Abstract
While most endosymbiotic bacteria are transmitted only vertically, Holospora spp., an alphaproteobacterium from the Rickettsiales order, can desert its host and invade a new one. All bacteria from the genus Holospora are intranuclear symbionts of ciliates Paramecium spp. with strict species and nuclear specificity. Comparative metabolic reconstruction based on the newly sequenced genome of Holospora curviuscula, a macronuclear symbiont of Paramecium bursaria, and known genomes of other Holospora species shows that even though all Holospora spp. can persist outside the host, they cannot synthesize most of the essential small molecules, such as amino acids, and lack some central energy metabolic pathways, including glycolysis and the citric acid cycle. As the main energy source, Holospora spp. likely rely on nucleotides pirated from the host. Holospora-specific genes absent from other Rickettsiales are possibly involved in the lifestyle switch from the infectious to the reproductive form and in cell invasion.
Keywords: Holospora, Rickettsiales, endosymbionts, paramecia, ciliates, nuclear symbionts, genome
Introduction
Symbiotic associations with bacteria are common to many, if not all, eukaryotes. Endosymbiotic bacteria are well studied in plants, insects, and some other organisms (Moran et al., 2005; Kawaguchi and Minamisawa, 2010; McCutcheon and Moran, 2010; Bennett and Moran, 2013; Becker et al., 2015; Wu et al., 2015; Wexler et al., 2016; Zipfel and Oldroyd, 2017). By the original definition (Oulhen et al., 2016), a symbiotic relationship does not imply benefits for the host. Nevertheless, in symbiotic relationships, symbiont explores its host as a habitat. In turn, host can acquire some necessary products and/or properties which it unable to synthesize, e.g., ammonium produced for plants by nitrogen fixing bacteria, essential amino acids produced by endosymbionts of sap-sucking insects (Moran et al., 2005; Archibald, 2015; Sabater-Muñoz et al., 2017), or vitamins for blood-feeding tsetse fly (Dale and Welburn, 2001). Some symbionts, like some strains of Wolbachia spp., protect the host from pathogenic viruses (Hedges et al., 2008); or are necessary for the maturation of the immune system, as e.g., Wigglesworthia for tsetse flies (Weiss et al., 2011). Caedibacter creates growth advantages for infected paramecia and helps them to out-compete uninfected ones (Kusch et al., 2000). An endosymbiont of ciliates from the genus Euplotes is essential for the host cell proliferation (Vannini et al., 2012, 2017). However, most relations are not well understood, and an advantage for the host is not always demonstrated, especially for facultative endosymbionts (Lu et al., 2016; Yañez et al., 2016).
Endosymbiotic bacteria and endonuclear parasites typically have several characteristic features, such as small genome size, low GC content, and short intergenic spacers (reviewed in McCutcheon and Moran, 2012; Batut et al., 2014; Martínez-Cano et al., 2015). Another important trait of these bacteria is an accelerated rate of genome evolution, caused by the minimal gene flow and substantial genetic drift due to the small effective population size of endosymbionts (Marais et al., 2008; Boscaro et al., 2017; Sabater-Muñoz et al., 2017). This leads to formation of pseudogenes and gene loss, and an overall decrease of the metabolic capacity (McCutcheon and Moran, 2012; Boscaro et al., 2017). The loss of genes involved in the reparation drives an even faster evolution of symbiotic species (McCutcheon and Moran, 2012). It also can lead to the decrease in the GC content (Horst et al., 1999; Moran et al., 2005; Long et al., 2018). A stable environment in the host cell does not require complicated regulatory systems, yielding reduction of intergenic regions (McCutcheon and Moran, 2012).
Holospora spp. are endonuclear symbiotic Alphaproteobacteria from the order Rickettsiales that inhabit either macro- or micronucleus of Paramecium spp. It has been suggested that Holospora spp. are parasites, as other Rickettsiales. A recent analysis of 16S rRNA lead some researchers to separate the basal rickettsial lineage of Holospora-like bacteria in a separate order Holosporales (Ferla et al., 2013; Szokoli et al., 2016), but for convenience we discuss both orders here together as Rickettsiales, because they have similar lifestyle and metabolic capacities. The genus Holospora currently is comprised of nine species, each showing clear nuclear and host specificity (Görtz and Schmidt, 2015). Holospora are not able to reproduce outside the host cell but can leave the host in order to invade a new one. Unlike most of the studied symbiotic species, Holospora have a complex life cycle involving two morphologically different forms. The short reproductive form exists only in the host nucleus and multiplies by binary fission, while the long infectious form does not multiply and is capable of infecting new host cells (Gromov and Ossipov, 1981; Fokin and Görtz, 2009). This form shows a unique cytological organization with a pronounced polarity (a large perinuclear space and a special tip), which seems to be of functional significance for the infection process. The infectious form enters new host via Paramecium food vacuole together with food bacteria, but escapes digestion, exits the vacuole and enters the cytoplasm followed by nuclear invasion. Two main mechanisms of this process have been proposed: Holospora either disrupt the digestive vacuole or enter the transport vacuoles (Schweikert et al., 2013). Then Holospora reach the nucleus by utilizing the host’s actin cytoskeleton (Fokin and Görtz, 2009; Sabaneyeva et al., 2009). In Holospora obtusa, the 89 kDa periplasmic protein was shown to be associated with cell invasion (Iwatani et al., 2005). The invasion of macronucleus by H. obtusa is associated with release of the 63 kDa periplasmic protein into the macronucleus of the host (Abamo et al., 2008). Additionally, 15 and 39 kDa periplasmic proteins are released from the cell tip during the macronucleus infection (Fujishima et al., 1997). Mechanisms underlying the transition from the reproductive to the infectious form and back are not well understood, but the 5.4 kDa protein with a signal peptide is only detected in the intermediate and infectious forms (Dohra et al., 1997).
In the presence of Holospora, Paramecium is able to grow, divide and mate (Schweikert et al., 2013). Holospora contribute to the heat-shock resistance in Paramecium caudatum, as cells infected with H. obtusa express high levels of hsp70 mRNA (Fujishima et al., 2005). They may also assist in acquiring high-salt and osmotic-shock resistance to the host (Fujishima, 2009). The presence of H. caryophila in the Paramecium biaurelia nucleus is advantageous in several cell lines during the exponential growth (Bella et al., 2016). Despite these observations of benefits for the host, Holospora have been shown to negatively affect host cells. For example, the presence of Holospora elegans in Paramecium leads to the formation of dysfunctional macronucleus during conjugation (Görtz and Fujishima, 1983). Holospora undulata increases mortality of the host, especially at low-food treatment (Restif and Kaltz, 2006), and high concentrations of the infectious form in the macronucleus inhibit the host cell growth (Fujishima, 2009). The relationship between Holospora spp. and their paramecia hosts seems to be a complex system in which benefits or damages for the host can be highly context-dependent.
The metabolism of Rickettsiales, and in particular of Rickettsia spp., has been studied in detail (Andersson et al., 1998; Hotopp et al., 2006; Fuxelius et al., 2007; Georgiades et al., 2011). Rickettsia export at least 51 metabolites from the host (Driscoll et al., 2017). They are not able to synthesize amino acids, nucleotides, lack glycolysis, and have to import such compounds as coenzyme A, pyruvate, FAD, biotin, etc.
So far, three Holospora genomes have been sequenced, all of which are endosymbionts of Paramecium caudatum: macronucleus-specific H. obtusa and micronucleus-specific H. elegans and H. undulata (Dohra et al., 2013, 2014). Analysis of common orthologous genes has yielded 572 single-copy core genes shared by the three genomes, and Holospora have been shown to rely on the host for energy production (Dohra et al., 2014). However, no detailed metabolic pathway reconstruction has been performed.
Here, we report a comparative analysis of four Holospora species, including Holospora curviuscula, a newly sequenced macronuclear endosymbiont of P. bursaria. We propose that Holospora use host-produced nucleotides as its energy source. We also identify the essential compounds that Holospora are likely able to synthesize, and compare their metabolism to that of other Rickettsiales.
