Abstract
The present study aimed to investigate the effect and possible mechanism of action of crocetin on the high cholesterol diet (HCD) induced atherosclerosis rat. The Wistar rats were used in the current investigation. The rats were divided into following group, Group I: control, Group II: HCD induced AS, Group III: AS + crocetin (25 mg/kg), Group IV: AS + crocetin (50 mg/kg) and Group V: AS + Simvastatin, respectively. AS was induced in the rats using the vitamin D3 and HCD. The rats received the pre-determined treatment for the 10 weeks. After the study period, the level of lipid profile, malonaldehyde (MDA) and superoxide dismutase (SOD) were also estimated. The proinflammatory cytokines viz., tumor necrosis factor (TNF)-α and interleukin (IL)-6 were scrutinized using the ELISA kits. We also estimated the expression of phosphorylated p38 (p-p38) MAPK using the Western blot techniques. The results revealed that the AS was successfully induced in the rats. The AS control group rats showed the modulated level of lipid profile, and decreased the level of the SOD and boost the level of the MDA as compared with the normal control. However, crocetin thrived in enhancing the lipid profile toward the standard value in the normal control group rats. The crocetin and simvastatin group rats significantly inhibited the expression of the p-p38 MAPK as compared to the AS group rats. In conclusion, the current investigation revealed that the crocetin reduced the HCD induced dyslipidemia in the Wistar rats, the possible mechanism of action may be connected to the antioxidative, down regulating of p-p38 MAPK and antiinflammatory effect by crocetin.
Keywords: Crocetin, Atherosclerosis inflammation, Dyslipidemia, p38 MAPK, Oxidative stress
1. Introduction
Atherosclerosis (also known as arteriosclerotic vascular disease or ASVD) is a specific form of arteriosclerosis in which an artery-wall thickens as a result of invasion and accumulation of white blood cells (WBCs) (foam cell) and proliferation of intimal-smooth-muscle cell creating a fibrofatty plaque. The accumulation of the white blood cells is termed “fatty streaks” early on because of the appearance being similar to that of marbled steak. These accumulations contain living, active WBCs (producing inflammation) and remnants of dead cells, including cholesterol and triglycerides. The remnants eventually include calcium and other crystallized materials within the outermost and oldest plaque. The “fatty streaks” reduce the elasticity of the artery walls. However, they do not affect blood flow for decades because the artery muscular wall enlarges at the locations of plaque. The wall stiffening may eventually increase pulse pressure; widened pulse pressure is one possible result of advanced disease within the major arteries. Atherosclerosis (AS) is a clinical condition of the cholesterol, it shows the hardening and narrowing the arteries as the consequence of manufacturing the fatty material (Wang et al., 2014a, Wang et al., 2014b). It generally occurs due to the cardiovascular diseases viz., acute coronary disease, strokes and heart attack. While, the mechanism of action and occurrence of the AS still remain not properly explained, few researchers claim that the few risk factors such as hypotension, diabetes and dyslipidemia take part in the initiation of AS. Among the different risk reasons, dyslipidemia have an imperative role in the pathogenesis of the AS, during the dyslipidemia, the patients often confirm the high concentration of low density protein, which can be oxidized through vessels cell and changed into the oxidized LDL. The oxidized LDL level starts the pathological changes such as inflammation, oxidative stress and endothelium damage.
For the treatment of the AS, statins are commonly used due to their excellent efficacy in minimizing the LDL level in the serum and inhibiting the vascular risk, but the continuous use of statins lead to more side effects viz., rhabdomyolysis, myopathy, liver injury, muscle toxicity and acute renal failure (Jose et al., 2014, Hopewell et al., 2014). Medicinal plant derived metabolites were known for various biological activities (Antonisamy et al., 2015, Balamurugan, 2015, Rathi et al., 2015, Nandhini and Stella Bai, 2015, Kalaiselvi et al., 2016, Neelamkavil and Thoppil, 2016, Valsan and Raphael, 2016). Consequently, the plant flavonoids having anti-artherosclerotic effect gained the more popularity and also proved to inhibit the risk of the CVS including AS in a large number of clinical and fundamental status (Lo et al., 2012, Shen et al., 2014, Xing et al., 2013, Noorudheen and Chandrasekharan, 2016, Santhosh et al., 2016, Sreeshma et al., 2016, Puthur, 2016, Serasanambati and Chilakapati, 2016).
