Abstract
To investigate the interrelation between economic, marital, and known histopathologic/therapeutic prognostic factors in presentation and survival of patients with lung cancer in nine different ethnic groups. A retrospective review of the SEER database was conducted through the years 2007–2012. Population differences were assessed via chi‐square testing. Multivariable analyses (MVA) were used to detect overall survival (OS) differences in the total population (TP, N = 153,027) and for those patients presenting with Stage IV (N = 70,968). Compared to Whites, Blacks were more likely to present with younger age, male sex, lower income, no insurance, single/widowed partnership, less squamous cell carcinomas, and advanced stage; and experience less definitive surgery, lower OS, and lung cancer‐specific (LCSS) survival. White Hispanics presented with younger age, higher income, lower rates of insurance, single/widowed partnership status, advanced stage, more adenocarcinomas, and lower rates of definitive surgery, but no difference in OS and LCSS than Whites. In the TP and Stage IV populations, MVAs revealed that OS was better or equivalent to Whites for all other ethnic groups and was positively associated with insurance, marriage, and higher income. Blacks presented with more advanced disease and were more likely to succumb to lung cancer, but when adjusted for prognostic factors, they had a better OS in the TP compared to Whites. Disparities in income, marital status, and insurance rather than race affect OS of patients with lung cancer. Because of their presentation with advanced disease, Black and Hispanics are likely to have increased benefit from lung cancer screening.
Keywords: Disparities, insurance, lung cancer, marriage, metastatic lung cancer, outcomes, race, socio‐economic factors
Introduction
In the United States, lung cancer occurs in approximately 225,000 patients and is associated with over 160,000 deaths annually 1. However, despite the prevalence of this malignancy, the influence and/or interrelation between economic and insurance factors as well as ethnicity have been poorly studied. One recent study did investigate racial/ethnic differences in lung cancer incidence and mortality in women, but found no differences in fully adjusted models 2. Additionally, another report demonstrated lung cancer rates have dropped faster in Black women than the rates in Whites since the 1990s 3. Using the Christiana Care Tumor Registry (CCTR) in Delaware, disparities in survival were related to lower socioeconomic status and having Medicaid insurance, but there were no such differences related to race or sex 4. A National Cancer Database project 5 found income and race (White, Black, and Asian) were not related to survival, but patients with Medicaid or who were uninsured had worse outcomes 5.
The purpose of our analysis was to study whether marital status, household income, insurance type, and ethnicity play a role in the presentation and prognosis of all patients with NSCLC and those presenting with metastatic disease in the United States. We feel this investigation is unique because we investigate the racial groups in terms of their presenting economic, histopathologic, and marital status and assess whether racial differences account for differing prognoses.
Materials and Methods
Data source/cohort selection
The “SEER‐18” database was available since the year 2000 6 and covers approximately 28% of the American population 6. The years 2007–2012 were queried to identify patients with microscopically confirmed NSCLC as their first primary tumor.
Outcome and presenting characteristics were examined for all patients (153,027) and patients with metastatic disease (70,968) for whom sufficient information was collected to assess the outcomes in relation to patient, economic, histopathologic, and insurance variables.
Outcome variables and other covariates
The main purpose of our analysis was to examine whether there were differences in presenting characteristics and outcome in nine different ethnic groups by examining marital status, household income, and insurance type in addition to established histopathologic (tumor location, size, differentiation, stage, and histology), treatment factors (radiation and definitive surgical procedure), and patients factors (age, gender, marital status, presenting year, and SEER registry site). The patients with lung cancer were split into nine different ethnic groups as follows: White non‐Hispanic (White), Black, White Hispanic (Hispanic), American Indian/Alaskan native (AI/AN), Chinese, Japanese, South Asian (Asian Indian and Pakistani), other Asians (OA, Filipino, Thai, Vietnamese, Korean, Kampuchean, Laotian, and Hmong), and other races (OR, Chamorran, Fiji Islander, Guamanian, Hawaiian, Melanesian, Micronesian, New Guinean, Pacific Islander, Polynesian, Samoan, Tahitian, Tongan, unknown, and others) in both the TP and Stage IV populations. The number of Black Hispanic patients was scant in both the TP (1.0%) and the Stage IV groups (0.7%), thus precluding the possibility of considering a separate patient category, and thus, Black Hispanic patients are included in the Black category, similar to a past study 7. Throughout this manuscript, the term population(s) will refer to total population of patients with lung cancer and those with Stage IV disease, while group(s) will refer to the nine different ethnicities.
At the time of our analysis, SEER did not contain information regarding whether systemic therapy was given, nor does SEER contain information regarding the systemic agents that were used. SEER does contain information including the following: year of diagnosis (1975–2014), sex, patient age (1–84 and 85+), SEER registry location, median household income, insurance, marital status, origin recode (Hispanic, non‐Hispanic), race/ethnicity, tumor location, primary site, sequence number, grade, laterality, tumor size, tumor extension, number of nodes examined, number of nodes positive, TNM stages, Mets at diagnosis, type of surgery, cause of death, vital status, and survival months.
Statistical analysis
Chi‐square and t‐tests compared differences between the ethnic groups with respect to treatment and patient/tumor characteristics. Cox proportional hazards models (Therneau, Grambsch) 8 were used to calculate adjusted hazard ratios with their 95% confidence intervals and to show how treatment and other covariates were related to overall survival OS and LCSS. Medicare eligibility was controlled through use of two strata for age at diagnosis (≥65 years old vs. <65 years) because individual cases will change when they enroll in Medicare.
Results
Median follow‐up time was calculated by the methods of Schemper and Smith in which death becomes a censored follow‐up time and was noted to be 35 and 31 months in the TP and Stage IV groups, respectively 9.
