Abstract
Lung cancer is the commonly diagnosed cancer and one of the most important avoidable causes of death around the world. We conducted the study to investigate the pattern of lung cancer incidence worldwide. Joinpoint analysis was used to extend international lung cancer incidence rates by the latest data from Cancer Incidence in Five Continents over the 35‐year period 1973–2007 from 24 populations from Americas, Asia, Europe, and Oceania. Age‐standardized incidence rates (ASRs) of lung cancer were from 33.3 to 66.8 per 100,000 among males and 10.5 to 37.4 per 100,000 among females in most of Americas, Europe, and Oceania populations during the period 2003–2007. In Asia, ASRs in China (Hong Kong) were the highest, up to 53.3 per 100,000 in males and 21.9 per 100,000 in females during the period 2003–2007. The international trends between 1973 and 2007 showed that ASRs of lung cancer among males were declining in 13 of 18 selected Americas, Oceania, and Europe populations, with AAPC from −0.7% to −2.9%, whereas the rates among females in 18 selected populations were increasing, with AAPC from 1.3% to 5.0%. The increasing and decreasing trends of ASRs of lung cancer in Asia have a geographic variation but no gender differences. Although the decreasing trends in ASRs of lung cancer for males were observed, the ASRs were higher than females. The declining trends in males were mainly attributed to tobacco control, whereas the increasing trends in females should be given more concern and need to be further studied in etiology factors.
Keywords: Average annual percentage change, incidence, international trends, joinpoint analysis, lung neoplasms
Introduction
Lung cancer is the commonly diagnosed cancer and one of the most important avoidable causes of death around the world. According to GLOBOCAN 2012, lung cancer is estimated to cause 1.6 million deaths in the world, which were quantitatively close to the corresponding 1.8 million cases 1. Lung cancer is more common in males than females with a cumulative life time (0–74) risk of 4% and 1%, respectively 1. Whereas the incidence rates for males have been declining in most areas of North America, the Nordic countries, Europe, and Oceania, while rates for females have been rising rapidly virtually everywhere 2. Tobacco smoking may be attributable to lung cancer pattern. Blot reported that the majority (85–90%) of lung cancer among women like in men are considered to be caused by smoking 3.
The major histological subtypes of lung cancer comprise adenocarcinoma, squamous carcinoma, small cell carcinoma, and large cell carcinoma, which in aggregate, account for approximately 99% cases of primary lung cancer 4. The predominant form of lung cancer has been squamous carcinoma in males and adenocarcinoma in females, although adenocarcinoma has surpassed squamous cell carcinoma in frequency among males in several populations in recent years 2.
Some studies have described the international lung cancer trends by histologic subtypes except for Asia 2, 5, 6. The patterns and trends in lung cancer incidence have varied because of difference in smoking patterns and exposures to other lung carcinogens 7, 8. Herein, we examined the international trends in overall lung cancer incidence among males and females in 24 selected populations from Americas, Asia, European, and Oceania during 35‐year period (1973–2007) and described the distribution of lung cancer incidence by histological subtypes based on the 2003–2007 incidence data.
Materials and Methods
Incidence data
To examine the changing trends in the incidence of lung cancer over time, age‐standardized (by world standard population 9) incidence rates (ASRs) by sex were obtained from Volumes 4–10 of Cancer Incidence in Five Continents (CI5) 10, 11, 12, 13, 14, 15, 16 from the Web site of International Agency for Research on Cancer (IARC). Volumes 4–10 of CI5 generally provided data by 5‐year period: 1973–1977, 1978–1982, 1983–1987, 1988–1992, 1993–1997, 1998–2002, and 2003–2007. Incidence data by histologic subtypes (adenocarcinoma, squamous carcinoma, small cell carcinoma, and large cell carcinoma) from 2003 to 2007 were collected from 24 populations in four continents from Vol. 10 of CI5. Classification of lung cancer from Vols. 4, 5–8, and 9–10 of CI5 was coded according to the International Classification of Diseases (ICD) 8th (163), 9th (163), and 10th (C33‐C34) revisions, respectively.
Populations were chosen for inclusion in our study on the basis of the following criteria: (1) incidence for time periods at least as far back as 1983–1987; (2) no changes in population coverage or warnings regarding data quality reported in CI5 Vols. 4–10; and (3) a sufficiently large number of registered cases in CI5 Vol. 10 to enable analyses of recent rates by histologic subtypes (trends by histologic subtypes were not included in our study). Only one registry from each country was selected; if more than one registry met the basic criteria, the registry with the largest population was included in the analysis (except for China, Hong Kong, and Shanghai). A total of 24 populations were selected: four from the Americas (Canada, British Columbia (BC); Colombia, Cali; USA, Black/White), six from Asia (China, Hong Kong; China, Shanghai; India, Mumbai; Israel, Jews; Japan, Osaka Prefecture; Singapore, Chinese), five from Northern Europe (Denmark; Finland; Norway; Sweden; UK, England, North Western Region (NWR)), three from Western Europe (France, Bas‐Rhin; Germany, Saarland; Switzerland, Geneva), four from elsewhere in Europe 17 (Southern and Central & Eastern Europe including Italy, Varese Province; Poland, Cracow; Slovakia; Spain, Navarra), and two from Oceania (Australia, New South Wales (NSW); New Zealand). No African populations met all the inclusion criteria. However, four African populations (Algeria, Setif Wilaya; Egypt, Gharbiah; Uganda, Kyadondo; Zimbabwe, Harare: African) were chosen to describe the lung incidence in the last time interval (2003–2007).
Incidence data for white and black populations in United States were not included in CI5 vols. 4 (1973–1977) and 5 (1978–1982), so we further referred to the U.S. Surveillance Epidemiology and End Results (SEER) dataset 18. SEER program is a population‐based cancer registration system covering 18 registries and 28% of the U.S. population. Long‐term data from 1973 to 2010 were available from nine registries that included approximately 9.4% of the U.S. population (based on 2010 census).