In the current investigation, we confirmed the protective effect of the crocetin in the HCD induced AS rats. We hypothesized that crocetin attenuates the expression and instigation of AS by reducing p38 MAPK pathway and inflammatory response. We also estimated the crocetin effect on the pathogenesis of the AS rats. Our data confirmed that the crocetin treatment considerably prevented the expansion of AS.
2. Materials and methods
2.1. Animal treatment
In the current study, we used the Swiss Albino Wistar rats (150–210 g). The rats were procured from the Departmental animal house. The rats were kept in separate cages under normal laboratory conditions (temperature 24 ± 2 °C, air humidity 50 ± 15%, 12 h light–dark cycle) and had free access to drinking water and were fed the normal pellet diet for 7 days. The rats were acclimatization for 7 days, after acclimatization of rats in the laboratory conditions, the Wistar rats were divided into the following groups: Group I: normal control, Group II: normal control treated with crocetin, Group III: atherosclerosis, Group IV: received crocetin (25 mg/kg), Group V: received crocetin (50 mg/kg) [23] and Group VI: treated with simvastatin. The high-cholesterol diet (HCD) was induced using the vitamin D3 (70 U/kg) for continuous administration for 3 days. For the HCD, the ingredients such as normal diet (80.3%), animal oil (11%), cholesterol (4.5%), sodium cholate (1.5%), propylthiouracil (0.7%) and refined sugar (4%) were added at the end of the study (9th week). After 9 weeks, half of the animals were selected from the normal control group and AS group for the estimation of whether the AS was successfully induced. The serum sample of the rats were collected and used for the scrutinization of the lipid profile such as TG, TC, LDL, HDL and for the histopathological examination the tissue was selected from the aortic arch vessel. After replacing the HCD diet with the normal laboratory diet all group rats received the predetermined treatment for 10 weeks. After the 10 week treatment of the rats, all group rats were sacrificed and the blood samples of all group rats were collected. The blood samples were centrifuged at 15 g rpm for 15 min and the serums were used to estimate the different biochemical assay. At the end of the study, all group rats were sacrificed and the aortic arch vessel quickly removed for scrutinizing the histopathological characters, western blot analyses and gene expression.
2.2. Aortic fatty streak staining
For the Aortic Streak Staining the thoracic aortas were sliced to describe the intimal surface. The tissue samples were immediately washed with the normal saline and stained using Sudan IV for 10 min.
2.3. Biochemical assays estimation
The level of the biochemical parameters viz., TG, TC, LDL, HDL and antioxidant parameter viz., SOD and MDA were scrutinized using the available kit using the method provided by the manufacture.
2.4. Estimation of the inflammatory mediator
The proinflammatory parameters viz., TNF-α and IL-6 were also estimated using the ELISA kits.
2.5. Western blot analysis
For the estimation of the western blot sample, the thoracic samples were homogenized in the homogenizer containing protease inhibitor and vortexed for ½ h. The tissue samples were centrifuged at a high speed (15,000× g for 15 min) and the protein extract (supernatant) was collected, and the concentration of the protein was estimated using the bicinchoninic acid (BCA) method. The equal quantity of the samples were placed on the SDS–PAGE (10%) gels for maintaining the current 120V for 2 h and after that the samples were transferred onto the PVDF membranes for 1 h at the 100 V. After that the membrane was blocked for 60 min at room temperature (37 °C) with non fat dry milk (5%). The primary antibodies were incubated at 4 °C with the membrane, and followed by the secondary antibodies which were also incubated at the room temperature (37 °C) for 60 min.
2.6. Statistical analysis
The results of the current investigation were presented as mean standard (SD). In the current study the one-way analysis of variance (ANOVA) was applied for multiple comparisons and the P value less than 0.05 was considered to be statistically significant.
3. Result
3.1. Estimation of AS on animal
As presented in the Fig. 1, the weight of the AS control group had less weight gain as compared to the normal group rats after the end of the study (9th week). The AS control group rats demonstrated the increased level of the lipid profile in the blood such as TG, TC, LDL and decreased level of the HDL as compared to the normal control group rats (Fig. 2).
Figure 1.
