Main Text
Increasing evidence has linked the aggressiveness of non-Hodgkin’s lymphoma, in particular activated B cell-like type diffuse large B cell lymphomas (ABC-DLBCL), to signaling by toll-like receptor 9 (TLR9)/MyD88 and STAT3. In this issue of Molecular Therapy, Zhao et al.1 describe a dual function molecule comprising a clinically-relevant TLR9 agonist (CpG7909) fused to a STAT3 inhibitor in the form of a high-affinity decoy oligodeoxynucleotide (dODN). CpG-STAT3dODN blocked STAT3 DNA binding and activity, thus reducing expression of downstream target genes, such as MYC and BCL2L1, in human and mouse lymphoma cells. These effects led to the generation of lymphoma cell-specific CD8/CD4-dependent T cell immunity that could protect mice from tumor rechallenge.
STAT3 is a transcription factor known for its master immune-regulatory activity in both tumor cells and in tumor microenvironment-associated immune cells,2 as well as for its role in cancer cell proliferation, survival, and angiogenesis.3 STAT3 is frequently activated in solid tumors and hematological malignancies such as myeloma and lymphoma.3, 4 Different approaches have been tested to inhibit STAT3 over the past few years, yet no inhibitory drug or small molecule has been approved by the Federal Drug Administration (FDA). Therapeutic agents employed to block STAT3 include small molecules5, 6, 7 and oligonucleotides.8, 9 While small molecules have not yet proven successful in clinical cancer therapy trials, the STAT3 antisense oligonucleotides continue to be tested in combination with immune checkpoint inhibitors for therapy of solid tumors, such as non-small cell lung, colorectal and head and neck cancers (http://clinicaltrails.gov). The results from many of these clinical trials remain to be disclosed. Toxicity and poor tumor targeting might be important caveats to consider in future therapeutic strategies that are aimed at blocking STAT3.
TLR9 is expressed in cells of the immune system, including dendritic cells, macrophages, natural killer cells, and other antigen presenting cells. TLR9 binds preferentially to CpG-enriched DNA sequences10 in viral and bacterial DNA, which triggers signals that initiate a pro-inflammatory cytokine response. Tumors, infection, and tissue damage can all affect TLR9 expression and activation. TLR9 agonist-based therapies are being pursued for cancer immunotherapy, and there are already some promising results in preclinical settings and early clinical trials.11, 12, 13, 14
In the current study from Zhao et al.,1 the authors describe an attractive approach to inhibit STAT3 in B cell lymphoma that employs a molecule comprising a TLR9 agonist fused to a STAT3 decoy oligodeoxinucleotide (dODN). This approach targets STAT3 inhibition in B cell lymphomas by inducing a dual effect on malignant cells (Figure 1). Gene-expression profiling using NanoString technology revealed that treatment with CpG(B)-STAT3dODN induced the expression of both pro-apoptotic and antitumor immune response-related genes. With a single therapeutic reagent, the authors induced tumor death and concurrently activated the immune system with a TLR9 agonist and STAT3 blockade.
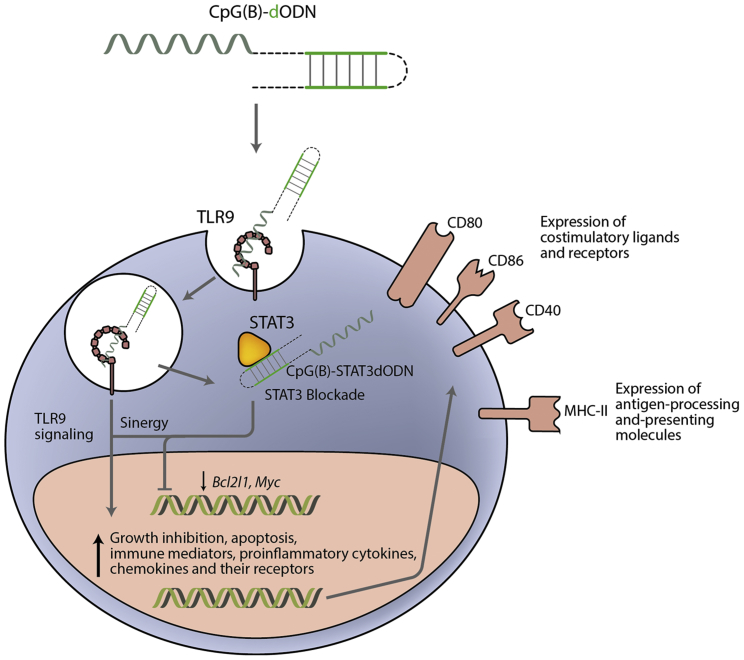
Figure 1.