Materials and Methods
Growth Conditions and Genomic DNA Preparation
Holospora curviuscula has an obligate association with its host, Paramecium bursaria, and is therefore uncultivable, so the bacteria have been grown inside host cells. Holospora curviuscula strain NRB217 from Paramecium bursaria isolated in the Novosibirsk Akademgorodok were obtained from the infected clones maintained in the CCCS (Culture Collection of Ciliates and their Symbionts), Research Park, Saint-Petersburg State University. The host cells were cultivated at the room temperature on the lettuce medium inoculated with Enterobacter aerogenes as a food resource for paramecia. The culture of P. bursaria bearing H. curviuscula was concentrated by centrifugation (10 min at 4500 g) and then homogenized using 10% solution of detergent Nonidet P-40 (Sigma-Aldrich Cat No. 21-3277 SAJ). The infectious forms of H. curviuscula were isolated from the homogenate by centrifugation in Percoll density gradient (Sigma-Aldrich Cat No. P1644) as described previously (Rautian and Wackerow-Kouzova, 2013). Genomic DNA was isolated with the DNeasy Blood and Tissue kit (QIAGEN Cat No. 69504) using a modified protocol — the time of incubation of cell homogenate with ATL buffer and proteinase K was increased to 16 h. All the subsequent steps were performed according to the standard Quick-Start Protocol.
Genome Assembly and Annotation
Two libraries were generated, paired-end MiSeq Illumina library (2 × 250) (PE), and mate-pair library with insertion size 4 kbp (MP). The initial MP library size was 2.5 million reads, and the PE library size, 4.5 million. MP reads were filtered with NextClip v1.3 (Leggett et al., 2014) and only category A pairs of reads (both reads in a pair contain adapters) were selected for further analysis. Both MP and PE were filtered by quality with trimmomatic version 0.33 (Leading 15, sliding -15, slidingwindow -4:25), after filtering 1.3 million MP reads and 1.7 million PE reads were retained for assembly. Processed reads were assembled with platanus version 1.2.1. The Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession PHHC00000000. The version described in this paper is version PHHC01000000.
To estimate the completeness of the Holospora assemblies, we used HMMer to search for essential PFAM domains (Rinke et al., 2013). A domain was considered to be present, if it was found by hmmsearch (with default parameters) (Mistry et al., 2013) and the bias was lower than the hit score. For all missing genes we checked whether they were present in 60 genomes of Rickettsiales and 23 other endosymbiotic Proteobacteria with tiny genomes less than 0.8 Mb (the complete list of genomes is given in Supplementary Table 1).
The resulting contigs were annotated with the PROKKA pipeline (Seemann, 2014). Metabolic pathways were reconstructed with SEED (Overbeek et al., 2005) and BioCyc (Caspi et al., 2016). To search for known bacteriocins we used APD3 (Wang et al., 2016), BAGEL3 (van Heel et al., 2013), and searched for known bacteriocin-associated PFAM domains. AntiSMASH (Weber et al., 2015) was used to predict biosynthesis of secondary metabolites. Transmembrane helices in proteins were predicted with TMHMM1. Transporter protein specificity was predicted by TCDB (Saier et al., 2016). SignalP and SecretomeP were used to predict signal peptides and secreted proteins, respectively (Bendtsen et al., 2005a; Nielsen, 2017). To predict the twin-arginine motif we utilized TatP server (Bendtsen et al., 2005b). Autotransporter proteins were predicted by search for PFAM domains PF03797, PF12951, PF05662, PF05658, PF11924, PF11557, PF16168, PF15403, PF03895 with HMMer package. To find phage-like regions in the genome assemblies, we used PHAST (Zhou et al., 2011). Additionally, we searched for all PFAM-domains with the keywords “transposase” and “phage” (328 PFAM domains).
16S rRNA-Based Phylogeny
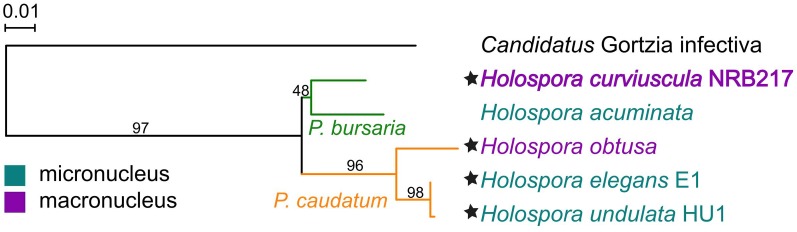
To reconstruct the phylogenetic tree of Holospora spp., the PhyML software (Guindon et al., 2010) was used. 16S rRNAs from H. curviuscula NRB217, H. elegans E1, H. obtusa, and H. undulata HU1 were extracted from the full genome sequences, while 16S rRNAs from H. acuminata and Candidatus Gortzia infectiva were obtained from the SILVA database (accession numbers: KC164379 and HE797907, respectively). To root the tree, we used 16S rRNA of Rickettsiales (SILVA accession number: CP009217). The phylogenetic tree was constructed with PhyML v. 3.1 with 100 bootstrap replicates, GTR substitution model, and -o tlr parameter.
Analysis of Repeats
To estimate the fraction of repetitive DNA in the Holospora genome assemblies, reads were realigned with Bowtie 2 (–end-to-end mode) (Langmead and Salzberg, 2012) back to the respective assemblies, and the coverage for each nucleotide was calculated. In the case of H. curviuscula, we used PE reads generated in this study, reads for H. obtusa and H. undulata were downloaded from DDBJ (accession numbers DRP001203 and DRA001008, respectively). A fragment was presumed to be duplicated if it was longer than 20 bp, and its coverage was at least 1.6-fold higher than the median contig coverage.
Orthologous Groups and Genome Comparisons
To investigate the phylogenetic relations between H. undulata and H. elegans, pairwise genome alignments of all Holospora genomes were constructed with MAUVE (snapshot 2015-02-13) (Darling et al., 2010). Alignments of orthologous genes from the essential list (see above) were extracted from the MAUVE output, and all gaps were removed. The number of substitutions was calculated for each gene for each pair of genomes.
To investigate the gene repertoire of all Holospora and other Rickettsiales, we constructed groups of orthologous genes using OrthoMCL v. 2.0.9 (Li et al., 2003) with the MCL software v. 14-137. The output gene clusters were grouped in five subgroups using an ad hoc perl script which considered orthologs, co-orthologs and inparalogs. All Rickettsiales and other Holospora genomes (for the complete list see Supplementary Table 2) were downloaded from NCBI Genbank (Benson et al., 2013).
Results
Holospora curviuscula Genome
The H. curviuscula draft genome sequenced here is comprised of 152 scaffolds (210 contigs) with N50 of ∼39 kbp, total length of 1.7 Mb, and GC content of 37.6% (Table 1). The longest scaffold is 153367 bp and contains 126 CDSs. We predicted 1594 genes, including 1555 protein-coding genes with the average encoded protein length of 218 aa (Supplementary Figure 1). We assigned protein function to 683 genes. The genome assembly contains all rRNA genes and a set of 36 tRNAs necessary to recognize all codons. The 16S rRNA analysis revealed that the sequenced genome indeed belongs to the genus Holospora, and specifically to H. curviuscula, and is closer to Holospora acuminata, another endosymbiont of P. bursaria, than to Holospora from P. caudatum (Figure 1). The H. curviuscula genome is the largest Holospora genome sequenced so far (Table 1). Its GC content is slightly higher than that of most other non-Rickettsiales obligate endosymbionts (McCutcheon and Moran, 2012), but is typical for Rickettsiales (Supplementary Figure 2).
Table 1.
Results of genome assembly and annotation of H. curviuscula, and comparison with other Holospora species.
| H. curviuscula | H. obtusa | H. undulata | H. elegans | |
|---|---|---|---|---|
| Genome (bp) | 1715500 | 1334837 | 1402636 | 1268333 |
| No. of contigs | 210 | 91 | 203 | 152 |
| GC content (%) | 37.6% | 35.2% | 36.1% | 36.0% |
| CDS | 1594 | 1117 | 1224 | 1212 |
FIGURE 1.
