Abstract
Significance: MicroRNAs (miRNAs) are important regulators of gene expression and define part of the epigenetic signature. Their influence on every realm of biomedicine is established and progressively increasing. The impact of environment on human health is enormous. Among environmental risk factors impinging on quality of life are those of chemical nature (toxic chemicals, heavy metals, pollutants, and pesticides) as well as those related to everyday life such as exposure to noise or mental and psychosocial stress.
Recent Advances: This review elaborates on the relationship between miRNAs and these environmental risk factors.
Critical Issues: The most relevant facts underlying the role of miRNAs in the response to these environmental stressors, including redox regulatory changes and oxidative stress, are highlighted and discussed. In the cases wherein miRNA mutations are relevant for this response, the pertinent literature is also reviewed.
Future Directions: We conclude that, even though in some cases important advances have been made regarding close correlations between specific miRNAs and biological responses to environmental risk factors, a need for prospective large-cohort studies is likely necessary to establish causative roles. Antioxid. Redox Signal. 28, 773–796.
Keywords: : environmental chemicals, air pollution, heavy metals, pesticides, noise exposure, deafness, hearing loss, mental stress, neuropsychiatric disorders
Introduction
Since their discovery in 1993, the importance of microRNAs (miRNAs) on post-transcriptional regulation has become commonly accepted, and now miRNA research has exploded upon a massive swell of interest because of the enormous range and potential in almost every biological discipline. The miRNAs are short (∼22 nucleotides [nt]), evolutionarily conserved, single-stranded RNAs that control the expression of complementary target mRNAs, usually leading to their transcript destabilization, translational inhibition, or both. As such, they are crucial for the development and maintenance of tissues, both in healthy and diseased states (3). The human genome encodes >2000 miRNAs, which are predicted to target about 60% of protein-coding genes. Each miRNA is transcribed by RNA polymerase II as a long precursor RNA, called primary miRNA (pri-miRNA), which is then subjected to nuclear processing by the Drosha–DGCR8 “microprocessor” complex. The resulting intermediate, a hairpin-shaped precursor miRNA (pre-miRNA) of ∼70 nt in length, is exported to the cytoplasm and then further shortened by Dicer, yielding a ∼22 nt mature miRNA. In the cytoplasm, miRNAs associate with specific mRNAs within a multiprotein complex of Argonaute proteins, known as the RNA-induced silencing complex (RISC), providing sequence-specific silencing activity (8). A single miRNA may regulate the expression of numerous genes associated with the same physiological process, suggesting that specific miRNAs are key participants in regulating gene regulatory networks. The final effect of a particular miRNA on gene expression depends on its relative cell- and tissue-specific expression levels as well as on its specificity toward its targets and the abundance of these targets (5).
The miRNAs are encoded in different locations in the genome, including intronic and intergenic regions. Interestingly, intronic miRNAs often control the expression of genes associated with the same cellular functions regulated by the host gene where they are encoded. This elegant mechanism of gene regulation is exemplified by the sterol response element binding protein (SREBPs)/miR-33ab gene loci. SREBP2 and SREBP1 regulate the synthesis and uptake of cholesterol and synthesis of fatty acid, respectively. Coinciding with the transcription of SREBP2 and SREBP1, miR-33a and miR-33b are cotranscribed and negatively regulate the expression of a number of genes involved in regulating cholesterol efflux and fatty acid oxidation. Both negative feedback loops cooperate to enhance intracellular cholesterol and fatty acid levels by simultaneously balancing transcriptional activation and post-transcriptional repression of lipid homeostasis genes (156). In addition to lipid metabolism, most of cellular processes have been shown to be regulated by miRNAs. Importantly, recent work has demonstrated that miRNAs are able to “fine-tune” the regulation of redox signaling, by direct interaction with nuclear factor erythroid 2 like 2 (NFE2L2; also known as Nrf2), the major transcriptional regulator of defense against reactive oxygen species (ROS) (63, 103). The miRNAs may also interact with its coregulators Kelch-like erytroid cell-derived protein with cap ‘h’ collar homology (ECH)-associated protein 1 (Keap1) and Broad-complex, Tramtrack and Bric a brac (BTB) domain and cap ‘h’ collar (CNC) homolog 1 (Bach1), or regulate the generation of ROS. These new subsets of miRNAs that either regulate redox pathways or are themselves regulated by the cellular redox state have been termed “redoximiRs” (23). Redox regulation affects gene expression as well as translational processes at multiple levels, including the classical pathways (activity of transcription factors, mRNA stability) but also epigenetic processes (miRNA signaling, DNA methylation, histone modifications) and DNA damage/repair, all of which contribute significantly to overall genome stability (Fig. 1) (125).
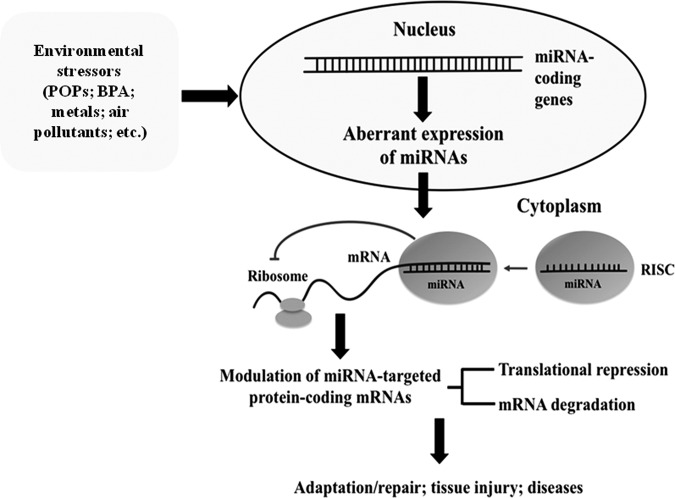
FIG. 1.
