Abstract
Epidemiologic studies indicate that elevated levels of γ-glutamyltransferase (GGT), a key enzyme of glutathione metabolism, might be associated with increased cancer risk. Furthermore, preclinical studies support a role for GGT in tumor invasion and progression. However, the relationship between GGT and risks of cervical intraepithelial neoplasia III (CIN-III) and invasive cervical cancer (ICC) have not been evaluated. We investigated the association of enzymatically determined GGT in blood serum with subsequent incidence of CIN-III and ICC in a prospective population-based cohort of 92,843 women ages 18 to 95, of whom 79% had at least one gynecologic examination including Pap smear testing during follow-up. Cox regression was used to compute adjusted hazard ratios (HR) with 95% confidence intervals for the association of GGT with CIN-III and ICC. During median follow-up of 13.8 years, 702 CIN-III and 117 ICC diagnoses were observed. Compared with normal low GGT (<17.99 units/L), risk of ICC was significantly elevated for all other baseline GGT categories, with adjusted HRs of 2.31 (1.49–3.59) for normal high GGT (18.00–35.99 units/L), 2.76 (1.52–5.02) for elevated GGT (36.00–71.99 units/L), and 3.38 (1.63–7.00) for highly elevated GGT [>72.00 units/L; P trend < 0.0001, HR log unit increase 3.45 (1.92–6.19)]. In contrast, associations between GGT serum levels and CIN-III risk were not statistically significant in the main analysis. Exclusion of the first 2 or 5 years of follow-up did not change the results. Effects did not differ by age, body mass index, or socioeconomic status. Our findings implicate GGT in the progression of premalignant cervical lesions to invasive cancer.
Introduction
Cervical cancer is second to breast cancer in incidence and mortality among women worldwide (1). In many developing countries, it still remains the major cause of death in women of reproductive age (2). Although great strides in the understanding of the epidemiologic and molecular context of cervical cancer have been made in recent years, there are large differences in incidence rates of this cancer among populations. These differences may largely reflect variability in screening by Papanicolaou tests, treatment of preinvasive lesions, and prevalence of virulent high-risk human papillomavirus (HPV) that is known to be a necessary cause of cervical cancer, as well as its immediate precursor cervical intraepithelial neoplasia III (CIN-III; refs. 3, 4). However, biological cofactors that operate in the presence of oncogenic HPV to increase the risk of cervical tumorigenesis need further study.
In clinical practice, serum γ-glutamyltransferase (GGT) is a commonly used diagnostic test, mainly as an indicator for hepatobiliary disease and a marker of alcohol intake (5–8). However, recent experimental models have further elucidated the ability of cellular GGT to modulate crucial redoxsensitive functions, such as antioxidant/antitoxic defenses and cellular proliferative/apoptotic balance, and its role in tumor progression, invasion, and drug resistance has been proposed (9–12).
We previously reported a significant association of GGT with overall cancer incidence and several lifestyle-triggered cancers in both men (13) and women (14) from the general Austrian population. This novel association was recently confirmed in the third U.S. National Health Examination and Nutrition Examination Survey, further demonstrating elevated GGT to significantly increase risk of overall cancer death (15).
In the present study, we report on the associations of serum GGT with risk of subsequent CIN-III and invasive cervical cancer (ICC) in a population-based cohort of 92,843 Austrian women across a wide age range, free from malignancies at baseline. To our knowledge, this is the first epidemiologic investigation to explore the association between GGT and risk of CIN-III and ICC.
Materials and Methods
Study population
The Vorarlberg Health Monitoring and Promotion Program (VHM&PP; ref. 16) is one of the world's largest ongoing population-based risk factor surveillance programs. The cohort was initiated in 1985 and is conducted by the Agency for Social and Preventive Medicine in Vorarlberg, the westernmost province of Austria. All adults in the region were invited to participate through a combination of different measures including written invitations, television, radio, and newspaper reports. Participants were enrolled continuously through the end of follow-up in 2003. Followup was determined based on subject's home addresses using a recall system of written biennial re-invitation letters. Loss to follow-up (e.g., due to migration) was <1%.
Sociodemographic data were recorded, and a voluntary physical examination was conducted regularly in a standardized manner by trained local physicians and internists. During the exam, a fasting blood sample was taken. Costs were covered by the participant's (compulsory) health insurance. A more detailed description of the program methodology has been reported elsewhere (16).
Between 1985 and 2003, 94,628 female Vorarlberg residents (ages >18 y) were enrolled in the VHM&PP. We excluded 1,734 participants (1.8%) with missing or incomplete data on GGT at enrollment or with history of malignancies prior to enrollment. To eliminate possible effects of preclinical cancer by producing/altering GGT, we further excluded participants with baseline GGT serum values >600 units/L (n = 51), resulting in a total of 92,843 women eligible for analyses for the current investigation. All participants signed informed consents to have personal data stored and processed. For this study, institutional review board approval was obtained by the Ethics Committee of the province of Vorarlberg.