Complete demographic and histologic details of the TP (153,027) and Stage IV (70,968) populations can be seen in Table 1. Median age in the TP and Stage IV are 68.0 and 67.0, respectively. There was a male predominance to both populations (54.1%‐ TP, and 55.8%‐ Stage IV). The three largest ethnic groups in the TP and Stage IV population were White, Black, and Hispanic and were 74.4%, 12.3%, and 5.7%; and 72.3%, 13.2%, and 6.3%, respectively. A similar proportion of the Stage IV (31.7%) and TP (32.3%) patients presented with a low median family income (<$50,000). The majority were married with 51.6% and 51.2% in the TP and Stage IV, respectively. 82.3% and 80.1% of TP and Stage IV patients were insured. Adenocarcinoma was the predominant histology in both populations (52.6%, TP; and 55.5%, Stage IV).
Table 1.
Demographic characteristics of both the TP and Stage IV patients
| All Patients (TP) N = 153,027 | Stage IV patient N = 70,968 | |
|---|---|---|
| Age—year, median | 68.0 | 67.0 |
| Sex | ||
| Female | 70,212 (45.9%) | 31,353 (44.2%) |
| Male | 82,815 (54.1%) | 39,615 (55.8%) |
| Race | ||
| White Hispanic | 8579 (5.6%) | 4441 (6.3%) |
| White non‐Hispanic | 114,013 (74.5%) | 51,296 (72.3%) |
| Black | 18,852 (12.3%) | 9360 (13.2%) |
| Chinese | 2413 (1.6%) | 1261 (1.8%) |
| Japanese | 1229 (0.80%) | 567 (0.80%) |
| South Asian | 451 (0.29%) | 238 (0.34%) |
| Other Asians | 4831 (3.2%) | 2544 (3.6%) |
| Other Races | 1957 (1.3%) | 943 (1.3%) |
| American Indian/Alaskan Native | 702 (0.46%) | 318 (0.45%) |
| SEER registry | ||
| Alaska Natives | 199 (0.13%) | 76 (0.11%) |
| Atlanta | 4629 (3.0%) | 2226 (3.1%) |
| California excl SF/SJM/LA | 30,007 (19.6%) | 14,304 (20.2%) |
| Connecticut | 8088 (5.30%) | 3639 (5.1%) |
| Detroit | 9852 (6.4%) | 4632 (6.5%) |
| Greater Georgia | 14,260 (9.3%) | 6380 (9.0%) |
| Hawaii | 2375 (1.6%) | 1159 (1.6%) |
| Iowa | 6805 (4.4%) | 3205 (4.5%) |
| Kentucky | 13,916 (9.1%) | 5980 (8.4%) |
| Los Angeles | 11,437 (7.5%) | 5789 (8.2%) |
| Louisiana | 10,783 (7.0%) | 4767 (6.7%) |
| New Jersey | 17,451 (11.4%) | 7796 (11.0%) |
| New Mexico | 2610 (1.7%) | 1215 (1.7%) |
| Rural Georgia | 353 (0.23%) | 140 (0.20%) |
| San Francisco–Oakland | 7081 (4.6%) | 3469 (4.9%) |
| San Jose–Monterey | 3203 (2.1%) | 1635 (2.3%) |
| Seattle | 8271 (5.4%) | 3726 (5.3%) |
| Utah | 1707 (1.1%) | 830 (1.2%) |
| Income | ||
| <$50,000 | 49,407 (32.3%) | 22,524 (31.7%) |
| $50,000–74,000 | 81,027 (52.9%) | 37,933 (53.5%) |
| ≥75,000 | 22,593 (14.8%) | 10,511 (14.8%) |
| Marital status | ||
| Divorced | 18,851 (12.3%) | 8815 (12.4%) |
| Married | 78,957 (51.6%) | 36,349 (51.2%) |
| Separated | 1785 (1.2%) | 895 (1.3%) |
| Single | 21,126 (13.8%) | 10,872 (15.3%) |
| Unknown | 6032 (3.9%) | 2866 (4.0%) |
| Domestic Partner | 126 (0.082%) | 60 (0.084%) |
| Widowed | 26,150 (17.1%) | 11,111 (15.7%) |
| Tumor stage | ||
| Unknown | 3174 (2.0%) | 0 |
| I | 34,255 (22.3%) | 0 |
| II | 7825 (5.1%) | 0 |
| III | 39,979 (26.1%) | 0 |
| IV | 70,968 (46.3%) | 70,968 (100.0%) |
| Insurance | ||
| Insured | 125,876 (82.3%) | 56,859 (80.1%) |
| Medicaid | 20,741 (13.6%) | 10,324 (14.5%) |
| Uninsured | 5272 (3.4%) | 3145 (4.4%) |
| Unknown | 1138 (0.74%) | 640 (0.96%) |
| Lateral location | ||
| Bronchus, Left | 2427 (1.6%) | 1311 (1.8%) |
| Bronchus, Right | 2427 (2.2%) | 1875 (2.6%) |
| Bronchus, Unknown | 168 (0.11%) | 103 (0.15%) |
| Left Lower | 17,384 (11.4%) | 7266 (10.2%) |
| Left Upper | 35,216 (23.0%) | 14,827 (20.9%) |
| Left NOS | 4733 (3.1%) | 2993 (4.2%) |
| Lung, NOS | 6207 (4.1%) | 5382 (7.6%) |
| Left Overlapping | 507 (0.32%) | 249 (0.35%) |
| Right Lower | 22,457 (14.7%) | 9476 (13.4%) |
| Right Middle | 6376 (4.2%) | 2848 (4.0%) |
| Right Upper | 45,478 (29.7%) | 19,134 (27.0%) |
| Right NOS | 7460 (4.9%) | 4921 (6.9%) |
| Right Overlapping | 1268 (0.83%) | 583 (0.82%) |
| Histology—No. (%) | ||
| Adenocarcinoma | 80,499 (52.6%) | 39,415 (55.5%) |