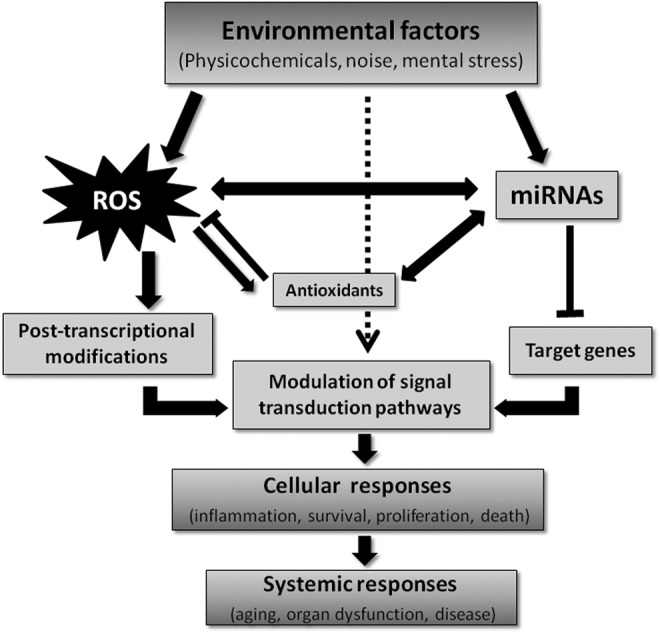
Interaction between redox, environmental factors, miRNAs, and gene/protein expression. Environmental exposure leads to redox–miRNA networks reprogramming modulating pathophysiology state. Physicochemical agents, noise, or mental stress can induce alteration on redox state or miRNAs expression, which can also regulate redox pathways or be themselves regulated by the cellular redox, modulating gene/protein expression, and cellular responses. miRNAs, microRNAs.
Environmental Risk Factors and Scope of the Review
It has become increasingly recognized that chronic human diseases are primarily associated with environmental factors as opposed to genetic factors (155). Environmental risk factors comprise a large number of determinants related to populations sharing common living or working spaces. They can be of physicochemical or social nature and it is now quite clear that their influence on individual and social health differences is a concern of increasing magnitude (1). Nearly all human diseases result from a complex interaction between an individual's genetic profile and his/her exposure to environmental factors. Since adverse regulation of miRNA has a substantial impact on the development and progression of cardiovascular disease, environmental changes of miRNA expression and activity likely affect cardiovascular health (31). The concept of the “exposome” has been recently established to study gene–environment interactions, identify novel biomarkers, and discover key regulators of adaptive response after exposure to environmental risk factors (126). Importantly, environmental exposure may lead to epigenetic reprogramming (including changes in miRNA signatures), which can be a contributor to disease development later in life (Fig. 2). Specific miRNA expression profiles have been linked to several toxic environmental risk elements, including radiation, air pollution, and cigarette smoke (128). Efforts have been made to model the complexity of the networks and regulatory mechanisms of miRNAs involved in environmental gene regulation using computational tools (104, 176). In this review, we delineate three specific settings of environmental risk factors and the reciprocal influence of miRNAs as well as their connection to oxidative stress: (i) namely environmental chemicals, (ii) noise-induced hearing loss (NIHL), and (iii) mental stress.
FIG. 2.
Environmental risk factors modulate specific miRNA expression profiles. Exposure to environmental stressors, noise or mental stress, causes changes in the levels of specific miRNAs, which then globally modulate the expression of targeted protein-coding mRNAs through translation repression, or mRNA degradation via the RISC. The consequences of this modulation may include changes in the levels of adaptive/repair proteins, antioxidant defense, tissue inflammation, and injury or persistent disease states. RISC, RNA-induced silencing complex.
Environmental chemicals
In this section, we discuss the critical involvement of miRNAs in xenobiotic-induced disease pathogenesis (Fig. 3). The important environmental risk determinants to be covered in this section include persistent environmental chemicals (polychlorinated biphenyls [PCBs], polybrominated diphenyl ethers [PBDEs], perfluorocarboxylic acids [PFCAs], and perfluorooctanesulfonate [PFOS]), benzene, bisphenol A (BPA), heavy metals, air pollution, and pesticides.
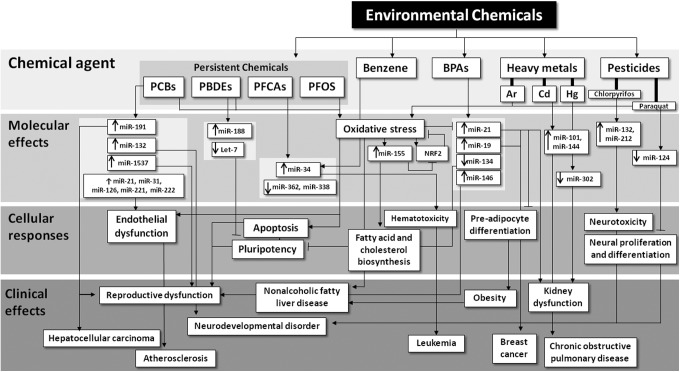
FIG. 3.
Environmental chemicals exposure toxicity is mediated by alterations of redox state and miRNA expression. Exposure to environmental chemicals, such as POPs (e.g., PCBs, PBDEs, PFCAs, and PFOS), endocrine disrupting chemicals such as BPA, heavy metals (e.g., Cd, Pb, As, and Hg), and pesticides induces changes in the levels of specific miRNAs and oxidative stress that alter cellular functions, leading to organ dysfunction. BPA, bisphenol A; PBDEs, polybrominated diphenyl ethers; PCBs, polychlorinated biphenyls; PFCAs, perfluorocarboxylic acids; PFOS, perfluorooctanesulfonate; POPs, persistent organic pollutants.
PCBs are environmental toxicants that produce a wide spectrum of toxicities in humans such as reproductive dysfunction (183), neurodevelopmental disorders (166, 167, 211), and obesity (53). Although the production of PCBs was banned in the United States in 1979, these chemicals are still widespread in food, drinking water, and soil because of their highly persistent nature, raising great safety concerns (54, 66). PCBs are activators for xenobiotic-sensing transcription factors, which, in turn, upregulate their target genes such as cytochrome P450s involved in xenobiotic biotransformation. Whereas the coplanar (also known as “dioxin-like”) PCBs are activators for the aryl hydrocarbon receptor (AhR), the noncoplanar PCBs are activators for the pregnane X receptor and the constitutive androstane receptor (4, 55).
PBDEs were used as flame retardants incorporated into plastics, rubbers, and textiles, and were recently banned because of their wide spectrum of toxicities such as thyroid hormone disorders (50, 58, 186, 232, 233), neurotoxicity (95), oxidative stress in liver (2, 227), and carcinogenesis (132). However, owing to their highly persistent and bioaccumulative nature, PBDEs still raise growing safety concerns, as certain PBDE congeners are enriched in seafood, breast milk, household dust, and in electric waste dismantling sites (110, 116, 169, 212).
The persistent perfluorinated compounds, especially PFCAs and PFOS, have been detected in humans and wildlife, raising health concerns (18, 35, 45, 170, 174). PFCAs have been extensively used in Scotchgard products and in making Teflon brand products. PFOS is used in industrial and consumer applications as surfactants and building material components (136).