Data collection and GGT measurements
Age, height, weight, smoking status (current/former, never), and GGT were routinely obtained for each study participant. Individuals who reported smoking of at least one cigarette per day during the year before examination were classified as current smokers. Occupational status (blue collar, white collar, or self-employed) was determined by the insurance number of participants and used as a surrogate measure of socioeconomic status. Participants who were retired at baseline were classified according to their former occupation, and housewives were classified according to their husband's occupation.
Two central laboratories undergoing regular internal and external quality procedures enzymatically determined serum GGT concentrations on fasting blood samples. It has been shown that GGT display a considerable intraindividual stability and strong “tracking” pattern (tracking coefficient ~0.70; ref. 16). Within 60 to 240 min after venous blood sample collection from a cubital vein, serum was obtained by centrifugation for 15 min at 4,000 rotations per minute. Subsequently, GGT concentrations were measured at 37°C and were given as units per liter (units/L). To check calibration, three daily control samples were included. If average values of the control samples of each run were not within 3% of the true value, the run was repeated. Day-by-day variation had to be within 5%. Intrabatch correlation was 3.3 for 27 units/L (mean GGT), 1.6 for 74 units/L, and 1.2 for 107 units/L; interbatch correlation was 2.1 for 51 units/L (mean GGT).
Cervical cytology and cancer ascertainment
Independent from participation in the routine health examination program, women were offered annual gynecologic examinations. Women received biennial re-invitation letters if their last gynecologic examination was >2 y ago. Gynecologic examinations including conventional Papanicolaou (Pap) smear testing with Szalay Cyto-Spatula (CSM Graf GmbH; refs. 17, 18) were conducted by gynecologists and trained medical staff following a standardized procedure. The conventional spatula pap test has similar sensitivity and specificity as liquid-based cervical cytology for the detection of high-grade CIN and a lower number of false-positive results (19). Pap smears were reviewed by a cytotechnologist and all smears showing abnormalities and all biopsies were reviewed by the study pathologists (U. Gruber-Moesenbacher and F. Offner).
Pap smear results were reported according to the modified (Munich) Papanicolaou Classification System, consisting of five classes: class I, normal; class II, benign atypical inflammation; class III, dysplasia (including IIId, mild dysplasia; and IIIg, endometrial cell); class IV, carcinoma in situ; and class V, invasive carcinoma. Class III-V findings were considered positive results (20). All women with positive cytologic results were subjected to immediate colposcopy. In case of a Pap III, the smear was repeated immediately. In case of Pap IIID, the smear was repeated after 3 mo. All patients with persistent Pap IIID (>6 mo) or with Pap IV or V were referred to histologic evaluation either by punch biopsy followed by conization or by primary conization. In all cases of CIN-II or CIN-III in the punch biopsy, conization was performed. Directed punch biopsies and cone biopsies were fixed in formalin, embedded in paraffin, and processed into 5-µm-thick H&E-stained sections for light microscopy, following routine procedures. Biopsy tissue was diagnosed using the commonly agreed-upon CIN-I, CIN-II, or CIN-III nomenclature (21). Incident cancers of the cervix (ICD-10:C53) were further recorded by the Vorarlberg cancer registry, which has been accepted for IARC publication since 1993 (22) and has high completeness of recording (23). Nearly all cancers (96.7%) were histologically confirmed. Cohort data were linked with the Vorarlberg Death Index to identify deaths and to calculate person-years at risk.
Statistical analyses
Cox proportional hazards models, adjusted for age, body mass index (BMI), smoking status (never/former, current), year of entry into the cohort, occupational status (blue collar, white collar, self-employed), number of gynecologic examinations during follow-up, and baseline cytology result (obtained within ± 6 mo from baseline GGT measurement) were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association of GGT with CIN-III and ICC. The proportional hazards assumption was checked using Schoenfeld residuals (24) and visual inspection of the hazard plots. Follow-up for women started at enrollment in the cohort for all analyses, and ended at CIN-III and/or ICC diagnosis or at censoring. For the analysis of CIN-III outcomes, censoring events were ICC diagnosis, death, end of study, loss to follow-up, and emigration. For the analysis of ICC outcomes, censoring events were CIN-III diagnosis, death, end of study, loss to followup, and emigration. For the CIN-III analysis, only women with at least one gynecologic examination were included.