Maximum likelihood phylogenetic tree of Holospora spp. reconstructed by PhyML (see Materials and Methods). Violet, macronucleus endosymbiont; cyan, micronucleus endosymbiont. Genomes marked with stars have been sequenced.
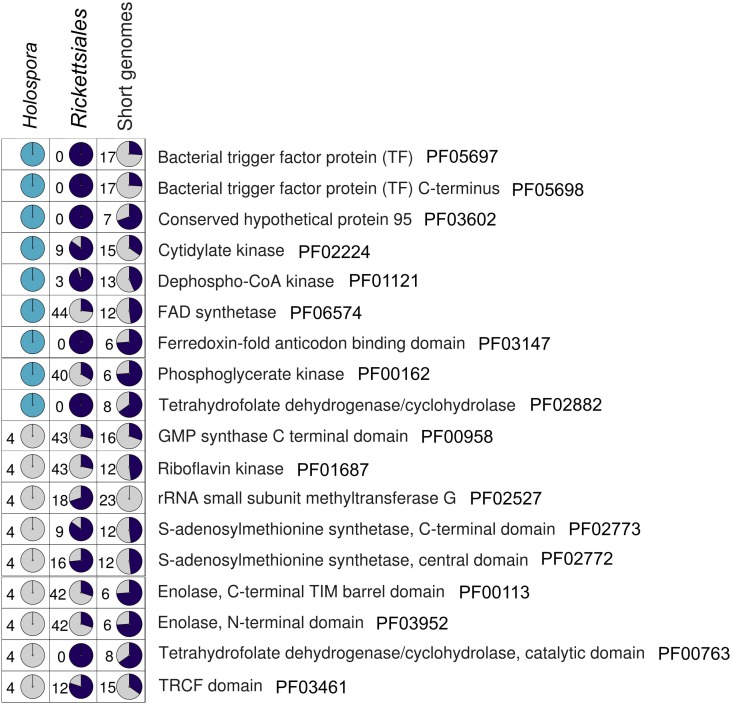
We estimated the quality of the assembly by searching for nearly universal bacterial genes (see Materials and Methods). Among the 139 universal PFAM domains, 121 are present in the H. curviuscula genome. Nine of the 18 missing domains have been found with a lower similarity threshold, which makes their presence uncertain. The remaining nine of the 18 missing domains are absent in all other Holospora as well. Moreover, these domains are also absent either in some endosymbiotic bacteria with small genomes or in some Rickettsiales genomes (Figure 2), meaning that their absence can be tolerated. We further searched for these 18 missing domains in unassembled sequencing reads of H. curviuscula (14% of all reads), and found no indication of their presence.
FIGURE 2.
The presence/absence pattern of 18 essential PFAM domains in Holospora, endosymbiotic bacteria with tiny genomes, and Rickettsiales (Supplementary Table 1). Color code: dark blue, the domain is present; gray, the domain is absent; cyan represents domains found with decreased similarity threshold (see Materials and Methods). The number in each cell is the number of genomes where the domain has not been found.
We manually checked 615 ORFs for frameshifts, and found only nine ORFs with frameshifts. This number may be an underestimate, because H. curviuscula genome contains short ORFs with unknown functions that have no homologs outside Holospora and can be remnants of some ancestral genes. Still, it implies that the H. curviuscula genome contains few pseudogenes.
Rickettsiales have been reported to have an atypical rRNA operon (Andersson et al., 1998). However, all Holospora have a standard rRNA operon, with 23S and 16S genes located close to each other and separated by several tRNA genes.
Reanalysis H. undulata and H. elegans Genomes
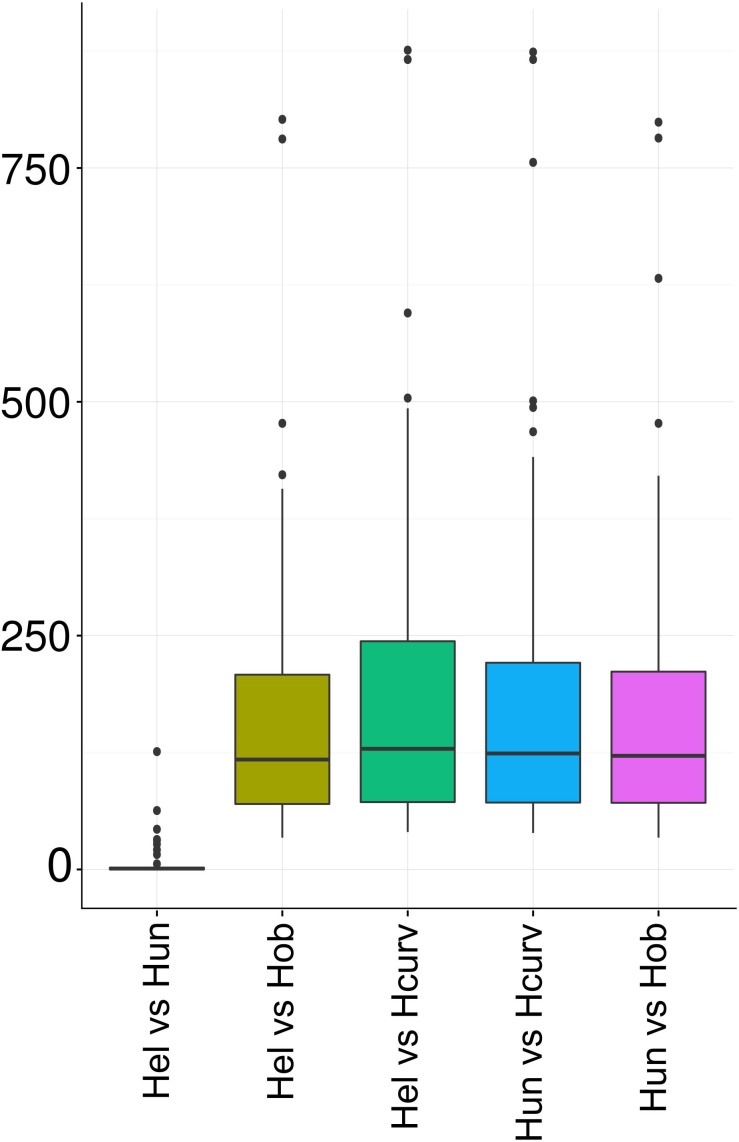
Holospora undulata HU1 and H. elegans E1 extracted from the micronucleus of P. caudatum have been recognized as separate species based on differences in the cell morphology (Görtz and Schmidt, 2015). However, our comparison of 16S rRNA genes from the available genome assemblies (Genbank IDs NZ_ARPM00000000 and NZ_BAUP00000000, respectively) of these species revealed just a single-nucleotide difference (Figure 1), yielding > 99.9% sequence identity, which is above the common thresholds of 97 or 98.7% used to define species (Janda and Abbott, 2007). That led us to inquire whether the genomes of H. undulata and H. elegans indeed represented different species or just strains of the same species. We constructed whole-genome pairwise alignments of the Holospora genomes and counted mismatches in the essential genes (Figure 3). The H. elegans–H. undulata pair carried ∼100-fold fewer mismatches than all other genome pairs.
FIGURE 3.
The number of mismatches in pairs of Holospora species. Hcurv, H. curviuscula NRB217; Hel, H. elegans E1; Hob, H. obtusa F1; Hun, H. undulata HU1.
