration (Balavoine, 2014), the second is unusual, reported only for the syllids P. halleziana and (in some instances) T. antoni (Aguado, Helm et al., 2015; Allen, 1923). In this case, fragments of the midbody of P. halleziana can establish two SAZs (anterior and posterior), in which segment addition occurs following the posterior−anterior direction (Allen, 1923) (Figure 8C). As is well known for nereidids, posterior growth during juvenile development involves similar genetic pathways (as evidenced by similar gene expression patterns) to those occurring in the SAZ during posterior regeneration (Balavoine, 2014; Prud'homme et al., 2003). Unfortunately, these animals do not regenerate the anterior body. Whether anterior growth could also share similar mechanisms to juvenile growth is a topic that still needs to be clarified.
Figure 8.
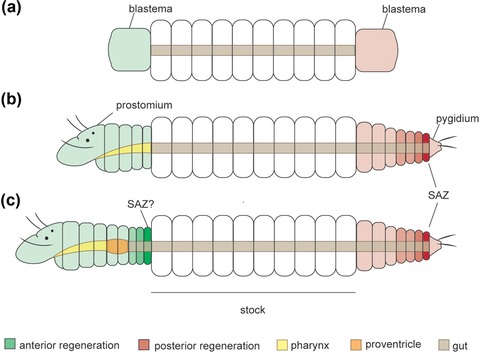
Schematic drawings of anterior and posterior resegmentation in syllids. (A) Blastema formation; generalized scheme for Syllidae. (B) Anterior and posterior pattern of resegmentation based on eusyllines and syllines (Aguado, Helm et al., 2015; Boilly, 1961, 1967c; Weidhase, Beckers et al., 2016; Weidhase et al., 2017). (C) Anterior and posterior patterns of resegmentation based on the autolytine Procerastea halleziana (Allen, 1923). Establishment of posterior SAZ (B), (C). Anterior regeneration: a SAZ is not detectable (B) or takes place in the region immediately attached to the stock segment (C). SAZ, segment addition zone
Boilly (1961) and Okada (1929) showed that the extent of regeneration depends on the region of dissection. Specimens of Eusyllis blomstrandi failed to regenerate anteriorly or died when dissected in the most posterior part of the body (Boilly, 1961). A similar limitation was observed in the posterior regeneration of Myrianida edwarsi, in which the anterior end needed a certain number of segments to regenerate a new tail (Okada, 1929). In general, posterior regeneration usually produces more new segments than anterior regeneration, with the exception of S. gracilis that is able to regenerate anteriorly up to 18 segments and posteriorly up to eight segments (Boilly & Thibaut, 1974; Mesnil, 1901) (Table 1).
Regarding the speed of regeneration, a new pygidium appears faster than a new prostomium according to the literature, even though huge differences in the pace of regeneration have been recorded (Table 1). The prostomium appears 4–35 days after dissection (dad) in the investigated syllid species (Allen, 1923; Boilly, 1967a, 1967c; Franke, 1980, 1983; Weidhase, Beckers et al., 2016; Weidhase et al., 2017) (Table 1). S. prolifera takes the longest time to regenerate a new prostomium (35 dad) (Franke, 1983), whereas T. antoni and P. halleziana need no more than 4 dad (Aguado, Helm et al., 2015; Allen, 1923; Weidhase, Beckers et al., 2016; Weidhase et al., 2017). The maximum number of regenerated segments recorded is 29 in P. halleziana. The appearance of pygidium varies between 3 and 9 dad (Allen, 1923; Boilly, 1967a, 1967c; Delye, 1962; Franke, 1980, 1983; Weidhase, Beckers et al., 2016; Weidhase et al., 2017) (Table 1). P. halleziana and T. zebra regenerate, respectively, 35 and 24 segments posteriorly; those are the highest number of regenerated segments recognized in syllids (Allen, 1923; Delye, 1962) (Table 1) .
6. CELLS, TISSUES AND ORGANS INVOLVED
Early stages of regeneration including cell dynamics, histogenesis, and renewal of organs were the focus of some investigations in syllids. The knowledge about cell dynamics and histogenesis was mainly provided by studies on S. amica (Boilly, 1962a, 1965, 1967a, 1967b, 1967c, 1967d, 1968a, 1968b; Wissocq, 1970a, 1970b). Punctual observations about organ regeneration are available (Allen, 1923; Boilly, 1967a; Boilly & Thibaut, 1974; Delye, 1962; Okada, 1929; Weidhase, Beckers et al., 2016; Weidhase et al., 2017).
Anterior and posterior regeneration appear to share similar mechanisms of cell proliferation and cells forming the regenerated tissues seem to have the same origin (Boilly, 1962a, 1962b, 1965a, 1965b, 1967a, 1967b, 1967c, 1967d, 1968a, 1968b; Boilly & Thibaut, 1974). The first signs of re‐establishment of tissues in the regeneration of S. amica are an invagination and retraction of the intestine (Boilly, 1967a). Both anterior and posterior fragments exhibit wound healing, followed by blastema formation by bulging of the scarring epidermis in 2–3 dad (Boilly, 1962b, 1967a). As a result of several studies, three phases of cell dynamics during regeneration were observed: (1) cellular dedifferentiation, loss of the elements that configure the characteristics of each cellular type; (2) activation and transformation of the regenerative cells related to intense synthesis of RNA; and (3) differentiation, characterized by an acquisition of a new structure and specific proteins (Boilly, 1962a, 1962b, 1965a, 1965b, 1967a, 1967b, 1967c, 1967d, 1968a, 1968b).
Blastema formation is the main characteristic of the dedifferentiation stage. The cells involved in blastema formation and renewing tissues derive from the last segment preceding the section site (Boilly, 1962a, 1962b, 1965). In contrast to clitellates, migratory regenerative cells have so far not been found in Syllidae (Boilly, 1967a). A quantitative antero‐posterior gradient has been observed in blastemal tissues of anterior regeneration in M. edwarsi (Okada, 1929). The more posterior the dissection region, the smaller blastema, and the total quantity of tissue derived from blastema is reduced (Berrill, 1952; Okada, 1929). The activation stage is supposed to be dependent on nervous fibers close to the wound region, and it begins once the signals of activation appear in the cells near the nerve chain (Boilly, 1967a, 1967b). This process does not occur immediately after section, due to the time required to heal the axon that elongates towards the microenvironment of the blastema and stimulates regeneration (Boilly, 1967a; Boilly, Faulkner et al., 2017). Neuropeptides, neurotransmitters, growth factors, and morphogens were suggested as mediators in this activation process (Boilly, Boilly‐Marer et al., 2017). Finally, during the differentiation stage, the regenerative cells experience changes in cytoplasmic elements to acquire specialized functions, e.g., muscular and epidermal cells (Boilly, 1967a, 1968b). During this process, ribosomes are abundant and proteins associated with cell proliferation and differentiation are clearly observed (Boilly, 1968b).