| Squamous Cell Ca | 41,573 (27.2%) | 14,239 (20.0%) |
| Non‐small‐cell Ca, NOS | 22,886 (15.0%) | 13,603 (19.2%) |
| Large Cell Ca | 4519 (3.0%) | 2301 (3.2%) |
| Adenosquamous Cell Ca | 2442 (1.6%) | 921 (1.3%) |
| Others | 1108 (0.72%) | 489 (0.69%) |
| Grade | ||
| Well, I | 8365 (5.4%) | 1795 (2.5%) |
| Moderately, II | 30,010 (19.6%) | 7974 (11.2%) |
| Poorly, III | 45,364 (29.6%) | 19,445 (27.3%) |
| Undifferentiated, IV | 2497 (1.6%) | 1142 (1.6%) |
| Unknown | 66,791 (43.8%) | 40,612 (57.2%) |
| Definitive surgical procedure | 39,105 (25.6%) | |
| Radiation given | 64,552 (41.8%) | 33,689 (47.5%) |
| Year of diagnosis | ||
| 2007 | 25,396 (16.6%) | 11,589 (16.3%) |
| 2008 | 25,529 (16.7%) | 11,572 (16.3%) |
| 2009 | 25,650 (16.8%) | 11,848 (16.7%) |
| 2010 | 25,631 (16.7%) | 12,135 (17.1%) |
| 2011 | 25,459 (16.6%) | 11,807 (16.6%) |
| 2012 | 25,362 (16.6%) | 12,017 (16.9%) |
Table S1 supplemental contains the unadjusted demographic, histologic, and treatment details in the TP and used Whites as the reference group. Blacks were presented with younger age, more males, lower median household income, more uninsured, higher stages, lower percentage of squamous cell carcinomas, lower rates of definitive surgery, and lower OS/LCSS. Hispanics were presented at a younger age, higher median household income, more uninsured, higher percentage of metastatic disease, higher percentage of adenocarcinomas, and lower rates of definitive surgery, but had a similar OS/LCSS. The Japanese were presented with a highest mean age (72.8), the only female predominance (51.2%), and the highest rates of insurance (96.4%), but there were a similar OS and LCSS compared to Whites. Whites were presented with the higher percentage of Stage I disease (23.4%) than all except for the South Asian and AI/AN. South Asians were presented with the highest percentage of metastatic disease at 52.8%. The Chinese were presented with the highest percentage of adenocarcinomas (69.4%), while AI/AN were presented with the highest percentage of squamous cell carcinomas (30.8%). Whites had significantly higher rates of definitive surgical procedures except for the Chinese, Japanese, and South Asians. As compared to the White population, OS and LCSS were significantly greater in the Chinese, South Asians, other Asians, and other racial groups. Blacks had a lower OS and LCSS. Unadjusted OS by ethnic group can be found in Figure 1A.
Figure 1.
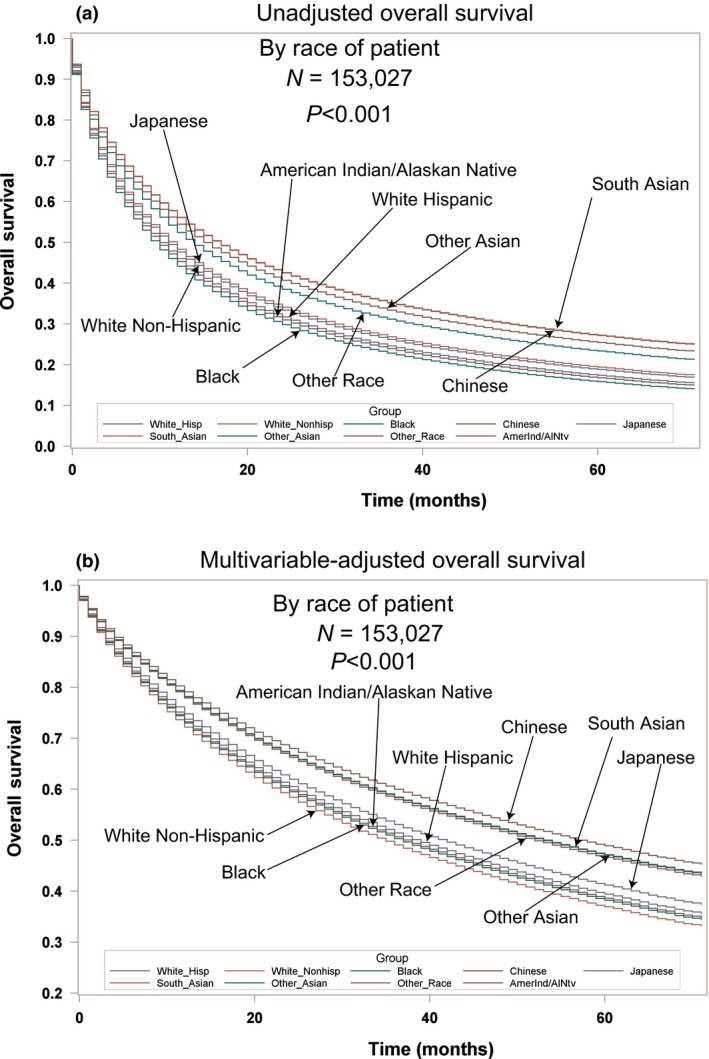
(A) Unadjusted overall survival by ethnic group in the total population. (B) Multivariable‐adjusted overall survival by ethnic group in the total population.
Multivariable analyses for OS and MVA‐adjusted OS in the TP can be seen in Table 2 and Figure 1B. Advancing age (P < 0.0001, HR = 1.185) and male sex (P < 0.0001, HR = 1.245) were associated with worse OS. Whites had a lower OS than all races (HR = 0.705–0.977) except for AI/AN who had a similar OS (P = 0.4890, HR = 0.963). OS was lower for lower (P = 0.0097, HR = 1.024) and better for higher median household incomes (P < 0.001, HR = 0.936). Insured patients had a better OS than the uninsured, those on Medicaid and those with unknown insurance (all P < 0.0001, HR = 1.197–1.246). Married patients had a better OS than separated, single, widowed, and unknown (all P ≤ 0.0004, HR = 1.062–1.166). As compared to Stage I, Stages II‐IV had a worse OS with increasing HRs with stage (all P < 0.0001, HR = 1.622–3.290). The lower lobes and mainstem bronchi locations were associated with worse OS. All histologies had a worse OS (all P < 0.0001, HR = 1.113–1.536) than adenocarcinomas. Compared to well‐differentiated tumors, other tumor grades had worse OS (all P < 0.0001, HR = 1.372–1.731). Patients who received radiation (P < 0.0001, HR = 0.759) or definitive surgery (P < 0.0001, HR = 0.331) had a better OS. OS by insurance, income, and marital status for TP can be seen in Figure 2A–C.