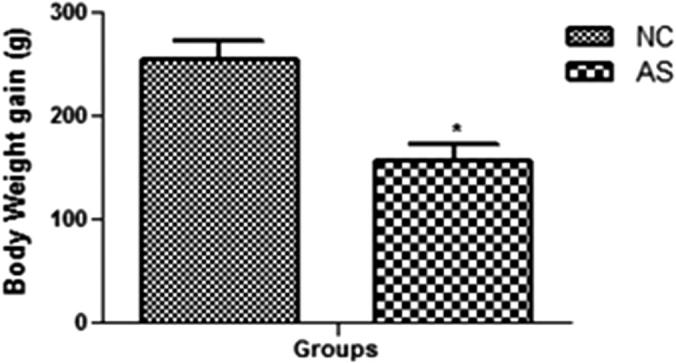
Body weight gain of normal control and AS control group rats. Values are mean ± SD; n = 10 AS = atherosclerosis. Compared with the control group *P < 0.01.
Figure 2.
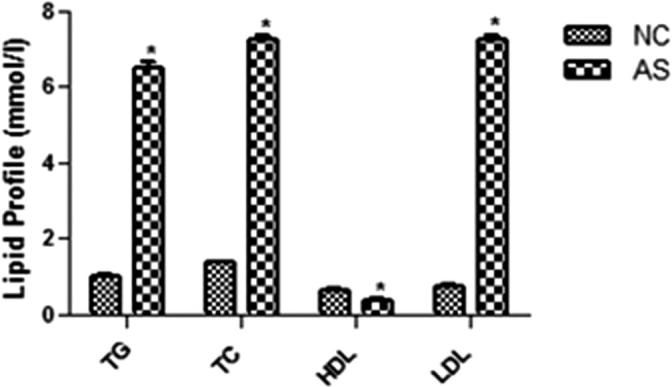
Lipid profile of the normal control and AS control group rats. Values are mean ± SD; n = 10 TG = triglyceride, TC = total cholesterol, LDL-C = low-density lipoprotein-cholesterol, HDL-C high-density lipoprotein-cholesterol, AS = atherosclerosis. Compared with the control group *P < 0.01.
3.2. Estimation of biochemical parameters
The figures confirmed the blood lipid profile in all group rats. Fig. 2 showed the increased level of TG (Fig. 3), TC (Fig. 4) and LDL (Fig. 5) in the AS control rats as compared with the normal control. Although, AS control rats received the crocetin significantly (P < 0.05) modulated the biochemical parameters and crocetin treated rats confirmed the recovery of the declined level of HDL at dose dependently (Fig. 6). The AS group rats explained the enhanced level of the MDA (Fig. 7) and declined level of the SOD (Fig. 8) which was significantly restored by the crocetin treatment in a dose dependent manner.
Figure 3.
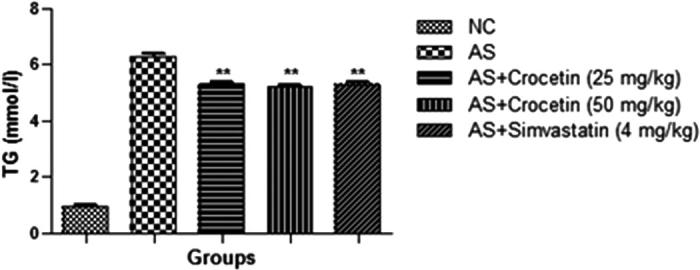
Effect of crocetin and simvastatin of the TG of all group rats. Values are mean ± SD; n = 10 TG = triglyceride, AS = atherosclerosis. Compared with the AS group **P < 0.01.
Figure 4.
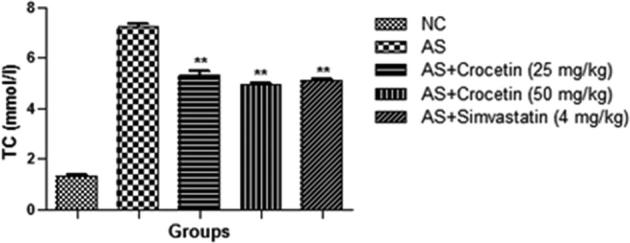
Effect of crocetin and simvastatin of the TC of all group rats. Values are mean ± SD; n = 10 TC = total cholesterol, AS = atherosclerosis. Compared with the AS group **P < 0.01.
Figure 5.
