Abstract
Background:
We conducted our first patient survey at the 2013 hereditary angioedema (HAE) patient summit and learned that, despite several novel therapies, the burden of disease was high.
Objective:
To determine, from the patient's perspective, if any improvements in the current state of HAE care occurred over a two-year period between HAE patient summits.
Methods:
A patient survey was conducted at the 2015 Hereditary Angioedema Association conference by using paper surveys that aimed at understanding the current state of HAE care. Questions included patient characteristics, burden of disease, and satisfaction with care and treatment options. Comparisons between patients with HAE with C1-inhibitor (HAE-C1INH) and patients with HAE with normal C1-inhibitor (HAE-nlC1INH), as well as between patients with HAE in 2013 and 2015, were performed by using χ2 tests.
Results:
There were 232 surveys distributed, and 143 surveys were identified as complete for inclusion and analysis from patients with self-reported HAE. Most patients had type I or type II HAE (67.5% [n = 106]), with a smaller number of patients with HAE-nlC1INH (23.6% [n = 37]). In 2015, almost half of the patients with HAE-C1INH (47.1%) and 56.7% of the patients with HAE-nlC1INH experienced a delay of ≥10 years between initial symptoms and diagnosis. Among the patients with HAE-C1INH, 25% reported one or more attacks per week and another 48% reported experiencing one or more attacks per month (fewer than one attack per week). The patients with HAE-nlC1INH reported attacks more frequently than did the patients with HAE-C1INH (p = 0.002), with 59.5% who reported attacks at least once a week. Emergency care was reported one or more times per month in 5% of the patients with HAE-C1INH and in 24.3% of the patients with HAE-nlC1INH.
Conclusion:
Similar to 2013, although significant progress has been made, there is still a high burden of disease that faces patients with HAE.
Keywords: Angioedema, swelling, quality of life, survey, C1 inhibitor, burden of disease, prophylaxis, satisfaction
Hereditary angioedema (HAE) is a rare autosomal dominant disorder that results from mutations in alleles encoding for C1-inhibitor (C1-INH). Individuals with HAE present with recurrent episodes of swelling that most commonly affects the extremities, gastrointestinal tract, face, or larynx. Most angioedema attacks are self-limiting, but abdominal attacks cause severe pain, nausea, and vomiting; swelling that affects the throat or larynx may be fatal due to asphyxiation. HAE is classified as either type I or type II; both forms have a dysfunctional C1-INH protein. In 2000, HAE with normal C1-INH (HAE-nlC1INH), previously referred to as type III HAE, was initially described and remains poorly understood.1,2 Clinical symptoms of HAE-nlC1INH are indistinguishable from HAE type I and II (HAE-C1INH). However, HAE-C1INH patients have normal plasma levels of functional C1INH and complement levels.
In 2013, with several novel HAE therapies available (Cinryze, Berinert, Ecallantide and Icatibant), we conducted our first patient survey at a HAE patient summit.3 We learned that the majority of patients had access to effective on-demand treatment options at home, with an emergency plan of care in place. This represented significant progress compared with an historical lack of effective HAE acute therapy in the United States and underscored the impact of recent clinical advances and publications in the HAE field at that time. Although our initial study was not designed to specifically assess quality of life, it seemed that, compared with previous years, although more frequent attacks are associated with lower quality of life,4,5 the patients with HAE had better access to effective care and were more satisfied overall with the management of their condition.6,7 Further improvements are clearly needed, especially in the care of patients with HAE-nlC1INH. Our findings highlighted several continued difficulties faced by patients with HAE, including long delays in diagnosis, dissatisfaction with care during emergency department (ED) visits, and breakthrough attacks, despite prophylactic treatment, which emphasizes the need for continued research and educational efforts aimed at decreasing the burden of disease.
We repeated our patient survey, 2 years later, at the next HAE patient summit in 2015 to determine if there were any subsequent improvements in either quality of life or the current state of HAE care from the patient perspective. In this 2-year period, there was one additional U.S. Food and Drug Administration–approved therapy for acute treatment of HAE attacks (recombinant C1 esterase inhibitor). Several guidelines and publications that focused on improving care were developed, which we hypothesized would translate into improved HAE management and a reduction in the burden of disease.8–10
METHODS
Questionnaire
Similar to the 2013 survey, questions were developed in collaboration with HAE experts (A.B., P.B., M.R., S.C.) to characterize the current state of HAE care. Questions fell into several broad categories, including patient characteristics, burden of disease, satisfaction with care, and treatment options, including on-demand versus prophylactic treatment.
Data Collection
Data for this study were collected during one session of the 2015 U.S. Hereditary Angioedema Association (HAEA) National Patient Summit, held in Denver, Colorado, in October 2015. At the 2015 HAEA conference, paper surveys were distributed to patients who were attending the conference; patients were asked to fill out the survey and return it to a collection bin. This was in contrast to the 2013 HAEA conference where participant responses were captured by using an audience response system (Padgett Communications, Tampa, FL). The survey was optional, the patients who attended the summit were self-selected, and all data, including HAE diagnosis, were self-reported. The purpose of the data collection was explained to patients by the investigators before completion of the survey, and patients were encouraged to completely fill out the survey to the best of their ability. The paper survey responses were coded and data were analyzed at Massachusetts General Hospital. Incomplete surveys were excluded from analysis. The Partners Human Research Committee granted a waiver for this study because no identifying information was in the survey.