Endocrine disruptors such as BPA, dichlorodiphenyltrichloroethane (DDT), and phthalates interfere with the body's endocrine system through modulating the activities of hormonal receptors such as the estrogen receptor (ER), producing a wide spectrum of developmental, reproductive, neurological, and immune effects in both humans and wildlife (150). BPA is widely used as a plasticizer in epoxy resins and in thermal papers. Thus, humans are regularly exposed to BPA, and this may lead to chronic diseases such as hormone-dependent cancers. Of particular concern are exposures to BPA that occur early in life, a period of susceptibility that can have a life-long impact on disease risk (149, 150).
Heavy metals and metalloids such as lead, cadmium, mercury, selenium, chromium, and arsenic toxicity are highly diverse, and are dependent upon many factors including the organ targeted, exposure route, time of development, gender, and dietary factors (20). The body has mechanisms to deal with heavy metal exposures including their excretion through the upregulation of cationic transporters, sulfhydryl-based scavengers (e.g., glutathione and metallothionein) (94), reduction of metabolism and methylation (87) at the transcriptional, translational, and post-translational levels to deal with metal exposures (87). Ambient air pollution is a term used to describe outdoor air pollution, which most often is a mixture of particulate matter (PM), volatile organic compounds, ozone, oxides of nitrogen and sulfur, and in some cases industrial emissions rich in heavy metals and toxic organics. Exposure to ambient air pollution has been associated with adverse outcomes in many organ systems, including the lung, the cardiovascular system, the liver, and the central nervous system (CNS) (20). As with other complex mixtures, the effects are variable depending on the sources, distances from those sources, and variations in climate, sunlight, or traffic loads. Therefore, there has been a need to identify biomarkers of exposure that are convenient, robust, reproducible, and ideally specific of the exposure.
miRNAs and persistent environmental chemicals (PCBs, PBDEs, and PFCAs/PFOS)
Polychlorinated biphenyls
For the dioxin-like PCBs, in human peripheral blood mononuclear cells, miR-191 expression correlates with total blood concentrations of PCBs, and in particular with the dioxin-like congener PCB169 (57). The blood levels of PCB169 significantly correlate with miR-191 in pregnant women living in a PCB-contaminated area who have undergone therapeutic abortion because of fetal malformations. Of note, miR-191 is also known to be upregulated by dioxin in hepatocellular carcinoma cells in vitro (Table 1) (57).
Table 1.
List of Relevant Studies in Environmental Chemicals and MicroRNAs from 2015 to Present
| Environmental chemicals | miRNAs | Target genes or pathways | Species or cell types | References |
|---|---|---|---|---|
| PBDEs | miR-188-5p (positively associated with BDE-209) Let-7c (inversely associated with BDE-99) |
N/A | Human placenta | (106) |
| PBDEs (BDE-209) | miR-145/miR-335 ↑ | Pluripotency, apoptosis, oxidative stress | HESCs (FY-hES-10 and FY-hES-26) | (39, 40) |
| PCBs | miR-1537 (positively associated with PCB levels) | N/A | Human placenta | (106) |
| PCBs (Aroclor 1260) | 557 miRNAs changed Validated: ↑ in miR-21, miR-31, miR-126, miR-221, miR-222 |
21 miRNAs associated with vascular diseases; cardiac injury (mir-21) Inflammation (miR-126, miR-31) |
Primary human endothelial cells | (191) |
| PFCA | miR-34a ↑ miR-362-3p ↓ miR-338-3p ↓ |
Fucosyltransferase 8 (miR-34a) Lactate dehydrogenase (miR-34a) |
Mouse liver | (196) |
| PFOS | miR-155 | Nrf2 and oxidative stress | HepG2 cells | (192) |
| BPA | miR-146a (positively associated with BPA levels) | Enzyme, cell cycle, signal transduction, transcription factors, cancer, nervous system | Human placenta | (34) |
| BPA | miR-21a-5p inhibits BPA-induced adipocyte differentiation | Adipogenic differentiation, MKK3/p38/MAPK | 3T3-L1 cells | (214) |
| Cd | miR-1537 (positively associated with exposure) | N/A | Human placenta | (106) |
| Hg | Multiple let-7c members ↓ | N/A | Human placenta | (106) |
| Hg | 17 miRNAs inversely associated with toenail Hg | N/A | Cervical tissue from pregnant women | (163) |
| Hg | miR-92a ↑ miR-486 ↑ |
N/A | Human plasma of poisoned workers | (37) |
| Pb | miR-575 and miR-4286 inversely associated with tibial bone Pb | N/A | Cervical tissue from pregnant women | (163) |
| Pb | Multiple let-7c members ↓ | N/A | Human placenta | (106) |
| Heavy metals | Many miRNAs (Table 1 in Yuan et al., 2016) | Please refer to the review | Please refer to the review | (224a) |
| Pesticides | 6 miRNAs positively associated with farmworkers status during postharvest season (five have a positive dose–response relationship with organophosphate pesticide metabolites) | N/A | Human urine from parent/child, farmworker/nonfarmworker pairs during two agricultural seasons | (206) |
| Pesticides (chlorpyrifos) | miR-132/miR-212 ↑ | Disruption of neurotrophin-mediated cognitive processes | CA1 region of the hippocampus of male Long–Evans rats | (101) |
BPA, bisphenol A; HESC, human embryonic stem cells; miRNAs, microRNAs; N/A, not available; PBDEs, polybrominated diphenyl ethers; PCBs, polychlorinated biphenyls; PFCAs, perfluorocarboxylic acids; PFOS, perfluorooctanesulfonate; MAPK, mitogen-activated protein kinase.
The nondioxin-like PCBs have been linked to neuropsychological dysfunction in children. Specifically, they increase spontaneous Ca2+ oscillations in neurons by stabilizing ryanodine receptor (RyR) calcium release channels in the open configuration, leading to cAMP response element-binding (CREB)-dependent dendritic outgrowth (122). The nondioxin-like congener PCB95 at nanomolar concentrations promotes synaptogenesis via RyR-dependent upregulation of miR-132 and inhibition of RyR, CREB, or miR-132 block PCB95-mediated effects. Interestingly, miR-132 is also dysregulated in Rett syndrome and schizophrenia. Therefore, miR-132 is an important risk factor for PCB-mediated neurodevelopmental disorders (102).
PCBs have also been correlated with multiple vascular complications such as endothelial cell dysfunction and atherosclerosis, through producing oxidative stress and induction of proinflammatory cytokines and cell adhesion proteins (65). In primary human endothelial cells, the commercial PCB mixture Aroclor 1260 alters the expression of 557 out of 6658 miRNAs, and 21 of them have been associated with vascular diseases according to the MetaCore database (191). Specifically, Aroclor 1260 increases the expression of miR-21, miR-31, miR-126, miR-221, and miR-222. Whereas miR-21 has been implicated in cardiac injury, miR-126 and miR-31 have been shown to modulate inflammation (191). Last but not least, the National Children's Study (NCS) has established the associations between miRNA expression profiles and various environmental pollutants including PCBs, and specifically, PCBs positively associate with miR-1537 expression in term placentas (106). These studies have suggested that miRNAs may serve as potential biomarkers to stratify distinct mechanisms of various diseases associated with PCB exposure.