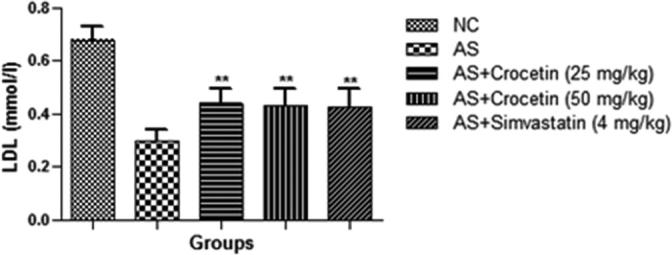
Effect of crocetin and simvastatin of the LDL of all group rats. Values are mean ± SD; n = 10 LDL = low density cholesterol, AS = atherosclerosis. Compared with the AS group ∗∗P < 0.01.
Figure 6.
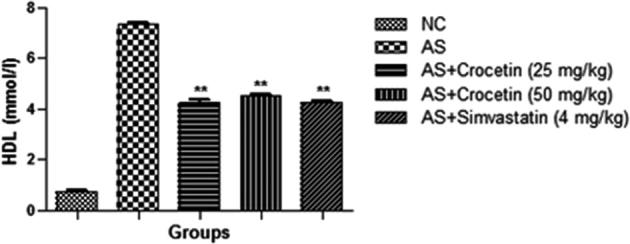
Effect of crocetin and simvastatin of the HDL of all group rats. Values are mean ± SD; n = 10 LDL = low density cholesterol, AS = atherosclerosis. Compared with the AS group **P < 0.01.
Figure 7.
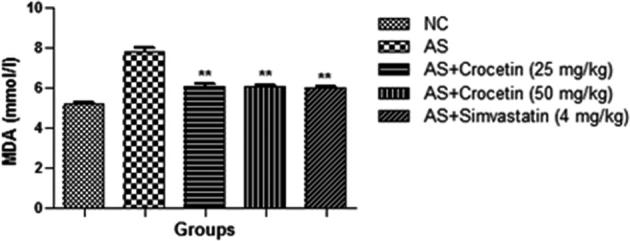
Effect of crocetin and simvastatin of the MDA of all group rats. Values are mean ± SD; n = 10 MDA = malonaldehyde, AS = atherosclerosis. Compared with the AS group **P < 0.01.
Figure 8.
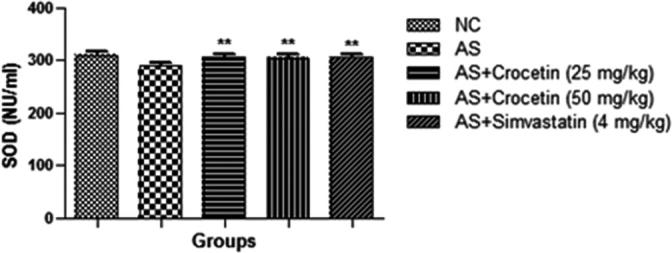
Effect of crocetin and simvastatin of the SOD of all group rats. Values are mean ± SD; n = 10 SOD = superoxide dismutase, AS = atherosclerosis. Compared with the AS group **P < 0.01.
3.3. Estimation of the inflammatory cytokines
The level of the inflammatory cytokines viz., TNF-α and IL-6 is presented in the Figure 9, Figure 10. Fig. 3 confirmed the increased level of the inflammatory cytokines (TNF-α and IL-6) as compared with the control group rats. The AS group rats treated with the crocetin significantly (P < 0.05) reduced as comparison with the AS control rats at dose dependently.
Figure 9.
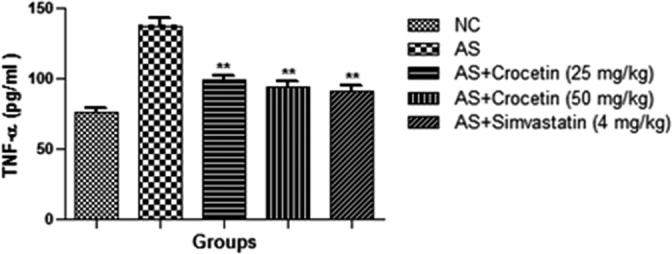
Effect of crocetin and simvastatin on the TNF-α of all group rats. Values are mean ± SD; n = 10 TNF-α = Tumor necrosis factor-α, AS = atherosclerosis. Compared with the AS group **P < 0.01.
Figure 10.