Mode of Action of CpG(B)-STAT3dODN in B Cell Lymphoma
CpG(B)-STAT3dODN engages scavenger receptors on the surface of target cells facilitating its internalization. Inside the endosome the CpG motif triggers TLR9 receptor activity and may facilitate the conjugate release into cytosol. Once in the cytoplasm, the dODN binds STAT3 and impedes its transcriptional function. Lack of STAT3 activity leads to reduced proliferation-associated and pro-tumorigenic gene expression. TLR9 pro-apoptotic signaling in synergy with STAT3 blockade leads to the upregulation of immune-related genes involved in antigen-processing and -presenting molecules, chemotaxis, interferons and costimulatory signaling, which in the end promote an inflamed tumor environment. This strategy shows a double-pronged approach inducing apoptotic cytotoxicity in tumor cells and a strong antitumor immune response.
A similar therapeutic strategy was previously utilized by the same group to target STAT3 inhibition in human myeloid cells resulting in a potent antitumor effect in acute leukemia.15 A few years earlier, they also pioneered the development of a strategy to specifically deliver STAT3 small-interfering RNAs (siRNAs) to TLR9+ immune and cancer-expressing cells by means of conjugation of a STAT3 siRNA to a CpG(A) oligonucleotide.16, 17, 18, 19 Target inhibition of STAT3 with CpG oligonucleotides has shown very promising results in several types of tumor models such as melanoma, prostate cancer, acute myeloid leukemia, or lymphoma.20, 21
Choosing the best-in-class inhibitor of STAT3 (STAT3 siRNA, or dODN) when designing a therapeutic strategy depends on factors such as stability, pharmacokinetics, and accessibility to the intracellular compartments, among others. The optimal choice of chemistry might even vary from one type of tumor to another. STAT3 dODN binds to the STAT3 protein itself and prevents its engagement with the DNA promoter region, thus precluding the induction of the STAT3-mediated immunosuppressive transcriptional program. STAT3 siRNA on the other hand will eliminate STAT3 mRNA in a Dicer-dependent manner. STAT3 dODN is a phosphorothioation (PS) modified DNA molecule of hairpin design and therefore is significantly more stable than siRNAs.
In addition, STAT3 dODN is smaller than siRNA, which would likely favor its escape from endosomes into the cytosol so as to block STAT3 inhibition. Future studies will likely show which of the designs of CpG-STAT3 inhibitors holds the most promise for clinical translation to therapy of various types of human cancers. First generation CpG(A)-STAT3dODN based on the class A CpG is very efficient at targeting different types of human and mouse myeloid cell. However, it is poorly internalized in both non-malignant B cells and B cell lymphoma cells.15 Zhao et al.1 used a previously described and extensively clinically tested class B CpG ODN sequence (CpG7909) comprising a fully PS-modified backbone, known for targeting B cells. The new CpG(B)-STAT3dODN design afforded a significantly increased half-life and a quicker and more efficient internalization in primary human and mouse B and myeloid cells. Indeed, the CpG(B)-STAT3dODN conjugate was internalized by malignant human ABC-DLBCL and A20 murine lymphoma cells within 6 hr. This optimized strategy enabled STAT3 targeting and the inhibition of its transcriptional activity, which directly leads to a strong reduction of the STAT3 pro-tumorigenic program, inducing important changes in the cell’s gene expression profile.