Polybrominated diphenyl ethers
In the NCS, it was reported that BDE-209 positively correlates with miR-188-5p, whereas PBDE-99 inversely correlates with the miRNA let-7c in term placentas (106). In human embryonic stem cell (ESC) lines (FY-hES-10 and FY-hES-26), BDE-209 at nanomolar concentrations reduces expression of pluripotent genes such as OCT4, SOX2, and NANOG and induces apoptosis (39). In addition, the downregulation of OCT4 is accompanied by hypermethylation of the OCT4 promoter and increased expression of miR-145 and miR-335, which inhibit OCT4 expression (106). BDE-209 also produces ROS and decreases superoxide dismutase (SOD)2 expression. Therefore, the authors have concluded that BDE-209 decreases pluripotent gene expression via epigenetic regulation (e.g., miRNAs) and induces apoptosis through ROS generation (39).
Perfluorocarboxylic acids
Among various types of PFCAs, perfluorononanoic acid (PFNA), which is a PFCA with a nine-carbon backbone, produces hepatomegaly, increases hepatic triglycerides and total cholesterol, and increases serum transaminases (154). Many miRNAs were differentially regulated by PFNA in a dose-dependent manner, including an upregulation of miR-34a and a downregulation of miR-362-3p and miR-338-3p at both doses tested, whereas miR-34a regulates fucosyltransferase 8 and lactate dehydrogenase expression. Therefore, the authors have concluded that PFNA exerts its hepatic effect at least partially through miRNA-mediated post-translational downregulation (196).
Perfluorooctanesulfonate
PFOS has been shown to induce adipogenesis and glucose uptake in preadipocytes and this was associated with activation of the oxidative stress responsive transcription factor Nrf2, which is important for upregulating antioxidant genes and metabolic reprogramming (219). In addition, it has been shown in rats that PFOS tends to accumulate in the liver, resulting in hepatomegaly, actin filament remodeling, endothelial permeability changes, and ROS production. This coincides with PFOS-mediated upregulation of miR-155, which appears to suppress Nrf2 signaling, because pretreatment of HepG2 cells with catalase (CAT) decreases miR-155 expression, increases Nrf2 expression and activation, and reduces PFOS-induced cytotoxicity and oxidative stress (192). Therefore, PFOS-induced oxidative stress is at least partially dependent on miRNA-mediated downregulation of Nrf2 signaling.
miRNAs and benzene
Exposure to benzene such as that of paint sprayers may result in hematological disorders of significant severity. The aberrant expression of miRNAs in workers exposed to benzene has been analyzed (7). It was found that miRs 34a, 205, 10b, let-7d, 185, and 423 5p-2 were upregulated and 133a, 543, has-130a, 27b, 223, 142-5p, and 320b were downregulated. Several pathways involved in cell proliferation and differentiation including vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), and Wnt signaling were shown to be affected, thus providing the opportunity to explore them as causative links or to exploit selected miRNAs as potential biomarkers.
miRNAs and BPA
In human placental cells, miRNA microarrays were used to identify several miRNAs that were significantly altered in response to BPA treatment, exemplified by a marked upregulation of miR-146 that leads to slower proliferation and higher sensitivity to the DNA damaging drug bleomycin (6, 171). Its expression correlated significantly with BPA accumulation in the placentas from pregnant women living in a polluted area and undergoing therapeutic abortion because of fetal malformations (34). Therefore, miR-146 expression may serve as a biomarker for developmental exposure to BPA.
In both ESCs and embryoid bodies (EBs) of mouse origin, BPA decreases the expression of miR-134, which is a suppressor of the pluripotency markers Oct4, Sox2, and Nanog, suggesting that miR-134 may play a role in BPA-mediated disturbances in pluripotency in ESCs and EBs (22).
In sheep, gestational BPA exposure at environmentally relevant doses altered the expression of steroidogenic enzymes Cyp19 and 5α-reductase in the ovaries at gestational day 65, and the expression of fetal ovarian miRNAs (45 downregulated at gestational day 65 and 11 downregulated at gestational day 90). Importantly, miRNAs that target Sry-related high-mobility-group box (SOX) family genes, kit ligand, and insulin-related genes were downregulated by BPA, suggesting that miRNAs may play a role in BPA-mediated disturbances in gonadal differentiation, folliculogenesis, and insulin homeostasis (190).
Exposure to BPA is also implicated in breast cancer. In the ER-positive and hormone-sensitive human MCF-7 breast cancer cell line, BPA potentiates ER transcriptional activity and this coincides with alterations in the expression profiles of certain miRNAs including miR-21, which is an onco-miR that is frequently upregulated in solid tumors (182). BPA also increases the expression of the onco-miRs miR-19a and miR-19b and dysregulates the expression of the miR-19-related downstream proteins such as PTEN, p-AKT, p-MDM2, and p53 in MCF-7 cells. Interestingly, the chemopreventive drug curcumin reverses these effects, suggesting that curcumin may suppress BPA-induced breast cancer through modulating the miR-19/PTEN/AKT/p53 axis (109). Regarding the effect of BPA on intermediary metabolism, in epidemiological and animal studies, BPA exposure has been associated with type-2 diabetes and especially gestational diabetes mellitus (GDM), and there is emerging evidence showing that placental-derived exosome miRNAs may serve as predictors for GDM (43). In rats, miR-21a-5p overexpression attenuated BPA-induced obesity in vivo (214). In preadipocytes, BPA-induced cell differentiation is suppressed by miR-21a-5p by targeting map2k3 in the MKK3/p38/mitogen-activated protein kinase (MAPK) pathway, suggesting that miR-21a-5p mimics may serve as a potential therapy for BPA-induced obesity (214).
miRNAs and heavy metals
Arsenic
Earlier work published by Marsit et al. (119) investigated the relationship between arsenic exposure and miRNA expression in TK6 cells (an immortalized human lymphoblast cell line). Treatment with sodium arsenite led to decreased miR-210 expression and increases in miR-22, miR-34a, miR-221, and miR-222, similar to those that would be observed with cellular nutritional stress such as folate deficiency. These effects were then confirmed in human peripheral blood samples similarly exposed to arsenite.