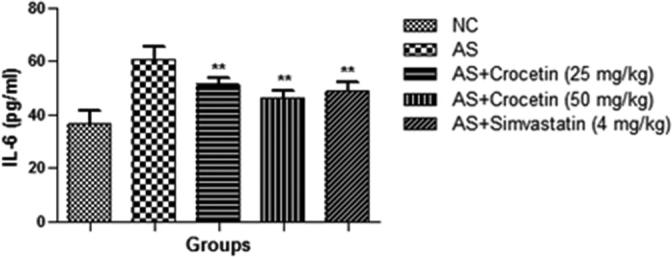
Effect of crocetin and simvastatin on the IL-6 of all group rats. Values are mean ± SD; n = 10 IL-6 = interlukin-6, AS = atherosclerosis. Compared with the AS group **P < 0.01.
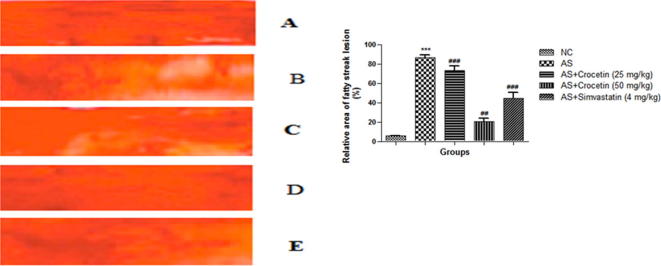
3.4. Aortic fatty streak staining
The Fig. 11 demonstrated the considerably enhanced aortic fatty streak lesions in AS group. Conversely, the AS group rats that received the crocetin showed considerable inhibition of the intensity of the aorta intima. The result of the crocetin group rats was almost similar to that of the simvastatin group rats.
Figure 11.
Aortic fatty streak in all group rats is at the end of the experimental period in different groups. (a) Normal control; (b) AS group; (c) AS + crocetin (25 mg/kg); d = AS + crocetin (50 mg/kg) e = AS + simvastatin (4 mg/kg); f relative area of fatty streak lesion. Compared with the control group, ***P < 0.001. Compared with the AS group, ##P < 0.01, ###P < 0.001.
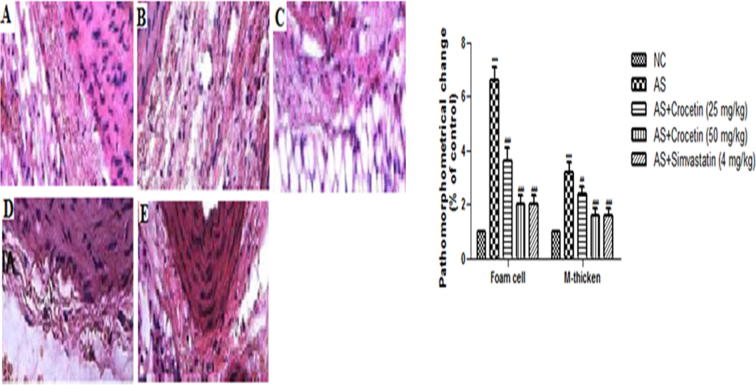
3.5. Histopathological observation
Fig. 12 demonstrates the histopathological examination of all group rats. The normal control group rat histopathology explained the intact and usual aortic intima with orderly arranged endothelial cells. In the AS control group rats, we observed the disarrangement of the endothelial cells along with the proliferation, deposition of the foam cells and thicken artery tunica as compared to the normal control group rats. AS control rats received a different dose of the crocetin and simvastatin that confirmed the thinner aortic tunica as compared to the AS group. The finding of the current investigation confirm the inhibitory effect of crocetin in the expansion of the aortic lesion in the AS control rats.
Figure 12.
Figure showed the different histopathological changes of the aortic intima in the different group of rats at the end of the experimental study. (a) Normal control; (b) AS group; (c) AS + crocetin (25 mg/kg); d = AS + crocetin (50 mg/kg) e = AS + simvastatin (4 mg/kg);. Five fields of vision were selected to count the number of foam cells in individual experiments (×200). The thickness of tunica media (M thickness) = (the ring diameter of external elastic membrane–the ring diameter of inner elastic)/2π. AS = atherosclerosis. Compared with the control group, ***P < 0.001. Compared with the AS group, ##P < 0.01, ###P < 0.001.