NanoString technology was used to analyze gene expression and to determine the genetic fingerprint elicited by each treatment. The gene expression pattern induced by decoy-mediated STAT3 inhibition revealed the dual mode of action of this strategy (antitumor proliferation checkpoints and immune-related genes) eventually leading to tumor-growth inhibition and increased survival of tumor-bearing mice. Local treatment with CpG(B)-STAT3dODN increased expression of apoptotic and immune genes, including interferons, pro-inflammatory cytokines, chemokines, and MHC class I and class II antigen presentation-related genes, T cell regulators, etc. These effects were not observed after treatment with CpG or STAT3 decoy oligonucleotides alone. It is worth noting that the immune gene-expression profile showed an increase in cytotoxic-related genes as well as a signature of Th1 immune responses. Treatment with CpG(B)-STAT3dODN in vivo essentially turned the B cell lymphoma into a sort of “endogenous vaccine” that triggered an antitumor immune response by increasing the inherent immunogenicity of the tumor. One could argue that this therapy effectively morphs the B cell lymphoma cells into activated antigen-presenting cells that favor induction of tumor immunity.
The importance of the immune system in this therapeutic approach is depicted in an experiment in which systemic treatment with CpG(B)-STAT3dODN showed a weak antitumor effect in immunodeficient mice (NSG). The authors confirmed the importance of CD8 T lymphocytes in this approach as CD4 depletion had only a modest effect on survival of the mice, while CD8 depletion accelerated lymphoma progression. The authors also assessed whether the higher grade of inflammation in the tumor induced following treatment with CpG(B)- STAT3dODN would favor the activity of immune-checkpoint blockade antibodies such as anti-PD1 therapy. Treatment with PD1-blockade antibody has elicited unprecedented results in clinical trials in non-Hodgkin’s lymphoma patients, but its effect is still restricted to only approximately one third cancer patients.22, 23, 24 While both CpG(B)-STAT3dODN and PD1-blockade used as monotherapy exerted similar antitumor activity against A20 B cell lymphoma in murine models, the combination of CpG(B)-STAT3dODN with anti-PD1 antibody resulted in 90% survival of treated mice. Phenotypic analysis revealed that CpG-STAT3dODN treatment triggers immunogenicity of A20 lymphoma cells, as measured by an increase in MHC class II complexes and costimulatory molecules. There was also higher CD8+ and CD4+ T cell tumor infiltration and significant reduction of Tregs (CD4+/FoxP3+).
It is also important to highlight the fact that this strategy seems to be safe and well tolerated since no toxicity has been detected at up to 60 mg/kg/week. Target therapeutic agents are also aimed to enhancing the therapeutic index, allowing stronger antitumor effects with lower doses and hence reducing toxic side effects.