The data for New Zealand between 1983–1987 and 1988–2002 were not included in CI5, but contained in CI5plus 19 which is a database of successive volumes of CI5 series. So we abstracted the data for New Zealand during the periods 1983–1987 and 1988–2002 from CI5plus and the latest time period 2003–2007 from CI5 vol.10.
Data analysis
Incidence trends in ASRs of lung cancer were analyzed using Joinpoint regression (Joinpoint regression software, version 3.5.3‐May 2012, available through the Surveillance Research Program of the US National Cancer Institute). The permutation method was used for significance tests. Changes in annual incidence rates from lung cancer were calculated as annual percentage change (APC) in each segment. In the final model, the Joinpoint analysis provided average annual percentage change (AAPC). The significant test of APC and AAPC to 0 was also conducted.
Age‐standardized incidence rates of lung cancer by histologic subtypes (adenocarcinoma, squamous carcinoma, small cell carcinoma, and large cell carcinoma) and sex for selected populations during the period 2003–2007 were integrated and calculated. Secular trends in ASRs of lung cancer were examined by registries, sexes for every five‐year period during 1973–2007. The trends in ASRs of lung cancer from New Zealand were described during five‐year time periods from 1983–1987 to 2003–2007. Figures displaying the incidence trends were prepared using a semilog scale to facilitate the comparison of temporal trends as well as magnitude. These data were plotted at the midpoint of each five‐year interval.
Results
Age‐standardized incidence rates of lung cancer in 2003–2007 were the highest in Americas and Europe (U.S. black, Poland, and Germany) and much lower in most of populations in Asia and Africa (India, Algeria, and Uganda; Tables 1 and 2). In most of Americas, Europe, and Oceania, ASRs of lung cancer ranged from 33.3 to 66.8 per 100,000 among males and 10.5 to 37.4 per 100,000 among females other than Colombia (19.0 per 100,000 in males and 8.9 per 100,000 in females, respectively). In Asia, ASRs in China (Hong Kong) were the highest, up to 53.3 per 100,000 in males and 21.9 per 100,000 in females. The variation of ASRs ranged from 29.8 per 100,000 in Israel (Jews) to 44.7 per 100,000 in Singapore (Chinese) among males and from 13.4 per 100,000 in Israel (Jews) to 18.1 per 100,000 in China (Shanghai) among females. In Africa, the extent of ASRs was between 10.1 per 100,000 and 19.9 per 100,000 in males and from 3.7 per 100,000 to 6.4 per 100,000 in females.
Table 1.
International variation in lung cancer incidence rates, from 1973–1977 to 2003–2007
| Country | Period of registry established | Mean of MV%a | Males | Joinpoint analyses (1973–2007) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1973–1977 | 2003–2007 | Trend 1 | Trend 2 | AAPC (%) | |||||||
| Cases | Rateb | Cases | Rateb | Period | APC (%) | Period | APC (%) | ||||
| Northern Europe | |||||||||||
| Denmark | 1953–1957 | 86.2 | 7585 | 51.8 | 10,822 | 45.0 | 1975~1985 | 0.9 | 1985~2005 | −1.3g | −0.7g |
| (1973–1976) | |||||||||||
| Finland | 1959–1961 | 85.6 | 11,523 | 74.4 | 8126 | 34.4 | 1975~1985 | −1.6 | 1985~2005 | −3.4g | −2.9g |
| (1971–1976) | |||||||||||
| Norway | 1959–1961 | 89.6 | 3710 | 25.4 | 7182 | 36.7 | 1975~2005 | 1.0g | 1.0g | ||
| Sweden | 1959–1961 | 97.9 | 8007 | 23.8 | 9087 | 20.7 | 1975~2005 | 0.4 | 1985~2005 | −1.1g | −0.7g |
| (1971–1975) | |||||||||||
| UK, England, NWR | 1973–1977 | 53.8 | 12,807 | 86.0 | 14,188 | 47.4 | 1975~1985 | −0.6 | 1985~2005 | −2.8g | −2.2g |
| Western Europe | |||||||||||
| France, Bas‐Rhin | 1975–1977 | 92.1 | 860 | 54.3 | 2098 | 53.2 | 1975~1990 | 1.5g | 1990~2005 | −1.7g | −0.1 |
| (1975–1977) | |||||||||||
| Germany, Saarland | 1968–1972 | 79.0c | 2629 | 74.4 | 3048 | 58.4 | 1975~1990 | 0.5 | 1990~2005 | −1.2g | −0.9g |
| Switzerland, Geneva | 1970–1972 | 95.1 | 703 | 69.4 | 743 | 42.9 | 1975~2005 | −1.8g | −1.8g | ||