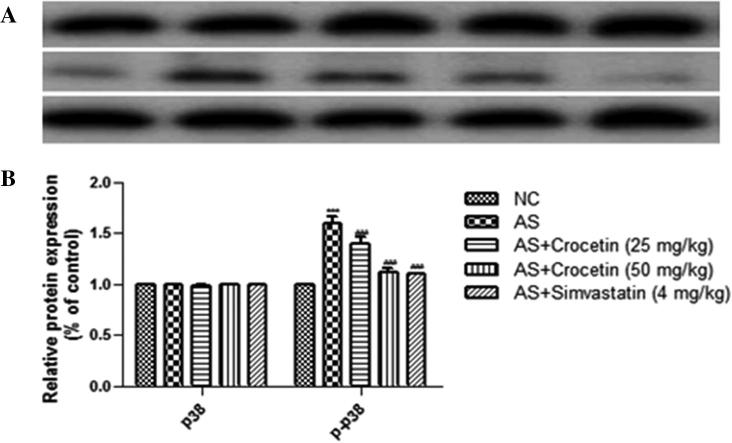
3.6. Western blot examination
For the determination of the phosphorylation concentration of the p38 MAPK, we used the western blot technique. The figure displayed that increased level of the phosphorylation concentration of the p38 MAPK in the aortas thoracic in the AS group as comparison with the normal control rats. The concentration of p38 MAPK significantly inhibited by the crocetin at concentration dependently. The data of the current study confirmed that the crocetin attenuates the AS induced pathogenesis via p38 MAPK signaling pathways (Fig. 13).
Figure 13.
Figure demonstrated the western blot data. Representative Western blots and p-p38 MAPK expression levels in the rat thoracic aorta of various groups. (A): Western blot assays for p38MAPK and p-p38MAPK expression. (B): p38MAPK and p-p38MAPK expression as a ratio to the control. (a) Normal control; (b) AS group; (c) AS + crocetin (25 mg/kg); d = AS + crocetin (50 mg/kg) e = AS + simvastatin (4 mg/kg). AS atherosclerosis, p38 MAPK p38 mitogen-activated protein kinase, p-p38 MAPK phosphorylated p38 MAPK. Compared with the control group, ***P < 0.001. Compared with the AS group, ###P < 0.001.
4. Discussion
Various researchers claim that the augmented level of the serum cholesterol is the imperative feature of the atherogenesis factor (Li et al., 2008). The epidemiological study confirms the correlation between the LDL, TC and the serve degree of the AS, and the elevated level of oxidized LDL was connected with the enhance frequency of hypertriglyceridemia (Goldstein et al., 1973). It already proved that the AS clinical complications were inhibited, and the lifetime was delayed via reducing the concentration of the blood lipid profile viz., TG, TC and LDL (Illingworth and Bacon, 1987). However, the concentration of the HDL was enhanced in the AS conditions, and it also showed that the HDL was inversely associated with the risk factor of the AS (Castelli et al., 1986, Gordon et al., 1989). The current investigation explained that the crocetin significantly enhanced the hyperlipidemia, confirming that the crocetin could be beneficial for the lipid profile via attenuating the progression of AS.
The lipid metabolism disorder and inflammatory mediators, also arise in the aorta of the hypercholesterolemic rats (Deng et al., 2006, Deng et al., 2015). The previous reports showed that the unusual inflammatory reaction may take part in the progression and expansion of the AS (Haddy et al., 2003). During the AS condition, proinflammatory inflammatory mediators viz., (IL-6 and TNF-α) was activated through the macrophage activation and the endothelial cell propagation. Consequently, for the treatment of atherogenesis disease, anti inflammatory therapy was also needed (Voloshyna et al., 2014). In the present investigation, we observed that the crocetin not only restored the level of the lipid profile in the HCD induced AS, but also attenuated the inflammatory cytokine (IL-6 and TNF-α) secretion, exposing that the vascular protecting effects of crocetin could be associated with the minimization of inflammation response.
It has been proved that the oxidative stress and vascular inflammation arises during AS expression, which plays a significant function in the promotion of the endothelial damage and expression of the AS disease (Rosenson and Stafforini, 2012, Wang et al., 2013). MDA and SOD, both are the oxidative stress markers. MDA is the significant indicator of the oxidative stress and its concentration in the serum can be utilized to identify the lipid peroxidation injury (Chen et al., 2014, Kumar and Bhandari, 2013). Another antioxidant marker such as SOD is utilized to protect the cell from the oxidative damage (Wang et al., 2014a, Wang et al., 2014b). In the current investigation, we observed the considerably reduce level of SOD and the considerably enhance concentration of the MDA in the HCD induced AS rats. The result of the current investigation showed that the crocetin enhanced the AS in the rat via attenuating the oxidative stress.