The available information about the regeneration of different organs and tissues is limited in syllids. Most detailed descriptions about digestive tube, muscular, and nervous tissue regeneration are available from former studies in S. amica and, more recently, T. antoni (Boilly, 1962a, 1962b, 1965a, 1965b, 1967a, 1967b, 1967c, 1967d, 1968a, 1968b; Weidhase, Beckers et al., 2016; Weidhase et al., 2017; Wissocq, 1970a, 1970b).
In S. amica, the digestive tube regenerates supposedly from ectodermal, mesodermal, and endodermal tissues (Boilly, 1967c, 1969). The pharynx has ectomesodermal origin and the intestinal epithelium originates from ectodermal and mesodermal regenerative cells (Boilly, 1967c, 1969). However, these studies employed limited methodologies to trace cell lineage during regeneration in S. amica; hence these results should be verified with state‐of‐the‐art methods. Curiously, specimens that after anterior amputation lack part of the pharynx or proventricle regenerate an intestine posteriorly, instead of the missing part of the pharynx or the proventricle (Boilly, 1967c). The regeneration of pharynx, proventricle, or, more occasionally, some anterior appendages is missing or incomplete in many syllids (Abeloos, 1950; Boilly, 1961; Michel, 1909b; Okada, 1929; Weidhase, Beckers et al., 2016) (Table 1). A few species restore the proventricle and the pharyngeal armature (tooth or trepan), such as P. halleziana (Allen, 1923), T. zebra (Delye, 1962), and S. gracilis (Boilly & Thibaut, 1974; and personal observation) (Figure 3).
Regeneration of the nervous system occurs by growth and elongation of the remaining nerve fibers (Weidhase et al., 2017). The nervous system of T. antoni begins to regenerate anteriorly during the blastema stage (2 dad) with an ingrowth of neurites originating in the remaining ventral nerve cord, and then neurites form loops in 4 dad together with the nerve cord elongation and development of a new brain (Weidhase et al., 2017). In S. amica, the ventral nerve cord was also observed in an elongation process from 3 dad and the prostomium was completely differentiated in 7 dad (Boilly, 1967a). The nervous system at the posterior end of T. antoni regenerates in a similar way, but the blastema is formed in 3 days, and a day after a new pygidium with cirri is present (Weidhase et al., 2017). This process is comparable to the posterior regeneration of S. amica, differing only in the appearance of the pygidium at 9 dad (Boilly, 1967a).
7. THE ROLE OF THE PROVENTRICLE DURING REGENERATION
Several studies in schizogamous species focused on the triggers of the stolonization process. Usually, experiments with removal of the proventricle indicated a potential role of this structure during reproduction (Durchon, 1957, 1959; Franke, 1980, 1983; Franke & Pfannenstiel, 1984; Weidhase, Beckers et al., 2016). Posterior ends of fragments without proventricle seem to increase the production of male stolons, suggesting that the proventricle inhibits the stolonization and is involved in sexual determination (Durchon, 1957, 1959; Durchon & Wissocq, 1964; Franke & Pfannenstiel, 1984; Heacox & Schroeder, 1982; Weidhase, Beckers et al., 2016; Wissocq, 1966). Experiments in which the proventricle is removed in S. prolifera have shown that stolonization stops when the structure is re‐implanted, indicating that the proventricle may inhibit stolonization while promoting regeneration (Franke & Pfannenstiel, 1984). However, no glandular structure was found in the proventricle of T. antoni, and a suggestion is that structures associated with this organ (e.g., nervous system) might be responsible for the regulation of reproductive and regenerative processes (Weidhase, Beckers et al., 2016).
In effect, stolonization is favored over regeneration in an individual unable to regenerate the proventricle and vice versa. Similar results were described regarding the interactions between paratomic fission and regeneration in other annelids and flatworms (e.g., Child, 1903; Zattara & Bely, 2013). This tendency of mutual inhibition between regeneration and stolonization or fission operates probably by a comparable machinery.
8. ABERRANT FORMS AND BRANCHING PATTERNS
Major phenotypic changes in syllids have been observed in experimental regeneration studies that report aberrations such as double heads and bifurcated forms (Aguado, Glasby et al., 2015; Allen, 1923; Okada, 1929; Weidhase, Beckers et al., 2016) (Figure 9A−F). An extraordinary case of heteromorphic regeneration happened in a specimen of M. edwarsi that restored a tail in the place of a head during anterior regeneration (Okada, 1929) (Figure 9A). Another related aberration is the development of double heads, as reported for P. picta after dissection between the segments 12 and 13 (Okada, 1929) (Figure 9B). Additionally, double heads were observed in stolons of P. halleziana, S. amica, S. prolifera, and T. antoni, during regeneration experiments of amputation with proventricle removal (Langhammer, 1928; Okada, 1934; Weidhase, Beckers et al., 2016).
Figure 9.