| Europe, Other | |||||||||||
| Italy, Varese Province | 1976–1977 | 73.6 | 642 | 70.4 | 2170 | 51.3 | 1975~1985 | 1.9 | 1985~2005 | −2.4g | −1.2g |
| (1976–1977) | |||||||||||
| Poland, Cracow | 1973‐1977 | 66.1 | 1138 | 74.6 | 1252 | 58.7 | 1975~2005 | −0.8g | −0.8g | ||
| (2003–2006) | |||||||||||
| Slovakia | 1973–1977 | 70.9 | 2549 | 43.8 | 9453 | 56.2 | 1975~1985 | 6.4g | 1985~2005 | −2.1g | 0.3g |
| Spain, Navarra | 1973–1977 | 84.8 | 357 | 23.5 | 1350 | 50.5 | 1975~2005 | 2.3g | 2.3g | ||
| Americas | |||||||||||
| Canada, BC | 1969–1972 | 78.1 | 3501 | 49.0 | 7097 | 38.3 | 1975~1985 | 2.1 | 1985~2005 | −2.3g | −0.8g |
| Colombia, Cali | 1967–1971 | 53.9 | 193 | 19.5 | 810 | 19.0 | 1975~1985 | 2.4 | 1985~2005 | −1.5g | −0.4 |
| (1972–1976) | |||||||||||
| USA, SEER: Black | 1973–1975 | 91.8d | 3043e | 87.1 | 4972 | 66.8 | 1975~1985 | 2.2g | 1985~2005 | −2.4g | −0.9g |
| USA, SEER: White | 1973–1975 | 90.7d | 28,365e | 60.4 | 35,816 | 44.8 | 1975~1985 | 0.8 | 1985~2005 | −1.9g | −1.1g |
| Oceania | |||||||||||
| Australia, NSW | 1973–1977 | 82.1 | 6756 | 51.4 | 9665 | 34.7 | 1975~1985 | 0.1 | 1985~2005 | −2.1g | −1.5g |
| New Zealand | 1960‐1962 | 75.7f | – | – | 5124 | 33.3 | 1985~2005 | −2.6g | −2.6g | ||
| Asia | |||||||||||
| China, Hong Kong | 1974–1977 | 63.5 | 3666 | 55.5 | 14,003 | 53.3 | 1975~1985 | 4.0g | 1985~2005 | −1.9g | −0.2 |
| (1974–1977) | |||||||||||
| China, Shanghai | 1975 | 44.0 | 1306 | 51.2 | 12,413 | 40.9 | 1975~2005 | −0.7 | −0.7 | ||
| (1975) | |||||||||||
| India, Mumbai | 1964–1966 | 60.0 | 714 | 14.2 | 2134 | 9.3 | 1975~2005 | −1.7g | −1.7g | ||
| (1973–1975) | |||||||||||
| Israel: Jews | 1960–1966 | 81.7 | 2254 | 29.3 | 5114 | 29.8 | 1975~2005 | 0.2g | 0.2g | ||
| (1972–1976) | |||||||||||
| Japan, Osaka Prefecture | 1970‐1971 | 70.9 | 4713 | 29.4 | 19,929 | 41.6 | 1975~1985 | 3.8g | 1985~2005 | 0.0 | 1.0g |
| Singapore: Chinese | 1950‐1961 | 79.6 | 1734 | 68.0 | 3338 | 44.7 | 1975~1985 | 0.2 | 1985~2005 | −2.2g | −1.5g |
| Africa | |||||||||||
| Algeria, Setif Wilaya | 1986–1989 | – | – | 466 | 19.9 | ||||||
| Egypt, Gharbiah | 1999‐2002 | – | – | 795 | 11.9 | ||||||
| Uganda, Kyadondo | 1954‐1960 | – | – | 59 | 5.2 | ||||||
| Zimbabwe, Harare: African | 1990‐1992 | – | – | 87 | 10.1 | ||||||
| (2003–2006) | |||||||||||
APC, annual percent change; AAPC, average annual percent change.
Average of MV% (Percentage of morphologically verified cases) was calculated from 1978 to 2007.
Rate is age‐standardized to the world population, per 100,000 person‐years.
Germany, Saarland (1983–2007).
USA, SEER: Black/White (1988–2007).
The data of USA, SEER: Black/White were from SEER nine registries database.
New Zealand (1993–2007).
APC/AAPC is significantly different from 0 (two‐sided P < 0.05).
Table 2.
International variation in lung cancer incidence rates, from 1973–1977 to 2003–2007
| Country | Period of registry established | Mean of MV%a | Females | Joinpoint analyses (1973–2007) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1973–1977 | 2003–2007 | Trend 1 | Trend 2 | AAPC (%) | |||||||
| Cases | Rateb | Cases | Rateb | Period | APC (%) | Period | APC (%) | ||||
| Northern Europe | |||||||||||
| Denmark | 1953–1957 | 86.5 | 1967 | 12.1 | 9252 | 35.2 | 1975~1985 | 6.7g | 1985~2005 | 2.1g | 3.4g |
| (1973–1976) | |||||||||||
| Finland | 1959–1961 | 85.0 | 1245 | 5.8 | 3250 | 10.8 | 1975~1985 | 2.5g | 1985~2005 | 1.7g | 1.9g |
| (1971–1976) | |||||||||||
| Norway | 1959–1961 | 89.0 | 881 | 5.4 | 4886 | 23.3 | 1975~1995 | 5.7g | 1995~2005 | 3.3g | 5.0g |
| Sweden | 1959–1961 | 97.6 | 2161 | 5.7 | 7791 | 17.2 | 1975~1985 | 5.2g | 1985~2005 | 2.9g | 3.6g |
| (1971–1975) | |||||||||||
| UK, England, NWR | 1973–1977 | 51.1 | 3194 | 16.8 | 11,280 | 30.8 | 1975~1985 | 4.5g | 1985~2005 | 0.7 | 1.8g |
| Western Europe | |||||||||||
| France, Bas‐Rhin | 1975–1977 | 89.5 | 94 | 4.2 | 670 | 14.5 | 1975~1985 | 3.2g | 1985~2005 | 5.1g | 4.6g |
