Abstract
Background and Purpose
Cognition and education influence functional trajectories, but whether associations differ with subclinical brain infarcts (SBI) or white matter hyperintensity volume (WMHV) is unknown. We hypothesized that SBI and WMHV moderated relationships between cognitive performance and education, and functional trajectories.
Methods
1290 stroke-free individuals underwent brain MRI and were followed for 7.3 years (mean) with annual functional assessments with the Barthel index (BI; range 0–100). MRI measurements included pathology-informed SBI (PI-SBI) and WMHV (% total cranial volume). Generalized estimating equation models tested associations between MRI variables and baseline BI and change in BI, adjusting for demographic, vascular, cognitive, and social risk factors, and stroke and myocardial infarction during follow-up. We tested interactions among education level, baseline cognitive performance (mini-mental state score), and functional trajectories, and ran models stratified by levels of MRI variables.
Results
Mean age was 70.6 (SD 9.0) years; 19% had PI-SBI and mean WMHV was 0.68%. Education did not modify associations between cognition and functional trajectories. PI-SBI modified associations between cognition and functional trajectories (p=0.04), with a significant protective effect of better cognition on functional decline seen only in those without PI-SBI. There was no significant interaction for WMHV (p=0.8). PI-SBI, and greater WMHV, were associated with 2- to 3-fold steeper functional decline, holding cognition constant.
Conclusions
PI-SBI moderated the association between cognition and functional trajectories, with threefold greater decline among those with PI-SBI (compared to no PI-SBI) and normal baseline cognition. This highlights the strong and independent association between “subclinical” markers and patient-centered trajectories over time.
Keywords: subclinical infarct, disability, epidemiology
Subclinical brain infarcts (SBI) are MRI-defined lesions of presumed vascular origin that are not associated with acute clinical events. Similarly, MRI white matter hyperintensities (WMH) are thought to represent structural damage due to small vessel disease. In the population-based Rotterdam Scan Study, among 1077 individuals 60–90 years of age, subclinical infarcts were at least 5 times as prevalent as clinical strokes.1 In several studies, including the Helsinki Ageing Study and Austrian Stroke Prevention Study and the population-based Cardiovascular Health Study and Rotterdam Study, WMH were present in >90% of individuals.2
SBI and WMH have been associated in multiple studies with cognitive impairment3 and reduced functional status.1, 4 Functional status here refers to performance in activities of daily living (ADL) and in some cases also instrumental ADLs, measured by standard scales such as the Barthel index or the Katz scale. In a prior analysis using the MRI sample of the Northern Manhattan Study (NOMAS),5 WMH volume (WMHV) was independently associated with poorer episodic memory, processing speed, and semantic memory. In previous analyses, we showed that SBI and WMHV are each independently associated with accelerated long-term functional decline.6
Education and cognitive performance are important determinants of functional status,7, 8 but the moderating effects of SBI and WMHV on the relationships between education and functional trajectories, and cognition and functional trajectories, are not known. We hypothesized that MRI measures (SBI and WMHV) moderated 1) the relationship between baseline cognitive performance and long-term functional trajectories, and 2) the relationship between education level and long-term functional trajectories, in those free of stroke at baseline.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Mitchell Elkind at Columbia University at mse13cumc.columbia.edu. The NOMAS MRI study is a sub-study of the NOMAS prospective cohort (as previously described9) that began in 2003 and included 1290 individuals: 1) ≥age 50 years, 2) without MRI contraindications, 3) without clinical stroke and 4) able to provide signed informed consent. MRI sequences (1.5T MRI system, Philips Medical Systems, Best, Netherlands) included: axial T1, axial T2, axial proton density, dual-spin echo, diffusion weighted imaging, and fluid-attenuated inversion recovery (FLAIR). Columbia University and University of Miami IRBs approved the study and all participants provided written informed consent.
Baseline Evaluation
Bilingual research assistants collected data from participants using standardized questions regarding the following conditions, as previously described: hypertension, diabetes, hypercholesterolemia, cigarette smoking, alcohol use, and cardiac conditions.10 All participants underwent a thorough baseline examination including comprehensive medical history, physical examination, review of medical records, functional status assessed by the Barthel index (BI), and fasting blood samples.
Follow-up
All participants were followed annually via phone screening to detect change in vital status, new neurological or cardiac symptoms and events, interval hospitalizations, and functional status via the BI. Only two subjects were lost to follow-up after their baseline examination, and the average annual contact rate was 99%.