Table 2.
Multivariate analysis for OS in TP, N = 153,207
| All (N = 153,207) | P‐value | Hazard ratio |
|---|---|---|
| Age—year | 1.185 | <0.0001 |
| Sex | ||
| Female | – | 1.0 |
| Male | <0.0001 | 1.245 |
| Race | ||
| White non‐Hispanic | – | 1.0 |
| White Hispanic | <0.0001 | 0.937 |
| Black | 0.0205 | 0.977 |
| Chinese | <0.0001 | 0.705 |
| Japanese | 0.0061 | 0.903 |
| South Asian | <0.0001 | 0.733 |
| Other Asians | <0.0001 | 0.762 |
| Other Races | <0.0001 | 0.792 |
| American Indian/Alaskan Native | 0.4890 | 0.963 |
| SEER Registry | ||
| Alaska Natives | 0.3002 | 1.111 |
| Atlanta | 0.1724 | 1.032 |
| California excl SF/SJM/LA | 0.0003 | 1.060 |
| Connecticut | – | 1.0 |
| Detroit | 0.3908 | 1.017 |
| Greater Georgia | <0.0001 | 1.095 |
| Hawaii | <0.0001 | 1.149 |
| Iowa | 0.0002 | 1.081 |
| Kentucky | <0.0001 | 1.135 |
| Los Angeles | 0.3514 | 0.983 |
| Louisiana | <0.0001 | 1.141 |
| New Jersey | 0.0117 | 1.044 |
| New Mexico | 0.6428 | 1.014 |
| Rural Georgia | 0.9719 | 0.998 |
| San Francisco–Oakland | 0.0325 | 1.046 |
| San Jose–Monterey | 0.1455 | 1.040 |
| Seattle | 0.2381 | 1.024 |
| Utah | 0.0011 | 1.114 |
| Income | ||
| <$50,000 | 0.0097 | 1.024 |
| $50,000–74,000 | – | 1.0 |
| ≥75,000 | <0.0001 | 0.936 |
| Insurance | ||
| Insured | 1.0 | |
| Medicaid | <0.0001 | 1.200 |
| Uninsured | <0.0001 | 1.246 |
| Unknown | <0.0001 | 1.197 |
| Marital status | ||
| Married | – | 1.0 |
| Divorced | <0.0001 | 1.144 |
| Separated | 0.0001 | 1.120 |
| Single | <0.0001 | 1.166 |
| Unknown | 0.0004 | 1.062 |
| Domestic Partner | 0.3785 | 1.124 |
| Widowed | <0.0001 | 1.147 |
| Tumor stage | ||
| I | – | 1.0 |
| II | <0.0001 | 1.622 |
| III | <0.0001 | 1.994 |
| IV | <0.0001 | 3.290 |
| Lateral location | ||
| Right upper | – | 1.0 |
| Bronchus, Left | <0.0001 | 1.232 |
| Bronchus, Right | <0.0001 | 1.297 |
| Bronchus, Unknown | 0.0169 | 1.226 |
| Left Lower | <0.0001 | 1.062 |
| Left Upper | 0.0874 | 1.015 |
| Left NOS | <0.0001 | 1.228 |
| Lung, NOS | <0.0001 | 1.211 |
| Left Overlapping | 0.0077 | 1.152 |
| Right Lower | <0.0001 | 1.083 |
| Right Middle | 0.2052 | 1.022 |
| Right NOS | <0.0001 | 1.253 |
| Right Overlapping | <0.0001 | 1.313 |
| Histology—No. (%) | ||
| Adenocarcinoma | – | 1.0 |
| Adenosquamous | <0.0001 | 1.196 |
| Large Cell | <0.0001 | 1.176 |
| Nonsmall Cell | <0.0001 | 1.149 |
| Others | <0.0001 | 1.536 |
| Squamous | <0.0001 | 1.113 |
| Grade | ||
| Well, I | – | 1.0 |
| Moderately, II | <0.0001 | 1.372 |
| Poorly, III | <0.0001 | 1.629 |
| Undifferentiated, IV | <0.0001 | 1.731 |
| Unknown | <0.0001 | 1.537 |
| Definitive Surgical Procedure | <0.0001 | 0.331 |
| Radiation | <0.0001 | 0.759 |
| Year of diagnosis | ||
| 2007 | – | 1.0 |
| 2008 | 0.0664 | 0.982 |
| 2009 | 0.0002 | 0.963 |
| 2010 | <0.0001 | 0.956 |
| 2011 | <0.0001 | 0.914 |
| 2012 | <0.0001 | 0.891 |
Figure 2.