Holospora spp. Have Multiple Repeat Sequences and Contain Prophages
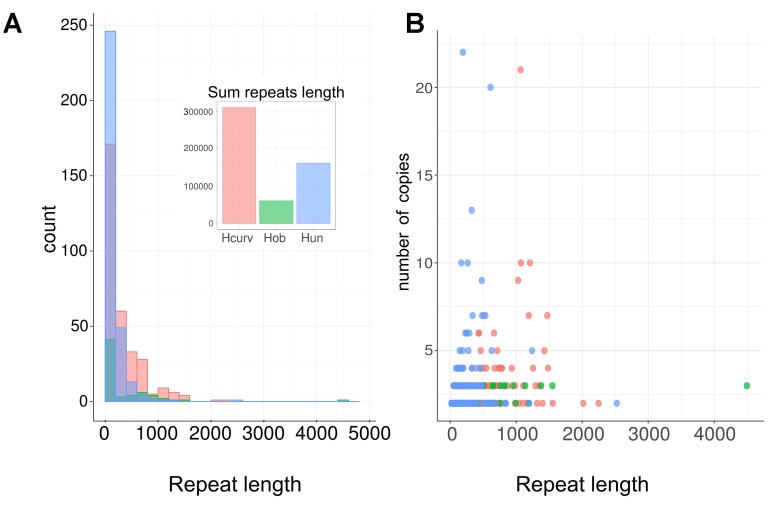
All available Holospora assemblies are comprised of multiple contigs, and reassembly has not led to better assemblies even with added genome coverage. Moreover, the genome length of H. curviuscula has been estimated at ca. 2 Mb (unpublished data), while the total assembly length is around 1.7 Mb. This suggests that Holospora genomes may contain a considerable fraction of repetitive DNA. To test this, we searched for fragments with high read coverage (see Materials and Methods). In H. curviuscula, we found ∼300 repeats with the total length of ∼300 kb (Figure 4A) and the estimated copy number varying from 2 to 21 (Figure 4B). In H. obtusa and H. undulata, the similarly estimated lengths of repetitive DNA were 60 and 161 kb, respectively. We were not able to estimate the fraction of repetitive DNA for H. elegans, as no raw sequencing data were available. The calculated repeat lengths could be underestimates, as we have applied strict coverage cutoffs yielding conservative repeat boundaries. For the same reason, we could overestimate the number of repeats by splitting long repeats into individual repeats in regions of low coverage. Still, the match between the difference in the assembly length and the genome length and the total coverage of repetitive DNA suggests that unassembled repeats comprise ∼15% of the genome in H. curviuscula. The high copy-number repetitive regions in H. curviuscula include short ORFs of possible transposases. In addition, the identified repeats include two copies of rRNA operons with the similar structure, and also genes encoding prophage-like proteins.
FIGURE 4.
Lengths and the copy number distribution of repeats in Holospora spp. (A) Lengths of repeated sequences in Holospora spp. (B) Relations between repeat the repeat copy number and length. Hcurv, H. curviuscula NRB217 (pink); Hob, H. obtusa F1 (green); Hun, H. undulata HU1 (blue).
To characterize prophages further, we searched for complete prophages and, additionally, for phage-related PFAM domains in all Holospora genomes. We found two possible prophage regions in H. elegans, one in H. obtusa, and two in H. undulata. One of the H. undulata prophages was intact, contained all proteins necessary for the phage replication and was surrounded by integration sequences (Supplementary Figure 3); the remaining copies were severely disrupted and lacked insertion sequences. Although no intact prophages were found in H. curviuscula, scaffold565 had a locus with two DDE superfamily endonucleases and two putative transposases, and other scaffolds carried genes encoding phage capsid and phage portal proteins similar to the ones found in other Holospora as well as some full-length and fragmentary transposases (data not shown).
Holospora spp. Cannot Synthesize Amino Acids and Some Other Essential Small Molecules
Holospora have a reduced metabolism even by the standards of the already gene-poor order Rickettsiales (Georgiades et al., 2011). In particular, Holospora lack genes encoding enzymes of the citric acid cycle (CAC) (Supplementary Figure 4). By contrast, Rickettsia and other Rickettsiales have at least some of these enzymes and use them to convert small molecules (Driscoll et al., 2017).
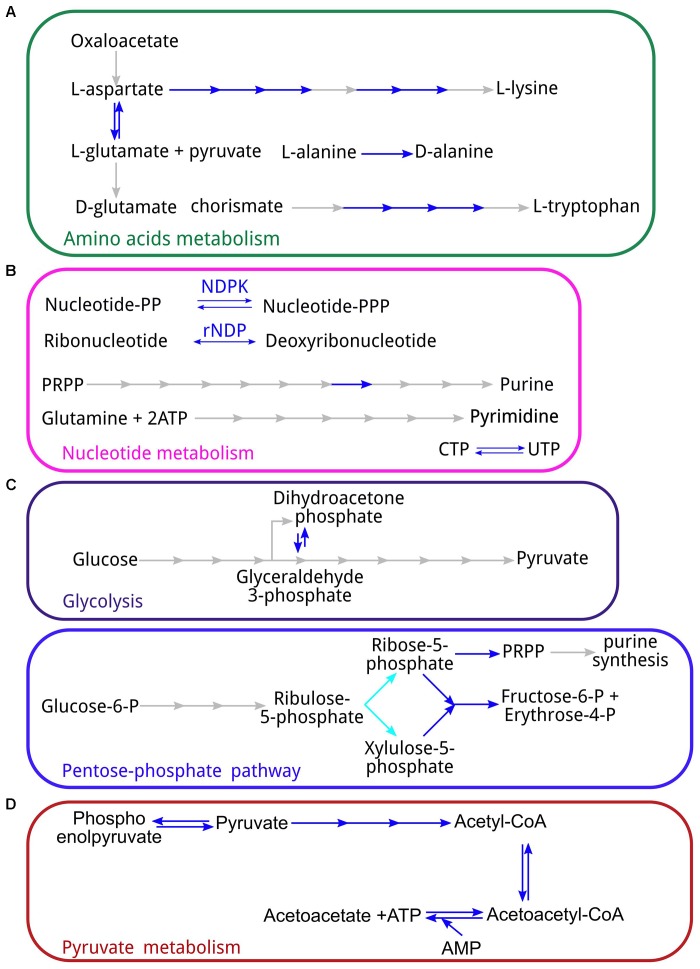
All sequenced Holospora lack amino acid synthesis pathways. For most amino acids, all enzymes of the pathway are missing. The exceptions are the partial pathways for the biosynthesis of L-tryptophane, L-lysine, and L-glutamate and for the conversion of L-alanine to D-alanine, an essential compound for formation of the cell wall (Figure 5A). This means that all amino acids have to be imported from the host. While Rickettsia also cannot produce amino acids and probably import them from the host (Driscoll et al., 2017), other Rickettsiales are capable of synthesizing some amino acids such as L-lysine and L-glutamine (Fuxelius et al., 2007). Similar to Rickettsia, Holospora cannot produce chorismate or use it as a substrate for downstream reactions, and need to import it or its derivates from the host.
FIGURE 5.
Some aspects of the Holospora metabolism: (A) amino acid metabolism; (B) metabolism of purines and pyrimedines; (C) energy metabolism (top – glycolysis, bottom – pentose phosphate pathway); (D) pyruvate metabolism. Blue arrows represent reaction for which the corresponding enzyme has been found in all Holospora spp.; cyan – enzymes absent in H. curviuscula, but present in H. undulata and H. obtusa. PRPP, phosphoribosyl pyrophosphate; NDPK, nucleoside-diphosphate kinase; rNDP, ribonucleotide reductase.
Like Rickettsia, Holospora are not capable of purine and pyrimidine synthesis (Figure 5B), although, also like Rickettsia, they can convert UTP to ÑTP. Therefore, they need to obtain all nucleoside triphosphates from the host. On the other hand, Holospora carry genes for ribonucleotide reductases and therefore can convert ribonucleotides into deoxyribonucleotides.
The ubiquinone biosynthesis pathway is also partial, as it is comprised of only dimethylallyl diphosphate (DMAPP) and the enzymes downstream of it (Supplementary Figure 6), similarly to what has been reported for Rickettsia (Driscoll et al., 2017). Unlike Rickettsia, Holospora are unable to convert DMAPP to isopentenyl diphosphate, although they carry geranyl-diphosphate synthase which is missing in Rickettsia. Therefore, DMAPP seems to be obligatorily imported from the host.
The only major metabolic pathway that is almost intact is the fatty acids synthesis (Supplementary Figure 5).
In order to determine how the missing compounds are imported from the host, we analyzed predicted transporters. In H. curviuscula, thirty transport-associated genes have been identified. As expected, Holospora genomes encode oligopeptide and amino acid transporters, as well as proteases, some of which are periplasmic. In particular, we have found arginine/glutamine, proline, acetyl-serine/cysteine, choline/glycine/betaine, and putative branched amino acids candidate transporters. In addition, we found putative transport systems for magnesium, ferric ions, ribose, purines, sulfoacetate, L-galactonate or other sugars, and putrescine. However, these transport systems are insufficient to deliver all missing compounds to Holospora; in particular, it is not clear, how the remaining amino acids are delivered, unless as components of oligopeptides.
