Abstract
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) has been traditionally delivered with two independent cannulae at different venous insertion sites. The bicaval dual lumen cannula has gained popularity as a cannula for simultaneous venous drainage and reinfusion of blood via the internal jugular vein (IJV). Its insertion requires special attention to details to prevent the occurrence of serious complications. In this review, we discuss different techniques of cannula insertion and tips for troubleshooting.
Keywords: Extracorporeal membrane oxygenation (ECMO), cannulation, dual lumen cannula, double lumen cannula
Background
In the past decade, the use of veno-venous extracorporeal membrane oxygenation (VV-ECMO) has regained attention as a result of advancement in technology and the proven benefits to patient survival in severe respiratory failure refractory to conventional ventilatory support. The provision of VV-ECMO involves the drainage of deoxygenated blood from the central venous system to a pump and an oxygenator, where after oxygenation of blood and carbon dioxide removal, the blood is returned to the patient’s venous system. Traditionally, this has been achieved by a two-cannulae technique—the cannula for drainage (typically 23–27 French) is inserted into the inferior vena cava (IVC) via the femoral vein, and the cannula for blood return (typically 17–19 French) is inserted into one of the internal jugular vein (IJV) or femoral or subclavian veins. However, the efficacy of gaseous exchange using this ECMO configuration is limited by the occurrence of recirculation, especially at high ECMO blood flow (1). Moreover, this technique limits patients from active mobilization and rehabilitation that is beneficial to certain subgroups, such as patients requiring long-term VV-ECMO support awaiting lung transplantation (2).
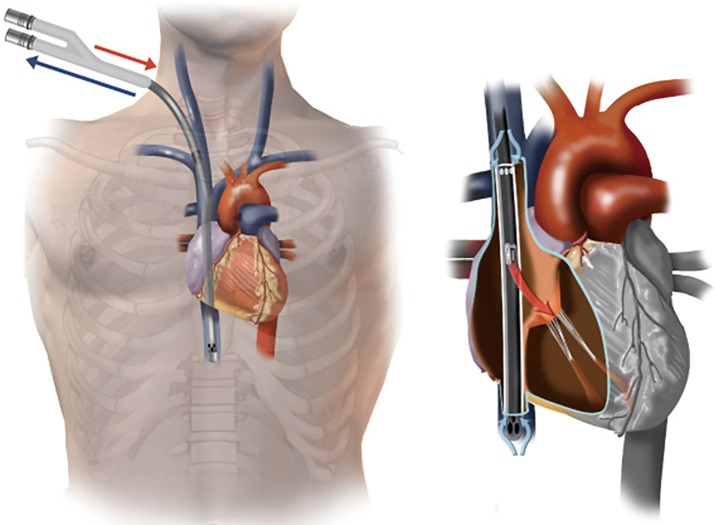
The introduction of the bicaval dual lumen cannula is a feasible solution to the above problems. With its special three-ports design, the cannula is introduced from the right IJV, passing through the right atrium (RA) down to the IVC. Deoxygenated blood is drained from the two drainage ports located at the superior vena cava (SVC) and IVC, and oxygenated blood is returned through the reinfusion port at the RA directing at the tricuspid valve (Figure 1) (3). The incidence of recirculation is reported to be as low as 2% in optimally-positioned cannulae (4). In addition, IJV cannulation allows easier patient mobilization and facilitates prone positioning during ECMO support (5).
Figure 1.
Correct position of Avalon cannula. Reproduced from Hirose et al. (3).
Proper insertion technique and precise positioning of the cannula is the key to patient safety and optimal functioning of the dual lumen cannula. Serious cannulation-related complications commonly reported include cardiac perforation and cardiac tamponade (3–15%) (6,7). In this review, we will discuss the various cannulation techniques, their advantages and disadvantages.