| (1975–1977) | |||||||||||
| Germany, Saarland | 1968–1972 | 76.8c | 276 | 5.4 | 1215 | 21.1 | 1975~1985 | 3.4g | 1985~2005 | 5.3g | 4.7g |
| Switzerland, Geneva | 1970–1972 | 93.1 | 121 | 8.5 | 405 | 18.7 | 1975~2005 | 2.6g | 2.6g | ||
| Europe, Other | |||||||||||
| Italy, Varese Province | 1976–1977 | 66.1 | 71 | 5.8 | 646 | 12.6 | 1975~1985 | 3.3g | 1985~2005 | 2.4g | 2.6g |
| (1976–1977) | |||||||||||
| Poland, Cracow | 1973‐1977 | 60.7 | 269 | 11.1 | 568 | 17.9 | 1975~1995 | 2.2g | 1995~2005 | 0.4 | 1.7g |
| (2003–2006) | |||||||||||
| Slovakia | 1973–1977 | 63.4 | 309 | 4.4 | 2465 | 10.5 | 1975~2005 | 2.2g | 2.2g | ||
| Spain, Navarra | 1973–1977 | 75.9 | 49 | 2.6 | 232 | 8.9 | 1975~2005 | 3.6g | 3.6g | ||
| Americas | |||||||||||
| Canada, BC | 1969–1972 | 78.9 | 1054 | 14.1 | 6440 | 32.1 | 1975~1985 | 7.3g | 1985~2005 | 0.7 | 2.5g |
| Colombia, Cali | 1967–1971 | 55.0 | 71 | 5.4 | 512 | 8.9 | 1975~1985 | 5.7g | 1985~2005 | −0.8g | 1.3g |
| (1972–1976) | |||||||||||
| USA, SEER: Black | 1973–1975 | 90.6d | 852e | 20.4 | 3919 | 37.4 | 1975~1985 | 4.2g | 1985~2005 | −0.2 | 2.0g |
| USA, SEER: White | 1973–1975 | 89.7d | 10,068e | 18.4 | 33,168 | 34.6 | 1975~1985 | 5.5g | 1985~2005 | 0.6g | 2.0g |
| Oceania | |||||||||||
| Australia, NSW | 1973–1977 | 81.6 | 1343 | 8.7 | 5824 | 19.2 | 1975~1985 | 4.5g | 1985~2005 | 1.7g | 2.5g |
| New Zealand | 1960‐1962 | 74.7f | – | – | 3873 | 23.5 | 1985~2005 | 1.3g | 1.3g | ||
| Asia | |||||||||||
| China, Hong Kong | 1974–1977 | 57.6 | 1942 | 23.4 | 6817 | 21.9 | 1975~1985 | 3.7g | 1985~2005 | −1.9g | −0.3 |
| (1974–1977) | |||||||||||
| China, Shanghai | 1975 | 39.0 | 539 | 18.1 | 6238 | 18.1 | 1975~2005 | 0.1 | 0.1 | ||
| (1975) | |||||||||||
| India, Mumbai | 1964–1966 | 57.6 | 154 | 4.0 | 824 | 3.5 | 1975~2005 | −0.3 | −0.3 | ||
| (1973–1975) | |||||||||||
| Israel: Jews | 1960–1966 | 79.8 | 725 | 9.0 | 2887 | 13.4 | 1975~2005 | 1.4g | |||
| (1972–1976) | |||||||||||
| Japan, Osaka Prefecture | 1970‐1971 | 64.5 | 1748 | 8.5 | 8408 | 13.9 | 1975~1985 | 3.3g | 1985~2005 | 0.9 | 1.6g |
| Singapore: Chinese | 1950‐1961 | 74.5 | 624 | 19.8 | 1713 | 17.6 | 1975~2005 | −0.7g | −0.7g | ||
| Africa | |||||||||||
| Algeria, Setif Wilaya | 1986–1989 | – | – | 69 | 2.6 | ||||||
| Egypt, Gharbiah | 1999‐2002 | – | – | 257 | 3.7 | ||||||
| Uganda, Kyadondo | 1954‐1960 | – | – | 55 | 5.1 | ||||||
| Zimbabwe, Harare: African | 1990‐1992 | – | – | 49 | 6.4 | ||||||
| (2003–2006) | |||||||||||
APC, annual percent change; AAPC, average annual percent change.
Average of MV% (Percentage of morphologically verified cases) was calculated from 1978 to 2007.
Rate is age‐standardized to the world population, per 100,000 person‐years.
Germany, Saarland (1983–2007).
USA, SEER: Black/White (1988–2007).
The data of USA, SEER: Black/White were from SEER nine registries database.
New Zealand (1993–2007).
APC/AAPC is significantly different from 0 (two‐sided P < 0.05).
In American, European, and Oceania populations, there were different trends in ASRs for lung cancer for both males and females. Among males, ASRs for lung cancer in 13 of 18 populations decreased significantly from 1973 to 2007, with AAPC from −0.7% to −2.9% (P < 0.05; Table 1 and Fig. 1A), of which nine populations including Denmark, Finland, Sweden, UK, England, Germany, Italy, Canada, USA: Black, USA: White and Australia kept declining after mid‐1990s. In contrast, ASRs for lung cancer in Norway, Slovakia, and Spain showed a significant increase trends from 1973 to 2007, with AAPC of 1.0%, 0.3%, and 2.3% (P < 0.05), respectively. ASRs for lung cancer in France and Colombia leveled off in the whole period, with AAPC of −0.1% and −0.4% (P > 0.05), respectively. Among females, ASRs for lung cancer in 18 populations showed a significant increase during all over the period, with AAPC from 1.3% to 5.0% (P < 0.05; Table 2 and Fig. 1B).
Figure 1.