Holospora spp. Use Nucleotides as the Main Energy Source
All Holospora lack most genes involved in energy production. In particular, all enzymes necessary for the glycolysis except phosphoglycerate mutase (Figure 5C) and all enzymes of the citric acid cycle except malate dehydrogenase (Supplementary Figure 4) are missing. The F1F0-ATPase is also missing. In addition, Holospora cannot produce coenzyme A from scratch, although as Rickettsia, they seem to have CoaE (PF01121, Figure 2) and thus are able to synthesize coenzyme A from dephospho-CoA (Driscoll et al., 2017). Of the pentose phosphate pathway, only the non-oxidative branch is present, as well as the downstream enzyme ribose-phosphate mutase that converts phosphorybosil pyrophosphate (PRPP) to sugars (Figure 5C); the energy-producing oxidative pathway leading to the ribulose-5-phosphate is missing. By contrast, all Holospora have the pyruvate dehydrogenase complex and are able to convert pyruvate to acetyl-CoA, and further to acetoacetyl-CoA and acetoacetate, with the production of ATP (Figure 5D). All Holospora have a set of ribonucleotide reductases, which would allow them to use either nucleotides or ribonucleotides as an energy source. No obvious source of energy other than nucleotides was found.
Secretory Systems and Putative Invasins
Although most Rickettsiales are parasites and their genomes are highly reduced, they retain secretory systems such as the Tat and Sec pathways, a type IV system, and the TolC protein from a type I secretion system (Gillespie et al., 2015). Moreover, the VirB protein from a type IV secretory system is thought to be essential for the host invasion in most Rickettsiales (Rennoll-Bankert et al., 2015; Gillespie et al., 2016). By contrast, all studied Holospora demonstrate a significant decay of secretion pathways. They still possess the complete Sec system, additional systems helping to translocate proteins to the outer membrane (LolA, LolD, and possibly LolE) or transport them outside the cell (BamA, BamB, BamD, and chaperone DegP), and a TolC-like protein. However, we found no proteins similar to components of the Tat-system (TatA, TatB, or TatC). Since Tat-system proteins may be hard to identify (Gillespie et al., 2015), we performed a genome-wide search for proteins with the twin-arginine signal required for the recognition by the Tat-system and found no significant hits. Together, these observations show that the Tat transport system is indeed missing, which means that Holospora are unable to export folded substrates.
In Rickettsia, invasion is associated with proteins RalF, RickA, and Sca (Gillespie et al., 2015) which are missing in Holospora, implying that Holospora use other mechanisms to invade the host cell. There are also no proteins with ankyrin domains assumed to play a role in the pathogenesis in Rickettsia (Gillespie et al., 2015). Although Sec proteins are present in all Holospora, only one protein with a putative autotransporter domain (PF03797) was found in H. curviuscula (HCUR_00103). Even though this gene has homologs in other Holospora, this domain was not predicted in them, implying that it can be a false positive. However, we found multiple (3–9 copies per genome) genes similar to ompA in all Holospora. OmpA has been previously shown to be involved in the pathogenesis by Rickettsiales (reviewed in Palmer and Noh, 2012).
Holospora-Specific Genes
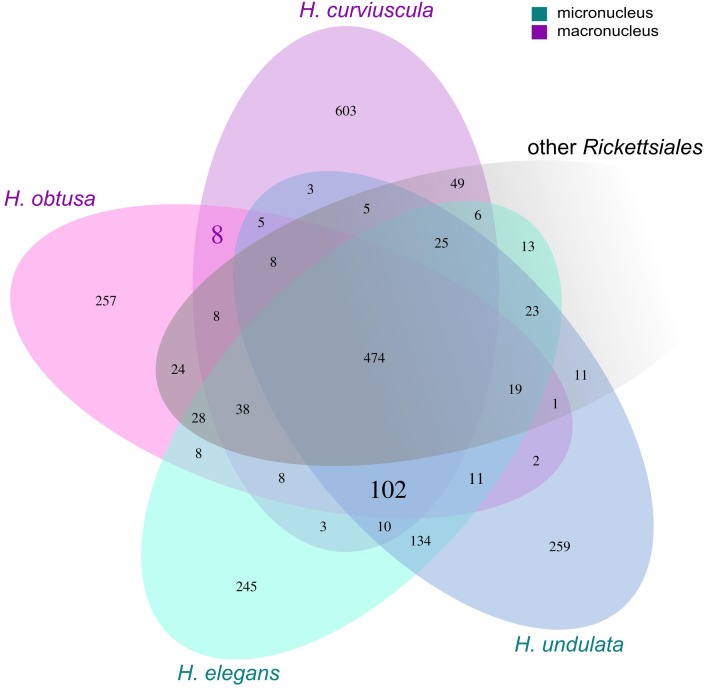
To search for genes that could be essential for the survival in the nucleus, we compared orthologous groups (OGs) for all Holospora with a set of all complete Rickettsiales genomes (Figure 6). We identified 102 OGs that occur in all Holospora and do not occur in any other Rickettsiales species (hereafter, Holospora-specific OGs, HOGs), 97 of HOGs contained no paralogs. HOGs encoded transporters, regulators, and hypothetical proteins. We expect proteins essential for survival and adaptation to the host either to be located on the surface of the bacterial cell or to be secreted. Therefore, we focused on those proteins that contained transmembrane helices or signal peptides. Thirty seven HOGs contained proteins with predicted transmembrane helices; 12 other OGs, proteins with predicted classic signal peptides; eighteen OGs were predicted to be secreted by the non-classical pathway (Supplementary Table 3). One of the latter HOGs contained the 89 kDa periplasmic protein which has been reported to be associated with cell invasion in H. obtusa (Iwatani et al., 2005). One HOG contained proteins similar to OmpA. These proteins are highly conserved among Holospora, and all Holospora except H. curviuscula carry two paralogs of this gene.
FIGURE 6.
Groups of orthologous proteins in Holospora spp. and Rickettsiales. Numbers in cells indicate either the number of orthologous groups, or, in the case of unique genes, the number of singletons. Numbers in bold represent groups of orthologous groups common for either macronuclear (8) or all (102) Holospora. OGs common for the macronuclear endosymbionts are two OGs encoding alpha/beta hydrolases; one, a possible aspartate/glutamate racemase or a malate isomerase; one, nucleotide sugar epimerase; one OG had an unknown function; and three remaining OGs encode short proteins, of which one was similar to the DDE superendonuclease, and another one has a predicted signal peptide. Of 102 Holospora-specific OGs: 30 are secreted, 37 contain putative transmembrane helices; 17 HOGs contain short peptides, of which 5 are potentially secreted.
Holospora genomes encode numerous short (<100 aa) peptides with unknown functions. Short proteins have been shown to be involved in spore formation, regulation of transport, regulation of transcription, and signal transduction, or to possess antimicrobial or other toxic activities (Wang et al., 2008; Storz et al., 2014). Of the 102 HOGs, seventeen contained short proteins, including five HOGs that contained proteins with secretory signal peptide sequences. One of these HOGs has been described earlier as the 5.4 kDa protein involved in the switch between the reproductive and infectious forms (Dohra et al., 1997).
To determine whether there are proteins that could determine the nuclear specificity of Holospora, we searched for HOGs specific to the two macronuclear species, H. curviuscula and H. obtusa. We found eight HOGs present in both H. curviuscula and H. obtusa and absent in H. undulata, H. elegans and in other studied Rickettsiales. Of these, two HOGs encoded alpha/beta hydrolases; one, a possible aspartate/glutamate racemase or a maleate isomerase; one, nucleotide sugar epimerase; one HOG had an unknown function; and three remaining HOGs encoded short proteins, one of which was similar to DDE superendonuclease, and another one had a predicted signal peptide.