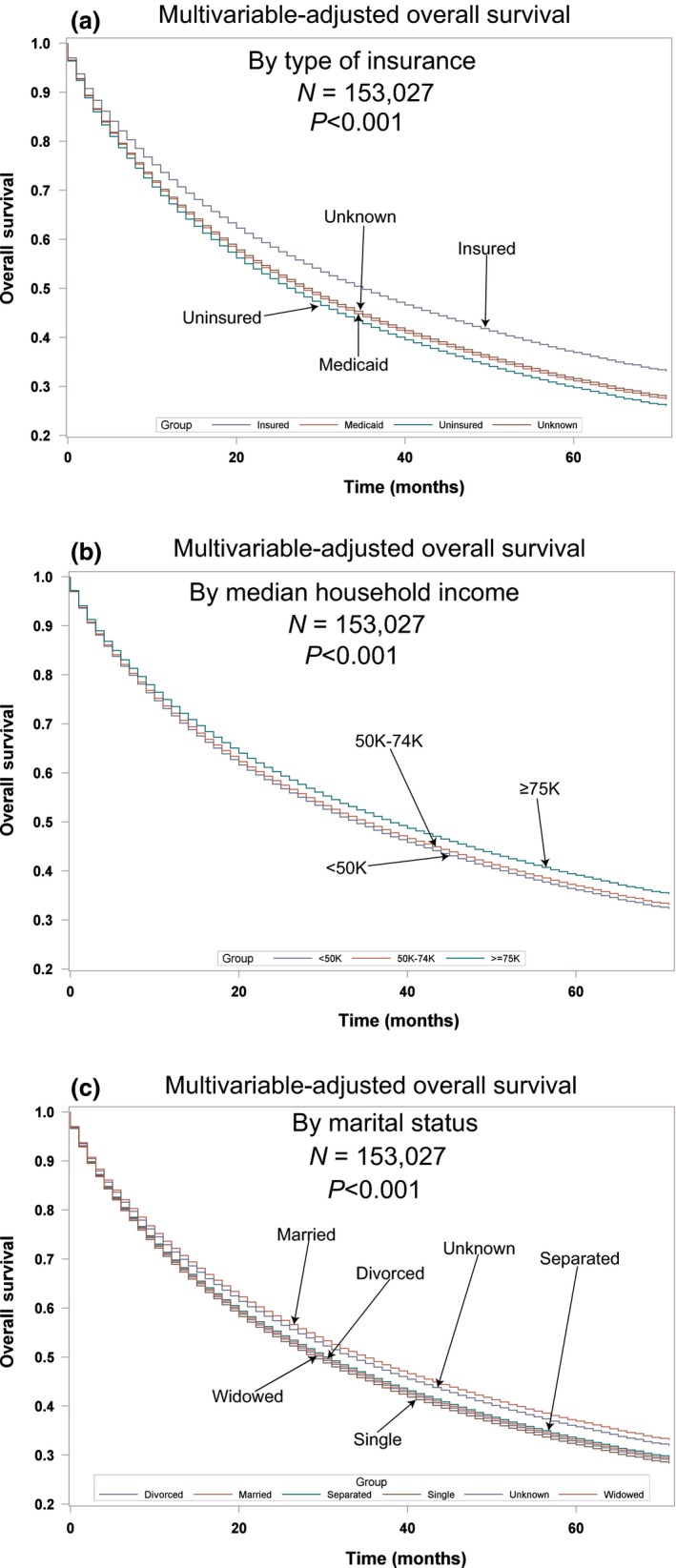
Multivariable‐adjusted overall survival in the total population. (A) by insurance; (B) by income; (C) by marital status.
Multivariable analyses for OS for the Stage IV population can be seen in Table 3. Age (P < 0.0001, HR = 1.017) and male sex (P < 0.0001, HR = 1.233) were associated with worse OS. All races had better OS than Whites (HR = 0.709–0.898) except for AI/AN and Blacks who had a similar OS. OS decreased for lower incomes (P = 0.0484, HR = 1.025) and increased for higher incomes (P < 0.001, HR = 0.934). Insured patients had a better OS than uninsured and those with Medicaid and unknown insurance (all P < 0.0001, HR = 1.195–1.277). Married patients or those with a domestic partner had better OS than those not living in a stable partner situation (divorced, P < 0.0001, HR = 1.154; widowed, P < 0.0001, HR = 1.149; separated, P = 0.0009, HR = 1.134; and unknown, P = 0.0023, HR = 1.069). Involvement of mainstem bronchi and right lower lobe was deleterious for OS. All other histologies were associated with a worse OS compared to adenosquamous cell carcinoma or adenocarcinoma (all P < 0.0001, HR = 1.107–1.482). All tumors differentiation compared to well‐differentiated had significantly worse OS (all P < 0.0001, HRs 1.258–1.702). Palliative radiation significantly improved OS (P < 0.0001, HRs 0.896). Starting in the year 2010, OS started to significantly improve with progressively lower HRs each year.
Table 3.