Statistical Analysis
Data were presented in frequencies and percentages. To make data statistically comparable between the 2013 and 2015 questionnaires, all percentages presented in this article included missing values if there were any associated with a specific question. The number of missing values varied with each question, and the complete data are summarized in the tables. The χ2 tests were performed among participants who completed the corresponding questions. To ensure that there was no bias associated with missing responses, we performed sensitivity analyses, in which “missing” was included as a category. By using sensitivity analysis (to include missing data), none of the results that compared HAE-C1INH and HAE-nlC1INH were different.
When comparing 2013 questionnaire responses with 2015, due to a higher response rate without missing data in 2015 compared with 2013, responses to three questions were significantly different among the patients with HAE-C1INH only if missing responses were included: the time between the first attack and a diagnosis, the frequency of HAE attacks during the past year, and if physicians discussed a treatment plan in a life-threatening HAE attack. After a deeper investigation into these questions, we observed that the participants who responded to these questions had almost identical distribution of responses and that the missing responses were not from the same group of participants. Thus, we did not expect bias concerning missing responses from respondents. Comparisons of demographics, burden of disease, satisfaction with care, and treatment options between patients with HAE-C1INH and patients with HAE-nlC1INH, as well as between patients with HAE in 20133 and 2015, were performed by using χ2 tests. A two-sided p value of <0.05 was considered to be significant. All analyses were performed by using SAS 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 232 surveys sequentially numbered and distributed to patients at the 2015 HAEA summit. Of these, 169 were returned to the investigators. Twelve of these surveys were excluded: four due to lack of completion (i.e., only the first page of the questions was answered) and eight due to reliability concerns (i.e., multiple and/or no answers circled to many questions, directions not followed, written comments such as “I am not sure what you mean”). In sum, 157 surveys were identified as complete for inclusion. The patients who reported their HAE type as “other or I do not know” were excluded from analysis due to reliability of categorization concerns (8.9% [n = 14]). The patients who self-reported type I HAE or type II HAE were grouped together (67.5% [n = 106]) and were referred to, in the survey, as having HAE-C1INH. The patients with HAE-C1INH were analyzed separately from the patients with HAE-nlC1INH (23.6% [n = 37]). Overall, 143 surveys from patients with self-reported HAE were included in the data analysis. The 2013 HAEA patient summit data3 included 186 patients with HAE, of whom 80% were self-reported as having HAE-C1INH and 20% as having HAE-nlC1INH.
Patient Characteristics
The patients with HAE-C1INH who attended the 2015 HAEA conference and completed this survey varied in age from adolescents (0–15 years of age [6.6%]) through senior citizens (>65 years of age [8.5%]). Most patients were between 46 and 65 years old (46–55 years old [23.6%], 56–65 years old [22.6%]), and younger adults, between 16 and 45 years of age, comprised 38.7% of the sample. Most patients with HAE-nlC1INH were between 46 and 65 years old, and were, on average, older than the patients with HAE-C1INH who attended the 2015 conference (p = 0.02) (Table 1). Almost half of the patients with HAE-C1INH (47.2%) lived in cities (population of 100,000 to 1 million) or large metropolitan areas (population of >1 million), whereas 23.6% lived in rural areas (population of <20,000) and 29.2% in small towns (population, 20,000–10,000). The age distribution and geographic distribution of patients with HAE-C1INH between the 2013 and 2015 attendees was similar. In contrast, whereas the patients with HAE-nlC1INH had similar age distribution between the 2 survey years, their geographic distribution was dissimilar, with more patients from small towns and cities in 2015 compared with 2013 (p = 0.04).
Table 1.
Characteristics of patients with HAE-C1INH and patients with HAE-nlC1INH
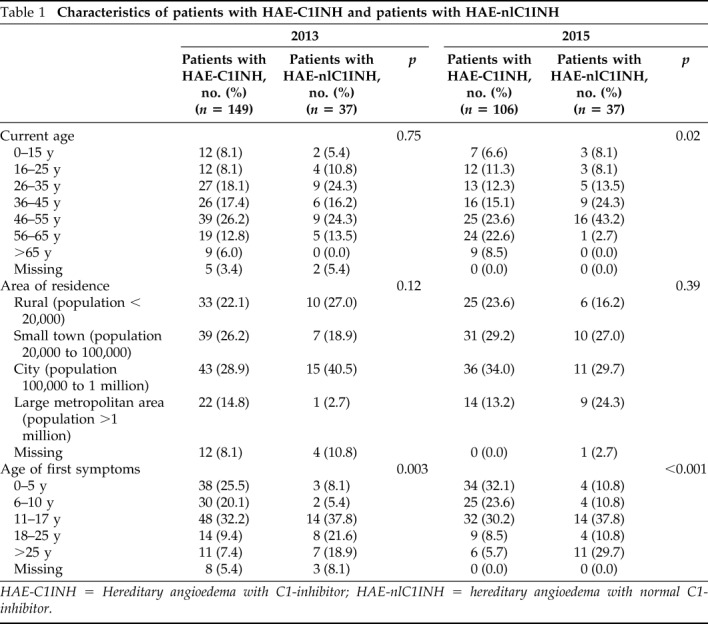
HAE-C1INH = Hereditary angioedema with C1-inhibitor; HAE-nlC1INH = hereditary angioedema with normal C1-inhibitor.