We first computed adjusted HRs with 95% CIs for baseline GGT levels as a categorical variable, using the groups <17.99 units/L (normal low), 18.00 to 35.99 units/L (normal high), 36.00 to 71.99 units/L (elevated), and >72.00 units/L (highly elevated; ref. 14). A test for log linear trend across categories of GGT was performed. We further calculated adjusted HRs with log transformed GGT as a continuous variable. To investigate possible effects of reverse causality, we repeated all analyses, excluding the first 2 and 5 y of follow-up.
We evaluated whether the GGT-CIN-III/ICC association was modified by age, BMI, smoking, socioeconomic status, number of gynecologic visits, differed by cervical cancer staging, and/or season of blood draw. We computed P values for interaction to assess heterogeneity of effects across strata. Age was used as the underlying time-metric in all analyses. Two-sided P < 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.2 statistical software.
Results
Characteristics of the study population
Demographic and clinical characteristics of the study cohort are shown in Table 1. Median follow-up was 13.8 years for CIN-III and 13.9 years for ICC, with a total of 1,110,330 person-years at risk. Most participants (93.3%) were followed-up for at least 2 years after baseline GGT measurement and 65.8% were followed 10 or more years. Mean age at study entry was 41.7 years. During follow-up, 702 CIN-III and 117 ICC diagnoses were observed. Mean age at CIN-III and ICC diagnoses was 31.1 ± 9.6 and 47.6 ± 15.7 years, respectively. Baseline GGT levels ranged from 3.0 to 590.7 units/L, with a median of 17.9 units/L.
Table 1.
Characteristics of study population (VHM&PP, 1985–2003)
| All female VHM&PP participants, 1985–2003 | 94,628 |
| Participants with data on GGT at enrollment | 92,894 |
| Participants eligible for analyses* | 92,843 |
| Participants with one or more gynecologic examination(s), no. (%) | 73,354 (79.0) |
| Participants with 0 gynecologic examinations, no. (%) | 19,489 (21.0) |
| Total gynecologic examinations, no. | 429,311 |
| Gynecologic examinations per participant, mean ± SD (median)† | 5.8 ± 3.9 (5.0) |
| Age, mean ± SD (range), y | 41.7 ± 15.9 (18–95) |
| BMI, mean ± SD (median), kg/m2 | 24.2 ± 4.6 (23.3) |
| Serum GGT, mean ± SD (median), units/L | 23.7 ± 26.9 (17.9) |
| Current or former smoker (%) | 21.5 |
| Occupational status | |
| Blue collar (%) | 36.9 |
| White collar (%) | 54.9 |
| Self-employed (%) | 8.2 |
| Incidence of CIN-III, no. (%)† | 702 (0.96) |
| Age at CIN-III diagnosis, mean ± SD, y | 31.1 ± 9.6 |
| Follow-up, mean ± SD (median), y | 12.2 ± 5.6 (13.8) |
| Incidence of ICC, no. (%) | 117 (0.13) |
| Age at ICC diagnosis, mean ± SD, y | 47.6 ± 15.7 |
| Follow-up, mean ± SD (median), y | 12.3 ± 5.5 (13.9) |
| Total person-years at risk | 1,110,330 |
Participants with baseline GGT concentrations >600 units/L or with history of malignancies prior to enrollment were excluded.
Among 73,354 women with one or more gynecologic examinations during follow-up.
Gynecologic examinations, cytology outcome and incidence of CIN-III and ICC
Seventy-three thousand and three hundred fifty-four women (79% of the total study population) had at least one gynecologic examination including Pap smear testing during follow-up (total 429,311 gynecologic examinations; median per participant, 5.0; Table 1). When categorizing participants according to their numbers of gynecologic visits using the groups 0, 1–2, 3–5, and ≥6, we found a significant positive trend (P < 0.0001) for positive cytology outcome (class III–V). However, given that our cohort was not regularly screened, the incidence of CIN-III decreased with increasing frequency of gynecologic examinations (P < 0.0001). Likewise, the incidence of ICC statistically significantly decreased with increasing screening frequency (Table 2). Median serum GGT concentrations were similar among strata of gynecologic visits, ranging from 16.1 units/L (≥3 visits) to 17.9 units/L (0–2 visits).
Table 2.
Incidence of CIN-III and ICC according to the number of gynecologic examinations during follow-up (VHM&PP, 1985–2003)
| Outcome | No. of gynecologic examinations | P trend* | |||
|---|---|---|---|---|---|
|
|
|||||
| 0 (n = 19,489) |
1–2 (n = 19,023) |
3–5 (n = 20,433) |
≥6 (n = 33,898) |
||
| Positive cytology (class III, IV, V), no. (%)* | n.a.† | 281 (1.5) | 579 (2.8) | 1,662 (4.9) | <0.0001 |
| CIN-III, no. (%) | n.a.† | 243 (1.3) | 266 (1.3) | 193 (0.6) | <0.0001 |
| ICC, no. (%) | 39 (0.2) | 47 (0.2) | 22 (0.1) | 9 (0.0) | <0.0001 |
Papanicolaou classification system.