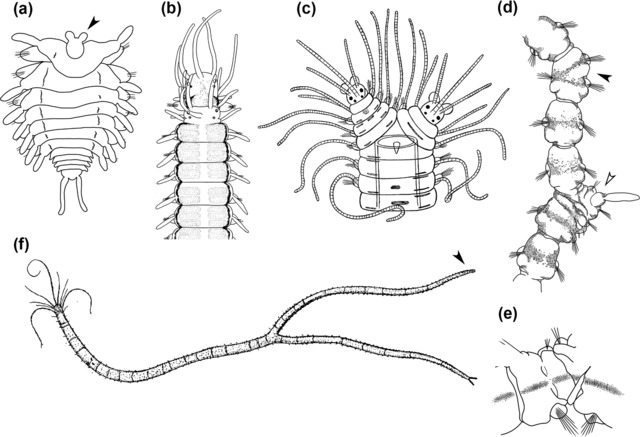
Aberrant forms in some species of Syllidae. (A) Myrianida edwarsi: arrow point at tail regenerated in anterior end after transversal dissection; modified after Okada (1929). (B) Proceraea picta: double head regenerated after transversal dissection between 12th and 13th segment, modified after Okada (1929). (C) Syllis variegata: specimen with bifurcated anterior end; modified after Andrews (1892). (D) Procerastea halleziana: filled arrow point at additional parapodium laterally projected in the right side of 16th segment, empty arrow point at lateral regeneration in 19th segment, dorsal view, and (E) ventral view of the regenerate; modified after Allen (1923). (F) Myrianida ornata specimen with bifurcated posterior ends: arrow point at abnormal tail without cirri; modified after Andrews (1892). All modified figures are in the public domain
Reports of bifurcated patterns in regeneration are known from Syllis variegata (Langerhans, 1879), P. halleziana (Allen, 1923; Langhammer, 1928), and Myrianida ornata (Andrews, 1891, 1892). A case of a bifurcated anterior end was observed in S. variegata under non‐experimental conditions by Langerhans (1879) (Figure 9C). The specimen probably had regenerated two heads after losing the original one (Andrews, 1892; Langerhans, 1879) (Figure 9C). Bifurcated tails are reported in a specimen of M. ornata found in nature (Figure 9F); the additional tail was interpreted as a lateral outgrowth (Andrews, 1891, 1892). A similar lateral projection was found in P. halleziana probably as a result of an error in the healing process after injury (Allen, 1923; Langhammer, 1928) (Figure 9D, E). Sometimes, bifurcated appendages such as antennae and pygidial cirri are observed during regeneration of several species (Durchon & Wissocq, 1964; Malaquin, 1893; Michel, 1909b).
Interestingly, as an error of the fragmentation process, P. halleziana exhibited a specimen with three regenerating regions, suggesting the activity of three different SAZs (Allen, 1923) (Figure 5D). Bifurcated or branching forms occurring in non‐gemmiparous species suggest that errors can arise during genetic control of regeneration and reproduction of syllids. It might be speculated that these kinds of “errors” may have led to the evolution of gemmiparity and branching species in Syllidae (Aguado, Glasby et al., 2015).
9. CONCLUSIONS AND FUTURE DIRECTIONS
Regenerative ability in syllids is linked to both sexual and asexual reproductive modes. During sexual reproduction by schizogamy, syllids regenerate the posterior body after release of a stolon, thereby being able to undergo another stolonization cycle. Asexual reproduction by architomic fission is known for species that can restore a complete body from fragments of the original individual. Stolonization in syllids and paratomic fission in annelids seem to share a regenerative mechanism in which mutual inhibition occurs, implying that the reproductive modes are comparable.
Studies in regeneration and fission facilitated another finding in syllid biology. Some species were able to split in specific areas during experiments of stress induction, including species that do not reproduce asexually. These events of fragmentation can be interpreted as an autotomic response rather than an architomic fission.
Considering whole‐body regeneration, syllids are able to regenerate anterior and posterior ends but often are limited in restoration of certain anterior organs (e.g., proventricle). Although this limited anterior regeneration is not complete (see Zattara & Bely, 2016), from a morphological point of view, the regenerating individuals are able to reproduce since the stolonization process is induced by proventricle removal. This organ seems to play an important role in reproduction and regeneration in syllids.
Some patterns regarding the speed and ability to regenerate segments and organs can be recognized in syllids. For instance, posterior regeneration is usually faster than anterior regeneration. Restoration of a new anterior end becomes easier when the bisection plane is close to the prostomium. Additionally, some species can renew the pharyngeal armature, and also show better overall anterior regenerative ability. Implications for ensuing studies can be a comparison of the genetic mechanisms during regeneration among syllids that reproduce by different modes.
The origin of regenerative cells in syllids has never been elucidated. Knowledge is limited to histological visualizations in different stages of regeneration, restricted to a single species. Studies of cell lineages during regeneration using fine‐scale methods and techniques of labeling and histology are missing.
Heteromorphic regeneration points out errors in the regenerative process that may have occurred and been established during the evolution of branching body patterns in syllids, such as those of R. multicaudata and S. ramosa. The occurrence of aberrant forms in stages of regeneration exemplifies an intriguing possibility of investigating the underlying genetic control processes. Therefore, syllid regeneration should be considered as a good model to study the evolution and development of body axes. At present, studies about gene expression patterns during regeneration or development in syllids are still missing. Emerging genomic methods will be useful to understand this process. By sequencing genomes and transcriptomes, genes involved in regeneration can be identified (Bhambri et al., 2017; Myohara et al., 2006; Nyberg et al., 2012), thereby opening up the possibility of a functional characterization, e.g., by CRISPR or RNAi methods (Boettcher & McManus, 2015). Different studies of annelid regeneration identified the expression of genes such as engrailed, wingless (Wnt), orthodenticle homologs, markers of germline cells (e.g. piwi, vasa and nanos), and Hox genes during regenerative processes (Bakalenko et al., 2013; Bely & Wray, 2001; Novikova et al., 2013; Özpolat & Bely, 2015; Prud'homme et al., 2003). Furthermore, morphogens (e.g., bone morphogenetic protein) and fibroblast growth factors might be involved in nervous activation of blastema formation (Boilly, Faulkner et al., 2017; Satoh, Makanae, Nishimoto, & Mitogawa, 2016; Takeo et al., 2013). These approaches are opportune to investigate the triggers of the regenerative response, the pathways that control growth and patterning of the new structures, and the re‐establishment of antero‐posterior polarity in syllids and other bilateral animals.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGMENTS
We are grateful to Michael Weidhase for providing figures of regenerating Typosyllis antoni individuals. We are grateful to Guillermo Ponz Segrelles for helpful comments on the manuscript. We acknowledge Guillermo Díaz‐Agras for providing facilities to perform experiments with a specimen of Proceraea picta at the Marine Biology Station of A Graña (Ferrol, Spain). The authors are very grateful to Benoni Boilly for interchange of ideas and sharing with us his experience and about syllids regeneration and aberrant ramifications. RPR is supported by the program “Contratos predoctorales para Formación de Personal Investigador, FPI‐UAM,” Universidad Autónoma de Madrid. This study is a contribution of the project “Macroevolutionary transitions in Syllidae” CGL2015‐63593‐P supported by MINECO/FEDER, UE funds, PI: MTA. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
Ribeiro RP, Bleidorn C, Aguado MT. Regeneration mechanisms in Syllidae (Annelida). Regeneration. 2018;5:26–42. https://doi.org/10.1002/reg2.98
Contributor Information
Christoph Bleidorn, Email: christoph.bleidorn@gmail.com.