In the 2015 survey, 81.1% of the patients with HAE-C1INH and 91.9% of the patients with HAE-nlC1INH reported that an allergist/immunologist was their main HAE health care provider. A majority of the patients with HAE-C1INH (85.9%) experienced their first HAE symptoms before the age of 18 years. Patients with HAE-nlC1INH were generally older when they experienced their first angioedema symptoms compared with the patients with HAE-C1INH, with only 59.4% who experienced their first symptoms before age 18 years (p = 0.003). There were no differences seen from 2013 when the majority of the patients with HAE surveyed also had an allergist/immunologist as the primary health care provider and reported a similar age of first angioedema symptoms.
Burden of Disease
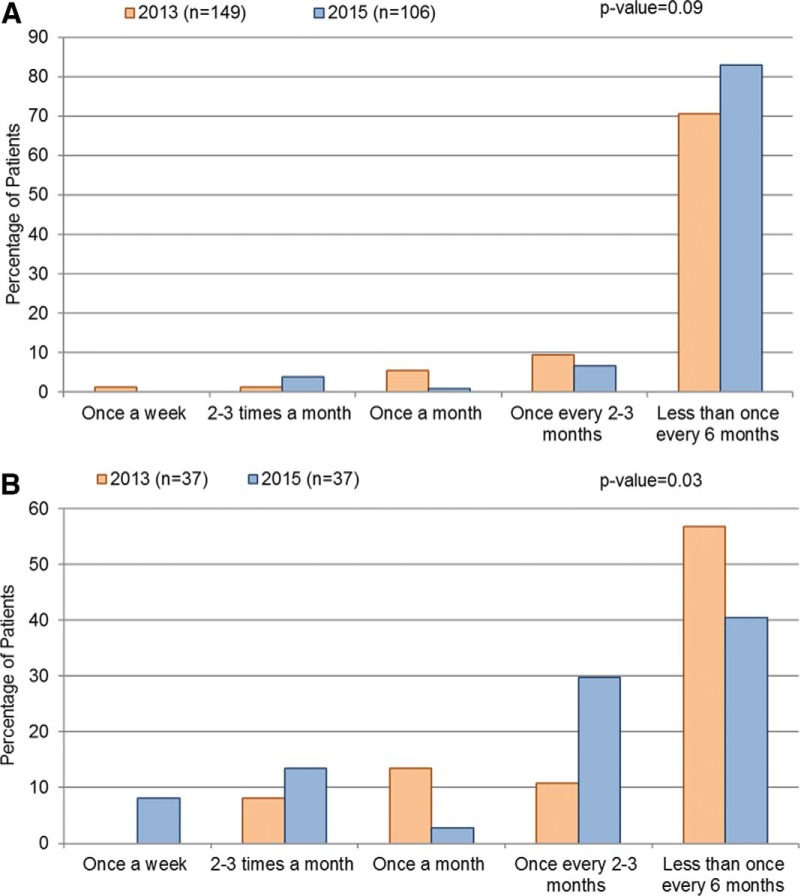
In 2015, one-fourth of the patients with HAE-C1INH were diagnosed within 1 year of onset of HAE symptoms, but almost half (47.1%) experienced a delay of ≥10 years between the initial symptoms and diagnosis. In 2013, 28.1% of patients had received a diagnosis within 1 year of their first HAE attack and 28.2% experienced a delay of ≥10 year between onset and diagnosis. For patients with HAE-nlC1INH, 18.9% were diagnosed within 1 year of symptoms onset, but 56.7% experienced a delay of ≥10 years. For the patients with HAE-nlC1INH, there was a significantly longer time to diagnosis compared with the patients with HAE-C1INH in 2013 (p = 0.02) but a similar delay in 2015 (p = 0.38) (Fig 1).
Figure 1.
The time between the first hereditary angioedema (HAE) attack and diagnosis (a) in 2013 and (b) in 2015. The comparisons between data of patients with HAE with C1-inhibitor (HAE-C1INH) and the patients with HAE with normal C1-inhibitor (HAE-nlC1INH) were conducted by using the χ2 test.