Screening detected.
Association of GGT and covariates with CIN-III and ICC
The association of age, BMI, occupational status, smoking status, and number of gynecologic examinations with CIN-III and ICC, estimated from Cox regression models, is shown in Table 3. For CIN-III, low BMI, infrequent gynecologic screening, and current or former cigarette smoking significantly increased risk (all P < 0.0001); for ICC, older age (P = 0.02), infrequent gynecologic screening (P < 0.0001), and current or former smoking (P = 0.01) were associated with increased risk (Table 3).
Table 3.
Association of sociodemographic, socioeconomic, and health behavior–related covariates with CIN-III and ICC (VHM&PP, 1985–2003)
| Covariate | Category | CIN-III (n = 702) | ICC (n = 117) |
|---|---|---|---|
|
|
|
||
| HR (95% CI)* | HR (95% CI)* | ||
| Age (y)† | <28 | 1.00 (Referent) | 1.00 (Referent) |
| 28–39 | 0.95 (0.77–1.17) | 0.56 (0.26–1.21) | |
| 39–53 | 1.13 (0.75–1.70) | 2.04 (0.70–6.00) | |
| >53 | 1.06 (0.45–2.50) | 8.49 (1.99–36.3) | |
| P trend* | 0.59 | 0.02 | |
| BMI (kg/m2)† | <20.9 | 1.00 (Referent) | 1.00 (Referent) |
| 20.9–23.3 | 0.88 (0.73–1.05) | 0.73 (0.41–1.31) | |
| 23.4–26.6 | 0.71 (0.57–0.88) | 0.88 (0.50–1.53) | |
| >26.6 | 0.60 (0.47–0.78) | 0.89 (0.51–1.55) | |
| P trend* | <0.0001 | 0.91 | |
| Gynecologic examinations (no.) | 0 | n.a‡ | 1.00 (Referent) |
| 1–2 | 1.00 (Referent) | 0.78 (0.47–1.29) | |
| 3–5 | 0.77 (0.65–0.92) | 0.30 (0.16–0.55) | |
| ≥6 | 0.28 (0.22–0.34) | 0.06 (0.03–0.14) | |
| P trend* | <0.0001 | <0.0001 | |
| Occupational status | White collar | 1.00 (Referent) | 1.00 (Referent) |
| Blue collar | 1.02 (0.86–1.20) | 1.20 (0.81–1.76) | |
| Self-employed | 0.79 (0.60–1.04) | 0.98 (0.48–1.98) | |
| P trend* | 0.28 | 0.43 | |
| Smoking | Never | 1.00 (Referent) | 1.00 (Referent) |
| Current/former | 2.05 (1.76–2.39) | 1.63 (1.07–2.50) | |
| P trend* | <0.0001 | 0.01 |
NOTE: Participants with baseline GGT >600 units/L or with a history of malignancies prior to enrollment were excluded.
Estimated from Cox proportional hazards regression adjusted for age, BMI, occupational status, smoking status, year of entry into the cohort, number of gynecologic examinations and baseline cytology (within ± 6 mo from baseline GGT measurement). Age was used as the underlying time-metric in all analyses.
For continuous covariates, quartiles of the empirical distribution were used as cutoff values.
Limited to women with one or more gynecologic examinations during follow-up.