Kong et al. (97) assessed microalbuminuria in adolescents, and its relationship among urinary metals (Hg, Pb, As, and Cd) and the levels of miR-21, miR-126, miR-155, and miR-221. They found no relationship between metal levels and microalbuminuria, but miR-21 and miR-221 were negatively associated with this arsenic and lead levels, and miR-21 was associated with microalbuminuria. Thus, miRNA levels were proposed as biomarkers of kidney function in the context of heavy metal exposure.
In a study of arsenic exposure to pregnant mothers and the consequences for their infants, Rager et al. (153) examined the relationship between arsenic in drinking water and maternal urine, and the expression of miRNAs in cord blood. There were significant associations between a number of cord blood miRNAs (let-7a, miR-107, miR-126, miR-16, miR-17, miR-195, miR-20a, miR-20b, miR-454, miR-96, and miR-98) and urinary arsenic. These miRNAs have been linked to cancer and diabetes. Furthermore, there was a depression in the expression of a number of immune response-related mRNAs that were predicted to be partially caused by changes in these miRNAs.
Bollati et al. (16) studied the expression of miR-21, miR-146a, and miR-222 in peripheral blood leukocytes (PBLs) obtained from steel workers occupationally exposed to PM containing arsenic, iron, nickel, lead, cadmium, chromium, and manganese. They also examined the impact of exposure on the oxidative stress biomarker 8-hydroxyguanine, and the effects the individual metals have on miRNA expression in isolated PBLs. Both miR-21 and miR-222 were increased when comparing baseline expression (start of work week) to 3 days of exposure, and miR-222 levels were correlated with lead exposure. Conversely, miR-146a was inversely correlated with exposure to lead and cadmium. Furthermore, miR-21 was associated with 8-hydroxyguanine levels. The authors thus suggested that the expression of these miRNAs could represent novel mechanisms of response to PM and its associated metals. Exposure to lead associated with atmospheric PM is also related to the expression of miRNAs that are involved in oxidative stress and inflammation (16), all of which contribute to cardiovascular risk and mortality (29).
Cadmium
Cadmium (Cd) is an especially toxic and persistent heavy metal. Although many studies have examined the effects of Cd on mRNA transcript levels, few have examined the miRNAs-mediated Cd effects. Fabbri et al. (44) investigated the effects of Cd exposure in HepG2 human hepatoma cells on global gene expression and miRNA levels. Transcriptional changes at higher Cd exposure included those related to cancer and depressed hepatic function, and a number of let-7 miRNA family members were differentially expressed by Cd, suggesting a connection between their tumor suppressor roles and cadmium carcinogenesis.
Cd is also a major component of cigarette smoke. In a study designed to assess the effects of cigarette smoke and Cd on cystic fibrosis transmembrane regulator (CFTR) function, Hassan et al. (62) showed that cigarette smoke and cadmium increased miR-101 and miR-144 expression in human airway epithelial cells, which suppressed the expression of CFTR protein. They also showed that cigarette smoke exposure caused similar changes in miR-101 in the lungs of mice. Moreover, chronic obstructive pulmonary disease (COPD) patients had increased pulmonary expression of miR-101 than patient controls, suggesting a link between cigarette smoking, Cd exposure, and suppression of CFTR in COPD.
Mercury
Mercury in its inorganic form (Hg++ ion) has deleterious effects on the kidney, whereas the methylated form of mercury (MeHg) targets the CNS, especially during embryonic and fetal development (20, 163). In an in vitro model of CNS differentiation, Pallocca et al. (141) treated mixed neuronal/glial cell cultures derived from NT2 cell ESC precursors with MeHg chloride during differentiation. As the cells differentiated, there was a decrease in the stem cell miRNA expression signature (downregulation of miR-302 cluster that reflects stem cell character) and an upregulation of miRNAs emblematic of neuronal differentiation (let-7, miR-125b, and miR-132). When exposed to MeHg, these cultures showed differential regulation of several miRNAs (miR-141, miR-196b, miR-302b, miR-367, and miR-372) whose targets were mapped to pathways important for axonal guidance, learning, and memory.
Changes in the expression of circulating miRNAs with occupational exposure to mercury were recently examined in a pilot case–control study by Ding et al. (37). High-level Hg exposure was associated with increases in the expression of miR-92a and miR-486, suggesting that these two miRNAs may be suitable biomarkers in larger cohorts occupationally exposed to Hg at lower levels.
miRNAs and air pollution
Changes in the expression of miRNAs have also been investigated as biomarkers of air pollution exposure (70, 83, 113, 146, 204) (Table 2). Susceptibility to particle health effects, microRNAs and exosomes (SPHERE) study (15) is focused on evaluating the adverse health effects of air pollution on obese subjects. This same group has shown that exposure to PM is associated with changes in the exosomal miRNA profile in humans, with similar changes in A549 human lung epithelial cells (14). In addition, Rodosthenous et al. (157) found an association between long-term exposure to ambient PM of <2.5 μm in diameter (PM2.5) and increased levels of extracellular vesicle miRNA circulating in the serum of subjects in the aging cohort study. These included miR-126-3p, miR-19b-3p, miR-93-5p, miR-223-3p, and miR-142-3p with 6 months of exposure, and miR-23a-3p, miR-150-5p, miR-15a-5p, miR-191-5p, and let-7a-5p with 1 year of PM2.5 exposure. Pathway analysis revealed gene targets of these miRNAs that were associated with cardiovascular disease, including oxidative stress, inflammation, and atherosclerosis.
Table 2.