Multivariate Analysis for OS in Stage IV population, N = 70,968
| All (n = 70,968) | P‐value | Hazard ratio |
|---|---|---|
| Age—year | <0.0001 | 1.017 |
| Sex | ||
| Female | – | 1.0 |
| Male | <0.0001 | 1.233 |
| Race | ||
| White non‐Hispanic | – | 1.0 |
| White Hispanic | <0.0001 | 0.924 |
| Black | 0.1704 | 0.982 |
| Chinese | <0.0001 | 0.709 |
| Japanese | 0.0281 | 0.898 |
| South Asian | <0.0001 | 0.729 |
| Other Asians | <0.0001 | 0.776 |
| Other Races | <0.0001 | 0.800 |
| American Indian/Alaskan Native | 0.9669 | 0.997 |
| SEER Registry | ||
| Alaska Natives | 0.3708 | 1.136 |
| Atlanta | 0.6764 | 0.987 |
| California excl SF/SJM/LA | 0.2900 | 1.022 |
| Connecticut | – | 1.0 |
| Detroit | 0.7774 | 1.007 |
| Greater Georgia | 0.0971 | 1.042 |
| Hawaii | 0.0229 | 1.097 |
| Iowa | 0.0442 | 1.057 |
| Kentucky | 0.0487 | 1.052 |
| Los Angeles | 0.0346 | 0.950 |
| Louisiana | 0.0044 | 1.078 |
| New Jersey | 0.9390 | 0.998 |
| New Mexico | 0.9163 | 1.004 |
| Rural Georgia | 0.9052 | 1.011 |
| San Francisco–Oakland | 0.5813 | 1.015 |
| San Jose–Monterey | 0.4499 | 0.975 |
| Seattle | 0.9550 | 1.001 |
| Utah | 0.6367 | 1.020 |
| Income | ||
| <$50,000 | 0.0484 | 1.025 |
| $50,000–74,000 | – | 1.0 |
| ≥75,000 | <0.0001 | 0.934 |
| Insurance | ||
| Insured | – | 1.0 |
| Medicaid | <0.0001 | 1.195 |
| Uninsured | <0.0001 | 1.273 |
| Unknown | <0.0001 | 1.277 |
| Marital status | ||
| Married | – | 1.0 |
| Divorced | <0.0001 | 1.154 |
| Separated | 0.0009 | 1.134 |
| Single | <0.0001 | 1.167 |
| Unknown | 0.0023 | 1.069 |
| Domestic Partner | 0.1157 | 1.283 |
| Widowed | <0.0001 | 1.149 |
| Lateral location | ||
| Right upper | – | 1.0 |
| Bronchus, Left | <0.0001 | 1.210 |
| Bronchus, Right | <0.0001 | 1.330 |
| Bronchus, Unknown | 0.0104 | 1.308 |
| Left Lower | 0.0607 | 1.029 |
| Left Upper | 0.5952 | 1.006 |
| Left NOS | <0.0001 | 1.181 |
| Lung, NOS | <0.0001 | 1.214 |
| Left Overlapping | 0.0241 | 1.168 |
| Right Lower | 0.0037 | 1.041 |
| Right Middle | 0.4196 | 0.982 |
| Right NOS | <0.0001 | 1.163 |
| Right Overlapping | 0.0002 | 1.186 |
| Histology—No. (%) | ||
| Adenocarcinoma | – | 1.0 |
| Adenosquamous | 0.0831 | 1.067 |
| Large Cell | <0.0001 | 1.174 |
| Nonsmall Cell | <0.0001 | 1.186 |
| Others | <0.0001 | 1.482 |
| Squamous | <0.0001 | 1.107 |
| Grade | ||
| Well, I | ||
| Moderately, II | <0.0001 | 1.258 |
| Poorly, III | <0.0001 | 1.627 |
| Undifferentiated, IV | <0.0001 | 1.702 |
| Unknown | <0.0001 | 1.645 |
| Radiation | <0.0001 | 0.896 |
| Number of Nodes examined | ||
| Year of Diagnosis | ||
| 2007 | – | 1.0 |
| 2008 | 0.4704 | 0.990 |
| 2009 | 0.0684 | 0.976 |
| 2010 | 0.0332 | 0.971 |
| 2011 | <0.0001 | 0.937 |
| 2012 | <0.0001 | 0.899 |
Discussion
A major finding of our analysis is Blacks often present at a younger age, have worse prognostic characteristics, and a lower OS/LCSS than Whites. However, after multivariable adjustment, Blacks have a better OS in the TP and similar OS in the Stage IV patients as compared to Whites. Blacks present with many poor prognostic factors including the following: lower median household income, single/widowed partnership status, higher male predominance, more uninsured, higher stages, and lower rates of definitive surgery. However, Blacks did present at a younger age and have a lower percentage of squamous cell carcinomas, both of which are associated with a better prognosis. As insurance, presentation stage, and surgical eligibility can be altered, there is hope that outcomes for Blacks can be improved with better access to insurance and by increased CT screening 10. In comparison with Whites, Hispanics presented with a higher proportion of several risk factors associated with poor prognosis including more uninsured, a lower proportion of Stage I/II tumors, and lower rates of definitive surgery, but there was no detrimental effect on the unadjusted OS or LCSS in the TP. Furthermore, MVAs demonstrated OS was significantly better for Hispanics compared to Whites in both the TP and Stage IV populations. It should be noted that previous analysis demonstrated this preferential OS benefit associated with Hispanics may be limited to those who are foreign‐born as compared to those born in the United States 11. Because the East Asian populations are enriched for the EGFR mutation tumors 12, it is not surprising the Chinese, South Asian, other Asians, and Japanese had a better adjusted OS in the TP/Stage IV populations, although this analysis lacks details on the mutational status of tumors.
In both MVAs for OS in the TP and Stage IV populations, male sex, poorer tumor differentiation, higher tumor stage, and advanced age were shown to be poor prognostic features and have been well established 13, 14. Furthermore, palliative radiation therapy was found to be important for OS in the Stage IV population. Involvement of the mainstem bronchi and lower lobes was associated with worse OS. Although it can be hypothesized the involvement of the mainstem bronchi can contribute to an increased mortality due to postobstructive pneumonia and/or hypoxia, the survival decrement noted with the lower lobe locations may be due to a greater involvement of normal lung volumes. Because of the known ability of radiation to alleviate symptoms in Stage IV lung cancer 15, we feel the OS benefit noted with radiation in this study may be due to its palliation of central‐based obstructive masses.
Since 2010 (2009 in TP), a consistent improvement in OS was noted in both populations. Although our analysis is unable to identify reasons for this progressive improvement, we feel the reasons are multifactorial. We speculate better staging with frequent use of CT/PET scanning 16 is associated with better outcomes. However, the benefits in the Stage IV population may have also been due to the recognition of chemotherapeutic regimens based upon histology 17 and benefits of targeted therapeutic agents for EGFR mutations 18 and EML4‐ALK translocations 19. Unfortunately, SEER does not contain information regarding the mutations or systemic therapy.