Discussion
Here we report a comparative analysis of the newly sequenced genome of H. curviuscula NRB217 and other available Holospora genomes. The phylogenetic analysis of all available Holospora has confirmed (Rautian and Wackerow-Kouzova, 2013) that they are clustered by the host species rather than by micro- vs. macronuclear specificity, so that H. curviuscula is the outgroup to the previously analyzed genomes. Furthermore, the fact that we found only one substitution in the 16S rRNA gene and few substitutions genome-wide between the sequenced samples of H. elegans and H. undulata suggests that these are in fact strains of the same species.
The previous genomic analysis of Holospora spp. (Dohra et al., 2014) included only a general description of the available COG categories and some reconstruction of the metabolism. It has been observed that many pathways are missing in Holospora. The pyruvate dehydrogenase complex that is present in Holospora has been proposed to be a possible relic of an ancestral pathway. Additionally, it has been suggested that Holospora strongly relies on the host for energy production.
Addition of H. curviuscula allows for a detailed comparative genomic analysis of the metabolism of Holospora spp. We found that all Holospora have reduced metabolic capacities and have to import many metabolites from the host.
Even though the genomes of Holospora are relatively large (Table 1) in comparison with other symbiotic species such as Buchnera, Candidatus Baumannia, or Candidatus Carsonella, Holospora are unable to produce most of the essential compounds. Furthermore, all available Holospora genomes seem to contain a large fraction of repetitive sequences, which complicates the genome assembly. Arguably, as all available Holospora genomes consist of multiple contigs, some of the enzymes could have been missed. However, quantitative analysis suggests that all universal genes are indeed present and that nearly all non-repetitive regions are contained in contigs; any potential missing part of the genome would have to be small, and cannot account for a large number of missing genes. Furthermore, our analysis is based on independent assemblies of multiple moderately related species, and it is unlikely that the same gene would be missing in several assemblies.
While smaller endosymbionts can produce at least some amino acids or retain some parts of the central metabolism, Holospora are unable to synthesize any amino acid, and have to acquire them from the host, despite the fact that some relics of pathways are seen, such as a partial pathway for the tryptophan synthesis. Holospora lack glycolysis, the Entner-Doudoroff pathway, and the pentose phosphate pathway. Surprisingly, Holospora have no enzymes of the citric acid cycle, which is unusual for Rickettsiales, as even the most reduced Rickettsia retain it.
Although we have not performed a complete metabolic reconstruction, it is evident that a broad range of compounds, including amino acids, DMAPP, and chorismate derivates have to be imported from the host for Holospora to survive. It is unlikely that these compounds are accumulated by Holospora during its brief residence outside the nucleus. Indeed, the process of Holospora infection is a rapid one, whereas Holospora stay in the nucleus for long periods of time, which means that all these compounds have to be acquired from the host. Amino acids can be obtained by degrading host nuclear proteins, and indeed the Holospora infection is associated with increased proteolytic activity in the macronucleus (Freiburg, 1985). The situation with other compounds is more intriguing. All these small molecules can passively diffuse through the nuclear pore complex (Knockenhauer and Schwartz, 2016). Further, Holospora alter the host’s gene expression and increase RNA synthesis (Freiburg, 1985; Fujishima, 2009). These alterations may help bacteria to acquire the necessary nutrients. To understand how the missing nutrients are delivered to Holospora, we searched for transport proteins and attempted to predict their specificity. This has explained some, but not all of the missing nutrients, and this topic has to be investigated further.
We propose that Holospora not only relies on the host for the energy production, but specifically use nucleotides or ribonucleotides as the energy source. Indeed, they are able to interconvert them and to convert UTP to CTP, and putative ribose transport proteins, which can also transport nucleotides, are present. We suggest that ribonucleotides are the preferred energy source, as the intracellular abundance of ribonucleotides can be 10- to 100-fold higher than that of dNTPs (Traut, 1994). This seems natural, given the nuclear habitat of Holospora.
The interactions between Holospora and their hosts have been studied in some depth. Holospora infection can either decrease or increase the Paramecium viability under a variety of conditions (Fujishima et al., 2005; Hori et al., 2008; Fujishima, 2009; Bella et al., 2016). Addition of Holospora curviuscula to the analysis allowed us not only to investigate proteins specific to Holospora in a particular host, but also to find proteins that may play a role in the macronucleus infection. While the details of the mechanism remain unclear, it is likely that the interaction and stable infection rely on Holospora-specific proteins, in particular, secreted short conserved peptides. The 89 kDa periplasmic protein shown to be involved in the H. obtusa infection (Iwatani et al., 2005) is conserved in all studied Holospora spp., which indicates its importance. Finally, we propose that OmpA-like proteins may be involved in the Holospora invasion.
Overall, the analysis of the Holospora genomes demonstrates that their metabolic capabilities are unusually restricted, especially given the genome size, and provides a list of candidate genes essential for their unique lifestyle.
Author Contributions
SG, MR, and MG designed the research. AB and MR isolated and cultivated bacteria. ML prepared the sequencing libraries and sequenced the genome. ML and SG assembled the genome. SG, AB, DM, and MG annotated the genome and performed the comparative analysis. SG, AB, MR, and MG wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Georgii Bazykin (IITP, Skoltech) for useful discussions. Scientific research was performed in part at the Research Park of the St. Petersburg State University Center for Molecular and Cell Technologies and the Center for Culturing Collection of Microorganisms. We are grateful to students of the Faculty of Bioengineering and Bioinformatics, Lomonosov Moscow State University (classes of 2016 and 2017) who have annotated fragments of the H. curviuscula genome as a part of the regular course work.
Funding. This study was supported by the Russian Science Foundation under grant 14-50-00150.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00738/full#supplementary-material
References
- Abamo F., Dohra H., Fujishima M. (2008). Fate of the 63-kDa periplasmic protein of the infectious form of the endonuclear symbiotic bacterium Holospora obtusa during the infection process. FEMS Microbiol. Lett. 280 21–27. 10.1111/j.1574-6968.2007.01023.x [DOI] [PubMed] [Google Scholar]
- Andersson S. G. E., Zomorodipour A., Andersson J. O., Sicheritz-Pontén T., Alsmark U. C. M., Podowski R. M., et al. (1998). The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396 133–140. 10.1038/24094 [DOI] [PubMed] [Google Scholar]
- Archibald J. M. (2015). Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 25 R911–R921. 10.1016/j.cub.2015.07.055 [DOI] [PubMed] [Google Scholar]
- Batut B., Knibbe C., Marais G., Daubin V. (2014). Reductive genome evolution at both ends of the bacterial population size spectrum. Nat. Rev. Microbiol. 12 841–850. 10.1038/nrmicro3331 [DOI] [PubMed] [Google Scholar]
- Becker M. H., Walke J. B., Murrill L., Woodhams D. C., Reinert L. K., Rollins-Smith L. A., et al. (2015). Phylogenetic distribution of symbiotic bacteria from Panamanian amphibians that inhibit growth of the lethal fungal pathogen Batrachochytrium dendrobatidis. Mol. Ecol. 24 1628–1641. 10.1111/mec.13135 [DOI] [PubMed] [Google Scholar]