The first step of cannulation is the puncturing of the IJV. Similar to central venous catheter insertion, ultrasound (USG) guidance is recommended to prevent complications such as pneumothorax, carotid artery injury and nerve injury. After successful venous access, a guidewire is advanced sequentially into the SVC, RA and then IVC with imaging guidance, either by transesophageal echocardiography (TEE) or fluoroscopy. Subsequently, the skin and subcutaneous tissue is dilated with serial dilators, and the cannula is inserted into the final position.
TEE-guided technique
During TEE-guided cannulation, a bicaval view is commonly used to visualize the guidewire entering the SVC, RA and IVC. However, it may be difficult to image the sub-diaphragmatic IVC due to anatomical limitations. Supplementing TEE with transthoracic echocardiography (subcostal views) usually overcomes this limitation (8).
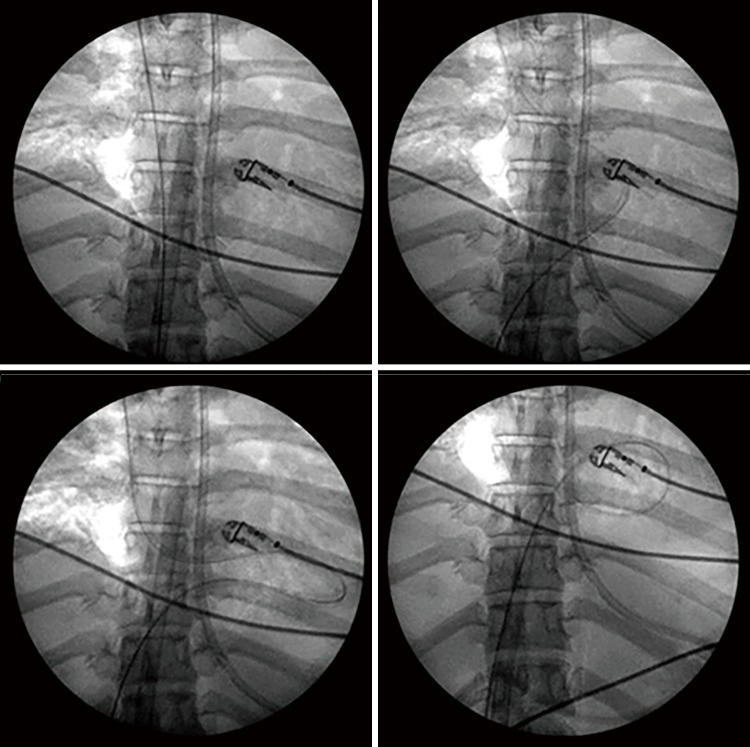
Regardless, catastrophic complications during cannulation have been reported when TEE is used as the sole method of image guidance. The guidewire in the commercial insertion kit is a 0.038 inch ×200 cm J-tipped guidewire which is comparatively soft, with the intended advantage of providing flexibility and minimizing the risk of injury to the heart and venous structures. This feature may become a disadvantage during the placement of the guidewire into the IVC and during the advancement of the dilators and the cannula. Firstly, a softer guidewire may be difficult to advance into the IVC. Instead, it may preferentially go into the RA and right ventricle (RV) especially when a large Eustachian valve is encountered in the RA. There are reported cases of looped guidewire inside the RV (Figure 2) before its advancement into the IVC (9,10). This can only be detected with a high index of suspicion and repeated echocardiographic examinations in order to prevent serious complications. Even if the guidewire is successfully positioned into the IVC, a soft guidewire may be dislodged from the IVC during dilatation of the skin tissue and subsequent cannula insertion, especially when the maneuvers are forceful.
Figure 2.
Looping of guidewire in right ventricle before entering the IVC. Reproduced from Teman et al. (9) (with permission).