In both patient populations, MVA demonstrated higher income was positively associated with OS. Lower socioeconomic status was previously shown to affect cancer mortality and to be associated with modifiable risk factors such as smoking, diet, BMI, and lower levels of physical activity 20. Unfortunately, these modifiable risk factors are not contained within SEER‐18, but information concerning insurance is available and is more strongly correlated with OS than income. Furthermore, cigarette smoking is noted to be more prevalent in lower socioeconomic groups 21 and could account for the lower OS associated with economic factors. The effects of not being insured have greater effect on OS not only in this population group, but in our companion article concerning surgical patients in these same ethnic groups. It is interesting to note patients with Medicaid have similar hazard ratios for adverse outcomes as compared to those without insurance. We hypothesize the poor outcomes noted in the Medicaid population may be due to the socioeconomic conditions of individuals who have this coverage or possibly due to provider differences. Similar poor outcomes of patients who are receiving Medicaid or who are uninsured have recently been reported in patients with testicular cancers, glioblastomas, and head and neck cancers 22, 23, 24. Nevertheless, hopefully, Medicaid expansion will provide better health outcomes for patients with cancer and has already been associated with increases in medication adherence, preventive care, and healthcare quality 25. In a database of 75 countries obtained from the World Bank and WHO (1990–2010), unemployment was associated with increased lung cancer mortality, but only in men 26. The effects of unemployment on cancer mortality appeared to be mitigated by universal health coverage. Our results suggest the type of insurance can affect the prognosis of patients with short expected survivals, that is, Stage IV. Although higher lung cancer mortality was recently noted in the mid‐South 27 and our analysis indicates there is worse OS in Kentucky and Louisiana in both patient populations, the effects of geography on poor prognosis in our study were not limited to those areas. Our results show married patients or those with a domestic partner have a significantly longer survival, even in metastatic disease. Although our results conflict with those of a past investigation 28 in patients with lung cancer, other investigators have noted unmarried patients with lung cancer had a greater incidence of depression, less social support, and a survival decrement 29.
SEER‐18 lacks many variables including smoking, diet, BMI, levels of physical activity, type of chemotherapeutic agents, radiation doses/volumes, surgical complications, medical office visits, and patient comorbidities. Therefore, our analysis cannot account for these variables.
It should be noted that there are past studies that have shown that Blacks have uniformly worse outcomes than Whites 30, 31, 32, 33, our study is more comprehensive in that we assess nine different ethnic groups and because we adjust for marital, economic, histopathologic, and insurance variables. Our comprehensive analysis allows for a unique finding that Blacks may have better (TP) or similar (Stage IV) outcomes as compared to the White population. Therefore, because the White Hispanic and Black populations present at more advanced stages and have better outcomes, we feel that increased lung cancer screening would be preferentially better in these patients. Such a clear pathway for survival improvement in the White population cannot be ascertained in our population. Unfortunately, SEER does not contain genomic information by race or otherwise. However, at present, genomic information in patients with lung cancer is not prevalent enough (5% or 10% frequency) in ethnic groups other than Whites in the Cancer Genome Atlas (TCGA) 34.
Conclusion
In summary, our analysis does demonstrate racial disparities do exist in the presentation of the Black and Hispanic populations with lung cancer. Both groups were presented with lower rates of insurance, higher stages, and lower rates of definitive surgery. Blacks had a lower OS/LCSS, but when adjusted for histopathologic, therapeutic, marital status, and economic factors, they had a better OS in the TP than Whites. Disparities in income, marital status, and insurance rather than ethnicity affect OS of patients with lung cancer. Because of their more common presentation with advanced disease, the Black and Hispanic groups may benefit preferentially from screening. Our analyses support the expansion of lung cancer screening to people at higher risk of presenting with advanced stage secondary to limited access to health care due to lower income and lack of insurance, particularly in the Black and Hispanic groups. Specifically, affordable and quality healthcare needs to be provided to these at‐risk populations possibly by education/health literacy and care navigators/coordinators. However, the outcome improvement in the White population may need attention in areas other than just screening.
Conflict of Interest
None declared.
Supporting information
Table S1. Contains the demographic, histologic, and treatment details in the TP for the nine different ethnic groups.
Cancer Medicine 2018; 7(4):1211–1220
References
- 1. Miller, K. D. , Siegel R. L., Lin C. C., Mariotto A. B., Kramer J. L., Rowland J. H., et al. 2016. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 66:271–289. [DOI] [PubMed] [Google Scholar]
- 2. Patel, M. , Wang A., Kapphahn K., Desai M., Chlebowski R. T., Simon M. S., et al. 2016. Racial and ethnic variations in lung cancer incidence and mortality: results from the Women's Health Initiative. J. Clin. Oncol. 34:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeSantis, C. E. , Siegel R. L., Sauer A. G., Miller K. D., Fedewa S. A., Alcaraz K. I., et al. 2016. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 66:290–308. [DOI] [PubMed] [Google Scholar]
- 4. Caposole, M. Z. , Miller K., Kim J. N., Steward N. A., and Bauer T. L.. 2014. Elimination of socioeconomic and racial disparities related to lung cancer: closing the gap at a high volume community cancer center. Surg. Oncol. 23:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. A National Cancer Database project in lung cancer patients reported by Shi et al.(2) found that income and race(white, Black, Asian) were not related to survival, but patients with Medicaid or uninsured had a worse outcome.
- 6. Surveillance, Epidemiology, and End Results (SEER) Program . 2012. SEER Public‐use Data (1973–2012). Bethesda, Md: National Cancer Institute, DCCPS, Surveillance Research Statistics Branch. Available at http://seer.cancer.gov/registries/list.html (accessed 16 March 2016).