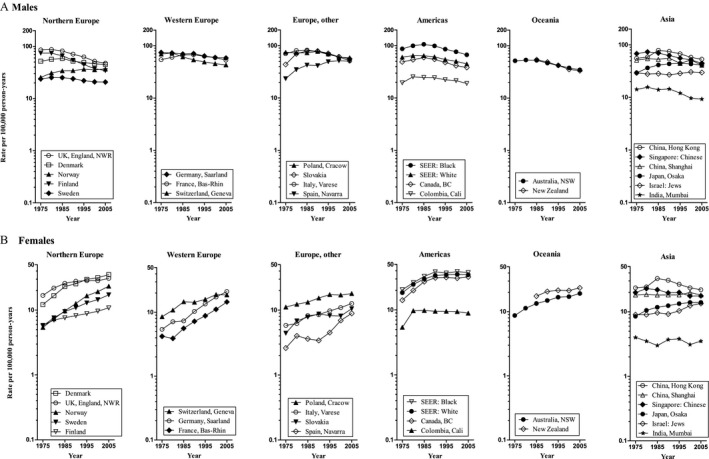
Trends in age‐standardized lung cancer incidence rates by continent and area for the time period 1973–2007: (A) Males, (B) Females.
In Asia, there were similar trends in ASRs for lung cancer for both males and females except for India (Tables 1 and 2 and Fig. 1). The increasing trends in Israel and Japan were observed from 1973 to 2007 (AAPC = 0.2% and 1.0% in males, 1.0% and 1.6% in females, respectively; P < 0.05), whereas decreasing trends in Singapore (AAPC = −1.5% in males, −0.7% in females, respectively; P < 0.05). ASRs for lung cancer in China (Hong Kong) and China (Shanghai) showed a stable trends from 1973 to 2007 (AAPC = −0.2% and −0.3% in males, −0.7% and 0.1% in females, respectively; P > 0.05).
Age‐standardized incidence rates for lung cancer by histologic subtypes from 2003 to 2007 suggested that adenocarcinoma for males was the dominant histologic subtypes in 17 of 24 selected populations, whereas squamous carcinoma in the seven populations, and the main histologic subtypes for females were adenocarcinoma in all selected populations, followed by squamous carcinoma, small cell carcinoma, and large cell carcinoma (Fig. 2). The highest incidence of adenocarcinoma was observed in USA: Black (19.1 per 100,000 in males and 13.0 per 100,000 in females, respectively) and China (Hong Kong; 19.1 per 100,000 in males and 12.6 per 100,000 in females, respectively), and the lowest one was shown in India (2.2 per 100,000 in males and 1.1 per 100,000 in females, respectively). The squamous carcinoma was frequent histologic subtypes in the seven populations by males including Slovakia (21.9 per 100,000), Germany (17.7 per 100,000), France (17.6 per 100,000), Poland (16.8 per 100,000), Spain (13.5 per 100,000), UK, England (10.9 per 100,000), and Finland (7.9 per 100,000). The incidence of squamous carcinoma for females in the seven selected populations showed an extent from 1.0 per 100,000 to 4.6 per 100,000. The small cell carcinoma incidence rates for both males and females were higher than large cell carcinoma except for Spain (13.9 per 100,000 in males, 2.3 per 100,000 in females, respectively), Italy (10.3 per 100,000 in males, 2.4 per 100,000 in females, respectively), Australia (6.6 per 100,000, 3.7 per 100,000 in females, respectively), Sweden (4.5 per 100,000, 3.4 per 100,000 in females, respectively), and Colombia (2.8 per 100,000, 1.4 per 100,000 in females, respectively).
Figure 2.
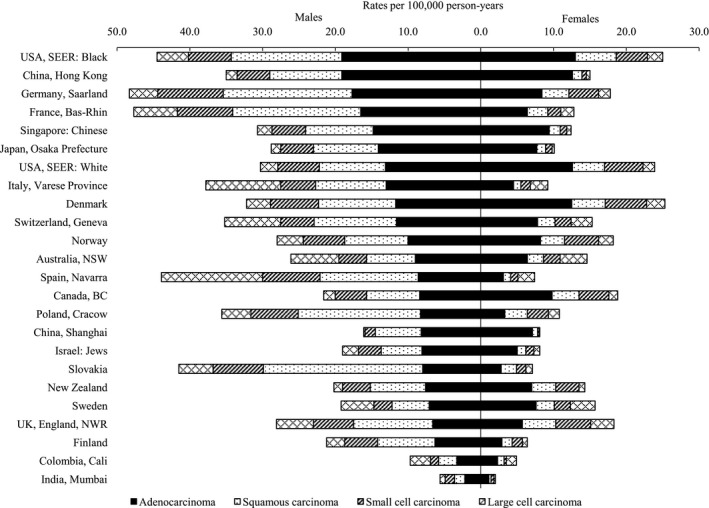
Age‐standardized lung cancer incidence rates by histologic subtypes for selected populations for the time period 2003–2007.
Discussion
Age‐standardized incidence rates for lung cancer have been declining among males in most of Americas, Europe, and Oceania, while ASRs among females have been rising rapidly in these populations. Meanwhile, the increasing and decreasing trends were seen among males and females but no gender difference in Asian populations.
The overwhelming cause of lung cancer is tobacco smoking. The association between cigarette smoking and lung cancer was first established in 1950 20, and about 85% of lung cancer cases had a tobacco smoking history 21. Decreasing lung cancer incidence trends for males and increasing trends for females in some developed countries were observed which may be in part because of the variation of the prevalent smoking. Forey B et al. 22 suggested that the prevalence of tobacco smoking among males has declined since the 1960s in virtually all the areas except Spain, and incidence rate of lung cancer in Spain was continuing to increase. In our study, the increasing trend in Spain (Navarra) was observed across all the period. The reason of the phenomenon in Spain (Navarra) was not fully clear, but tobacco smoking might be a main reason. In several western countries including the United States, the United Kingdom, Canada, and Australia, the tobacco epidemic was established and peaked by the middle of the last century when lung cancer incidence rates had been decreasing in males and plateauing in females 23, 24. However, in our study, ASRs of lung cancer for females in USA: Black after 1988–1992 kept plateauing. This directly reflects the patterns of smoking prevalence among women in the past. These differences in smoking habits between North American and European women were observed. In North America, women started smoking earlier and consumed more cigarettes than in Frances 25. Smoking prevalence among women in the United States peaked at 33.3% in 1965 and remained at that level throughout the late 1970s. Since that time, the rate has declined to a level of 23.5% 21. Tobacco consumption started increasing in French women in the middle 1960s 26. The increasing trends of lung cancer incidence among females in European countries all over period were observed in our study. Besides smoking habit, the variation in lung cancer and its occurrence among females and males has been also hypothesized to be related to genetic susceptibility and biological indicators 27, 28, hormonal factors 29, and so on. Furthermore, the air pollution induced by industrialization in more developed countries is another possible cause. Traffic‐related Nox and CO showed significant correlations with the lung cancer incidence in females but not in males 30.