In the 2015 survey, among the patients with HAE-C1INH, 25% reported one or more attacks per week, 48% reported experiencing one or more attacks per month (but fewer than one per week), and 26% reported experiencing attacks fewer than once a month (Fig 2a). This overall attack frequency reported in 2015 was similar to data from 2013 (p = 0.06). In 2015, the patients with HAE-nlC1INH reported attacks more frequently than did the patients with HAE-C1INH (p = 0.002), with 59.5% reporting attacks at least once a week. Overall, the attack frequency in the patients with HAE-nlC1INH was similar in 2013 and 2015 (p = 0.31) (Fig 2b).
Figure 2.
Frequencies of hereditary angioedema (HAE) attacks reported during the past 1 year (a) among the patients with HAE with C1-inhibitor (HAE-C1INH) and (b) among the patients with HAE with normal C1-inhibitor (HAE-nlC1INH). The comparisons between 2013 and 2015 data were conducted by using the χ2 test.
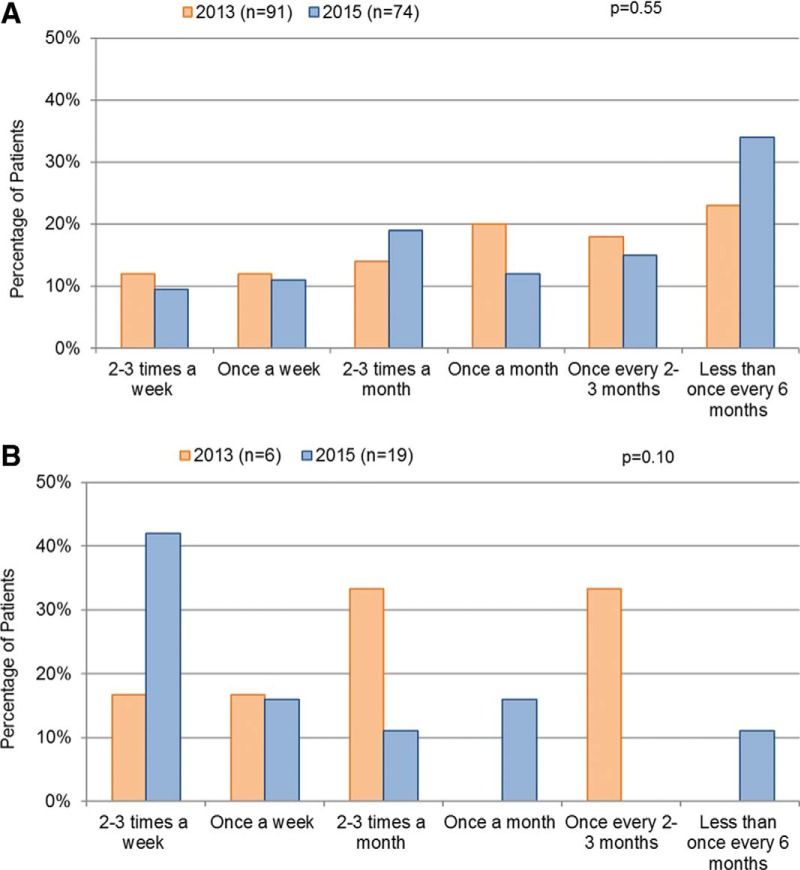
The patients were asked to estimate, on average, how frequently they went to the urgent care center or the ED, or were hospitalized for HAE. Among the patients with HAE-C1INH, 83% reported emergency care fewer times than once every 6 months, and 5% reported emergency care more than once a month. Similarly, in 2013, 71% of the patients with HAE-C1INH reported going to the ED or being hospitalized fewer than once every 6 months, with only a small minority (8%) averaging one or more visits per month. However, the 2013 survey did not ask about urgent care centers. In the current survey, 24.3% of the patients with HAE-nlC1INH reported emergency care at least once a month, 29.7% reported once every 2–3 months, and 40.5% reported fewer than once every 6 months. Compared with 2013, in 2015, the patients with HAE-nlC1INH sought out emergency care more frequently (p = 0.05) (Fig 3).
Figure 3.
The frequency of urgent care center, emergency department, or hospitalization visits for hereditary angioedema (HAE) attacks among (a) the patients with HAE with C1-inhibitor (HAE-C1INH) and (b) the patients with HAE with normal C1-inhibitor (HAE-nlC1INH). The comparisons between 2013 and 2015 data were conducted by using the χ2 test.
Satisfaction with Care
In 2015, of the patients with HAE-C1INH, 46% reported that they were not satisfied with the care they received in the emergency setting; however, in 2013, 62% of patients reported that they were not satisfied (p = 0.01) (Table 2). Most of the patients with HAE-nlC1INH (70%) were not satisfied with their ED care; this number was not significantly different from 2013 (77%). Although most patients were unsatisfied with their emergency care, 90% of the patients with HAE-C1INH reported that they were overall happy with the care that their physician gave to help manage their HAE; this has increased from 70% in 2013 (p = 0.003). A majority of patients with HAE-nlC1INH reported that they were happy with care from their physician, again, significantly higher than in 2013 (81 versus 41%; p = 0.007). In 2015, most patients with HAE-C1INH reported being “very satisfied” or “moderately satisfied” with current on-demand and prophylactic treatment options. A significantly lower number of patients with HAE-nlC1INH were happy or satisfied with emergency care (p = 0.04), the physician who provided care (p = 0.04) on demand (p = 0.02), and prophylactic treatment options (p = 0.002) compared with the patients with HAE-C1INH in the current survey.