- 7. Iqbal, J. , Ginsburg O., Rochon P. A., Sun P., and Narod S. A.. 2015. Differences in breast cancer stage at diagnosis and cancer‐specific survival by race and ethnicity in the United States. JAMA 313:165–173. [DOI] [PubMed] [Google Scholar]
- 8. Therneau, T. M. , and Grambsch P. M.. 2000. Modeling survival data: extending the cox model. P. 350 Springer, New York. [Google Scholar]
- 9. Schemper, M. , and Smith T. L.. 1996. A note on quantifying follow‐up in studies of failure time. Control. Clin. Trials 17:343–346. [DOI] [PubMed] [Google Scholar]
- 10. National Lung Screening Trial Research Team , Aberle, D. R. , Adams A. M., Berg C. D., Black W. C., Clapp J. D., et al. 2011. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. New Engl. J. Med. 365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel, M. I. , Schupp C. W., Gomez S. L., Chang E. T., and Wakelee H. A.. 2013. How do social factors explain outcomes in non‐small‐cell lung cancer among Hispanics in California? Explaining the Hispanic paradox. J. Clin. Oncol. 31:3572–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shigematsu, H. , Lin L., Takahashi T., Nomura M., Suzuki M., Wistuba I. I., et al. 2005. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 97:339–346. [DOI] [PubMed] [Google Scholar]
- 13. Chansky, K. , Sculier J. P., Crowley J. J., Giroux D., Van Meerbeeck J., Goldstraw P., et al. 2009. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non‐small cell lung cancer. J. Thorac. Oncol. 4:792–801. [DOI] [PubMed] [Google Scholar]
- 14. Hsu, C. P. , Hsia J. Y., Chang G. C., Chuang C. Y., Shai S. E., Yang S. S., et al. 2009. Surgical‐pathologic factors affect long‐term outcomes in stage IB (pT2 N0 M0) non‐small cell lung cancer: a heterogeneous disease. J. Thorac. Cardiovasc. Surg. 138:426–433. [DOI] [PubMed] [Google Scholar]
- 15. Fairchild, A. , Harris K., Barnes E., Wong R., Lutz S., Bezjak A., et al. 2008. Palliative thoracic radiation for lung cancer: a systemic review. J. Clin. Oncol. 26:4001–4011. [DOI] [PubMed] [Google Scholar]
- 16. Fischer, B. , Lassen U., Mortensen J., Larsen S., Loft A., Bertelsen A., et al. 2009. Preoperative staging of lung cancer with combined pet‐ct. New Engl. J. Med. 361:32–39. [DOI] [PubMed] [Google Scholar]
- 17. Scaggliotti, G. V. , Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. 2008. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J. Clin. Oncol. 26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 18. Maemondo, M. , Ioue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., et al. 2010. Gefitinib or chemotherapy for non‐small cell lung cancer with mutated EGFR. New Engl. J. Med. 362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 19. Solomon, B. J. , Mok T., Kim D. W., Wu Y. L., Nakagawa K., Mekhail T., et al. 2014. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. New Engl. J. Med. 371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 20. Hastert, T. A. , Ruterbusch J. J., Beresford S. A., Sheppard L., and White E.. 2016. Contribution of health behaviors to the association between area‐level socioeconomic status and cancer mortality. Soc. Sci. Med. 148:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mackenbach, J. P. , Huisman M., Andersen O., Bopp M., Borgan J. K., Borrell C., et al. 2004. Inequalities in lung cancer mortality by the educational level in 10 European populations. Eur. J. Cancer 40:126–135. [DOI] [PubMed] [Google Scholar]
- 22. Markt, S. C. , Lago‐Hernandez C. A., Miller R. E., Mahal B. A., Bernard B., Albiges L., et al. 2016. Insurance status and disparities in disease presentation, treatment, and outcome for men with germ cell tumors. Cancer 122:3127–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rong, X. , Yang W., Garzon‐Muvdi T., Caplan J. M., Hui X., Lim M., et al. 2016. Influence of insurance status on survival of adults with glioblastoma multiformed: a population based study. Cancer 122:3157–3165. [DOI] [PubMed] [Google Scholar]
- 24. Naghavi, A. O. , Echevarria M. I., Grass D. I., et al. 2016. Having Medicaid insurance negatively impacts outcomes in patients with head and neck malignancies. Cancer 122:3529–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sommers, B. D. , Blendon R. J., Orav J., and Epstein A. M.. 2016. Changes in utilization and health among low‐income adults after Medicaid expansion or expanded private insurance. JAMA Intern. Med. 176:1501–1509. [DOI] [PubMed] [Google Scholar]
- 26. Maruthappu, M. , Watkins J., Noor A. M., Williams C., Ali R., Sullivan R., et al. 2016. Economic downturns, universal health coverage, and cancer mortality in high‐income and middle‐income countries, 1990‐2010: a longitudinal analysis. Lancet 388:684–695. [DOI] [PubMed] [Google Scholar]
- 27. Torre, L. A. , Siegel R. L., and Jemal A.. 2016. Lung cancer statistics. Adv. Exp. Med. Biol. 893:1–19. [DOI] [PubMed] [Google Scholar]
- 28. Jatoi, A. , Novotny P., Cassivi S., Clark M. M., Midthun D., Patten C. A., et al. 2007. Does marital status impact survival and quality of life in patients with non‐small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist 12:1456–1463. [DOI] [PubMed] [Google Scholar]
- 29. Sullivan, D. R. , Forsberg C. W., Ganzini L., Au D. H., Gould M. K., Provenzale D., et al. 2016. Depression symptoms and health domains among lung cancer patients in CanCORS study. Lung Cancer 100:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hunt, B. , and Balachandran B.. 2015. Black: White disparities in lung cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 39:908–916. [DOI] [PubMed] [Google Scholar]
- 31. Coughlin, S. S. , Matthews‐Juarez P., Juarez P. D., Melton C. E., and King M.. 2014. Opportunities to address lung cancer disparities among African Americans. Cancer Med. 3:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richards, T. B. , Henley S. J., Puckett M. C., Weir H. K., Huang B., Tucker T. C., et al. 2017. Lung cancer survival in the United States by race and stage(2001‐2009): Findings from the CONCORD‐2 study. Cancer 123(Suppl. 24):5079–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sin, M. K. 2017. Lung cancer disparities and African‐Americans. Public Health Nurs. 34:359–362. [DOI] [PubMed] [Google Scholar]
- 34. Spratt, D. E. , Chan T., Waldron L., Speers C., Feng F. Y., Ogunwobi O. O., et al. 2016. Racial/Ethnic disparities in genomic sequencing. JAMA Oncol. 2:1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Contains the demographic, histologic, and treatment details in the TP for the nine different ethnic groups.