M. Teresa Aguado, Email: maite.aguado@uam.es.
REFERENCES
- Abeloos, M. (1950). Régénération et stolonisation épigame chez l'Annélide Syllis prolifera Khron. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 230, 1899–1900. [PubMed] [Google Scholar]
- Abeloos, M. (1952). Régénération chez l'Annélide Pterosyllis formosa Clap. Comptes Rendus de la Société de Biologie, 146, 1750–1752. [PubMed] [Google Scholar]
- Agata, K. , Saito, Y. , & Nakajima, E. (2007). Unifying principles of regeneration I: Epimorphosis versus morphallaxis. Development Growth and Differentiation, 49(2), 73–78. https://doi.org/10.1111/j.1440-169X.2007.00919.x [DOI] [PubMed] [Google Scholar]
- Aguado, M. T. , Glasby, C. J. , Schroeder, P. C. , Weigert, A. , & Bleidorn, C. (2015). The making of a branching annelid: an analysis of complete mitochondrial genome and ribosomal data of Ramisyllis multicaudata . Scientific Reports, 5(12072), 1–13. https://doi.org/10.1038/srep12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado, M. T. , Helm, C. , Weidhase, M. , & Bleidorn, C. (2015). Description of a new syllid species as a model for evolutionary research of reproduction and regeneration in annelids. Organisms Diversity and Evolution, 15(1), 1–21. https://doi.org/10.1007/s13127-014-0183-5 [Google Scholar]
- Aguado, M. T. , Nygren, A. , & Siddall, M. E. (2007). Phylogeny of Syllidae (Polychaeta) based on combined molecular analysis of nuclear and mitochondrial genes. Cladistics, 23, 552–564. https://doi.org/10.1111/j.1096-0031.2007.00163.x [DOI] [PubMed] [Google Scholar]
- Aguado, M. T. , & San Martín, G. (2009). Phylogeny of Syllidae (Polychaeta) based on morphological data. Zoologica Scripta, 38(4), 379–402. https://doi.org/10.1111/j.1463-6409.2008.00380.x [Google Scholar]
- Aguado, M. T. , San Martín, G. , & Siddall, M. E. (2012). Systematics and evolution of syllids (Annelida, Syllidae). Cladistics, 28(3), 234–250. https://doi.org/10.1111/j.1096-0031.2011.00377.x [DOI] [PubMed] [Google Scholar]
- Albert, F. (1887). Über die Fortpflanzung von Haplosyllis spongicola Gr. Mittheilungen aus der Zoologie Station zu Neapel, 7, 1–26. [Google Scholar]
- Allen, E. J. (1923). Regeneration and reproduction of the syllid Procerastea . Philosophical Transactions of the Royal Society B: Biological Sciences, 211(382–390), 131–177. https://doi.org/10.1098/rstb.1923.0003 [Google Scholar]
- Allen, E. J. (1927a). Fragmentation in the genus Autolytus and in other syllids. Journal of the Marine Biological Association of the United Kingdom, 14(4), 869–876. [Google Scholar]
- Allen, E. J. (1927b). Regeneration and reproduction of syllids. Annual Report and Proceedings, South Western Naturalists’ Union, 1(3), 14–21. [Google Scholar]
- Alvarado, A. S. , & Tsonis, P. A. (2006). Bridging the regeneration gap: genetic insights from diverse animal models. Nature Reviews Genetics, 7(11), 873–884. https://doi.org/10.1038/nrg1923 [DOI] [PubMed] [Google Scholar]
- Álvarez‐Campos, P. , Martín, G. S. , & Aguado, M. T. (2013). A new species and new record of the commensal genus Alcyonosyllis Glasby & Watson, 2001 and a new species of Parahaplosyllis Hartmann‐Schröder, 1990, (Annelida: Syllidae: Syllinae) from Philippines Islands. Zootaxa, 3734(2), 156–168. https://doi.org/10.11646/zootaxa.3734.2.4 [DOI] [PubMed] [Google Scholar]
- Andrews, E. A. (1891). Report upon the Annelida Polychaeta of Beaufort, North Carolina. Proceedings of the United States National Museum, 14, 277–302. [Google Scholar]
- Andrews, E. A. (1892). Bifurcated annelids. The American Naturalist, 26(309), 725–733. [Google Scholar]
- Bakalenko, N. I. , Novikova, E. L. , Nesterenko, A. Y. , & Kulakova, M. A. (2013). Hox gene expression during postlarval development of the polychaete Alitta virens . EvoDevo, 4(1), 13 https://doi.org/10.1186/2041-9139-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balavoine, G. (2014). Segment formation in annelids: patterns, processes and evolution. International Journal of Developmental Biology, 58(6–8), 469–483. https://doi.org/10.1387/ijdb.140148gb [DOI] [PubMed] [Google Scholar]
- Bely, A. E. (2006). Distribution of segment regeneration ability in the Annelida. Integrative and Comparative Biology, 46(4), 508–518. https://doi.org/10.1093/icb/icj051 [DOI] [PubMed] [Google Scholar]
- Bely, A. E. (2014). Early events in annelid regeneration: a cellular perspective. Integrative and Comparative Biology, 54(4), 688–699. https://doi.org/10.1093/icb/icu109 [DOI] [PubMed] [Google Scholar]
- Bely, A. E. , & Nyberg, K. G. (2010). Evolution of animal regeneration: re‐emergence of a field. Trends in Ecology and Evolution, 25(3), 161–170. https://doi.org/10.1016/j.tree.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Bely, A. E. , & Wray, G. A. (2001). Evolution of regeneration and fission in annelids: insights from engrailed‐ and orthodenticle‐class gene expression. Development, 128(14), 2781–2791. Retrieved from https://doi.org/11526083 [DOI] [PubMed] [Google Scholar]
- Bely, A. E. , Zattara, E. E. , & Sikes, J. M. (2014). Regeneration in spiralians: evolutionary patterns and developmental processes. International Journal of Developmental Biology, 58(6–8), 623–634. https://doi.org/10.1387/ijdb.140142ab [DOI] [PubMed] [Google Scholar]
- Berrill, N. J. (1952). Regeneration and budding in worms. Biological Reviews, 27(4), 401–438. [Google Scholar]
- Bhambri, A. , Dhaunta, N. , Patel, S. S. , Hardikar, M. , Srikakulam, N. , Shridhar, S. , ... Pillai, B. (2017). Insights into regeneration from the genome, transcriptome and metagenome analysis of Eisenia fetida . bioRxiv, 180612. https://doi.org/https://doi.org/10.1101/180612 [Google Scholar]