A positive screen for potential cardiac or neurological events was followed by an in-person confirmatory assessment. In addition, all admissions and discharges were screened for hospitalizations and outcomes that may not have been captured by telephone interview. Nearly 70% of vascular events lead to hospitalizations at Columbia University Medical Center. Hospital records were reviewed to classify outcomes as previously reported.11 Stroke included ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. At least 2 stroke neurologists verified and classified all stroke cases. MI diagnosis was adjudicated by cardiologists, based on criteria adapted from the Cardiac Arrhythmia Suppression trial12 and the Lipid Research Clinics Coronary Primary Prevention trial,13 requiring ≥2 of the 3 following criteria: (a) ischemic cardiac pain determined to be typical angina; (b) cardiac marker abnormalities defined as abnormal CK-MB fraction or troponin I values; and (c) ischemic EKG abnormalities.
Study outcome
The BI14, 15 measures performance in 10 activities of daily living (ADLs) and ranges from 0–100 in 5-point increments, with 100 indicating normal physical functioning. Previous research has demonstrated the reliability of phone assessments of function using the BI.16 Although it is an ordinal scale, recent research has advocated analyzing the scale as a continuous variable due to increased power to detect associations, ability to describe the course of change over time in linear form, and avoidance of potential misclassification due to crude categorization.17–19 All BI measurements from the time of MRI forward were included in this analysis.
Explanatory variables
For brain images, an operator traced dura mater, and non-brain structures were manually removed. Using a custom-designed image analysis package (QUANTA 6.2 using a Sun Microsystems Ultra 5 workstation), modeling of pixel-intensity histograms for cerebrospinal fluid (CSF) and brain white and gray matter was performed. Semi-automated measurements of pixel distributions were made to identify the optimal pixel-intensity threshold to distinguish CSF from brain matter. Total cranial volume (TCV) constituted the sum of whole brain volume voxels from the T1 segmentation process. WMHV was calculated as the sum of voxels ≥3.5 standard deviations above mean image intensity multiplied by pixel dimensions and section thickness.5 For analysis, WMHV was divided by TCV and standardized.
MRI sequences were read for cavitated infarcts and perivascular spaces (PVS), according to previously described criteria.20 Briefly, lesions with an effective cross-sectional diameter of >3 mm were characterized as large PVS or infarcts according to parameters such as to location, shape, and T1 and FLAIR appearance, validated in imaging-pathological correlational studies from other cohorts.20 The assignation of a lesion as pathology-informed SBI (PI-SBI) was further sub-classified with the understanding of the stroke mechanism as follows: lacunes of presumed vascular origin (i.e. cavitated lesions thought to be infarcts) were identified in the territory of penetrating arteries, subcortical but likely embolic infarcts were identified in the territory of medullary penetrating arteries, cortical infarcts were likely embolic, and cerebellar infarcts were located in the cerebellum. The PI-SBI variable was dichotomized into present versus absent.
Cognitive performance was measured with the mini-mental state examination (MMSE),21 a common, reliable, and valid 30-point scale that measures cognitive performance, with higher scores denoting better performance. MMSE values were analyzed continuously and centered at the mean. Education level was measured as years of education achieved and was dichotomized into at least high school education versus less than high school education.
Covariates
Analytic models were adjusted for the following variables: demographic variables (age, sex, race/ethnicity), medical risk factors (hypercholesterolemia [defined by self-report, lipid lowering therapy use, or fasting total cholesterol level >240 mg/dL], diabetes mellitus [defined by self-report, fasting blood glucose level ≥126 mg/dL, or insulin/oral hypoglycemic use], hypertension [defined as a systolic blood pressure recording ≥140 mmHg or a diastolic blood pressure recording ≥90 mm Hg based on the average of two blood pressure measurements or the patient's self-report of a history of hypertension or antihypertensive]), smoking (defined as either nonsmoker or smoker within the last year), alcohol use (with moderate alcohol use classified as 1 drink/month to 2 drinks/day), any physical activity (versus none), insurance status (classified uninsured/Medicaid versus Medicare/private insurance), and depressed mood. Covariates not significant at p<0.05 were removed from final models.
Statistical analysis
We first calculated the distributions of PI-SBI, WMHV, baseline covariates, and BI. Due to correlations among repeated measures of BI in the same individual, we used regression models using generalized estimating equations (GEE) with an identity link function to assess the association between predictors and repeated measurements of BI, adjusting for baseline demographic variables, medical risk factors, alcohol use and physical activity, and insurance status, as defined above. A variable for the time trend was included to estimate the annual change in BI.