According to the few researches, p38 MAPK signaling pathway is connected with the inflammatory response (Craig et al., 2000, Wang et al., 2014a, Wang et al., 2014b). The level of the IL-6 and TNF-α was enhanced during the expansion of p38 MAPK signaling pathway (Craig et al., 2000, Illingworth and Bacon, 1987, Kazi and Qian, 2009). In the present investigation, we found almost alike results with the above discussed studies. In the current investigation we observed the enhanced concentration of the inflammatory mediators, which revealed the activation of the p-p38 MAPK signaling. We also scrutinized the expression of the p-p38 MAPK, which was considerably amplified in the HCD induced AS rats (Wang et al., 2015). But, in our experiment we observed the down regulating expression of p-p38 MAPK in crocetin received rats. The current data confirmed that the changes in the concentration of the inflammatory mediators were strongly connected to change the p-p38 MAPK. Crocetin received group rats also influence the proinflammatory cytokines expression via changeable the p-p38 MAPK pathways. The current effect was connected to the antioxidant effect of the crocetin because oxidative stresses encourage the p-p38 MAPK expression.
5. Conclusion
In conclusion, the current investigation claims that the crocetin reduced the AS expression via inhibiting the inflammatory response and oxidative stress connected with the p38 MAPK pathway. Our result in addition tinted the importance of crocetin in the treatment of different CVS including AS.
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in wistar rats. South Ind. J. Biol. Sci. 2015;1:34–37. [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci. 2015;1:47–51. [Google Scholar]
- Castelli W.P., Garrison R.J., Wilson P.W., Abbott R.D., Kalousdian S. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- Chen Z., Li S., Zhao W., Chen X., Wang X. Protective effect of co-administration of rosuvastatin and probucol on atherosclerosis in rats. Can. J. Physiol. Pharmacol. 2014;92:797–803. doi: 10.1139/cjpp-2014-0169. [DOI] [PubMed] [Google Scholar]
- Craig R., Larkin A., Mingo A.M., Thuerauf D.J., Andrews C. P38MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000;275:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- Deng F.T., Chen S.R., Wu X.Q., Wang T.Q., Chen J.W. Hypercholesterolemia accelerates vascular calcification induced by excessive vitamin D via oxidative stress. Calcified Tissue Inter. 2006;79:326–339. doi: 10.1007/s00223-006-0004-8. [DOI] [PubMed] [Google Scholar]
- Deng Z.Y., Hu M.M., Xin Y.F., Gang C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015;64(5):321–332. doi: 10.1007/s00011-015-0810-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Hazzard W.R., Schrott H.G., Bierman E.L., Motulsky A.G. Hyperlipidemia in coronary heart disease. I. Lipid levels in 500 survivors of myocardial infarction. J. Clin. Invest. 1973;52:1533–1543. doi: 10.1172/JCI107331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.J., Probstfield J.L., Garrison R.J., Neaton J.D., Castelli W.P. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- Haddy N., Sass C., Droesch S., Zaiou M., Siest G. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170:277–283. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]
- Hopewell J.C., Reith C., Armitage J. Pharmacogenomics of statin therapy: any new insights in efficacy or safety? Curr. Opin. Lipidol. 2014;25(6):438–445. doi: 10.1097/MOL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Illingworth D.R., Bacon S. Hypolipidemic effects of HMG-CoA reductase inhibitors in patients with hypercholesterolemia. Am. J. Cardiol. 1987;60:33G–42G. doi: 10.1016/0002-9149(87)90589-3. [DOI] [PubMed] [Google Scholar]
- Jose J., Al-Tamimi F.A., Helal M.M., Jimmy B., Al Riyami Q. Statin associated hepatic adverse effects: a retrospective review from a regional hospital in sultanate of Oman. Oman Med. J. 2014;29:351–357. doi: 10.5001/omj.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaiselvi V., Binu T.V., Radha S.R. Preliminary phytochemical analysis of the various leaf extracts of Mimusops elengi L. South Ind. J. Biol. Sci. 2016;2:24–29. [Google Scholar]