- Boettcher, M. , & McManus, M. T. (2015). Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Molecular Cell, 58(4), 575–585. https://doi.org/10.1016/j.molcel.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boilly, B. (1961). Contribution à l’étude de la régénération antérieure chez Eusyllis blomstrandi Malgren (Annélide Polychète). Bulletin de la Société Zoologique de France, 86, 216–229. [Google Scholar]
- Boilly, B. (1962a). Inhibition de la régeneration caudale par irradiation X chez Syllis amica Quatrefages (Annélide Polychète). Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 255, 1414–1416. [Google Scholar]
- Boilly, B. (1962b). Origine des cellules dans la régénération postérieure de Syllis amica Quatrefages (Annélide Polychète). Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 255, 2198–2200. [Google Scholar]
- Boilly, B. (1965a). Origine des cellules de régénération antérieure chez Syllis amica Quatrefages (Annélide Polychète). Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 260, 6714–6716. [Google Scholar]
- Boilly, B. (1965b). Origine du mésoderme dans la régénération postérieure chez Syllis amica Quatrefages (Annélide Polychète). Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 261(6), 1561–1564. [Google Scholar]
- Boilly, B. (1967a). Étude histologique des premiers stades de l'histogenèse dans la régénération caudale et céphalique chez une Annélide Polychète (Syllis amica Quatrefages). Considérations sur l'origine des cellules de régénération. Archives d'Anatomie Microscopique, 56(2), 167–204. [PubMed] [Google Scholar]
- Boilly, B. (1967b). Role du mésoderme dans la différenciation du blastême de régénération chez une annélide polychète (Syllis amica Quatrefages). Bulletin de la Société Zoologique de France, 92, 331–335. [Google Scholar]
- Boilly, B. (1967c). Sur la régénération d'un intestin dans la zone pharyngienne chez Syllis amica Quatrefages (Annélide Polychète). Cahiers de Biologie Marine, 8, 221–231. [Google Scholar]
- Boilly, B. (1967d). Utilisation du “Thorotrast” dans l’étude de l'origine des cellules de régénération mésodermiques chez une Annéllide Polychète. Journal de Microscopie, 6, 40. [Google Scholar]
- Boilly, B. (1968a). Étude ultrastructurale de l’évolution des tissus impliqués dans la régénération céphalique et caudale de Syllis amica Q. (Annélide Polychète). I. La dédifférenciation. Journal de Microscopie, 7, 865–876. [Google Scholar]
- Boilly, B. (1968b). Étude ultrastructurale de l’évolution des tissus impliqués dans la régénération céphalique et caudale de Syllis amica Q. (Annélide Polychète). II. L'activation et la différenciation. Journal de Microscopie, 7, 877–894. [Google Scholar]
- Boilly, B. (1969). Sur l'origine des cellules régénératrices chez les annélides polychètes. Archives de Zoologia Expérimentale et Générale, 110(1), 127–143. [Google Scholar]
- Boilly, B. , Boilly‐Marer, Y. , & Bely, A. E. (2017). Regulation of dorso‐ventral polarity by the nerve cord during annelid regeneration: a review of experimental evidence. Regeneration, 4, 54–68. https://doi.org/10.1002/reg2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boilly, B. , Faulkner, S. , Jobling, P. , & Hondermarck, H. (2017). Nerve dependence: from regeneration to cancer. Cancer Cell, 31(3), 342–354. https://doi.org/10.1016/j.ccell.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Boilly, B. , & Thibaut, N. (1974). Etude histologique de la regeneration pharyngienne de Syllis gracilis Grube (Annelide polychete). Canadian Journal of Zoology, 52, 169–177. [Google Scholar]
- Brockes, J. P. , & Kumar, A. (2008). Comparative aspects of animal regeneration. Annual Review of Cell and Developmental Biology, 24(1), 525–549. https://doi.org/10.1146/annurev.cellbio.24.110707.175336 [DOI] [PubMed] [Google Scholar]
- Caullery, M. (1925). Schizogenèse et schizogamie de Procerastea halleziana Malaquin. Parasitisme de ce syllidien sur les tubulaires. Bulletin Biologique de La France et de La Belgique, 50, 204–208. [Google Scholar]
- Child, C. (1903). Studies on regulation. III. Regulative destruction of zooids and parts of zooids in Stenostoma. Archiv fur Entwickelungsmechanik der Organismen, 17, 1–40. [Google Scholar]
- Dehorne, L. (1917). Formation du sac ovigère et fecondation des oeufs chez la Myrianida pinnigera Montagu. Bulletin Biologique de La France et de La Belgique, 51, 431–440. [Google Scholar]
- Delye, P. (1962). Recherches sur la régénération de la trompe pharyngienne chez les syllinae (Syllidiens, Annélides Polychètes). Annales des Sciences Naturelles, Zoologie, 12(4), 527–541. [Google Scholar]
- Durchon, M. (1951). Stolonisation et hermaphrodisme successif chez Syllis amica quatrefages. Archives de Zoologie Expérimentale et Générale, 88, 96–100. [Google Scholar]
- Durchon, M. (1957). Rôle du proventricule dans le déterminisme de la stolonisation chez les Syllidiens (Annélides Polychètes). Comptes Rendus de l'Académie Des Sciences, 244, 1283–1286. [Google Scholar]
- Durchon, M. (1959). Contribution à l’étude de la stolonisation chez les syllidiens (Annélides, Polychètes) I. Syllinae. Bulletin Biologique de La France et de La Belgique, 93, 155–219. [Google Scholar]
- Durchon, M. , & Wissocq, J.‐C. (1962). Involution expérimentale de la tête stoloniale chez Autolytus pictus (Annelide Polychaeté). Comptes Rendus des Séances de la Société de Biologie et de ses Filiales, 156, 666–668. Retrieved from https://www.biodiversitylibrary.org/item/33991 [Google Scholar]
- Durchon, M. , & Wissocq, J.‐C. (1964). Contribution à l’étude de la stolonisation chez les syllidiens (Annélides, Polychètes) II. Autolytinae. Annales des Sciences Naturelles, Zoologie, 12(6), 159–212. [Google Scholar]