- Bella C., Koehler L., Grosser K., Berendonk T. U., Petroni G., Schrallhammer M. (2016). Fitness impact of obligate intranuclear bacterial symbionts depends on host growth phase. Front. Microbiol. 7:2084. 10.3389/fmicb.2016.02084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen J. D., Kiemer L., Fausbøll A., Brunak S. (2005a). Non-classical protein secretion in bacteria. BMC Microbiol. 5:58. 10.1186/1471-2180-5-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen J. D., Nielsen H., Widdick D., Palmer T., Brunak S. (2005b). Prediction of twin-arginine signal peptides. BMC Bioinformatics 6:167. 10.1186/1471-2105-6-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. M., Moran N. A. (2013). Small, smaller, smallest: the origins and evolution of ancient dual symbioses in a Phloem-feeding insect. Genome Biol. Evol. 5 1675–1688. 10.1093/gbe/evt118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D. J., Ostell J., et al. (2013). GenBank. Nucleic Acids Res. 41 D36–D42. 10.1093/nar/gks1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscaro V., Kolisko M., Felletti M., Vannini C., Lynn D. H., Keeling P. J. (2017). Parallel genome reduction in symbionts descended from closely related free-living bacteria. Nat. Ecol. Evol. 1 1160–1167. 10.1038/s41559-017-0237-0 [DOI] [PubMed] [Google Scholar]
- Caspi R., Billington R., Ferrer L., Foerster H., Fulcher C. A., Keseler I. M., et al. (2016). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 44 D471–D480. 10.1093/nar/gkv1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C., Welburn S. C. (2001). The endosymbionts of tsetse flies: manipulating host–parasite interactions. Int. J. Parasitol. 31 628–631. 10.1016/S0020-7519(01)00151-5 [DOI] [PubMed] [Google Scholar]
- Darling A. E., Mau B., Perna N. T. (2010). progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohra H., Suzuki H., Suzuki T., Tanaka K., Fujishima M. (2013). Draft genome sequence of Holospora undulata strain hu1, a micronucleus-specific symbiont of the ciliate Paramecium caudatum. Genome Announc. 1:e664-13. 10.1128/genomeA.00664-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohra H., Tanaka K., Suzuki T., Fujishima M., Suzuki H. (2014). Draft genome sequences of three Holospora species (Holospora obtusa, Holospora undulata, and Holospora elegans), endonuclear symbiotic bacteria of the ciliate Paramecium caudatum. FEMS Microbiol. Lett. 359 16–18. 10.1111/1574-6968.12577 [DOI] [PubMed] [Google Scholar]
- Dohra H., Yamamoto K., Fujishima M., Ishikawa H. (1997). Cloning and sequencing of gene coding for a periplasmic 5.4 kDa peptide of the macronucleus-specific symbiont Holospom obtusa of the ciliate Paramecium caudatum. Zoolog. Sci. 14 69–75. 10.2108/zsj.14.69 [DOI] [PubMed] [Google Scholar]
- Driscoll T. P., Verhoeve V. I., Guillotte M. L., Lehman S. S., Rennoll S. A., Beier-Sexton M., et al. (2017). Wholly Rickettsia! Reconstructed metabolic profile of the quintessential bacterial parasite of eukaryotic cells. mBio 8:e859-17. 10.1128/mBio.00859-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferla M. P., Thrash J. C., Giovannoni S. J., Patrick W. M. (2013). New rRNA gene-based phylogenies of the Alphaproteobacteria provide perspective on major groups, mitochondrial ancestry and phylogenetic instability. PLoS One 8:e83383. 10.1371/journal.pone.0083383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokin S. I., Görtz H.-D. (2009). “Diversity of Holospora bacteria in Paramecium and their characterization,” in Endosymbionts in Paramecium Microbiology Monographs, ed. Fujishima M. (Berlin: Springer; ), 161–199. 10.1007/978-3-540-92677-1_7 [DOI] [Google Scholar]
- Freiburg M. (1985). Isolation and characterization of macronuclei of Paramecium caudatum infected with the macronucleus-specific bacterium Holospora obtusa. J. Cell Sci. 73 389–398. [DOI] [PubMed] [Google Scholar]
- Fujishima M. (2009). “Infection and maintenance of Holospora species in Paramecium caudatum,” in Endosymbionts in Paramecium, ed. Fujishima M. (Berlin: Springer; ), 201–225. 10.1007/978-3-540-92677-1_8 [DOI] [Google Scholar]
- Fujishima M., Dohra H., Kawai M. (1997). Quantitative changes in periplasmic proteins of the macronucleus-specific bacterium Holospora obtusa in the infection process of the ciliate Paramecium caudatum. J. Eukaryot. Microbiol. 44 636–642. 10.1111/j.1550-7408.1997.tb05971.x [DOI] [PubMed] [Google Scholar]
- Fujishima M., Kawai M., Yamamoto R. (2005). Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol. Lett. 243 101–105. 10.1016/j.femsle.2004.11.053 [DOI] [PubMed] [Google Scholar]
- Fuxelius H.-H., Darby A., Min C.-K., Cho N.-H., Andersson S. G. E. (2007). The genomic and metabolic diversity of Rickettsia. Res. Microbiol. 158 745–753. 10.1016/j.resmic.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Georgiades K., Merhej V., Karkouri K. E., Raoult D., Pontarotti P. (2011). Gene gain and loss events in Rickettsia and Orientia species. Biol. Direct 6:6. 10.1186/1745-6150-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. J., Kaur S. J., Rahman M. S., Rennoll-Bankert K., Sears K. T., Beier-Sexton M., et al. (2015). Secretome of obligate intracellular Rickettsia. FEMS Microbiol. Rev. 39 47–80. 10.1111/1574-6976.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. J., Phan I. Q. H., Driscoll T. P., Guillotte M. L., Lehman S. S., Rennoll-Bankert K. E., et al. (2016). The Rickettsia type IV secretion system: unrealized complexity mired by gene family expansion. Pathog. Dis. 74:ftw058. 10.1093/femspd/ftw058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görtz H. D., Fujishima M. (1983). Conjugation and meiosis of Paramecium caudatum infected with the micronucleus-specific bacterium Holospora elegans. Eur. J. Cell Biol. 32 86–91. [PubMed] [Google Scholar]
- Görtz H.-D., Schmidt H. J. (2015). “Holospora,” in Bergey’s Manual of Systematics of Archaea and Bacteria. Hoboken, NJ: John Wiley & Sons, Ltd. [Google Scholar]
- Gromov B. V., Ossipov D. V. (1981). Holospora (ex Hafkine 1890) nom. rev., a genus of bacteria inhabiting the nuclei of paramecia. Int. J. Syst. Evol. Microbiol. 31 348–352. 10.1099/00207713-31-3-348 [DOI] [Google Scholar]
- Guindon S., Dufayard J.-F., Lefort V., Anisimova M., Hordijk W., Gascuel O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Hedges L. M., Brownlie J. C., O’Neill S. L., Johnson K. N. (2008). Wolbachia and virus protection in insects. Science 322:702. 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Hori M., Fujii K., Fujishima M. (2008). Micronucleus-specific bacterium Holospora elegans irreversibly enhances stress gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 55 515–521. 10.1111/j.1550-7408.2008.00352.x [DOI] [PubMed] [Google Scholar]
- Horst J.-P., Wu T., Marinus M. G., Horst J.-P., Wu T., Marinus M. G., et al. (1999). Escherichia coli mutator genes. Trends Microbiol. 7 29–36. 10.1016/S0966-842X(98)01424-3 [DOI] [PubMed] [Google Scholar]
- Hotopp J. C. D., Lin M., Madupu R., Crabtree J., Angiuoli S. V., Eisen J., et al. (2006). Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2:e21. 10.1371/journal.pgen.0020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatani K., Dohra H., Lang B. F., Burger G., Hori M., Fujishima M. (2005). Translocation of an 89-kDa periplasmic protein is associated with Holospora infection. Biochem. Biophys. Res. Commun. 337 1198–1205. 10.1016/j.bbrc.2005.09.175 [DOI] [PubMed] [Google Scholar]
- Janda J. M., Abbott S. L. (2007). 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J. Clin. Microbiol. 45 2761–2764. 10.1128/JCM.01228-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M., Minamisawa K. (2010). Plant–microbe communications for symbiosis. Plant Cell Physiol. 51 1377–1380. 10.1093/pcp/pcq125 [DOI] [PubMed] [Google Scholar]