The relationships among cognition, education, and functional status were examined in a multivariable model incorporating the time trend of annual change in BI score (time), centered MMSE score, education level (defined as at least high school education versus less than high school education), and interaction terms between MMSE × education, and between time × MMSE × education. We used the results of this model to determine whether to include the education term in subsequent models.
Next, in order to assess whether the association of MMSE with functional trajectories differed by PI-SBI, we ran a fully adjusted model including the interaction term: time × centered MMSE × PI-SBI, tested at a p-value cutoff of 0.1. This model also included 2-way interaction terms of its components. We then ran fully adjusted models stratified by the presence of PI-SBI, with the following main terms: annual change in BI (time), difference in baseline BI per point of MMSE, and additional annual change in BI per point of MMSE (time × MMSE). A similar series of models were run with WMHV (dichotomized at the mean) instead of PI-SBI.
Various model diagnostics including tests of linearity, residual plots, and goodness of fit measures were used to evaluate the final model. There was no evidence to suggest lack of linearity in the final models when quadratic time terms were tested. We chose the exchangeable (intraclass) working correlation structure after comparing the quasi-likelihood under the independence model criterion (QIC) obtained with this model with one using the unstructured working correlation structure. In order to control for the effect of vascular events on functional status, stroke and MI were included as time-varying covariates.
In order to illustrate significant interactions, we graphed estimated functional trajectories for an exemplar with mean values for covariates and diabetes (due to its known association with functional trajectories22), using the interaction models for PI-SBI and WMHV.
Results
Among 1290 participants, mean age was 70.6 years, vascular risk factors were common, and 46% received at least a high school education (Table 1). 1136 individuals (88.8%) had a BI score of 95 or 100 at baseline. 246 (19.1%) had PI-SBI (range up to 7), 3.0% were cerebellar, 2.9% cortical, 11.1% in the territory of medullary arteries, and 6.6% in the territory of penetrating arteries. Mean WMHV (as % of TCV) was 0.68 (SD 0.84, median 0.36). Mean follow-up time was 7.3 years (SD 2.1). There were 53 first MIs and 64 first strokes occurring during follow-up.
Table 1.
Baseline characteristics
| Variable | No. (%)* |
|---|---|
|
| |
| Number of participants | 1290 (36.9) |
|
| |
| Age, mean (SD), y | 70.6 (9.0) |
|
| |
| Body mass index, mean (SD), kg/m2 | 28.0 (4.8) |
|
| |
| Male | 510 (39.5) |
|
| |
| Race/ethnicity: | |
| Non-Hispanic white | 191 (14.8) |
| Non-Hispanic black | 223 (17.3) |
| Hispanic | 847 (65.7) |
| Other | 29 (2.3) |
|
| |
| Received at least high school education | 592 (45.9) |
|
| |
| Marital status, married | 543 (42.1) |
|
| |
| Health insurance | |
| Medicaid or no insurance | 613 (47.5) |
| Medicare or private insurance | 677 (52.5) |
|
| |
| Hypertension | 861 (66.7) |
|
| |
| Alcohol consumption: | |
| Never Drank | 264 (20.5) |
| Past Drinker | 256 (19.8) |
| Light Drinker | 163 (12.6) |
| Moderate Drinker | 530 (41.1) |
| Intermediate Drinker | 49 (3.8) |
| Heavy Drinker | 28 (2.2) |
|
| |
| Physical activity: | |
| None | 564 (44.3) |
| Any | 710 (55.7) |
|
| |
| Diabetes mellitus | 245 (19.0) |
|
| |
| Smoking: | |
| Never | 612 (47.4) |
| Former | 496 (38.5) |
| Current | 182 (14.1) |
|
| |
| Hypercholesterolemia | 797 (61.8) |
|
| |
| History of coronary heart disease | 177 (13.7) |
|
| |
| Hamilton depression scale score, mean (SD) | 3.1 (3.8) |
|
| |
| Mini mental state score, mean (SD) | 26.7 (3.3) |
unless otherwise indicated
The moderating effect of education level on the relationship between cognitive ability and functional trajectories was examined. There was no primary association of education (p=0.2), no interaction of education with cognitive ability (p=0.3), and no interaction between education and cognitive ability when examining slope of functional decline (p=0.8). Hence, we did not include education level in subsequent models.