Several methods to prevent guidewire dislodgement and looping during cannula insertion have been reported. Firstly, the guidewire should be inserted deep into the IVC down to the level of renal (11) or iliac vein bifurcation (12). Secondly, stiffer guidewires, e.g., Amplatz Super Stiff 0.035/0.038 inch (11) can be used in the first instance after venous puncture, or exchanged in place of the Avalon guidewire using a Berenstein directional catheter after the IVC is reached. The Berenstein catheter is an angled-tip catheter which helps to direct the guidewire away from the RV (9). Regardless of the strategy, ECMO physicians should be alerted to the risks and benefits of using a stiffer guidewire, which bears a higher risk of perforating vascular structures.
TEE-guided dual lumen cannula insertion is still adopted in many centers despite the above limitations. It is advantageous in critically ill patients unfit for transport or during emergencies, as it does not require transfer to fluoroscopic facilities which may not be readily available at the ICU bedside. Contingency plans and facilities should be available in the occurrence of complications, such as preparation for bedside surgical pericardial space decompression and operating theatre availability.
In summary, the ECMO physician should bear in mind the following safety points in order to minimize complications during TEE-guided Avalon cannula insertion.
Guidewire looping in RA/RV is a well-reported phenomenon;
The visualization of the guidewire in SVC and IVC does not exclude guidewire looping in RA/RV (7);
Guidewire kinking or dislodgement can occur during the advancement of serial dilators and cannula insertion;
Presence of premature ventricular complexes may signify dislodgement of the guidewire into the RV (3);
Advancement of dilators or cannulae should be abandoned when there is loss of guidewire visualization, and whenever resistance is encountered during insertion.
Fluoroscopic-guided technique
Fluoroscopic guidance allows continuous surveillance of the course and position of the guidewire, and hence possesses a superior safety profile when compared with TEE-guided insertion (the techniques in cannulation, in particular the choice and handling of different guidewires, is no different from the TEE-guided approach). The major limitation is the availability of fluoroscopic facilities in the ICU setting, and the risks of transporting a critically ill patient. As a result, this technique is not suited for patients requiring urgent cannulation.
We have developed a simulation training tool for fluoroscopic-guided cannulation (see https://www.youtube.com/watch?v=dr02RAMRk1A for details).
Alternative insertion sites
The dual lumen cannula is designed as a straight cannula and the recommended insertion configuration is through the right IJV. However, vascular anomalies such as stenosis and venous thrombosis may prohibit the use of such insertion site. Alternative sites of cannulation by using the left IJV (13) and the left subclavian vein have been reported in case series (14,15). Nevertheless, robust data and safety reports are still lacking, hence, their routine use cannot be recommended.
Alignment of the cannula reinfusion port
The next key step to ensure the efficiency of the dual lumen cannula is the optimal positioning of the reinfusion port. As aforementioned, the cannula should be manipulated to a position where the reinfusion port is directed at the tricuspid valve, so oxygenated blood can go through the RV and be pumped to the systemic circulation, minimizing recirculation in the RA. This usually requires minor manipulations and rotational maneuvers of the cannula guided by transthoracic or TEE with or without agitated saline contrast (16). Shafii et al. also reported a technique of using the pulmonary artery catheter to locate the position of tricuspid valve under fluoroscopic guidance (15).
Conclusions
The bicaval dual lumen cannula has a clear advantage over conventional cannulation configurations in being a single site device. However, its insertion requires special skills and safety measures. We recommend fluoroscopy as the preferred choice of image guidance in all patients suitable for transfer to such facilities. The ECMO physician should be aware of the potential complications, and contingency support should always be available during cannulation.