List of Relevant Studies in Air Pollution and MicroRNAs from 2015 to Present
| Air pollutant | miRNAs | Target genes or pathways | Species or cell types | References |
|---|---|---|---|---|
| Air pollution | miR-144 ↓ | Orcogene Zeb1 | Human nonsmall cell lung cancers | (142) |
| Air pollution (volatile organic compounds) | 467 miRNAs for toluene, 211 miRNAs for xylene, 695 miRNAs for ethylbenzene as a characteristic, discernible exposure indicator | N/A | Human whole blood | (177a) |
| Air pollution (diesel exhaust particles) | miR-21 ↑ | PTEN/PI3K/AKT pathway | Human bronchial epithelial cells | (229, 231) |
| Air pollution (PM) | mir-128 ↑ miR-302 ↑ |
Coronary artery disease pathways (miR-128); coronary artery disease, cardiac hypertrophy, heart failure (miR-302c) | Plasma cell-derived microvesicles; A549 pulmonary cell line | (14) |
| Air pollution (PM2.5) | miR-21, miR-146a, and miR-222 inversely associated with PM2.5 during the second trimester; Mir-20a and miR-21 positively associated with first trimester | PTEN | Human placenta | (185) |
| Air pollution (PM2.5) | 6 month window: ↑ in mir-126-3p, miR-19b-3p, miR-93-5p, mir-223-3p, mir-142-3p 1-year window: ↑ miR-23a-3p, miR-150-5p, miR-15a-5p, mir-191-5p, let-7a-5p |
Cardiovascular disease-related pathways (oxidative stress, inflammation, and atherosclearosis) | Human serum | (157) |
| Air pollution (PM2.5) | MiR-1228(*) prevents PM2.5-induced cell apoptosis | Inhibit apoptosis | Human alveolar epithelial cells (A549) | (108) |
| Air pollution (second-hand smoke) | 9 miRNAs ↑ by in utero exposure | Proasthmatic genes (miR-155-5p, miR-21-3p, and miR-18a-5p), tumor suppressor genes | Mouse lung | (213) |
| Air pollution (PM10) | miR-21 ↓, miR-222 ↓ | Inflammatory and oxidative stress pathways | Venous blood | (115) |
| Air pollution (PM10) | 9 miRNAs associated with PM10 levels 48 h after exposure MiR-101 mediates PM-10-induced increase in BP | BP | Human peripheral blood | (129) |
| Air pollution (black carbon) | Association of XPO5 rs11077 with miR-9 and miR-96 | Blood carbon–cognition associations | Blood from older men | (27) |
| Air pollution (PM2.5) elemental carbon, PM10 | 12 miRNAs associated with PM10 in office workers 46 human miRNAs associated with elemental carbon (short term) | Cellular proliferation/differentiation (truck drivers), inflammation (office workers) | Blood from truck drivers and office workers | (73) |
| Air pollution (PM2.5 and PM10) | miR-146a (PM10) ↑, miR-29c (PM2.5) ↑ | Inflammation (miR-146a) Epigenetic modification (miR-29c) |
Human bronchial BEAS-2B cells | (113) |
| Air pollution (PM2.5) | 37 miRNA altered by water PM2.5 62 miRNA altered by organic PM2.5 |
Nutrients, biosynthetic processes, nucleic acid metabolism; DNA replication, cell cycle | Human alveolar epithelial cells (A549) | (84) |
BP, blood pressure; PM, particulate matter.
Although human epidemiology studies are clearly important for evaluating such risks, mechanistic information has often come from cell culture and animal studies. Several in vitro studies have examined the effects of various air pollutants on miRNA expression. Bleck et al. (11) found that diesel exhaust and ambient particulate exposures were associated with miR-375-mediated regulation of thymic stromal lymphopoietin (TSLP; a potent proinflammatory chemokine important in both innate immunity and tail homology 2 (TH2)-adaptive immunity) in human bronchial epithelial cells. This effect was likely mediated via miR-375 targeting the AhR, thus relieving AhR suppression of TSLP. Zhou et al. (229) examined the effects of diesel exhaust particles on miRNA-21 in human bronchial epithelial cells and the potential carcinogenic mechanisms associated with such exposures. Diesel particle exposure caused an increase in the expression of miR-21, which, in turn, upregulated PTEN/PI3K/AKT signaling, a pathway that is often activated in cancer cells.
In recent studies, Li et al. (108) found that miR-1228(*) was able to inhibit apoptosis in A549 human alveolar epithelial cells exposed to fine PM. Using the same cell line, Jeong et al. (84) conducted an integrative analysis of mRNA and miRNA expression of these cells exposed to aqueous and organic-soluble extracts from PM2.5. This comprehensive analysis found that a large number of miRNAs were altered (37 and 62 miRNAs for aqueous and organic extracts, respectively), which mapped to a number of pathways important for nutrient sensing, nucleic acid synthesis, DNA synthesis, and cell cycle regulation.
Animal studies have also been conducted to investigate the roles of miRNAs in the adverse effects of PM exposure (213). Farraj et al. (47) exposed rats to ambient PM and noted changes in cardiac functional parameters (ST-segment depression in the electrocardiogram, arrhythmia, and vagal dominance), which was associated with a general decrease in the expression of miRNAs in cardiac tissue.
An earlier human study by Wilker et al. (209) noted associations between exposure to ambient black carbon (BC; a marker of traffic-related air pollution), blood pressure (BP) changes, and single nucleotide polymorphisms (SNPs) in several miRNA processing genes (DICER, GEMIN4, and DGCR8). This study did not evaluate the potential mechanisms by which these SNPs might be causative for the association between BC exposure and increased BP. In the normative aging study, Wilker et al. (208) investigated the relationships between ambient air pollutants, polymorphisms associated with miRNA processing, and the concentrations of circulating soluble cellular adhesion molecules, which are correlates of atherosclerosis and cardiovascular disease. Seven-day moving averages of PM2.5 exposure were associated with higher soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1) levels. Sulfates and sulfur dioxide 7-day moving averages were associated with higher sICAM-1 and a suggestive association was observed with sVCAM-1 in aging men (117). They also noted that SNPs in miRNA-processing genes may modify associations between ambient air pollutants and sICAM-1 and sVCAM-1. Of note, miRNA polymorphisms have also been encountered in patients with esophageal squamous cell carcinoma heavily exposed to environmental smoke (202).
In follow-on studies, Fossati et al. (49) investigated the relationships between miRNAs in PBLs and PM exposure. The mRNAs miR-1, miR-126, miR-135a, miR-146a, miR-155, miR-21, miR-222, and miR-9 were all associated with PM exposure. When mapped to targeting pathways, miR-126, miR-146a, miR-155, miR-21, and miR-222 were strongly associated with changes in the high-mobility group chromatin proteins. The relationships between the expression of these mRNAs and PM exposures were also influenced by polymorphisms in the RNA processing genes GEMIN4 and DGCR8. More recently, Motta et al. (129) found that miRNAs are a likely molecular mechanism underlying the BP-related effects of air pollution exposure, and indicated that changes in miR-101 expression are important as an epigenetic mechanism for this relationship.
Although less commonly investigated than the effects on cardiopulmonary function, a recent study found an association between miRNA expression, air pollution exposure, and lung cancer. Pan et al. (142) found that miR-144 was downregulated in air pollution-related lung cancer, and this could be related to the fact that it targets the oncogene Zeb1.
PM is also known to adversely impact vascular function, which has been elucidated, by measures of BP and flow-mediated vessel dilation (99). Imaging of the retina provides another measure of vascular function. Louwies et al. (115) measured microvascular responses to PM with retinography and investigated the roles that oxidative stress-associated miR-21 and miR-222 might have on PM-induced changes in these microvessels. Both miR-21 and miR-222 were found to correlate with PM-induced abnormalities in retinal microvessel diameter, suggesting a role for oxidative stress and inflammation in these effects.