- Fauchald, K. , & Rouse, G. (1997). Polychaete systematics: past and present. Zoologica Scripta, 26(2), 71–138. https://doi.org/10.1111/j.1463-6409.1997.tb00411.x [Google Scholar]
- Franke, H.‐D. (1980). Zur Determination der zeitlichen Verteilung von Fortpflanzungsprozessen in Laborkulturen des Polychaeten Typosyllis prolifera . Helgoländer Meeresunters, 84, 61–84. [Google Scholar]
- Franke, H.‐D. (1983). Endocrine mechanisms mediating light‐temperature effects on male reproductive activity in Typosyllis prolifera (Polychaeta, Syllidae). Wilhelm Roux Archives of Developmental Biology, 192(2), 95–102. [DOI] [PubMed] [Google Scholar]
- Franke, H.‐D. (1986). The role of light and endogenous factors in the timing of the reproductive cycle of Typosyllis prolifera and some other polychaetes. American Zoologist, 26, 433–445. [Google Scholar]
- Franke, H.‐D. (1999). Reproduction of the Syllidae (Annelida: Polychaeta). Hydrobiologia, 402, 39–55. [Google Scholar]
- Franke, H.‐D. , & Pfannenstiel, H.‐D. (1984). Some aspects of endocrine control of polychaete reproduction. Fortschritte der Zoologie, 29, 53–72. [Google Scholar]
- Glasby, C. J. (1993). Family revision and cladistic analysis of the Nereidoidea (Polychaeta: Phyllodocida). Invertebrate Systematics, 7(6), 1551–1573. https://doi.org/10.1071/IT9931551 [Google Scholar]
- Glasby, C. J. , Schroeder, P. C. , & Aguado, M. T. (2012). Branching out: a remarkable new branching syllid (Annelida) living in a Petrosia sponge (Porifera: Demospongiae). Zoological Journal of the Linnean Society, 164(3), 481–497. https://doi.org/10.1111/j.1096-3642.2011.00800.x [Google Scholar]
- Heacox, A. E. , & Schroeder, P. C. (1982). The effects of prostomium and proventriculus removal on sex determination and gametogenesis in Typosyllis pulchra (Polychaeta: Syllidae). Wilhelm Roux's Archives, 191, 84–90. [DOI] [PubMed] [Google Scholar]
- Hill, S. D. , Ferkowicz, M. J. , & Grassle, J. P. (1988). Effect of tail regeneration on early fecundity in Capitella sp. I and II. The Biological Bulletin, 175, 311. [Google Scholar]
- Hyman, L. H. (1940). Aspects of regeneration in annelids. The American Naturalist, 74, 513–527. [Google Scholar]
- Johnson, H. (1901). A new type of budding in annelids. The Biological Bulletin, 2(6), 336–337. [Google Scholar]
- Johnson, H. (1902). Collateral budding in annelids of the genus Trypanosyllis . The American Naturalist, 36, 295–315. [Google Scholar]
- Jong, D. M. , & Seaver, E. C. (2017). Investigation into the cellular origins of posterior regeneration in the annelid Capitella teleta . Regeneration, 4(4), 1–17. https://doi.org/10.1002/reg2.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqua, C. (1957). Stolonisation et polycéphalie expérimentales chez Trypanosyllis zebra Grube (Annélide Polychète). Annales des Sciences Naturelles, Zoologie, 19, 59–68. [Google Scholar]
- Kozin, V. V. , Filippova, N. A. , & Kostyuchenko, R. P. (2017). Regeneration of the nervous and muscular system after caudal amputation in the polychaete Alitta virens (Annelida: Nereididae). Russian Journal of Developmental Biology, 48(3), 198–210. https://doi.org/10.1134/S1062360417030079 [Google Scholar]
- Langerhans, P. (1879). Die Wurmfauna von Madeira. Zeitschrift für Wissenschaftliche Zoologie, 32, 513–592. [Google Scholar]
- Langhammer, H. (1928). Teilungs‐ und Regenerations‐Vorgänge bei Procerastea halleziana und ihre Beziehungen zu der Stolonisation von Autolytus prolifer . Wissenschaftliche Meeresuntersuchungen/Abteilung Helgoland, 17(1), 1–44. [Google Scholar]
- Malaquin, A. (1893). Recherches sur les syllidiens. Mémoires de la Société des Sciences et Arts de Lille, 18, 1–477. [Google Scholar]
- Marion, M. M. A. F. , & Bobretzky, N. (1875). Étude des annélides di Golfe de Marseille. Annales de Sciences Naturelles. Zoologie et Paléontologie, 6(2), 1–106. [Google Scholar]
- McIntosh, W. (1879). On a remarkably branched Syllis dredged by H.M.S. Challenger. The Journal of the Linnean Society of London, 14, 720–724. [Google Scholar]
- McIntosh, W. (1885). Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–1876. Challenger Reports, 12, 1–554. [Google Scholar]
- Mesnil, F. , & Caullery, M. (1919). Sur un processus normal de fragmentation, suivie de regeneration, chez un annelide polychete, Syllis gracilis Gr. Comptes Rendu de l'Academie des Sciences, Paris, 169, 926–929. [Google Scholar]
- Mesnil, M. M. F. (1901). Sur un cas de régénération de la partie antérieure du corps et de la trompe chez un syllidien. Comptes Rendus des Séances de la Société de Biologie et de ses Filiales, 53, 268–270. [Google Scholar]
- Mesnil, M. M. F. , & Caullery, M. (1919). Sur un processus normal de fragmentation, suivie de régénération, chez un Annélide polychéte Syllis gracilis . Comptes Rendus des Séances de la Société de Biologie et de ses Filiales, 169, 926–929. [Google Scholar]
- Michel, M. A. (1909a). Sur la formation du corps par la réunion de deux moitiés indépendantes, d'après l'origine de la queue de la souche chez les Syllidés. Comptes Rendu de l'Academie des Sciences, Paris, 148, 1421–1423. [Google Scholar]
- Michel, M. A. (1909b). Sur la valeur paire de parties impaires et sur la dissymétrie de parties paires, d'après des Syllidiens en stolonisation et en régénération. Comptes Rendus de l'Académie des Sciences, 149, 161–163. [Google Scholar]
- Michel, M. A. (1909c). Sur les divers types de stolons chez les Syllidiens, spécialement sur une nouvelle espèce (Syllis cirropunctata, n. sp.) à stolon acéphale et sur la réobservation du stolon tétracère de Syllis amica Qfg. Comptes Rendus de l'Académie des Sciences, 148, 318–320. [Google Scholar]