In Asia, the decreasing trends were observed in Singapore (Chinese) and India (Mumbai) males from 1973 to 2007 and China (Hong Kong) after mid‐1990s, although there was nonsignificant AAPC in China (Hong Kong) males and females. The declining trends for males in these populations can be explained by the gradual decline in smoking prevalence. However, the prevalence of smoking for females is low in Asia, which is different from western countries. It could reflect the effect of nonsmoking‐related risk factors more clearly. In Hong Kong, the prevalence of daily smokers decreased from 39.7% in 1982 to 22% in 2000 in males and from 5.6% to 3.5% in females 31. The other risk factors relating to lung cancer have changed especially indoor air pollution (IAP) including indoor fuel pollution and cooking. IAP is a major concern in less developed countries where biomass fuels are used for cooking and heating. Coal burning has been strongly associated with lung cancer risk in previous studies of rural and developing populations with high exposure 32, 33, and poor home ventilation can increase exposure to carcinogenic particulates which elevates lung cancer risk 34. The various possible etiologies of lung cancer have been probed in nonsmoking Chinese women living in Hong Kong 35. It is reported that the diet acts as an epidemiological confounder in the assessment of association between air pollution and female lung cancer in Hong Kong. Other risk factors for lung cancer including exposure to occupational and environmental carcinogens can also increase the risk of lung cancer 36. The lung cancer incidence rates for females in Shanghai kept stable in all period. Shanghai is a highly developed city, where the people's lifestyle is relatively close to the western developed countries. The pattern of lung cancer incidence may be likely because of the westernized lifestyle. Meanwhile, the use of rapeseed oil is common in Shanghai, which may be one of reasons. The association between lung cancer and frying pan fumes caused by rapeseed oil has been studied in Shanghai 37. A study from Shanghai had showed the stable level in lung cancer incidence for females and was likely to continue to increase in the future barring interventions were carried out for smoking cessation 38. In contrast, the lung cancer incidence in Japan and Israel (Jews) kept increasing trends. Lung cancer incidence reached a peak in the mid‐1990s for both males and females in Japan. As 70% of male lung cancer cases were reported because of active tobacco smoking 39, the observed peaks in lung cancer incidence might be a result of the high smoking prevalence in the 1960s or 1970s. The screening programs for lung cancer were implemented in 1987 40. Also, some changes in lifestyle, such as an increase in the proportion of dietary fat in energy intake 41, could be another explanation.
The occurrence of lung cancer is a slow process. The status of lung cancer resulted from different living environment and different way of life in the past 10 years or longer. With the decrease in the number of smoking, lung cancer incidence is still high. Besides exposure to occupational and environmental carcinogens, outdoor pollution needs to be focused 42. Since the 1970s, the United States 43 and Europe 44 have confirmed that lung cancer is associated with PM2.5. In October 2013, IARC classified particulate matter (PM) from outdoor air pollution as carcinogenic to humans and causes lung cancer 45. The surveillance of PM2.5 has begun in China, we cannot probe the relationship between the health problems and PM2.5 because of the shortage of data. Therefore, it is concluded that environmental pollution was one of an important risk factor. More than one‐half of the lung cancer deaths attributable to ambient fine particles were projected to have been in China and other East Asian countries 46.
This study has several strengths in that the data were abstracted from large, well‐established registries throughout the world. For the first time, data covering 35 years were analyzed to describe the variation of international trends in lung cancer incidence rates, which may stimulate further etiologic studies. In addition, this study can provide the update data of lung cancer for researchers in the world. This study, however, has limitations in that the trends in histologic subtypes of lung cancer were not investigated. Our study was also limited by the lack of nationwide cancer registries in some countries, where the registration data may not accurately reflect the true patterns in their nations.
In summary, our analysis on published CI5 data suggested that lung cancer incidence rates were declining among males and increasing among females in western populations where the highest incidence rate was still seen from 1973 to 2007. In Asia, there were increasing trends among males and females in some of countries and decreasing in the rest of populations. The international patterns of ASRs of lung cancer incidence were mainly attributed to tobacco smoking. However, other risk factors especially in females, including smoking habits, genetic susceptibility, and biological indicators, need to be further studied. The ongoing global tobacco reform is necessary to reduce the international burden of lung cancer.
Conflict of Interest
The authors declare that they have no competing interests.
Supporting information
Figure S1. The trends in age‐standardized smoking prevalence and ASRs of lung cancer for males.
Figure S2. The trends in age‐standardized smoking prevalence and ASRs of lung cancer for females.
Figure S3. The trends in mean annual consumption per capita and ASRs of lung cancer.
Acknowledgments
We are very grateful to Rong‐Shou Zheng from National Office for Cancer Prevention and Control, National Cancer Center / Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College for providing us with valuable opinions and suggestions for data interpretation. This study was funded by Cancer Screening Program in Urban China, Lung Cancer Early Detection Program (China) (No. CH‐L‐043), and National Key R&D Program of China (No. 2017YFC0907900, No. 2016YFC1302500, No. 2017YFC1308700, No. 2016YFC0905400).