The moderating effect of PI-SBI on functional trajectories and cognitive level was examined in a fully adjusted model. The interaction term between PI-SBI, MMSE score, and time was significant (p=0.04). Hence, we ran models stratified by presence of PI-SBI (Table 2). Among those with PI-SBI, there was a steeper estimated decline in functional status over time (−1.56 BI points per year, 95% CI −2.05, −1.07) compared to those without PI-SBI (−0.90, 95% CI −1.06, −0.73). Also, there was a significant relationship between MMSE and change in BI over time only among those without PI-SBI (reducing the rate of decline by 0.07 BI points per year, 95% CI 0.01, 0.12). There was no relationship between MMSE and baseline BI levels in either group (with or without PI-SBI).
Table 2.
Functional trajectories and relationships with cognition, stratified by presence of subclinical brain infarct*
| With PI-SBI (n=247) | Without PI-SBI (n=1044) | |||||
|---|---|---|---|---|---|---|
| Variable | Difference in BI score |
95% CI | p-value | Difference in BI score |
95% CI | p-value |
| Annual change in BI | −1.56 | −2.05, −1.07 | <0.0001 | −0.90 | −1.06, −0.73 | <0.0001 |
| Difference in baseline BI per point of MMSE | −0.20 | −0.87, 0.47 | 0.6 | 0.16 | −0.10, 0.43 | 0.2 |
| Additional annual change in BI per point of MMSE | −0.09 | −0.23, 0.06 | 0.2 | 0.07 | 0.01, 0.12 | 0.02 |
BI=Barthel index score; SD=standard deviation; PI-SBI=pathology-informed subclinical brain infarct; MMSE=mini-mental state examination score. Model additionally adjusted for: age at time of MRI, sex, race/ethnicity, diabetes, hypertension, physical activity, alcohol use, body mass index, insurance status, depression, and stroke and myocardial infarction occurring during follow-up
Next, we tested the moderating effect of WMHV on functional trajectories and cognitive level in a fully adjusted model. Although the interaction term between WMHV (dichotomized at the mean), MMSE score, and time was not significant (p=0.8), we ran models stratified by mean WMHV in order to estimate the overall time trend in each group (Table 3). Among those with WMHV greater than or equal to the mean, there was a steeper estimated decline in functional status over time (−1.92 BI points per year, 95% CI −2.36, −1.47) compared to those with WMHV less than the mean (−0.69, 95% CI −0.84, −0.55). Despite the