Table 2.
Satisfaction with care in the patients with HAE-C1INH and the patients with HAE-nlC1INH

HAE-C1INH = Hereditary angioedema with C1-inhibitor; HAE-nlC1INH = hereditary angioedema with normal C1-inhibitor; HAE = hereditary angioedema.
*Only included participants who reported using on-demand medication, including 94 patients with HAE-C1INH and 35 patients with HAE-nlC1INH.
#Only included participants who reported using prophylactic treatment, including 75 patients with HAE-C1INH and 19 patients with HAE-nlC1INH.
Treatment Options
In 2015, 75% of the patients with HAE-C1INH and 73% of the patients with HAE-nlC1INH discussed an individual treatment plan with their physician for life-threatening HAE attacks (Table 3). In 2013, a smaller percentage of the patients with HAE-C1INH (66%) and those with HAE-nlC1INH (43%) reported developing individual treatment plans with their physicians. In 2015, 89% of the patients with HAE-C1INH had access to on-demand medication, and, of these, 96% had on-demand medication available at home. The patients with HAE-C1INH on-demand availability and use were similar between 2013 and 2015, whereas the patients with HAE-nlC1INH had increased access to on-demand care from 2013 to 2015 (68 versus 95%; p = 0.02). More than half of the patients with HAE-C1INH (55%) and 48.6% of the patients with HAE-nlC1INH used on-demand medication ≥90% of the time to treat attacks.
Table 3.
Treatment and management in the patients with HAE-C1INH and patients with HAE-nlC1INH
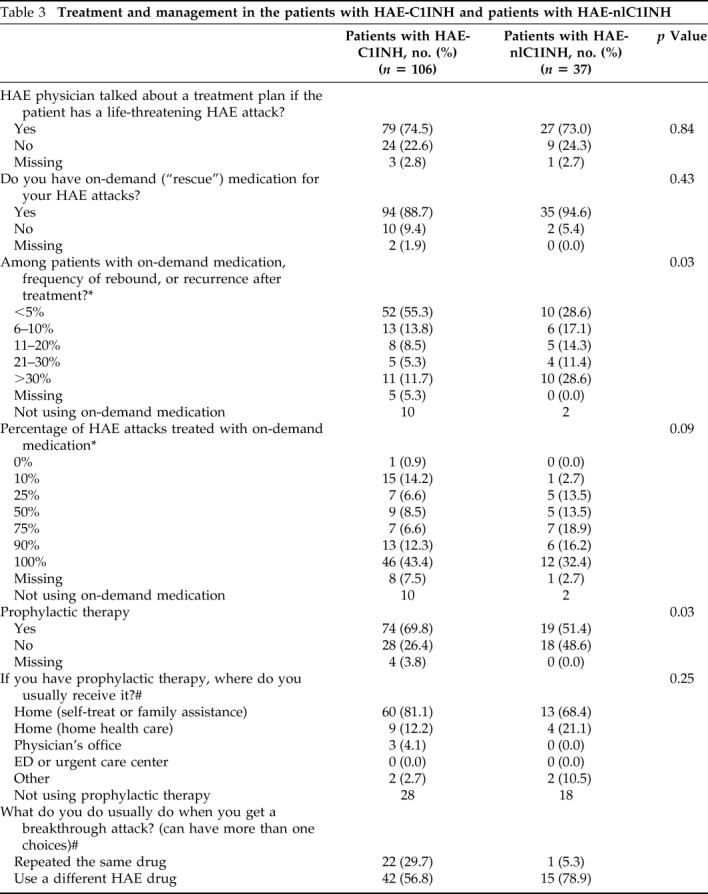
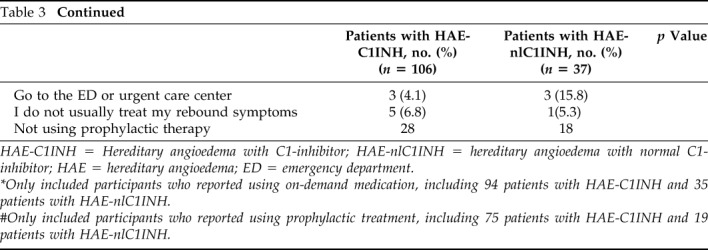
HAE-C1INH = Hereditary angioedema with C1-inhibitor; HAE-nlC1INH = hereditary angioedema with normal C1-inhibitor; HAE = hereditary angioedema; ED = emergency department.
*Only included participants who reported using on-demand medication, including 94 patients with HAE-C1INH and 35 patients with HAE-nlC1INH.