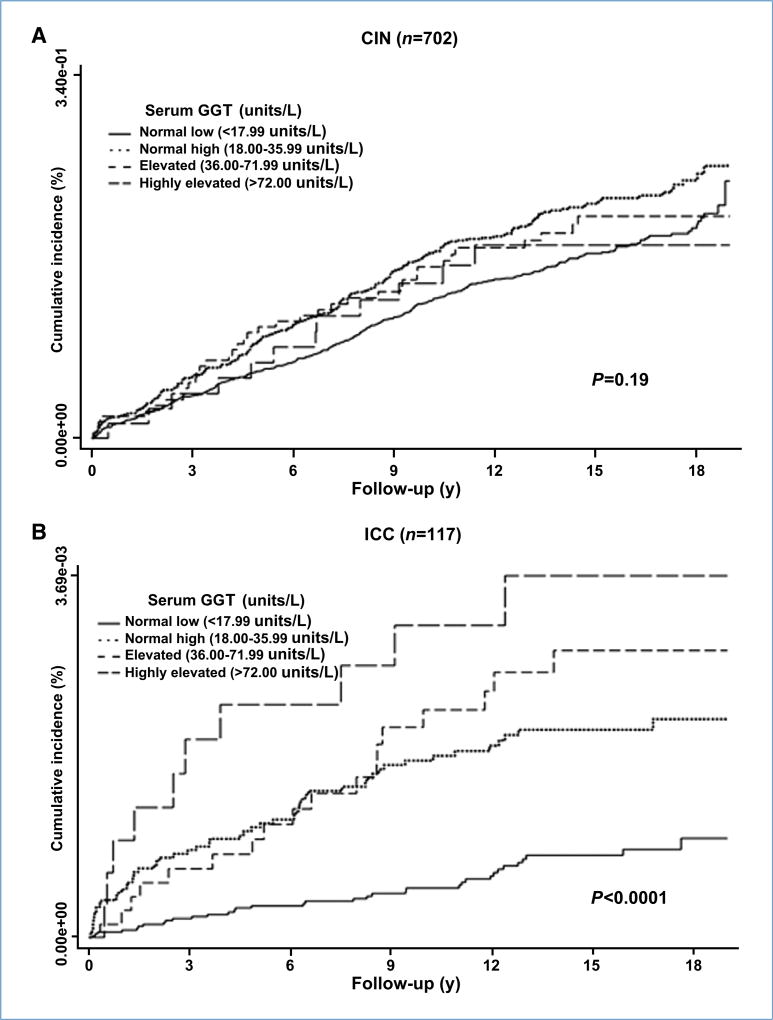
The associations of baseline GGT with subsequent CIN-III and ICC are shown in Table 4. Compared with normal low GGT (<17.99 units/L), risk of ICC was significantly elevated for all other GGT categories, with multivariate-adjusted HRs (95% CI) equalling 2.31 (1.49–3.59) for normal high GGT (18.00–35.99 units/L), 2.76 (1.52–5.02) for elevated GGT (36.00–71.99 units/L), and 3.38 (1.63–7.00) for highly elevated GGT (>72.00 units/L). The HRs show a strong dose-response relationship (P for trend < 0.0001), that is also apparent from Fig. 1, plotting the cumulative crude incidence of ICC according to categories of baseline GGT. When GGT was modeled as a continuous variable, the HR (95% CI) for ICC per GGT log unit increase was 3.45 (1.92–6.19, P < 0.0001; Table 4). When considering only women with at least one gynecologic visit during follow-up, the HR (95% CI) for ICC per GGT log unit increase was 4.30 (2.07–8.90, P < 0.0001).
Table 4.
Estimated adjusted HRs (95% CI) for CIN-III and ICC according to baseline categories of serum GGT (VHM&PP, 1985–2003)
| Serum GGT | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Normal low (<17.99 units/L) |
Normal high (18.00–35.99 units/L) |
Elevated (36.00–71.99 units/L) |
Highly elevated (>72.00 units/L) |
P for trend across GGT categories* |
HR for GGT log unit increase† |
P for GGT log unit increase† |
|
| CIN-III (n = 702)‡ | |||||||
| Events, no. (%) | 431/43,588 (1.0) | 222/22,230 (1.0) | 37/5,375 (0.7) | 12/2,161 (0.6) | 0.02 | 1.25 (0.89–1.76) | 0.19 |
| HR (95% CI)† | 1.00 (Referent) | 1.31 (1.11–1.54) | 1.20 (0.85–1.69) | 1.10 (0.62–1.98) | |||
| ICC (n = 117) | |||||||
| Events, no. (%) | 35/53,506 (0.1) | 54/28,915 (0.2) | 18/7,364 (0.2) | 10/3,058 (0.3) | <0.0001 | 3.45 (1.92–6.19) | <0.0001 |
| HR (95% CI)† | 1.00 (Referent) | 2.31 (1.49–3.59) | 2.76 (1.52–5.02) | 3.38 (1.63–7.00) | |||
NOTE: Participants with baseline GGT > 600 units/L or with a history of malignancies prior to enrollment were excluded. GGT measurements at first visit were used in the analyses.
P values for log linear trend were calculated using baseline GGT categories as an ordinal variable in Cox proportional hazards regression adjusted for age, BMI, occupational status, smoking status, year of entry into the cohort, number of gynecologic examinations, and baseline cytology (within ± 6 mo from baseline GGT measurement).
Estimated from Cox proportional hazards regression adjusted for age, BMI, occupational status, smoking status, year of entry into the cohort, number of gynecologic examinations, and baseline cytology (within ± 6 mo from baseline GGT measurement).
Among 73,354 women with one or more gynecologic examinations during follow-up.
Figure 1.
Kaplan-Meier estimates of cumulative incidence of CIN-III (A) and ICC (B) according to categories of serum GGT, measured at baseline. For the analysis of CIN-III, women with one or more gynecologic examinations during follow-up were included (n = 73,354); for the ICC analyses, all women were included (n = 92,843).