nonsignificant 3-way interaction term, there appeared to be a significant relationship between MMSE and change in BI over time among those with WMHV less than the mean, reducing the rate of decline by 0.06 BI points per year, 95% CI 0.01, 0.12). As with PI-SBI, there was no relationship between MMSE and baseline BI levels in either group (with or without WMHV).
Table 3.
Functional trajectories and relationships with cognition, stratified by mean white matter hyperintensity volume*
| WMHV ≥mean (n=357) | WMHV <mean (n=923) | |||||
|---|---|---|---|---|---|---|
| Variable | Difference in BI score |
95% CI | p-value | Difference in BI score |
95% CI | p-value |
| Annual change in BI | −1.92 | −2.36, −1.47 | <0.0001 | −0.69 | −0.84, −0.55 | <0.0001 |
| Difference in baseline BI per point of MMSE | 0.38 | −0.18, 0.94 | 0.2 | 0.05 | −0.20, 0.30 | 0.7 |
| Additional annual change in BI per point of MMSE | 0.001 | −0.13, 0.13 | 0.9 | 0.06 | 0.01, 0.12 | 0.03 |
BI=Barthel index score; SD=standard deviation; WMHV=white matter hyperintensity volume; MMSE=mini-mental state examination score. Model additionally adjusted for: age at time of MRI, sex, race/ethnicity, diabetes, hypertension, physical activity, alcohol use, body mass index, insurance status, depression, and stroke and myocardial infarction occurring during follow-up
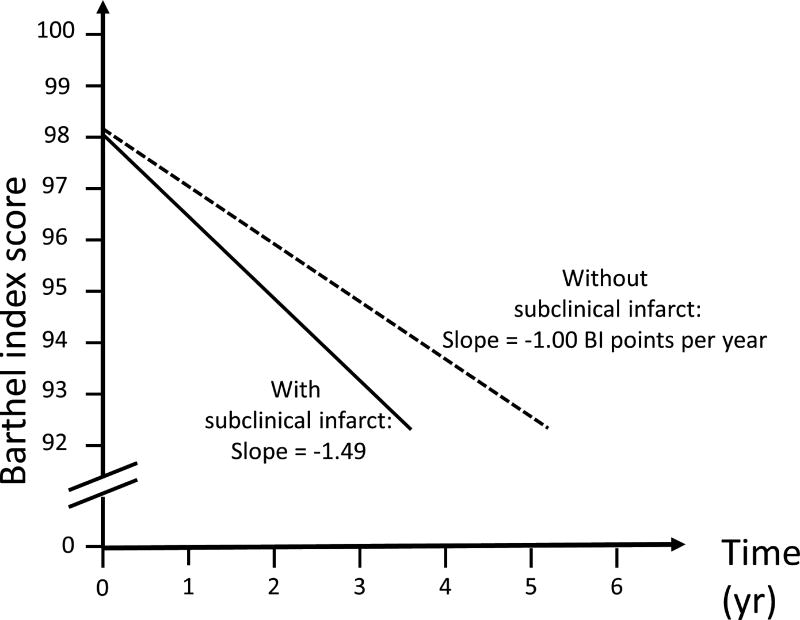
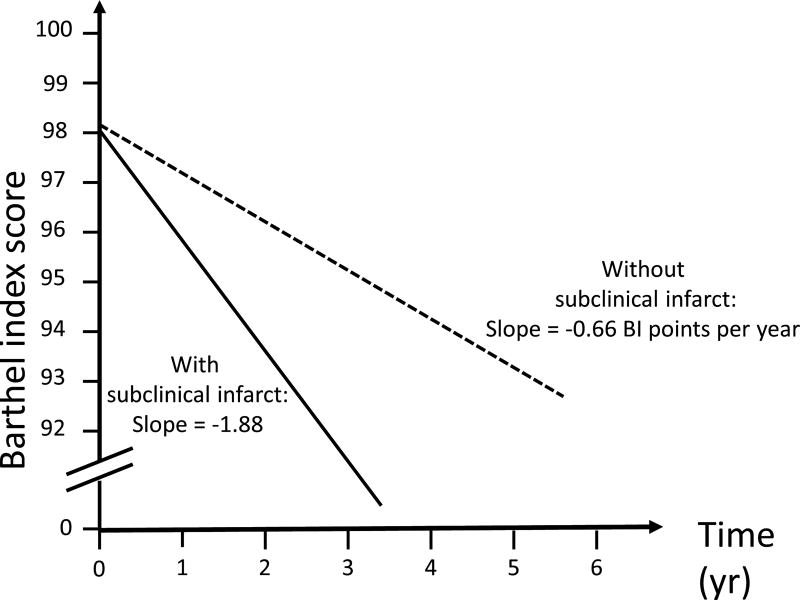
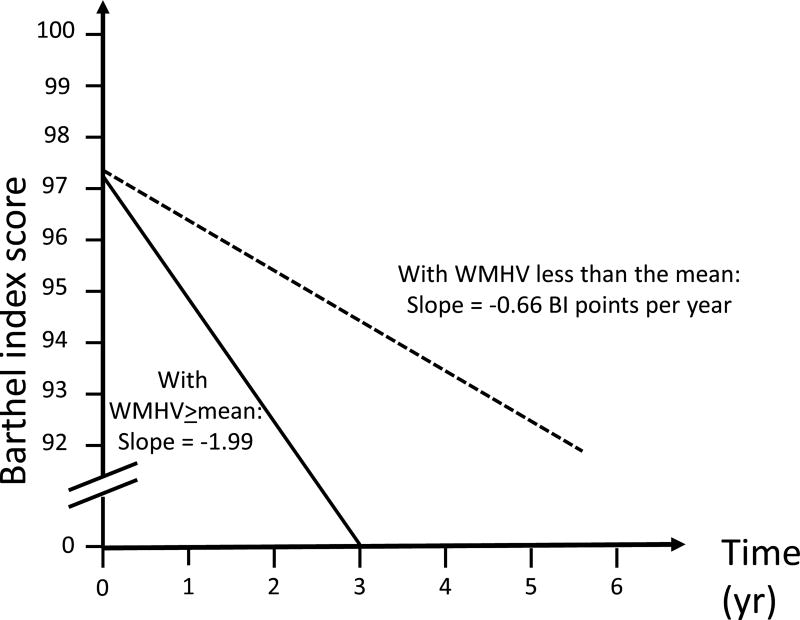
Figure 1 displays estimated functional trajectories for a Hispanic female with diabetes at the mean age and BMI. Figure 1A shows functional trajectories if the individual’s MMSE was 25, and depicts 2 curves, one if the individual did not have PI-SBI (slope= −1.00 BI points per year) and one if she had PI-SBI (slope=−1.49 BI points per year), showing a 50% greater decline with PI-SBI compared to without. Figure 1B displays estimated trajectories for a MMSE of 30, and shows a threefold greater functional decline in the presence of PI-SBI (−1.88 BI points per year) compared to without (−0.66 BI points per year). Figure 2 shows trajectories for a similar exemplar, applying to any baseline cognitive level. With WMHV greater than or equal to the mean (−1.99 BI points per year), there was a more than threefold greater functional decline compared to having WMHV less than the mean (−0.66 points per year).