Cancer Medicine 2018; 7(4):1479–1489
The first two authors contributed equally to this article.
References
- 1. Ferlay, J. , Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., et al. 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer, Lyon, France. Available at http://globocan.iarc.fr (accessed 20 January 2014).
- 2. Devesa, S. S. , Bray F., Vizcaino A. P., and Parkin D. M.. 2005. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer 117:294–299. [DOI] [PubMed] [Google Scholar]
- 3. Blot, W. J. , and McLaughlin J. K.. 2004. Are women more susceptible to lung cancer? J. Natl Cancer Inst. 96:812–813. [DOI] [PubMed] [Google Scholar]
- 4. Wahbah, M. , Boroumand N., Castro C., El‐Zeky F., and Eltorky M.. 2007. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann. Diagn. Pathol. 11:89–96. [DOI] [PubMed] [Google Scholar]
- 5. Janssen‐Heijnen, M. L. G. , and Coebergh J.‐W. W.. 2001. Trends in incidence and prognosis of the histological subtypes of lung cancer in North America, Australia, New Zealand and Europe. Lung Cancer 31:123–137. [DOI] [PubMed] [Google Scholar]
- 6. Lortet‐Tieulent, J. , Soerjomataram I., Ferlay J., Rutherford M., Weiderpass E., and Bray F.. 2014. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 84:13–22. [DOI] [PubMed] [Google Scholar]
- 7. Parkin, D. , Bray F. I., and Devesa S.. 2001. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 37:S4–S66. [DOI] [PubMed] [Google Scholar]
- 8. Blot, W. J. , and Fraumeni J. J.. 1996. Cancers of the lung and pleura Pp. 637–665 in Schotten‐feld D. and Fraumeni J. F., Jr, eds. Cancer epidemiology and prevention, 2nd ed Oxford Univ. Press, New York, NY. [Google Scholar]
- 9. Segi, M. 1960. Cancer mortality for selected sites in 24 countries (1950–57). Tohuku Univ. School of Public Health, Sendai. [Google Scholar]
- 10. Waterhouse, J. , Muir C. S., Shanmugaratnam K., and Powell J.. 1982. Cancer Incidence in Five Continents, Vol. IV. IARC Scientific Publications No. 42. IARC, Lyon.
- 11. Muir, C. S. , Waterhouse J., Mack T., Powell J., and Whelan S. L.. 1987. Cancer Incidence in Five Continents, Vol. V. IARC Scientific Publications No. 88. IARC, Lyon.
- 12. Parkin, D. M. , Muir C. S., Whelan S. L., Gao Y. T., Ferlay J., and Powell J.. 1992. Cancer Incidence in Five Continents, Vol. VI.IARC Scientific Publications No. 120. IARC, Lyon.
- 13. Parkin, D. M. , Whelan S. L., Ferlay J., Raymond L., and Young J.. 1997. Cancer Incidence in Five Continents, Vol. VII.IARC Scientific Publications No. 143. IARC, Lyon.
- 14. Parkin, D. M. , Whelan S. L., Ferlay J., Teppo L., and Thomas D. B.. 2002. Cancer Incidence in Five Continents, Vol. VIII. IARC Scientific Publications No. 155. IARC, Lyon.
- 15. Curado, M. P. , Edwards B., Shin H. R., Storm H., Ferlay J., Heanue M., et al. 2007. Cancer Incidence in Five Continents, Vol. IX. IARC Scientific Publications No. 160. IARC, Lyon.
- 16. Forman, D. , Bray F., Brewster D. H., Gombe M. C., Kohler B., Piñeros M., et al. 2013. Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon, IARC. Available at http://ci5.iarc.fr (accessed 28 October 2015).
- 17. Lortet‐Tieulent, J. , Renteria E., Sharp L., Weiderpass E., Comber H., Baas P., et al. 2015. Convergence of decreasing male and increasing female incidence rates in major tobacco‐related cancers in Europe in 1988–2010. Eur. J. Cancer 51:1144–1163. [DOI] [PubMed] [Google Scholar]
- 18. Surveillance, Epidemiology, and End Results (SEER) Program (November 2012 submission). Public use database (1973–1982). Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version < Version 8.0.4>, Released April 15, 2013.
- 19. Ferlay, J. , Parkin D. M., Curado M. P., Bray F., Edwards B., Shin H. R., et al. 2010. Cancer Incidence in Five Continents, Volumes I to IX: IARC CancerBase No. 9 [Internet]. International Agency for Research on Cancer, Lyon, France. Available at http://ci5.iarc.fr (accessed 30 May 2014).
- 20. Fukumoto, K. , Ito H., Matsuo K., Tanaka H., Yokoi K., Tajima K., et al. 2015. Cigarette smoke inhalation and risk of lung cancer: a case‐control study in a large Japanese population. Eur. J. Cancer Prev. 24:195–200. [DOI] [PubMed] [Google Scholar]
- 21. Olak, J. , and Colson Y.. 2004. Gender differences in lung cancer: have we really come a long way, baby? J. Thorac. Cardiovasc. Surg. 128:346–351. [DOI] [PubMed] [Google Scholar]
- 22. Forey, B. , Hamling J., Lee P., and Wald N.. 2002. International smoking statistics, 2nd ed Oxford Univ. Press, Oxford. [Google Scholar]
- 23. Jemal, A. , Thun M. J., Ries L. A., Howe H. L., Weir H. K., Center M. M., et al. 2008. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J. Natl Cancer Inst. 100:1672–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peto, R. , Lopez A., Boreham J., and Thun M.. 2006. Mortality from smoking in developed countries 1950–2000, 2nd ed Available at http://www.ctsu.ox.ac.uk/~tobacco/SMK_All_PAGES.pd. [Google Scholar]
- 25. Charloux, A. , Rossignol M., Purohit A., Small D., Wolkove N., Pauli G., et al. 1997. International differences in epidemiology of lung adenocarcinoma. Lung Cancer 16:133–143. [DOI] [PubMed] [Google Scholar]
- 26. European Bureau for Action on Smoking Prevention . 1990. Europe for smoking prevention. Newsletter 8. BASP, Brussels.