In a study that investigated the relationship between air pollutants and potential adverse perinatal effects, Tsamou et al. (185) measured the expression of six candidate miRNAs in placental tissue from 210 mother–newborn pairs. Of the six miRNAs examined, miR-22, miR-146a, and miR-222 expression were negatively associated with PM2.5 exposure, and the tumor suppressor PTEN was identified as a common target of these miRNAs. Importantly, its expression was increased with exposure to PM2.5 in the third trimester, suggesting a mechanistic link between PM2.5, miRNAs, and PTEN expression.
Regarding the potential role of miRNAs in the effects of PM on CNS function, long-term exposure to BC was shown to be associated with cognitive impairment in older men, which was also associated with SNPs in miRNA processing genes (27).
Finally, there is recently published evidence that PM is associated with suppression of innate immunity and decreased clearance of viruses. Hou et al. (73) investigated associations of short-term PM2.5, EC, and PM10 with miRNA expression. They found correlations between EC exposure and viral miRNA expression, suggesting that latent viral miRNAs are potential mediators of air pollution-associated health effects.
miRNAs and pesticides
Pesticides are substances intended for preventing, destroying, repelling, or mitigating pests such as insects, rodents, weeds, and many other unwanted organisms (20) and adversely impact human health ranging from skin irritation to more severe effects such as neurological disorders, reproductive problems, and cancer. Many pesticides can modulate the expression of miRNAs associated with certain diseases (28).
For organophosphates, which are a classic group of insecticides that inhibit acetylcholinesterase, urinary miRNAs have been suggested to be biomarkers for human exposure. Significant differences in miRNA profiles have been observed between farmworkers and nonfarmworkers, as well as between farmworkers during thinning and during postharvest agricultural seasons. Importantly, there is a positive dose–response relationship between certain miRNAs and organophosphate insecticide metabolites in farmworkers (206).
Subchronic exposure to the organophosphate insecticide chlorpyrifos is implicated in cognitive dysfunctions such as learning and memory deficits. In chlorpyrifos-exposed rats, miR-132 and miR-212 are elevated in the hippocampus CA1 region, and this has been suggested to play a role in the disruption of neurotrophin-mediated cognitive processes after chlorpyrifos exposure (101).
Another organophosphate, namely dichlorvos, can produce both neurotoxicity and non-neuronal toxicity. In porcine kidney epithelial cells, dichlorvos produces aberrant expression of miRNAs, and this coincides with inhibition in the cell proliferation in a dose- and time-dependent manner, which has been suggested to be a result of dichlorvos-induced apoptosis (107).
The phenylpyrazole insecticide fipronil and the broad-spectrum insecticide/miticide triazophos have been shown to alter miRNA expression in zebrafish and have been suggested to serve as biomarkers for toxicity (198).
The conazole fungicides triadimefon and propiconazole are mouse liver carcinogens, whereas another conazole fungicide myclobutanil is not. There are upregulated miRNAs in livers of mice that are treated with carcinogenic conazoles as compared with mice treated with noncarcinogeneic conazole, suggesting the important roles of miRNAs in certain conazole-mediated formation of liver cancer (158).
Paraquat is another extensively studied environmental chemical that is used as a herbicide. Paraquat produces toxicity in the lung through redox cycling and formation of superoxide anion and eventually hydroxyl radicals leading to lipid peroxidation (20). In human neural progenitor cells, 66 miRNAs have been found to be differentially regulated in proliferating cells upon paraquat treatment, and in silico analysis has shown that the targets of these miRNAs include genes involved in neural proliferation and differentiation, as well as cell cycle and apoptosis (75).
miRNAs regulation of NIHL
The mammalian inner ear comprises two main organs, the cochlea, responsible for hearing, which contains the organ of Corti, an extremely sensitive sensory epithelium, comprising specialized sensor hair cells, and the vestibule that is responsible for the perception of balance (51).
Since 2006 when miRNAs appeared in the mammalian inner ear field, they have exploded as an additional layer of gene regulation in both inner ear development and disease (172). miRNAs show particular expression patterns in the inner ear. Thus, 74 miRNAs have been differentially expressed in the auditory and vestibular portions, whereas the conserved miRNA cluster, which includes miR-96, miR-182, and miR-183, presents a well-defined pattern of expression along inner ear development (162, 207). In addition, mutations in the seed region of miR-96 are associated with hearing loss in humans and mice (123). Thus, miRNAs have also been described as effective elements in ear-related diseases and hearing loss (118).
The early tissue-specific deletion of the mouse Dicer1 gene, involved in the processing of mature miRNAs, results in gross inner ear malformations, suggesting that miRNAs are crucial for inner ear development (178). Inner ear-specific Dicer1 deletion decreases the expression of fibroblast growth factor (FGF) ligand FGF10, a critical signaling molecule of inner ear morphogenesis (144). These mice also have defects in prosensory cell proliferation and hair cell fate specification, which are likely because of derepression of Wnt signaling frizzled-related proteins Sfrp4 and Sfrp5. The expression of these genes in the developing cochlea is likely repressed by miR-124, which is selectively present in the differentiating auditory sensory epithelium (30, 78). In addition, Dicer1-deficient auditory prosensory cells do not properly exit the cell cycle, partially because of the loss of let-7 miRNA and the increased expression of its target genes N-Myc and cyclin D1(17). Furthermore, miR-200b, which is selectively expressed in cochlear and vestibular epithelial cells, regulates the critical processes for inner ear morphogenesis, epithelial-to-mesenchymal transition, and its reversal (mesenchymal-to-epithelial transition), underlying the negative feedback loop between members of miR-200 family and the transcription factors zinc finger E-box binding homeobox 1 (ZEB1) and ZEB2. Twirler mice, which have a noncoding point mutation in the first intron of the Zeb1 gene, have severe vestibular and auditory defects (67). Finally, the identification of specific miRNAs in age-related hearing loss (AHL) suggests that proapoptotic miRNAs and those promoting proliferation and differentiation are both involved in age-related degeneration of the organ of Corti (226).
Noise toxicity key factors on hearing loss
NIHL is a complex disease that results from the interaction of genetic and environmental factors with a susceptibility that differs remarkably among individuals. Long or repeated exposure to sounds at or >85 dB can cause hearing loss. Shearing forces from an excessive sound can cause cell death of the hair cells of the basilar membrane in the cochlea when they impact on the stereocilia. Hair cells are completely differentiated, and after becoming apoptotic, it is impossible to regenerate them. For that reason, an excessive noise exposure causes an irreversible degeneration in the basilar membrane of the cochlea (151). Different association studies have identified a cohort of genes involved in NIHL. These genes can be classified into different pathways: oxidative stress, potassium recycling, monogenic deafness, and heat shock protein (HSP)-related genes. In addition, post-translational regulation through miRNAs has also emerged as an additional layer of noise-induced gene regulation (173).