#Only included participants who reported using prophylactic treatment, including 75 patients with HAE-C1INH and 19 patients with HAE-nlC1INH.
A rebound and/or recurrence of symptoms after on-demand treatment was relatively rare, with 55% of the patients with HAE-C1INH reported to have experienced rebound symptoms ≤5% of the time, and only 12% reported rebound symptoms >30% of the time. However, when rebound symptoms occurred, almost all of the patients with HAE-C1INH treated the symptoms; only 9% did not treat rebound symptoms, 34% treated symptoms by using the same drug used for on-demand care, and 17% used a different HAE drug. In the 2015 survey, 28.6% of the patients with HAE-nlC1INH reported rebound symptoms <5% of the time. Among the patients with HAE-nlC1INH, 20% did not treat rebound symptoms, 60% reused the same HAE drug, and 14% opted for a different HAE drug.
The majority of the patients with HAE-C1INH (70%) were using prophylactic therapy (Table 3). Among the patients with HAE-C1INH who received prophylaxis, 93% received treatment in their home, either by self or family administration of medication, or via home health care visits; these results were comparable with the 2013 information.3 More than one-third (39%) reported that they were “very satisfied” with their current prophylactic treatment; 22% were “moderately satisfied,” 6% were “mildly satisfied,” and 2% reported they were “minimally satisfied” with current prophylaxis (Table 2). Among the patients with HAE-C1INH who received prophylaxis, 20% reported breakthrough attacks at least once per week, 31% reported breakthrough attacks one to three times per month, 15% reported breakthrough attacks once every 2–3 months, and 34% reported breakthrough attacks fewer than once every 6 months (Fig 4).
Figure 4.
The frequency of a hereditary angioedema (HAE) breakthrough after prophylactic therapy (a) among the patients with HAE with C1-inhibitor (HAE-C1INH) and (b) among the patients with HAE with normal C1-inhibitor (HAE-nlC1INH). The comparisons between 2013 and 2015 were conducted by using the χ2 test.
The patients with HAE-nlC1INH were less likely than the patients with HAE-C1INH to be on prophylaxis (51 versus 70%; p = 0.02). Among the patients with HAE-nlC1INH on prophylaxis, the majority (89%) received in-home treatment. The proportion of the patients with HAE-nlC1INH on prophylaxis increased markedly from 2013 to 2015; only 16% of the patients with HAE-nlC1INH reported prophylactic treatment in 2013 (p = 0.002). Breakthrough attacks despite prophylaxis were reported by 42.1% of the patients with HAE-nlC1INH at least two to three times a week, and another 15.7% reported attacks at least once a week.
DISCUSSION
Our 2015 survey encompassed data from 106 patients with HAE-C1INH and 37 patients with HAE-nlC1INH who attended a national U.S. patient summit. A comparison with our report from 2013 revealed that important progress had been made; however, the patients with HAE still face a significant burden of disease.11–13 Despite rapid advances in medicine and technology, there are still long delays in the time to HAE diagnosis even after the initial onset of angioedema symptoms. Patients are still experiencing attacks with significant frequency with and without prophylaxis, which negatively impacts quality of life. Although ED utilization was not high, the care experienced by patients with HAE in the ED needs to be improved for a substantial number of patients.14 On a positive note, an increasing number of the patients with HAE had individualized treatment plans and a significant majority had access to on-demand therapy available at home, which confirmed our hypothesis that the publication of multiple guidelines targeted to improve HAE management may have had a favorable impact. Overall, advances in patient care are clear but improvements in disease recognition, care options, and management continue to represent an unmet need for HAE.
Patients with HAE are most frequently managed by allergist/immunologists, and patients with HAE are reporting strong satisfaction with the overall care they are receiving. It is important to note that this satisfaction has improved significantly even within 2 years from 2013. There have been numerous online educational programs, presentations at national allergy conferences, and publications geared toward the practicing allergist/immunologists that we suspect have contributed to these positive findings. Recently published guidelines focus on the importance of HAE action plans,8,10 and this seems to have influenced care delivery reflected by our findings, which depicted a high frequency of individualized treatment plans. It will be important to continue these educational efforts as the field advances but consider innovative ways to target physicians in disciplines that would encounter patients with HAE.
The improvement in the satisfaction of care in the ED reported by the patients with HAE-C1INH in the ED is commendable but more work is needed. The existing disparity between the level of satisfaction with ED care versus specialist physician care points to the need for greater coordination of care with local EDs for patients with HAE, which could include telephone discussions by a specialist physician with ED directors about individualized plans of care for patients with HAE by detailing specifics. When possible, flagging a medical record to indicate the rare condition and the unique specific treatment required may be beneficial.15 Patients with HAE report long wait times before proper treatment is administered.14 Written HAE action plans carried by patients with HAE and calling the ED in advance of the patient's arrival for management of an ongoing HAE attack has been successful. The HAEA consensus document specifically concluded that a comprehensive individualized management plan developed between an expert HAE physician and the patient, in collaboration with local medical providers and the ED, can provide patients with the best opportunity to lead a normal life.10 Further research could examine, more specifically, measures that have been successful in the ED to manage similar chronic conditions with acute or life-threatening symptoms.