- Kazi H.A., Qian Z. Crocetin reduces TNBS-induced experimental colitis in mice by down regulation of NFkB. Saudi J. Gastroenterol. 2009;15(3):181–187. doi: 10.4103/1319-3767.54750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Bhandari U. Protective effect of Trigonella foenumgraecum Linn. on monosodium glutamate-induced dyslipidemia and oxidative stress in rats. Ind. J. Pharmacol. 2013;45:136–140. doi: 10.4103/0253-7613.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang Q.J., Zhu D.N., Yang Y. Reinioside C, a triterpene saponin of Polygala aureocauda Dunn, exerts hypolipidemic effect on hyperlipidemic mice. Phytother. Res. 2008;22:159–164. doi: 10.1002/ptr.2262. [DOI] [PubMed] [Google Scholar]
- Lo H.M., Wu M.W., Pan S.L., Peng C.Y., Wu P.H. Chrysin restores PDGF-induced inhibition on protein tyrosine phosphatase and reduces PDGF signaling in cultured VSMCs. J. Nutr. Biochem. 2012;23:667–678. doi: 10.1016/j.jnutbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Nandhini V.S., Stella Bai G.V. In-vitro Phytopharmacological effect and cardio protective activity of Rauvolfia tetraphylla L. South Ind. J. Biol. Sci. 2015;1:97–102. [Google Scholar]
- Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Ind. J. Biol. Sci. 2016;2:41–45. [Google Scholar]
- Noorudheen N., Chandrasekharan D.K. Effect of ethanolic extract of Phyllanthus emblica on captan induced oxidative stress in vivo. South Ind. J. Biol. Sci. 2016;2:95–102. [Google Scholar]
- Puthur J.T. Antioxidants and cellular antioxidation mechanism in plants. South Ind. J. Biol. Sci. 2016;2:14–17. [Google Scholar]
- Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci. 2015;1:60–65. [Google Scholar]
- Rosenson R.S., Stafforini D.M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 2012;53:1767–1782. doi: 10.1194/jlr.R024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh S.K., Venugopal A., Radhakrishnan M.C. Study on the phytochemical, antibacterial and antioxidant activities of Simarouba glauca. South Ind. J. Biol. Sci. 2016;2:119–124. [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of nuclear factor kappa B (NF-kB) in human diseases-a review. South Ind. J. Biol. Sci. 2016;2:368–387. [Google Scholar]
- Shen Y.J., Zhu X.X., Yang X., Jin B., Lu J.J. Cardamonin inhibits angiotensin II-induced vascular smooth muscle cell proliferation and migration by downregulating p38 MAPK, Akt, and ERK phosphorylation. J. Nat. Med. 2014;68:623–629. doi: 10.1007/s11418-014-0825-0. [DOI] [PubMed] [Google Scholar]
- Sreeshma P.S., Raphael K.R., Baby A.A. Pharmacognostic studies of leaves of Naravelia zeylanica (Linn) DC. South Ind. J. Biol. Sci. 2016;2:179–182. [Google Scholar]
- Valsan Al., Raphael K.R. Pharmacognostic profile of Averrhoa bilimbi Linn. leaves. South Ind. J. Biol. Sci. 2016;2:75–80. [Google Scholar]
- Voloshyna I., Littlefield M.J., Reiss A.B. Atherosclerosis and interferon-gamma: new insights and therapeutic targets. Trends Cardiovas. Med. 2014;24:45–51. doi: 10.1016/j.tcm.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhu H., Yang Z., Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Ind. J. Pharmacol. 2013;45:395–398. doi: 10.4103/0253-7613.115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pu H., Ma C., Jiang T., Wei Q. Adiponectin abates atherosclerosis by reducing oxidative stress. Med. Sci. Monit. 2014;20:1792–1800. doi: 10.12659/MSM.892299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang G.Z., Rabinovitch P.S., Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ. Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li Z., Zhang X., Xie X., Zhang Y. Rosuvastatin attenuates atrial structural remodelling in rats with myocardial infarction through the inhibition of the p38 MAPK signalling pathway. Heart Lung Circ. 2015;24:386–394. doi: 10.1016/j.hlc.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Xing J., Peng K., Cao W., Lian X., Wang Q. Effects of total flavonoids from Dracocephalum moldavica on the proliferation, migration, and adhesion molecule expression of rat vascular smooth muscle cells induced by TNF-alpha. Pharm. Biol. 2013;51:74–83. doi: 10.3109/13880209.2012.711839. [DOI] [PubMed] [Google Scholar]