Figure 1.
A. Trajectories of functional decline among those with mini-mental state score of 25: Estimated functional trajectories for a Hispanic female at the mean age and BMI with diabetes and mini-mental state score of 25
B. Trajectories of functional decline among those with mini-mental score of 30 Estimated functional trajectories for a Hispanic female at the mean age and BMI with diabetes and mini-mental state score of 30
Figure 2. Trajectories of functional decline among those with any mini-mental state score.
Estimated functional trajectories for a Hispanic female at the mean age and BMI with diabetes
Discussion
We found that PI-SBI but not WMHV significantly moderated the relationship between cognition and functional trajectories. Higher baseline cognitive ability was associated with less functional decline over time, a relationship that was not influenced by education. PI-SBI was independently associated with steeper functional decline, over and above the decline due to aging. Also, there was a significant interaction between cognition and PI-SBI, and a steeper decline in function in the presence of PI-SBI. For individuals with no cognitive impairment at baseline (MMSE score of 30), the presence of PI-SBI was associated with a threefold increased functional decline over time (−1.88 versus −0.66 BI points per year). Similarly, WMHV above the mean was associated with steeper functional decline, regardless of baseline cognitive function (−1.99 versus −0.66 BI points per year), demonstrating the large impact that even seemingly “subclinical” lesions may have on functional decline. Importantly, these declines were independent of incident vascular events during follow-up. Considering that 19% of the population had PI-SBI on imaging, despite 89% being functionally normal at the time of MRI, the population impact of subclinical brain lesions on disability is potentially large.
In a prior analysis in the NOMAS MRI sample,23 greater WMHV and smaller TCV were associated with poorer performance in learning a list of words. The current analysis expands upon this previous research by analyzing longitudinal trends of repeated measures of functional status and showing significant interactions between PI-SBI, cognition, and functional decline. We have also previously shown, in NOMAS, that periventricular WMHV is particularly associated with functional decline,24 and that right-left asymmetry of WMHV is associated with functional decline.25 Future studies in NOMAS will further explore relationships between WMHV in particular brain regions and functional and cognitive outcomes, as it is possible that the relationships among cognition, functional status, and WMHV may vary depending on the region involved..
Several previous studies have shown, as we have done in previous analyses,6 that greater WMHV was associated with greater disability, at 1 year26 and 2.42 years of follow-up,27 as well as with gait and balance28 and cognitive decline.29 Greater WMHV was associated with physical decline over 1 year, and possibly WMH in the deep frontoparietal and periventricular parietooccipital regions had the greatest impact on decline.30 SBI has also been previously associated with disability and cognitive impairment. Over 4 years of follow-up, WMHV and brain infarcts were associated with higher incidence of disability and accelerated decline in gait speed.4 Adjustment for incident stroke and dementia and mini-mental status score did not attenuate associations, but interactions with cognitive performance were not tested.
There are several brain structural changes associated with cerebrovascular disease that may have impacts on cognition and functional status. For example, brain infarcts have been associated with smaller hippocampal volumes, which are associated with poorer memory and cognitive function.31 SBI has also been associated with reduced gray matter volume and concomitant cognitive deficits.32 WMHV, SBI, microbleeds, and atrophy have been associated with declines in gait speed, cadence of gait, and length of steps,33, 34 which would affect the mobility aspects of ADL functioning. WMHV and progression of WMHV have been associated with neurological examination findings such as gait and stance abnormalities, upper motor signs, and slowing of fingertaps,35 and presence and number of neurological deficits have an independent impact on performance of ADLs.36 Deep SBI and WMHV have been associated with gait variability, which has been associated with falls and disability.37 In this study, although there was an association between WMHV and accelerated functional decline, WMHV did not moderate the relationship between cognitive performance and function, suggesting that WMHV may function through mechanisms distinct from SBI to affect ongoing functional decline independently of processes that determine cognitive ability.