Our survey found an increase in utilization of the ED by the patients with HAE-nlC1INH in 2015 compared with 2013. In contrast, patients with HAE-C1INH reported infrequent ED visits. The reasons for the increase among the patients with HAE-nlC1INH were not clear and were unfortunately not assessed in the survey. We know that, in contrast to HAE-C1INH, there are no quick definitive laboratory tests to confirm a diagnosis of HAE-nlC1INH or U.S. Food and Drug Administration–approved therapies, which makes the diagnosis and management of patients with HAE-nlC1INH in any setting more complex. Patients with recurrent episodes of angioedema and with normal laboratory test results are often labeled with HAE-nlC1INH; however, this diagnosis may be incorrect, and the patient could, in fact, have difficult-to-treat histaminergic angioedema. Understandably, when there is no obvious food or medication trigger, it is difficult to establish a precise cause of swelling in the ED, and any life-threatening component needs to be managed quickly and effectively. Along these lines, a group of experts in the field of emergency medicine and allergy/immunology created an evidence-based algorithm approach that is critical to effective care.9 These types of efforts are essential for improving the emergency care of patients who present with recurrent angioedema regardless of etiology.
The burden of disease for HAE remains high. Our survey found that patients are experiencing HAE attacks with some frequency despite long-term prophylaxis, and there are still significant delays in diagnosis. Published trials of long-term prophylaxis medications available to patients show significant but not complete prevention of symptoms, which leaves room for improvement in therapies. Promising data are emerging from trials of additional novel prophylactic therapies, which could help achieve the goal of patients with HAE to lead normal lives and improving quality of life.8,16,17 With various levels of confidence of patients administering on-demand medication,18 physicians must continue to manage patients with individualized treatment plans and encourage patients with HAE to treat all attacks, to treat the attack as soon as it is clearly recognized, and to always carry two doses of on-demand treatment.16 It is reassuring that patients with HAE who receive long-term prophylaxis are largely treated at home and are relatively satisfied with this approach, likely due to the reduced burden of treatment associated with home therapy.
Results of recent studies show an average delay in diagnosis between symptom onset and disease diagnosis of 8–10 years,19–21 and we also found significant delays in our survey. Although substantial efforts have aimed to educate providers about HAE, long diagnostic delays still exist for some patients. Testing family members of patients with identified HAE for C1 inhibitor deficiency could markedly decrease this delay. Not only is such family testing an effective screening method in autosomal dominant genetic conditions, but such an approach could be cost effective by decreasing the number of unnecessary tests and procedures ordered.22 Multiple published guidelines and consensus documents encourage testing of all family members,8,16 and this should be emphasized to patients with HAE and to their family.
The limitations of our study included population bias due to several factors. First, this was a self-selected population that took the initiative to travel to an HAE conference, which could indicate a group more engaged with and knowledgeable about their disease than the average patient with HAE. Thus, the results may not be generalizable to the overall population of individuals affected by HAE. Second, patients self-reported their HAE diagnosis, which was not confirmed by diagnostic testing. This could have led to both misreporting of the HAEA status and subtypes. It was of particular concern in patients who reported an HAE-nlC1INH diagnosis because no established confirmatory diagnostic tests exist outside of tests for factor XII mutations. There were likely differences in the 2015 versus 2013 HAE patient populations based on the location of the meeting and who chose to attend. These differences were difficult to assess. Also, similar to the 2013 survey, data were gathered at a single time point rather than longitudinally and not every patient answered all the questions. However, our study had several clear advantages, including simultaneous data collection from a large number of patients with HAE and assessing the current state of management from the perspective of patients with HAE.
CONCLUSION
We reported descriptive data from a patient-based survey of almost 150 patients with self-reported HAE. Similar to the data from 2013 and other published literature,23,24 our results indicated that significant progress was made in effectively managing HAE, but a high burden of disease remained for patients with HAE. Additional recent advances in HAE therapy that are more effective and easier to use are expected to further reduce the burden of disease. Treatment disparities and diagnostic challenges exist even more so for patients with HAE-nlC1INH compared with patients with HAE-C1INH. Our findings highlight the need for continued research and educational efforts aimed at improving quality of life for all patients with HAE.
ACKNOWLEDGMENTS
The authors thank the patients with HAE who participated in the survey.