- Miyamoto, N. , Shinozaki, A. , & Fujiwara, Y. (2014). Segment regeneration in the vestimentiferan tubeworm, Lamellibrachia satsuma . Zoological Science, 31(8), 535–541. https://doi.org/10.2108/zs130259 [DOI] [PubMed] [Google Scholar]
- Müller, M. C. M. , Berenzen, A. , & Westheide, W. (2003). Experiments on anterior regeneration in Eurythoe complanata (“Polychaeta”, Amphinomidae): reconfiguration of the nervous system and its function for regeneration. Zoomorphology, 122, 95–103. https://doi.org/10.1007/s00435-003-0073-4 [Google Scholar]
- Myohara, M. , Niva, C. C. , & Lee, J. M. (2006). Molecular approach to annelid regeneration: CDNA subtraction cloning reveals various novel genes that are upregulated during the large‐scale regeneration of the oligochaete, Enchytraeus japonensis . Developmental Dynamics, 235(8), 2051–2070. https://doi.org/10.1002/dvdy.20849 [DOI] [PubMed] [Google Scholar]
- Myohara, M. , Yoshida‐Nora, C. , Kobari, F. , & Tochinai, S. (1999). Fragmenting oligochaete Enchytraeus japonesis: a new material for regeneration study. Development, Growth & Differentiation, 41, 549–555. [DOI] [PubMed] [Google Scholar]
- Novikova, E. L. , Bakalenko, N. I. , Nesterenko, A. Y. , & Kulakova, M. A. (2013). Expression of Hox genes during regeneration of nereid polychaete Alitta virens (Annelida, Lophotrochozoa). EvoDevo, 4(1), 21 https://doi.org/10.1186/2041-9139-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, K. G. , Conte, M. A. , Kostyun, J. L. , Forde, A. , & Bely, A. E. (2012). Transcriptome characterization via 454 pyrosequencing of the annelid Pristina leidyi, an emerging model for studying the evolution of regeneration. BMC Genomics, 13(1), 287 https://doi.org/10.1186/1471-2164-13-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren, A. , & Sundberg, P. (2003). Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Molecular Phylogenetics and Evolution, 29(2), 235–249. https://doi.org/10.1016/S1055-7903(03)00095-2 [DOI] [PubMed] [Google Scholar]
- Ogawa, S. , Mizuno, M. , Suzuki, M. , Goto, K. , Hirose, Y. , Matsuda, A. , ... Furukawa, K. (2017). Isolation of a methylated mannose‐binding protein from terrestrial worm Enchytraeus japonensis . Glycoconjugate Journal, 34(5), 591–601. https://doi.org/10.1007/s10719-017-9778-3 [DOI] [PubMed] [Google Scholar]
- Okada, Y. K. (1929). Regeneration and fragmentation in the syllidian polychaetes. Wilhelm Roux' Archiv Fur Entwicklungsmechanik Der Organismen, 115(3), 542–600. [DOI] [PubMed] [Google Scholar]
- Okada, Y. K. (1933a). Syllidian miscellany. Journal of the Marine Biological Association of the UK, 18, 641–653. [Google Scholar]
- Okada, Y. K. (1933b). Two interesting syllids, with remarks on their asexual reproduction. Memoirs of the College of Science, Kyoto Imperial University, Series B, 8(3), 325–338. [Google Scholar]
- Okada, Y. K. (1934). Formation de têtes dans la stolonisation des polychètes syllidiens. Bulletin Biologique de La France et de La Belgique, 68(3), 388–405. [Google Scholar]
- Okada, Y. K. (1935). Stolonization in Myrianida . Journal of the Marine Biological Association of the United Kingdom, 20, 93–98. https://doi.org/10.1017/S0025315400010079 [Google Scholar]
- Okada, Y. K. (1938). An internal factor controlling posterior regeneration in syllid polychaetes. Journal of the Marine Biological Association of the United Kingdom, 23(1), 75–78. https://doi.org/10.1017/S0025315400053960 [Google Scholar]
- Özpolat, B. D. , & Bely, A. E. (2015). Gonad establishment during asexual reproduction in the annelid Pristina leidyi . Developmental Biology, 405(1), 123–136. https://doi.org/10.1016/j.ydbio.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Özpolat, B. D. , & Bely, A. E. (2016). Developmental and molecular biology of annelid regeneration: a comparative review of recent studies. Current Opinion in Genetics and Development, 40, 144–153. https://doi.org/10.1016/j.gde.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Özpolat, B. D. , Sloane, E. S. , Zattara, E. E. , & Bely, A. E. (2016). Plasticity and regeneration of gonads in the annelid Pristina leidyi . EvoDevo, 7(1), 22 https://doi.org/10.1186/s13227-016-0059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher, H. A. (1862). Untersuchungen über niedere Seethiere aus Cette. Zeitschrift für Wissenschaftliche Zoologie, 12, 265. [Google Scholar]
- Pfeifer, K. , Dorresteijn, A. W. C. , & Fröbius, A. C. (2012). Activation of Hox genes during caudal regeneration of the polychaete annelid Platynereis dumerilii . Development Genes and Evolution, 222(3), 165–179. https://doi.org/10.1007/s00427-012-0402-z [DOI] [PubMed] [Google Scholar]
- Potts, F. A. (1911). Methods of reproduction in the syllids. Fortschritte der Zoologie, 3(1911), 1–72. [Google Scholar]
- Potts, F. A. (1913). Stolon formation in certain species of Trypanosyllis . Quarterly Journal of Microscopical Science, 58, 411–446. [Google Scholar]
- Prud'homme, B. , Rosa, R. , Arendt, D. , Julien, J. F. , Pajaziti, R. , Dorresteijn, A. W. C. , ... Balavoine, G. (2003). Arthropod‐like expression patterns of engrailed and wingless in the annelid Platynereis dumerilii suggest a role in segment formation. Current Biology, 13(21), 1876–1881. https://doi.org/10.1016/j.cub.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Rouse, G. , & Pleijel, F. (2006). Reproductive biology and phylogeny of Annelida. Enfield: Science Publishers. [Google Scholar]
- Saint‐Joseph, M. (1886). Les annélides polychètes des cotes de Dinard. Annales des Sciences Naturelles, Zoologie, 9(4), 127–270. [Google Scholar]