“Functional reserve” refers to factors that protect functional capacities even in the setting of aging or other processes that would normally cause functional decline. Only when this reserve is depleted do functional limitations become manifest. This analysis supports the view that SBI depletes functional reserve and causes accelerated decline as individuals age, and that higher baseline cognitive function reduces the slope of decline. Although education has been posited as a factor influencing cognitive reserve, studies have not found a consistent moderating effect of education on age-related decline,38 or a relationship between education and decline over time,39 which parallels our findings.
Strengths of this study include the large population-based cohort, the accurate assessment of events during long-term follow-up of 7.3 years, minimal loss to follow-up, the use of state-of-the-art imaging and measurement of subclinical brain vascular disease, and the repeated measures of functional outcomes that allow trajectory analysis. Few prior studies have examined trajectories of these outcomes over time as we did, estimating not only change between 2 time-points but also slope of change over time. The analysis of repeated measures of functional status is more sensitive to longitudinal change than measurement at 2 or fewer time points. Due to reliable surveillance and tracking of events and regular and repeated measurements of functional status, this study can reliably estimate the likely central role that “subclinical” disease plays in functional ability and health over time. A limitation of this analysis is that the MRI NOMAS sample selects individuals who will be able to return for follow up and imaging and may reflect a healthy survivor bias, which may reduce power to detect declines in functional status. We lack pathological confirmation that our MRI findings are vascular in origin, leaving open the possibility of misclassification. However, postmortem MRI studies generally support our assumptions regarding the lesions identified here. We also did not have a comprehensive measure of cerebral microbleeds at the time of the analysis. Also, we relied on a single scale, the MMSE, to measure cognitive performance, and a more extensive assessment of cognitive domains was lacking. It is possible that by using the MMSE, we missed mild executive function impairment. Finally, the BI is subject to ceiling effects, and we may have underestimated the extent of functional decline over time. In particular, it is possible that the absence of a moderation by WMHV of the relationship between cognition and functional trajectories was due to ceiling effects of these measures.
More research is needed examining trajectories of cognition and disability over time, especially in relation to progressive imaging changes on repeated MRIs. Newer imaging and analytic techniques may be able to detect previously undetectable structural and functional brain dysfunction that could underlie the progressive disability and cognitive changes seen in epidemiological studies. This could lead to tests of interventions to prevent progression of subclinical cerebrovascular disease and hence possibly functional decline.
Acknowledgments
None
Funding: National Institute of Neurological Disorders and Stroke (R01 NS48134, MSVE; R01 NS29993, RLS/MSVE; K23NS079422, MSD).
Footnotes
Disclosures: none.
References
- 1.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 2.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat Rev Neurol. 2015;11:157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O'Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 4.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 5.Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, et al. Cognitive correlates of white matter lesion load and brain atrophy: The northern manhattan study. Neurology. 2015;85:441–449. doi: 10.1212/WNL.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.140th annual meeting american neurological association. Annals of Neurology. 2015;78:S1–S132. doi: 10.1002/ana.25865. [DOI] [PubMed] [Google Scholar]
- 7.Yu HW, Chen DR, Chiang TL, Tu YK, Chen YM. Disability trajectories and associated disablement process factors among older adults in taiwan. Archives of gerontology and geriatrics. 2015;60:272–280. doi: 10.1016/j.archger.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Liang J, Bennett JM, Botoseneanu A, Allore HG. Socioeconomic stratification and multidimensional health trajectories: Evidence of convergence in later old age. J Gerontol B Psychol Sci Soc Sci. 2015;70:661–671. doi: 10.1093/geronb/gbu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: The northern manhattan study. Stroke. 2006;37:13–19. doi: 10.1161/01.STR.0000195048.86810.5b. [DOI] [PubMed] [Google Scholar]
- 10.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, et al. The behavioral risk factor surveys: Ii. Design, methods, and estimates from combined state data. American journal of preventive medicine. 1985;1:9–14. [PubMed] [Google Scholar]
- 11.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: The northern manhattan study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 12.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid research clinics coronary primary prevention trial. Jama. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney FI, Barthel DW. Functional evaluation: The barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 15.Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: Analysis of repeated barthel index measures. Arch Phys Med Rehabil. 1979;60:14–17. [PubMed] [Google Scholar]