- Knockenhauer K. E., Schwartz T. U. (2016). The nuclear pore complex as a flexible and dynamic gate. Cell 164 1162–1171. 10.1016/j.cell.2016.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch J., Stremmel M., Breiner H.-W., Adams V., Schweikert M., Schmidt H. J. (2000). The toxic symbiontCaedibacter caryophila in the cytoplasm of Paramecium novaurelia. Microb. Ecol. 40 330–335. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett R. M., Clavijo B. J., Clissold L., Clark M. D., Caccamo M. (2014). NextClip: an analysis and read preparation tool for Nextera Long Mate Pair libraries. Bioinformatics 30 566–568. 10.1093/bioinformatics/btt702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Stoeckert C. J., Roos D. S. (2003). OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13 2178–2189. 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H., Sung W., Kucukyildirim S., Williams E., Miller S. F., Guo W., et al. (2018). Evolutionary determinants of genome-wide nucleotide composition. Nat. Ecol. Evol. 2 237–240. 10.1038/s41559-017-0425-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Hulcr J., Sun J. (2016). The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 47 487–505. 10.1146/annurev-ecolsys-121415-032050 [DOI] [Google Scholar]
- Marais G. A. B., Calteau A., Tenaillon O. (2008). Mutation rate and genome reduction in endosymbiotic and free-living bacteria. Genetica 134 205–210. 10.1007/s10709-007-9226-6 [DOI] [PubMed] [Google Scholar]
- Martínez-Cano D. J., Reyes-Prieto M., Martinez-Romero E., Partida-Martínez L. P., Latorre A., Moya A., et al. (2015). Evolution of small prokaryotic genomes. Front. Microbiol. 5:742 10.3389/fmicb.2014.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon J. P., Moran N. A. (2010). Functional convergence in reduced genomes of bacterial symbionts spanning 200 million years of evolution. Genome Biol. Evol. 2 708–718. 10.1093/gbe/evq055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon J. P., Moran N. A. (2012). Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol. 10 13–26. 10.1038/nrmicro2670 [DOI] [PubMed] [Google Scholar]
- Mistry J., Finn R. D., Eddy S. R., Bateman A., Punta M. (2013). Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 41:e121. 10.1093/nar/gkt263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A., Degnan P. H., Santos S. R., Dunbar H. E., Ochman H. (2005). The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc. Natl. Acad. Sci. U.S.A. 102 16919–16926. 10.1073/pnas.0507029102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. (2017). Predicting secretory proteins with SignalP. Methods Mol. Biol. 1611 59–73. 10.1007/978-1-4939-7015-5_6 [DOI] [PubMed] [Google Scholar]
- Oulhen N., Schulz B. J., Carrier T. J. (2016). English translation of heinrich anton de Bary’s 1878 speech, ‘Die Erscheinung der Symbiose’ (‘De la symbiose’). Symbiosis 69 131–139. 10.1007/s13199-016-0409-8 [DOI] [Google Scholar]
- Overbeek R., Begley T., Butler R. M., Choudhuri J. V., Chuang H.-Y., Cohoon M., et al. (2005). The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 33 5691–5702. 10.1093/nar/gki866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Noh S. M. (2012). Rickettsial entry into host cells: finding the keys to unlock the doors. Infect. Immun. 80 3746–3747. 10.1128/IAI.00836-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautian M. S., Wackerow-Kouzova N. D. (2013). Phylogenetic placement of two previously described intranuclear bacteria from the ciliate Paramecium bursaria (Protozoa, Ciliophora): “Holospora acuminata” and “Holospora curviuscula.” Int. J. Syst. Evol. Microbiol. 63 1930–1933. 10.1099/ijs.0.046631-0 [DOI] [PubMed] [Google Scholar]
- Rennoll-Bankert K. E., Rahman M. S., Gillespie J. J., Guillotte M. L., Kaur S. J., Lehman S. S., et al. (2015). Which way in? The RalF Arf-GEF orchestrates Rickettsia host cell invasion. PLoS Pathog. 11:e1005115. 10.1371/journal.ppat.1005115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restif O., Kaltz O. (2006). Condition-dependent virulence in a horizontally and vertically transmitted bacterial parasite. Oikos 114 148–158. 10.1111/j.2006.0030-1299.14611.x [DOI] [Google Scholar]
- Rinke C., Schwientek P., Sczyrba A., Ivanova N. N., Anderson I. J., Cheng J.-F., et al. (2013). Insights into the phylogeny and coding potential of microbial dark matter. Nature 499 431–437. 10.1038/nature12352 [DOI] [PubMed] [Google Scholar]
- Sabaneyeva E. V., Derkacheva M. E., Benken K. A., Fokin S. I., Vainio S., Skovorodkin I. N. (2009). Actin-based mechanism of Holospora obtusa trafficking in Paramecium caudatum. Protist 160 205–219. 10.1016/j.protis.2008.11.006 [DOI] [PubMed] [Google Scholar]
- Sabater-Muñoz B., Toft C., Alvarez-Ponce D., Fares M. A. (2017). Chance and necessity in the genome evolution of endosymbiotic bacteria of insects. ISME J. 11 1291–1304. 10.1038/ismej.2017.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Reddy V. S., Tsu B. V., Ahmed M. S., Li C., Moreno-Hagelsieb G. (2016). The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 44 D372–D379. 10.1093/nar/gkv1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikert M., Fujishima M., Görtz H.-D. (2013). “Symbiotic Associations Between Ciliates and Prokaryotes,” in The Prokaryotes. Berlin: Springer, 427–463. 10.1007/978-3-642-30194-0_18 [DOI] [Google Scholar]
- Seemann T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- Storz G., Wolf Y. I., Ramamurthi K. S. (2014). Small proteins can no longer be ignored. Annu. Rev. Biochem. 83 753–777. 10.1146/annurev-biochem-070611-102400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szokoli F., Castelli M., Sabaneyeva E., Schrallhammer M., Krenek S., Doak T. G., et al. (2016). Disentangling the taxonomy of Rickettsiales and description of two novel symbionts (“Candidatus Bealeia paramacronuclearis” and “Candidatus Fokinia cryptica”) sharing the cytoplasm of the ciliate protist Paramecium biaurelia. Appl. Environ. Microbiol. 82 7236–7247. 10.1128/AEM.02284-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. (1994). Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140 1–22. 10.1007/BF00928361 [DOI] [PubMed] [Google Scholar]
- van Heel A. J., de Jong A., Montalbán-López M., Kok J., Kuipers O. P. (2013). BAGEL3: automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 41 W448–W453. 10.1093/nar/gkt391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini C., Ferrantini F., Ristori A., Verni F., Petroni G. (2012). Betaproteobacterial symbionts of the ciliate Euplotes: origin and tangled evolutionary path of an obligate microbial association. Environ. Microbiol. 14 2553–2563. 10.1111/j.1462-2920.2012.02760.x [DOI] [PubMed] [Google Scholar]
- Vannini C., Sigona C., Hahn M., Petroni G., Fujishima M. (2017). High degree of specificity in the association between symbiotic betaproteobacteria and the host Euplotes (Ciliophora, Euplotia). Eur. J. Protistol. 59 124–132. 10.1016/j.ejop.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Wang F., Xiao J., Pan L., Yang M., Zhang G., Jin S., et al. (2008). A systematic survey of mini-proteins in bacteria and archaea. PLoS One 3:e4027. 10.1371/journal.pone.0004027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Li X., Wang Z. (2016). APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44 D1087–D1093. 10.1093/nar/gkv1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Blin K., Duddela S., Krug D., Kim H. U., Bruccoleri R., et al. (2015). antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 43 W237–W243. 10.1093/nar/gkv437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. L., Wang J., Aksoy S. (2011). Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 9:e1000619. 10.1371/journal.pbio.1000619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler A. G., Bao Y., Whitney J. C., Bobay L.-M., Xavier J. B., Schofield W. B., et al. (2016). Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc. Natl. Acad. Sci. U.S.A. 113 3639–3644. 10.1073/pnas.1525637113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., McNulty N. P., Rodionov D. A., Khoroshkin M. S., Griffin N. W., Cheng J., et al. (2015). Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350:aac5992. 10.1126/science.aac5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yañez O., Gauthier L., Chantawannakul P., Neumann P. (2016). Endosymbiotic bacteria in honey bees: Arsenophonus spp. are not transmitted transovarially. FEMS Microbiol. Lett. 363:fnw147. 10.1093/femsle/fnw147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang Y., Lynch K. H., Dennis J. J., Wishart D. S. (2011). PHAST: a fast phage search tool. Nucleic Acids Res. 39 W347–W352. 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Oldroyd G. E. D. (2017). Plant signalling in symbiosis and immunity. Nature 543 328–336. 10.1038/nature22009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.