References
- 1.Zhao X., Zhang Z., Moreira D., Su Y.-L., Won H., Adamus T., Dong Z., Liang Y., Yin H.H., Swiderski P. Mol Ther. 2018;26:695–707. doi: 10.1016/j.ymthe.2018.01.007. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 3.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 4.Kortylewski M., Kujawski M., Herrmann A., Yang C., Wang L., Liu Y., Salcedo R., Yu H. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res. 2009;69:2497–2505. doi: 10.1158/0008-5472.CAN-08-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh D.-Y., Lee S.-H., Han S.-W., Kim M.-J., Kim T.-M., Kim T.-Y., Heo D.S., Yuasa M., Yanagihara Y., Bang Y.-J. Phase I Study of OPB-31121, an Oral STAT3 Inhibitor, in Patients with Advanced Solid Tumors. Cancer Res. Treat. 2015;47:607–615. doi: 10.4143/crt.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong A.L., Soo R.A., Tan D.S., Lee S.C., Lim J.S., Marban P.C., Kong L.R., Lee Y.J., Wang L.Z., Thuya W.L. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann. Oncol. 2015;26:998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- 7.Ogura M., Uchida T., Terui Y., Hayakawa F., Kobayashi Y., Taniwaki M., Takamatsu Y., Naoe T., Tobinai K., Munakata W. Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci. 2015;106:896–901. doi: 10.1111/cas.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen M., Thomas S.M., Kim S., Yeh J.I., Ferris R.L., Johnson J.T., Duvvuri U., Lee J., Sahu N., Joyce S. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: Implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong, D., Kurzrock, R., Kim, Y., Woessner, R., Younes, A., Nemunaitis, J., Fowler, N., Zhou, T., Schmidt, J., Jo, M., et al. (2017). AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. 7, 314ra185. [DOI] [PMC free article] [PubMed]
- 10.Krieg A.M. Therapeutic potential of toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 11.Bode C., Zhao G., Steinhagen F., Kinjo T., Klinman D.M. CpG DNA as a vaccine adjuvant (author manuscript) Expert Rev. Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan M., Waxman D.J. CpG-1826 immunotherapy potentiates chemotherapeutic and anti-tumor immune responses to metronomic cyclophosphamide in a preclinical glioma model. Cancer Lett. 2016;373:88–96. doi: 10.1016/j.canlet.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link B.K., Ballas Z.K., Weisdorf D., Wooldridge J.E., Bossler A.D., Shannon M., Rasmussen W.L., Krieg A.M., Weiner G.J. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J. Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 14.Zent C.S., Smith B.J., Ballas Z.K., Wooldridge J.E., Brian K., Call T.G., Shanafelt T.D., Bowen D.A., Kay N.E., Thomas E. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. NIH Public Access. 2012;53:211–217. doi: 10.3109/10428194.2011.608451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q., Hossain D.M.S., Duttagupta P., Moreira D., Zhao X., Won H., Buettner R., Nechaev S., Majka M., Zhang B. Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood. 2016;127:1687–1700. doi: 10.1182/blood-2015-08-665604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortylewski M., Swiderski P., Herrmann A., Wang L., Kowolik C., Kujawski M., Lee H., Scuto A., Liu Y., Yang C. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat. Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang, Q., Hossain, D.S., Nechaev, S., Kozlowska, A., Zhang, W., Liu, Y., Kowolik, C.M., Swiderski, P., Rossi, J.J., Forman, S., et al. (2013). TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. 121, 1304–1315. [DOI] [PMC free article] [PubMed]
- 18.Hossain D.M.S., Dos Santos C., Zhang Q., Kozlowska A., Liu H., Gao C., Moreira D., Swiderski P., Jozwiak A., Kline J. Leukemia cell-targeted STAT3 silencing and TLR9 triggering generate systemic antitumor immunity. Blood. 2014;123:15–25. doi: 10.1182/blood-2013-07-517987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain D.M.S., Pal S.K., Moreira D., Duttagupta P., Zhang Q., Won H., Jones J., D’Apuzzo M., Forman S., Kortylewski M. TLR9-targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin. Cancer Res. 2015;21:3771–3782. doi: 10.1158/1078-0432.CCR-14-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira, D., Zhang, Q., Nechaev, S., Kortylewski, M., Immunology, T., and Immunology, T. (2016). SiRNA Delivery Methods. 1364, 183–196. [DOI] [PMC free article] [PubMed]
- 21.Kortylewski M., Moreira D. Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities. Cancer Immunol. Immunother. 2017;66:979–988. doi: 10.1007/s00262-017-1966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsirigotis P., Savani B.N., Nagler A. Programmed death-1 immune checkpoint blockade in the treatment of hematological malignancies. Ann. Med. 2016;48:428–439. doi: 10.1080/07853890.2016.1186827. [DOI] [PubMed] [Google Scholar]
- 23.Merryman R.W., Armand P., Wright K.T., Rodig S.J. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood. 2017;1:2643–2654. doi: 10.1182/bloodadvances.2017012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu-Monette Z.Y., Zhou J., Young K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]