- 16.Shinar D, Gross CR, Bronstein KS, Licata-Gehr EE, Eden DT, Cabrera AR, et al. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil. 1987;68:723–728. [PubMed] [Google Scholar]
- 17.Bath PM, Gray LJ, Collier T, Pocock S, Carpenter J. Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials. Stroke. 2007;38:1911–1915. doi: 10.1161/STROKEAHA.106.474080. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL. Optimal end points for acute stroke therapy trials: Best ways to measure treatment effects of drugs and devices. Stroke. 2011;42:2356–2362. doi: 10.1161/STROKEAHA.111.619122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song F, Jerosch-Herold C, Holland R, Drachler Mde L, Mares K, Harvey I. Statistical methods for analysing barthel scores in trials of poststroke interventions: A review and computer simulations. Clin Rehabil. 2006;20:347–356. doi: 10.1191/0269215506cr948oa. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: The northern manhattan study. Journal of hypertension. 2015;33:2115–2122. doi: 10.1097/HJH.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tombaugh TN, McIntyre NJ. The mini-mental state examination: A comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 22.Dhamoon MS, Moon YP, Paik MC, Sacco RL, Elkind MS. Diabetes predicts long-term disability in an elderly urban cohort: The northern manhattan study. Ann Epidemiol. 2014 doi: 10.1016/j.annepidem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glazer H, Dong C, Yoshita M, Rundek T, Elkind MS, Sacco RL, et al. Subclinical cerebrovascular disease inversely associates with learning ability: The nomas. Neurology. 2015;84:2362–2367. doi: 10.1212/WNL.0000000000001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhamoon MS, Cheung YK, Bagci A, Alperin N, Sacco RL, Elkind MSV, et al. Periventricular white matter hyperintensities and functional decline. J Am Geriatr Soc. 2017 doi: 10.1111/jgs.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhamoon MS, Cheung YK, Bagci A, Alperin N, Sacco RL, Elkind MSV, et al. Differential effect of left vs. Right white matter hyperintensity burden on functional decline: The northern manhattan study. Frontiers in aging neuroscience. 2017;9:305. doi: 10.3389/fnagi.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inzitari D, Simoni M, Pracucci G, Poggesi A, Basile AM, Chabriat H, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: The ladis study. Arch Intern Med. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 27.Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: Three year follow-up of ladis (leukoaraiosis and disability) study cohort. Bmj. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreisel SH, Blahak C, Bazner H, Inzitari D, Pantoni L, Poggesi A, et al. Deterioration of gait and balance over time: The effects of age-related white matter change--the ladis study. Cerebrovasc Dis. 2013;35:544–553. doi: 10.1159/000350725. [DOI] [PubMed] [Google Scholar]
- 29.Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T, et al. White matter changes and diabetes predict cognitive decline in the elderly: The ladis study. Neurology. 2010;75:160–167. doi: 10.1212/WNL.0b013e3181e7ca05. [DOI] [PubMed] [Google Scholar]
- 30.Zheng JJ, Delbaere K, Close JC, Sachdev P, Wen W, Brodaty H, et al. White matter hyperintensities are an independent predictor of physical decline in community-dwelling older people. Gerontology. 2012;58:398–406. doi: 10.1159/000337815. [DOI] [PubMed] [Google Scholar]
- 31.Blum S, Luchsinger JA, Manly JJ, Schupf N, Stern Y, Brown TR, et al. Memory after silent stroke: Hippocampus and infarcts both matter. Neurology. 2012;78:38–46. doi: 10.1212/WNL.0b013e31823ed0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo W, Jiang X, Wei X, Li S, Li M. A study on cognitive impairment and gray matter volume abnormalities in silent cerebral infarction patients. Neuroradiology. 2015;57:783–789. doi: 10.1007/s00234-015-1535-3. [DOI] [PubMed] [Google Scholar]
- 33.Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, et al. Brain structural change and gait decline: A longitudinal population-based study. J Am Geriatr Soc. 2013;61:1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- 34.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 35.Poggesi A, Gouw A, van der Flier W, Pracucci G, Chabriat H, Erkinjuntti T, et al. Cerebral white matter changes are associated with abnormalities on neurological examination in non-disabled elderly: The ladis study. J Neurol. 2013;260:1014–1021. doi: 10.1007/s00415-012-6748-3. [DOI] [PubMed] [Google Scholar]
- 36.Poggesi A, Gouw A, van der Flier W, Pracucci G, Chabriat H, Erkinjuntti T, et al. Neurological abnormalities predict disability: The ladis (leukoaraiosis and disability) study. J Neurol. 2014;261:1160–1169. doi: 10.1007/s00415-014-7332-9. [DOI] [PubMed] [Google Scholar]
- 37.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age-related cognitive decline: A review of recent research. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society. 2014 doi: 10.1111/psyg.12083. [DOI] [PubMed] [Google Scholar]
- 39.Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, et al. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. J Int Neuropsychol Soc. 2011;17:1039–1046. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]