- 27. Mollerup, S. , Ryberg D., Hewer A., Phillips D. H., and Haugen A.. 1999. Sex differences in lung CYP1A1 expression and DNA Adduct Lever among Lung Cancer Patients. Cancer Res. 59:3317–3320. [PubMed] [Google Scholar]
- 28. Schwartz, A. G. , Siegfried J. M., and Weiss L.. 1999. Familial aggregation of breast cancer with early onset lung cancer. Genet. Epidemiol. 17:274–284. [DOI] [PubMed] [Google Scholar]
- 29. Siegfried, J. M. 2001. Women and lung cancer: does oestrogen play a role? Lancet Oncol. 2:506–513. [DOI] [PubMed] [Google Scholar]
- 30. Liaw, Y. P. , Ting T. F., Ho K. K., and Yang C. F.. 2008. Cell type specificity of lung cancer associated with air pollution. Sci. Total Environ. 395:23–27. [DOI] [PubMed] [Google Scholar]
- 31. Au, J. S. , Mang O. W., Foo W., and Law S. C.. 2004. Time trends of lung cancer incidence by histologic types and smoking prevalence in Hong Kong 1983–2000. Lung Cancer 45:143–152. [DOI] [PubMed] [Google Scholar]
- 32. Armstrong, B. , Hutchinson E., Unwin J., and Fletcher T.. 2004. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta‐analysis. Environ. Health Perspect. 112:970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barone‐Adesi, F. , Chapman R. S., Silverman D. T., He X., Hu W., Vermeulen R., et al. 2012. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ 345:e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim, C. , Gao Y. T., Xiang Y. B., Barone‐Adesi F., Zhang Y., Hosgood H. D., et al. 2015. Home kitchen ventilation, cooking fuels, and lung cancer risk in a prospective cohort of never smoking women in Shanghai, China. Int. J. Cancer 136:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koo, L. C. , and Ho J. H.‐C.. 1996. Is it air pollution or diet?: Diet as an epidemiological confounder of the association between air pollution and female lung cancer in Hong Kong. Tumor 16:467–474 (Chinese). [DOI] [PubMed] [Google Scholar]
- 36. Spitz, M. R. , Wu X. C., Wilkinson A., and Wei Q.. 2006. Cancer of the lung Pp. 638–658 in Schottenfeld D. and Fraumeni J., Jr, eds. Cancer epidemiology and prevention, 3rd ed Oxford Univ. Press, New York, NY. [Google Scholar]
- 37. Chiang, T. A. , Wu P. F., and Ko Y. C.. 1999. Identification of carcinogens in Cooking Oil Fumes. Environ. Res. 81:18–22. [DOI] [PubMed] [Google Scholar]
- 38. Liao, M. L. , Chen Z. W., Zheng Y., Wu C. X., Lu S., Yu Y. F., et al. 2007. Incidence, time trend, survival, and predictive factors of lung cancer in Shanghai population. Natl. Med. J. China 87:1876–1880. [PubMed] [Google Scholar]
- 39. Katanoda, K. , Marugame T., Saika K., Satoh H., Tajima K., Suzuki T., et al. 2008. Population attributable fraction of mortality associated with tobacco smoking in Japan: a pooled analysis of three large‐scale cohort studies. J. Epidemiol. 18:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katanoda, K. , Matsuda T., Matsuda A., Shibata A., Nishino Y., Fujita M., et al. 2013. An updated report of the trends in cancer incidence and mortality in Japan. Jpn. J. Clin. Oncol. 43:492–507. [DOI] [PubMed] [Google Scholar]
- 41. Yoshimi, I. , Ohshima A., Ajiki W., Tsukuma H., and Sobue T.. 2003. A comparison of trends in the incidence rate of lung cancer by histological type in the Osaka cancer registry, Japan and in the surveillance, epidemiology and end results program, USA. Jpn. J. Clin. Oncol. 33:98–104. [DOI] [PubMed] [Google Scholar]
- 42. Hamra, G. B. , Guha N., Cohen A., Laden F., Raaschou‐Nielsen O., Samet J. M., et al. 2014. Outdoor particulate matter exposure and lung cancer: a systematic review and meta‐analysis. Environ. Health Perspect. 122:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beeson, W. L. , Abbey D. E., and Knutsen S. F.. 1998. Long term concentrations of ambient air pollutants and incident lung cancer in California adults:Results from the AHSMOG study. Environ. Health Perspect. 106:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harrison, R. M. , and Smith D.. 2004. What is responsible for the carcinogenicity of PM2.5? Occup. Environ. Med. 61:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loomis, D. , Grosse Y., Lauby‐Secretan B., El Ghissassi F., Bouvard V., Benbrahim‐Tallaa L., et al. 2013. The carcinogenicity of outdoor air pollution. Lancet Oncol. 14:1262–1263. [DOI] [PubMed] [Google Scholar]
- 46. Straif, K. , Cohen A., and Samet J.. 2013. Air pollution and cancer. IARC Scientific Pub. No. 161. IARC Press, Lyon, France. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The trends in age‐standardized smoking prevalence and ASRs of lung cancer for males.
Figure S2. The trends in age‐standardized smoking prevalence and ASRs of lung cancer for females.
Figure S3. The trends in mean annual consumption per capita and ASRs of lung cancer.