Footnotes
United States Hereditary Angioedema Association provided support for this study. This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102)
Aleena Banerji received research grant from Shire and member of Advisory Board for Shire, CSL, Biocryst, Pharming. Paula Busse received grant from Shire, CSL Behring, Pharming and Consult for Shire, CSL Behring. Marc Riedl Uncompensated medical advisory board for US Hereditary Angioedema Association. Bruce L Zuraw, Travel support at US Hereditary Angioedema Association. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Bork K, Barnstedt SE, Koch P, Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet. 2000; 356:213–217. [DOI] [PubMed] [Google Scholar]
- 2. Walford HH, Zuraw BL. Current update on cellular and molecular mechanisms of hereditary angioedema. Ann Allergy Asthma Immunol. 2014; 112:413–418. [DOI] [PubMed] [Google Scholar]
- 3. Banerji A, Busse P, Christiansen SC, et al. Current state of hereditary angioedema management: a patient survey. Allergy Asthma Proc. 2015; 36:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aabom A, Nguyen D, Fisker N, Bygum A. Health-related quality of life in Danish children with hereditary angioedema. Allergy Asthma Proc. 2017; 38:440–446. [DOI] [PubMed] [Google Scholar]
- 5. Nordenfelt P, Nilsson M, Lindfors A, Wahlgren CF, Björkander J. Health-related quality of life in relation to disease activity in adults with hereditary angioedema in Sweden. Allergy Asthma Proc. 2017; 38:447–455. [DOI] [PubMed] [Google Scholar]
- 6. Lumry WR, Miller DP, Newcomer S, Fitts D, Dayno J. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy Asthma Proc. 2014; 35:371–376. [DOI] [PubMed] [Google Scholar]
- 7. Christiansen SC, Bygum A, Banerji A, et al. Before and after, the impact of available on-demand treatment for HAE. Allergy Asthma Proc. 2015; 36:145–150. [DOI] [PubMed] [Google Scholar]
- 8. Cicardi M, Aberer W, Banerji A, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014; 69:602–616. [DOI] [PubMed] [Google Scholar]
- 9. Moellman JJ, Bernstein JA, Lindsell C, et al. A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014; 21:469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuraw BL, Bernstein JA, Lang DM, et al. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013; 131:1491–1493. [DOI] [PubMed] [Google Scholar]
- 11. Engel-Yeger B, Farkas H, Kivity S, Veszeli N, Kohalmi KV, Kessel A. Health-related quality of life among children with hereditary angioedema. Pediatr Allergy Immunol. 2017; 28:370–376. [DOI] [PubMed] [Google Scholar]
- 12. Jindal NL, Harniman E, Prior N, Perez-Fernandez E, Caballero T, Betschel S. Hereditary angioedema: health-related quality of life in Canadian patients as measured by the SF-36. Allergy Asthma Clin Immunol. 2017; 13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Longhurst H, Bygum A. The humanistic, societal, and pharmaco-economic burden of angioedema. Clin Rev Allergy Immunol. 2016; 51:230–239. [DOI] [PubMed] [Google Scholar]
- 14. Otani IM, Christiansen SC, Busse P, et al. Emergency department management of hereditary angioedema attacks: patient perspectives. J Allergy Clin Immunol Pract. 2017; 5:128–134.e4. [DOI] [PubMed] [Google Scholar]
- 15. Riedl MA. Creating a comprehensive treatment plan for hereditary angioedema. Immunol Allergy Clin North Am. 2013; 33:471–485. [DOI] [PubMed] [Google Scholar]
- 16. Zuraw BL, Banerji A, Bernstein JA, et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract. 2013; 1:458–467. [DOI] [PubMed] [Google Scholar]
- 17. Weller K, Maurer M, Fridman M, Supina D, Schranz J, Magerl M. Health-related quality of life with hereditary angioedema following prophylaxis with subcutaneous C1-inhibitor with recombinant hyaluronidase. Allergy Asthma Proc. 2017; 38:143–151. [DOI] [PubMed] [Google Scholar]
- 18. Jose J, Lehman EB, Craig T. Evaluating satisfaction of patients with hereditary angioedema with their past and present treatments: Implications for future therapies. Allergy Asthma Proc. 2018; 39:74–80. [DOI] [PubMed] [Google Scholar]
- 19. Zanichelli A, Magerl M, Longhurst H, Fabien V, Maurer M. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol. 2013; 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jolles S, Williams P, Carne E, et al. A UK national audit of hereditary and acquired angioedema. Clin Exp Immunol. 2014; 175:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christiansen SC, Davis DK, Castaldo AJ, Zuraw BL. Pediatric hereditary angioedema: onset, diagnostic delay, and disease severity. Clin Pediatr (Phila). 2016; 55:935–942. [DOI] [PubMed] [Google Scholar]
- 22. Lunn ML, Santos CB, Craig TJ. Is there a need for clinical guidelines in the United States for the diagnosis of hereditary angioedema and the screening of family members of affected patients? Ann Allergy Asthma Immunol. 2010; 104:211–214. [DOI] [PubMed] [Google Scholar]
- 23. Banerji A, Bas M, Bernstein JA, et al. Expert perspectives on hereditary angioedema: Key areas for advancements in care across the patient journey. Allergy Rhinol (Providence). 2016; 7:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohsawa I, Honda D, Nagamachi S, et al. Clinical manifestations, diagnosis, and treatment of hereditary angioedema: survey data from 94 physicians in Japan. Ann Allergy Asthma Immunol. 2015; 114:492–498. [DOI] [PubMed] [Google Scholar]