- San Martín, G. , Hutchings, P. , & Aguado, M. T. (2008). Syllinae (Polychaeta, Syllidae) from Australia. Part. 2. Genera Inermosyllis, Megasyllis n. gen., Opistosyllis, and Trypanosyllis . Zootaxa, 1840, 1–53. [Google Scholar]
- San Martín, G. , Hutchings, P. , & Aguado, M. T. (2010). Syllinae (Polychaeta: Syllidae) from Australia. Part 3. Genera Alcyonosyllis, genus A, Parahaplosyllis, and Trypanosyllis (Trypanobia). Zootaxa, 48(2493), 35–48. https://doi.org/10.5281/zenodo.195678 [Google Scholar]
- Satoh, A. , Makanae, A. , Nishimoto, Y. , & Mitogawa, K. (2016). FGF and BMP derived from dorsal root ganglia regulate blastema induction in limb regeneration in Ambystoma mexicanum . Developmental Biology, 417(1), 114–125. https://doi.org/10.1016/j.ydbio.2016.07.005 [DOI] [PubMed] [Google Scholar]
- Schroeder, P. C. , Aguado, M. T. , Malpartida, A. , & Glasby, C. J. (2017). New observations on reproduction in the branching polychaetes Ramisyllis multicaudata and Syllis ramosa (Annelida: Syllidae: Syllinae). Journal of the Marine Biological Association of the United Kingdom, 97(5), 1167–1175. https://doi.org/10.1017/S002531541700039X [Google Scholar]
- Schroeder, P. C. , & Hermans, C. O. (1975). Annelida: Polychaeta In Giese A. C. & Pearse J. S. (Eds.), Reproduction of marine invertebrates (Vol. 3, pp. 1–213). New York: Academic Express. [Google Scholar]
- Seaver, E. C. (2003). Segmentation: mono‐ or polyphyletic? International Journal of Developmental Biology, 47(7–8), 583–595. https://doi.org/10.1387/IJDB.14756334 [PubMed] [Google Scholar]
- Starunov, V. V. , Voronezhskaya, E. E. , & Nezlin, L. P. (2017). Development of the nervous system in Platynereis dumerilii (Nereididae, Annelida). Frontiers in Zoology, 14(1), 27 https://doi.org/10.1186/s12983-017-0211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo, M. , Chou, W. C. , Sun, Q. , Lee, W. , Rabbani, P. , Loomis, C. , … Ito, M. (2013). Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature, 499(7457), 228–232. https://doi.org/10.1038/nature12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguer‐Bocquet, M. (1979). Étude expérimentale de la régénération céphalique et étude infrastructurale de l'ceil régénéré chez Syllis amica (Annélide, Polychète). Archives de Biologie (Bruxelles), 90, 23–41. [Google Scholar]
- Viguier, C. (1884). Études sur les animaus inférieurs de la Baie d'Alger. Archives de Zoologie Expérimentale et Générale, 10, 69–110. [Google Scholar]
- Weidhase, M. , Beckers, P. , Bleidorn, C. , & Aguado, M. T. (2016). On the role of the proventricle region in reproduction and regeneration in Typosyllis antoni (Annelida: Syllidae) . BMC Evolutionary Biology, 16(1), 196 https://doi.org/10.1186/s12862-016-0770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhase, M. , Beckers, P. , Bleidorn, C. , & Aguado, M. T. (2017). Nervous system regeneration in Typosyllis antoni (Annelida: Syllidae). Zoologischer Anzeiger, 269, 57–67. [Google Scholar]
- Weidhase, M. , Bleidorn, C. , Beckers, P. , & Helm, C. (2016). Myoanatomy and anterior muscle regeneration of the fireworm Eurythoe cf. complanata (Annelida: Amphinomidae). Journal of Morphology, 277(3), 306–315. https://doi.org/10.1002/jmor.20496 [DOI] [PubMed] [Google Scholar]
- Weidhase, M. , Bleidorn, C. , & Helm, C. (2014). Structure and anterior regeneration of musculature and nervous system in Cirratulus cf. cirratus (Cirratulidae, Annelida). Journal of Morphology, 275(12), 1418–1430. https://doi.org/10.1002/jmor.20316 [DOI] [PubMed] [Google Scholar]
- Weidhase, M. , Helm, C. , & Bleidorn, C. (2015). Morphological investigations of posttraumatic regeneration in Timarete cf. punctata (Annelida: Cirratulidae). Zoological Letters, 1, 20 https://doi.org/10.1186/s40851-015-0023-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissocq, J.‐C. (1966). Rôle du proventricule dans le déterminisme de la stolonisation de Syllis amica Quatrefages (Annélide polychète). Comptes Rendus de l'Académie des Sciences, 262, 2605–2608. [Google Scholar]
- Wissocq, J.‐C. (1970a). Évolution de la musculature longitudinale dorsale et ventrale au cours de la stolonisation de Syllis amica Quatr. (Annélide Polychète). II. La dédifférenciation. Journal de Microscopie, 9, 1049–1074. [Google Scholar]
- Wissocq, J.‐C. (1970b). Évolution de la musculature longitudinale, dorsale et ventrale au cours de la stolonisation de Syllis amica Quatr. (Annélide Polychète) III. La dégénérescence. Journal de Microscopie, 9, 1075–1088. [Google Scholar]
- Yoshida‐Noro, C. , Myohara, M. , Kobari, F. , & Tochinai, S. (2000). Nervous system dynamics during fragmentation and regeneration in Enchytraeus japonensis (Oligochaeta, Annelida). Development Genes and Evolution, 210(6), 311–319. https://doi.org/10.1007/s004270050318 [DOI] [PubMed] [Google Scholar]
- Zattara, E. E. , & Bely, A. E. (2011). Evolution of a novel developmental trajectory: fission is distinct from regeneration in the annelid Pristina leidyi . Evolution and Development, 13(1), 80–95. https://doi.org/10.1111/j.1525-142X.2010.00458.x [DOI] [PubMed] [Google Scholar]
- Zattara, E. E. , & Bely, A. E. (2013). Investment choices in post‐embryonic development: quantifying interactions among growth, regeneration, and asexual reproduction in the annelid Pristina leidyi . Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 320(8), 471–488. https://doi.org/10.1002/jez.b.22523 [DOI] [PubMed] [Google Scholar]
- Zattara, E. E. , & Bely, A. E. (2016). Phylogenetic distribution of regeneration and asexual reproduction in Annelida: regeneration is ancestral and fission evolves in regenerative clades. Invertebrate Biology, 135(4), 400–414. https://doi.org/10.1111/ivb.12151 [Google Scholar]