Acknowledgements
The authors would like to acknowledge Mr. Peter C. K. Lai, Mr. Ricky W. K. Chan and Mr. Andy Y. T. Mok for their contribution to the development of the fluoroscopic-guided cannulation training tool.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J 2015;61:115-21. 10.1097/MAT.0000000000000179 [DOI] [PubMed] [Google Scholar]
- 2.Reeb J, Falcoz PE, Santelmo N, et al. Double lumen bi-cava cannula for veno-venous extracorporeal membrane oxygenation as bridge to lung transplantation in non-intubated patient. Interact Cardiovasc Thorac Surg 2012;14:125-7. 10.1093/icvts/ivr046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirose H, Yamane K, Marhefka G, et al. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg 2012;7:36. 10.1186/1749-8090-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Zhou X, Liu X, et al. Wang-Zwische double lumen cannula-toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J 2008;54:606-11. 10.1097/MAT.0b013e31818c69ab [DOI] [PubMed] [Google Scholar]
- 5.Kuhl T, Michels G, Pfister R, et al. Comparison of the Avalon Dual-Lumen Cannula with Conventional Cannulation Technique for Venovenous Extracorporeal Membrane Oxygenation. Thorac Cardiovasc Surg 2015;63:653-62. 10.1055/s-0035-1549359 [DOI] [PubMed] [Google Scholar]
- 6.Jayaraman AL, Cormican D, Shah P, et al. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations. Ann Card Anaesth 2017;20:S11-8. 10.4103/0971-9784.197791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berdajs D. Bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: Avalon(c) cannula in childhood disease. Perfusion 2015;30:182-6. 10.1177/0267659114544714 [DOI] [PubMed] [Google Scholar]
- 8.Tabak B, Elliott CL, Mahnke CB, et al. Transthoracic echocardiography visualization of bicaval dual lumen catheters for veno-venous extracorporeal membrane oxygenation. J Clin Ultrasound 2012;40:183-6. 10.1002/jcu.21873 [DOI] [PubMed] [Google Scholar]
- 9.Teman NR, Haft JW, Napolitano LM. Optimal endovascular methods for placement of bicaval dual-lumen cannulae for venovenous extracorporeal membrane oxygenation. ASAIO J 2013;59:442-7. 10.1097/MAT.0b013e31829a0102 [DOI] [PubMed] [Google Scholar]
- 10.Yastrebov K, Manganas C, Kapalli T, et al. Right ventricular loop indicating malposition of J-wire introducer for double lumen bicaval venovenous extracorporeal membrane oxygenation (VV ECMO) cannula. Heart Lung Circ 2014;23:e4-7. 10.1016/j.hlc.2013.05.643 [DOI] [PubMed] [Google Scholar]
- 11.Shaheen A, Tanaka D, Cavarocchi NC, et al. Veno-Venous Extracorporeal Membrane Oxygenation (V V ECMO): Indications, Preprocedural Considerations, and Technique. J Card Surg 2016;31:248-52. 10.1111/jocs.12690 [DOI] [PubMed] [Google Scholar]
- 12.Camboni D, Philipp A, Lubnow M, et al. Extracorporeal membrane oxygenation by single-vessel access in adults: advantages and limitations. ASAIO J 2012;58:616-21. 10.1097/MAT.0b013e31826a8a32 [DOI] [PubMed] [Google Scholar]
- 13.Abrams D, Brodie D, Javidfar J, et al. Insertion of bicaval dual-lumen cannula via the left internal jugular vein for extracorporeal membrane oxygenation. ASAIO J 2012;58:636-7. 10.1097/MAT.0b013e31826feda5 [DOI] [PubMed] [Google Scholar]
- 14.Bojic A, Steiner I, Gamper J, et al. Supraclavicular Approach to the Subclavian Vein as an Alternative Venous Access Site for ECMO Cannulae? A Retrospective Comparison. ASAIO J 2017;63:679-83. 10.1097/MAT.0000000000000529 [DOI] [PubMed] [Google Scholar]
- 15.Shafii AE, McCurry KR. Subclavian insertion of the bicaval dual lumen cannula for venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2012;94:663-5. 10.1016/j.athoracsur.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 16.Dolch ME, Frey L, Buerkle MA, et al. Transesophageal echocardiography-guided technique for extracorporeal membrane oxygenation dual-lumen catheter placement. ASAIO J 2011;57:341-3. 10.1097/MAT.0b013e3182179aae [DOI] [PubMed] [Google Scholar]