Although CIN-III risk was slightly elevated for high serum GGT levels, the effects were less pronounced and did not reach statistical significance in the main analysis [HR (95% CI) per GGT log unit increase, 1.25 (0.89–1.76); P = 0.19; Table 4; Fig. 1].
Similar associations of GGT with ICC were found when the first 2 or 5 years of follow-up were excluded [HR (95% CI) per GGT log unit increase, 4.16 (2.06–8.41); P < 0.001 and 5.25 (2.29–12.1); P < 0.001, respectively; Table 5]. However, we found no association of GGT with CIN-III risk, neither for latency periods of 2 nor 5 years (Table 5).
Table 5.
Estimated adjusted HRs (95% CI) for CIN-III and ICC according to baseline categories of serum GGT and period of follow-up (VHM&PP, 1985–2003)
| Serum GGT | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Normal low (<17.99 units/L) |
Normal high (18.00–35.99 units/L) |
Elevated (36.00–71.99 units/L) |
Highly elevated (>72.00 units/L) |
P for trend across GGT categories* |
HR for GGT log unit increase† |
P for GGT log unit increase† |
|
| CIN-III‡ | |||||||
| Follow-up >2 y (n = 560) | 1.00 (Referent) | 1.31 (1.09–1.58) | 1.14 (0.77–1.69) | 1.26 (0.67–2.37) | 0.03 | 1.27 (0.87–1.86) | 0.22 |
| Follow-up >5 y (n = 385) | 1.00 (Referent) | 1.27 (1.02–1.59) | 0.91 (0.55–1.53) | 1.32 (0.62–2.82) | 0.20 | 1.07 (0.67–1.72) | 0.77 |
| ICC | |||||||
| Follow-up >2 y (n = 80) | 1.00 (Referent) | 1.83 (1.08–3.09) | 2.98 (1.52–5.87) | 3.11 (1.25–7.74) | <0.001 | 4.16 (2.06–8.41) | <0.0001 |
| Follow-up >5 y (n = 56) | 1.00 (Referent) | 2.08 (1.11–3.91) | 3.89 (1.77–8.57) | 2.69 (0.77–9.39) | 0.001 | 5.25 (2.29–12.1) | <0.0001 |
NOTE: Participants with baseline GGT > 600 units/L or with history of malignancies prior to enrollment were excluded. GGT measurements at first visit were used in the analyses.
P values for log linear trend were calculated using baseline GGT categories as an ordinal variable in Cox proportional hazards regression runs adjusted for age, BMI, occupational status, smoking status, year of entry into the cohort, number of gynecologic examinations, and baseline cytology (within ± 6 mo from baseline GGT measurement).
Estimated from Cox proportional hazards regression adjusted for age, BMI, occupational status, smoking status, year of entry into the cohort, number of gynecologic examinations, and baseline cytology (within ± 6 mo from baseline GGT measurement).
Among 73,354 women with one or more gynecologic examinations during follow-up.
When we fitted separate models to “early stage ICC” (International Federation of Gynecology and Obstetrics stage I and II) and “late stage ICC” (International Federation of Gynecology and Obstetrics stage III and IV), GGT was significantly associated with both ICC stages but effects were slightly stronger for late stage tumors (heterogeneity of effects among strata, P = 0.01; Supplementary Table S1).
We found no evidence for effect modification of the GGT-CIN-III/ICC association by age, BMI, occupational status, or season of blood draw (P for interaction/heterogeneity of effects among strata, all P > 0.15). However, for the CIN-III analyses, we observed borderline effects of GGT in current/former smokers [HR (95% CI) per GGT log unit increase, 1.62 (1.00–2.64); P = 0.05], whereas no association was seen for never smokers [HR (95% CI) per GGT log unit increase, 0.98 (0.61–1.58); P = 0.92; heterogeneity among strata, P < 0.0001]. In our ICC models, we observed more prominent associations of GGT in more frequently screened women (P heterogeneity < 0.001); however, the GGT-ICC association remained statistically significant among all strata.
Discussion
The present study is the first epidemiologic investigation to explore the relation between GGT and subsequent CIN-III and ICC. Our data suggests a statistically significant association between GGT and ICC risk, persisting after adjustment for several confounding factors and under different modeling strategies. Conversely, associations of GGT serum levels with CIN-III risk did not reach significance in the main analysis.
It has been reported that cervical carcinogenesis is a multifactorial disorder, with other factors besides exposure to oncogenic HPV being involved to eventuate in invasive disease (25, 26). Infection with oncogenic HPV types is the necessary cause of cervical cancer; in addition, age at first intercourse, number of sexual partners, high parity, cigarette smoking, race, and low socioeconomic status consistently have been reported to affect cervical cancer risk (27). Many cases of HPV infection clear up spontaneously, and even in women with persistent infection, cervical cancer often only appears years or even decades later.
Experimental evidence has elucidated the ability of cellular GGT to modulate crucial redox-sensitive functions, such as antioxidant/antitoxic defenses and cellular proliferative/apoptotic balance, and its role in tumor progression, invasion, and drug resistance has repeatedly been suggested (9–12). The ability of cellular GGT to affect the catabolism of extracellular reduced glutathione (GSH) potentially reflects several aspects of cell metabolism, especially the modulation of redox status at cell surfaces and H2O2 production. It has been shown (28, 29) that several carcinogenic environmental pollutants, such as lead, cadmium, dioxins, or organochlorine pesticides increased serum GGT levels in the general population in the United States. Because cellular GGT is indispensable for the metabolism of GSH, higher serum GGT plausibly reflects increased cellular GGT activity to metabolize extracellular GSH conjugates. GSH has an important function in conjugating xenobiotics such as lead, cadmium, dioxins, or organochlorine pesticides to facilitate their excretion in the urine or bile, by rendering them more water-soluble. Thus, serum GGT levels increase with increasing exposure to xenobiotics with need to be conjugated to GSH.
From a previous analysis of women from the same cohort (14), we recently reported a significant dose-response association of serum GGT levels with overall cancer incidence and several site-specific cancer entities (including malignancies of the digestive organs, the respiratory system/intrathoracic organs, breast and female genital organs, and lymphoid and hematopoietic cancers), possibly suggesting GGT to be a general tumor marker.
Further research is needed to address the underlying biological mechanisms through which GGT might be related to cervical cancer, but not to its precursor CIN-III. One explanation that is consistent with our data, and with GGT having at least an indirect biological role in tumorigenesis, is that the relationship of GGT with risk of invasive cancer mainly involves the late stages in the multistage process of cervical tumorigenesis; specifically, the transformation from CIN-III to invasive cancer. For example, high GGT levels might reflect a response to high oxidative stress, a risk factor for cancer, in which case, GGT and oxidative stress are part of a biological pathway related to the incident development of cervical cancer. Cigarette smoking, e.g., by increasing oxidative stress, thereby possibly might unmask the relation between CIN-III and GGT, as observed in our data. Alternatively, elevated serum GGT might be due to the development of cancer and production of GGT from the tumor (30, 31) which would not occur with CIN-III, or be part of a physiologic response to the tumor, which might be particularly evident for later stage tumors. However, whereas reverse causation cannot be ruled out, it seems unlikely considering the results of our lag-time and tumor-stage analyses.
Our study had several strengths and limitations that should be considered. Major strengths were the prospective design, large sample size, length of follow-up, and the standardized study protocol. A limitation of our investigation was that information on some risks and confounding factors, including HPV infection, use of oral contraceptives, hormone replacement therapy, number of live births, other biological/serologic measures (e.g., certain inflammatory cytokines), and actual pack-years of cigarette smoking were not routinely available in our database; thus, some or even all of our results could be spurious. Additionally, we lacked complete information on hysterectomies and thus were unable to censor women when they had their uterus removed due to conditions other than ICC. However, we performed sensitivity analysis using partial hysterectomy data from a subsample of 5,645 women, indicating a rather minor effect on our estimates.
In summary, we aimed to investigate the association of GGT, an important redox-regulating enzyme in the blood, with subsequent incidence of CIN-III and ICC in a large population-based cohort of more than 92,000 Austrian women across a wide age range. Our findings identify GGT as a prognostic marker for cervical cancer, by indicating that increased levels of this enzyme are correlated with increased risk of progression of high-grade cervical dysplasia to frank carcinoma.
Supplementary Material
Acknowledgments
We thank all the participants and physicians of the VHM&PP, and Elmar Bechter, M.D. and Hans-Peter Bischof, M.D., at the Health Department of the Vorarlberg State Government. The members of the VHM&PP study group include Guntram Hinteregger, M.D., Karin Parschalk, M.D., Wolfgang Metzler, M.D., Elmar Stimpfl (Agency for Preventive and Social Medicine, Bregenz, Austria), Jochen Klenk, Ph.D., and Kilian Rapp, M.D. (Institute of Epidemiology, University of Ulm, Ulm, Germany).
Grant Support
Austrian National Bank grant OENB-12737 (H. Ulmer) and National Institute on Aging Intramural Research Program (L.J. Brant).
Footnotes
Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 3.Schoell WM, Janicek MF, Mirhashemi R. Epidemiology and biology of cervical cancer. Semin Surg Oncol. 1999;16:203–11. doi: 10.1002/(sici)1098-2388(199904/05)16:3<203::aid-ssu2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Zur Hausen H. Papillomaviruses in the causation of human cancers—a brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Whitfield JB. γ Glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 6.Meister A. Metabolism and transport of glutathione and other γ-glutamyl compounds. In: Larsson A, Orrenius S, Holmgren A, Mannervik B, editors. Functions of glutathione: biochemical, toxicological and clinical aspects. New York: Raven Press; 1983. pp. 1–22. [Google Scholar]
- 7.Rollason JG, Pincherle G,Robinson D. Serum γ glutamyltranspeptidase in relation to alcohol consumption. Clin Chim Acta. 1972;39:75–80. doi: 10.1016/0009-8981(72)90301-4. [DOI] [PubMed] [Google Scholar]
- 8.Skinner HA, Holt S, Schuller R, Roy J, Israel Y. Identification of alcohol abuse using laboratory tests and a history of trauma. Ann Intern Med. 1984;101:847–51. doi: 10.7326/0003-4819-101-6-847. [DOI] [PubMed] [Google Scholar]
- 9.Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. γ-Glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7:360–6. doi: 10.1016/j.coph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of γ-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71:231–8. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Franzini M, Corti A, Lorenzini E, et al. Modulation of cell growth and cisplatin sensitivity by membrane γ-glutamyltransferase in melanoma cells. Eur J Cancer. 2006;42:2623–30. doi: 10.1016/j.ejca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Dominici S, Valentini M, Maellaro E, et al. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of γ-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27:623–35. doi: 10.1016/s0891-5849(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 13.Strasak A, Rapp K, Brant LJ, et al. Association of γ-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970–7. doi: 10.1158/0008-5472.CAN-07-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasak A, Pfeiffer R, Klenk J, et al. Prospective study of the association of γ-glutamyltransferase with cancer incidence in women. Int J Cancer. 2008;123:1902–6. doi: 10.1002/ijc.23714. [DOI] [PubMed] [Google Scholar]
- 15.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and γ-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–85.e11. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Ulmer H, Kelleher C, Diem G, Concin H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: the Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J. 2003;24:1004–13. doi: 10.1016/s0195-668x(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 17.Szalay L. The first atlas with the new Bethesda terminology system. Maudrich Verlag; 1990. Cytoloty of the uterine cervix. [Google Scholar]
- 18. [Last accessed: June 8, 2009];CSM Graf Gmbh. Available from: http://www.csmgraf.ch/index.html?maine.htm.
- 19.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology. A systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–77. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 20.Jun JK, Choi KS, Jung KW, et al. Effectiveness of an organized cervical cancer screening program in Korea: results from a cohort study. Int J Cancer. 2009;124:188–93. doi: 10.1002/ijc.23841. [DOI] [PubMed] [Google Scholar]
- 21.Firnhaber C, Zungu K, Levin S, et al. Diverse and high prevalence of human papillomavirus associated with a significant high rate of cervical dysplasia in human immunodeficiency virus-infected women in Johannesburg, South Africa. Acta Cytol. 2009;53:10–7. doi: 10.1159/000325079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents. VIII. Lyon (France): IARC; 2003. [Google Scholar]
- 23.Oberaigner W, Vittadello F. Cancer mapping in alpine regions 1996–2000. Mammendorf: Pro Literature Verlag; 2006. [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 25.Ramsaroop R, Oei P, Ng D, Kumar N, Cotter PD. Cervical intraepithelial neoplasia and aneusomy of TERC: assessment of liquid-based cytological preparations. Diagn Cytopathol. 2009;37:411–5. doi: 10.1002/dc.21007. [DOI] [PubMed] [Google Scholar]
- 26.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–92. [PubMed] [Google Scholar]
- 27.Wang SS, Zuna RE, Wentzensen N, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev. 2009;18:113–20. doi: 10.1158/1055-9965.EPI-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DH, Lim JS, Song K, Boo Y, Jacobs DR. Graded associations of blood lead and urinary cadmium concentrations with oxidative-stress related markers in the U.S. population: results from the third National Health and Nutrition Examination Survey. Environ Health Perspect. 2006;114:350–4. doi: 10.1289/ehp.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and γ-glutamyltransferase: results from the National Health and Examination Survey 1999–2002. Clin Chem. 2006;52:1825–7. doi: 10.1373/clinchem.2006.071563. [DOI] [PubMed] [Google Scholar]
- 30.Hanigan MH, Pitot HC. γ-Glutamyltranspeptidase—its role in hepatocarcinogenesis. Carcinogenesis. 1985;6:165–72. doi: 10.1093/carcin/6.2.165. [DOI] [PubMed] [Google Scholar]
- 31.Hanigan MH, Gallagher BC, Townsend DM, Gabarra V. γ-Glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis. 1999;20:553–9. doi: 10.1093/carcin/20.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.