Acoustic contamination leads to sensory cell degeneration through well-differentiated pathways, resulting in severe apoptotic and/or necrotic phenotypes (13). For example, Taok1, involved in the stress response MAPK pathway, may be responsible of cochlear acoustic trauma via apoptosis modulation (195). Among the targets of miR-183 are early growth response 1 (Egr1) and insulin receptor substrate 1 (Irs1). Acoustic overstimulation in the rat cochlea increases the expression of Egr1 (24). However, even though Irs2-deficient mice develop sensorineural hearing loss (130), Irs1 has not been characterized in the cochlea.
Surprisingly, many predicted and validated target genes of NHIL-dependent miRNAs have been previously associated with sensorineural hearing loss. Xiap, a miR-186 target gene, has been involved in the protection against NHIL when it was overexpressed in a transgenic mouse model (194). The stress response P38α/MAPK is a predicted target of miR-124, and it has been linked to stress-related pathways in the cochlea and in apoptosis responses induced after acoustic damage (180). miR-124 target E2F3, this transcription factor, was upregulated 2 h postnoise exposure in the Chinchilla lanigera cochlea. This E2f3 increase was also involved with the p38/MAPK signaling pathway (82). Inhibition of Bcl11b, an antiapoptotic target of miR-124 and miR-381, both in vitro and in vivo, induces hair cell apoptosis (89).
Oxidative stress regulation
Recent publications have shown that increased oxidative stress in the inner ear is closely related to NIHL and AHL. These studies suggest that prevention of oxidative damage might be a solution to prevent development and progression of the majority of hearing loss cases (161).
During noise exposure, vasoconstriction increases after 8-isoprostane-F2a release (112). Then cochlear blood flow recovers to normal levels inducing ischemia reperfusion. This ischemia-reperfusion injury induces mitochondrial dysfunction and release of ROS in the cochlea. Antioxidant treatments have been shown to protect and decrease the progress of hearing loss in laboratory animals (181). Nrf2 is one of the master regulators of antioxidant genes. Although Nrf2 is not essential for normal development and cochlea function, Nrf2-/- mice seem to be more susceptible to noise exposure and have an impaired recovery and increased oxidative stress accumulation. Two groups of antioxidant enzymes, both tightly regulated by Nrf2, are active in the cochlea: glutathione metabolism enzymes and peroxide/superoxide scavengers, including CAT and SOD1 and SOD2 (72). Rabinowitz et al. have shown that noise-exposed workers that carry glutathione S transferase M1 (GSTM1) were protected from NIHL (152). Moreover, the SNP V16A in SOD2 in Chinese workers was associated with an enhanced sensitivity to NIHL. Some miRNAs expressed in the inner ear have been previously associated with impairment of Nrf2 function, as miR-34a and miR-200, but further investigation about their role in the Nrf2 pathway needs to be done.
Activation of NADPH oxidase enzymes, in particular NOX3, has been described in drug, noise, and AHL (160). Du et al. suggested that NOX3-associated oxidative stress may contribute to the accumulation of mtDNA mutations and activate a caspase 3-dependent apoptotic signalling pathway in the cochlea (40). However, the relationship between NOX3 and miRNAs has to be further investigated. Another interesting protein is pejvakin (PJVK). PJVK function is still unknown but PJVK-deficient mice are exceptionally vulnerable to sound. Noise damage in the cochlea induces upregulation of PJVK transcription and triggers peroxisome proliferation, resulting in an enhanced antioxidant effect in the auditory system (36).
AHL, also known as presbycusis, is also caused by cochlear hair cell degeneration and it is the most common form of hearing loss. Oxidative stress is also enhanced with aging, and has a fundamental role in inducing hair cell apoptosis in AHL. However, the mechanisms that mediate this effect need further investigation (92). One of the proteins previously related to hearing loss and aging is Sirtuin-1 (SIRT-1). SIRT-1 is a highly conserved NAD-(+)-dependent protein deacetylase, which has protective effects against age-related diseases. This protein acts like a sensor to regulate the internal oxidative stress by deacetylation of its substrates, like proliferator-activated receptor gamma coactivator 1α (PGC-1α) or the tumor suppressor protein p53 (147). SIRT-1 expression in cochlea has been well described and its reduction is associated with elevated hearing threshold and hair cell loss during aging, becoming a protective molecule (216). Several publications have shown differences in the expression of miR-29 and miR-34a families in the inner ear during aging (188). mir-34 has previously described to provide robustness to stress response programs by controlling noise in the DAF-16/FOXO-regulated gene network in Caenorhabditis elegans (79). Pang et al. described an increase in miR-34a in the cochlea, auditory cortex, and plasma from C57BL/6 mice during aging experiments and they corroborated these pieces of evidence in human patients with AHL. This was accompanied by a decrease in SIRT-1 and other miR-34a targets (143). Xiong et al. studied this mechanism by analyzing SIRT-1-dependent p53 acetylation. Their results showed that an increase in miR-34a and the consequent decrease in SIRT-1 lead to an increase in p53 acetylation and apoptosis (217). SIRT-1 and miR-34a have also been involved in other hearing pathologies as cisplatin-mediated hearing impairment, especially when together with SIRT-1 decreased expression; its function is also compromised by the reduction of intracellular NAD(+) (93).
miR-29b has been shown to be involved in aging, cellular senescence, and apoptosis, and one of its confirmed targets is also SIRT-1 (231). In this scenario, Xue et al. studied the correlation between miR-29 and SIRT-1 that also leads to hair cell apoptosis. They demonstrated that not only SIRT-1 but also PGC-1α, which plays an essential role in apoptosis and mitochondrial metabolism, are affected. Inhibition of miR-29b in in vitro studies with HE1-OCI cells, a hair cell line, increased the expression of these targets while decreasing apoptosis (220). However, the role of miR-34a and miR-29 in redox-dependent NIHL as well their utility as biomarkers in early detection of NIHL awaits further confirmation.
Finally, miR-451, a DICER-independent miRNA (222), previously described as protective against erythroid oxidative stress (224), was reported to be upregulated in a HEI-OC1 cell model of oxidative stress after treatment with tert-butyl-hydroperoxide (t-BHP) (203). This miRNA is one of the most upregulated in NIHL patients versus individuals exposed to noise, but it is significantly downregulated compared with nonexposure controls. These findings underscore its relevance in oxidative stress modulation in hearing loss pathology.
Potassium recycling pathway genes
The sensory cells of the inner ear are bathed in the endolymph, an extracellular fluid that is rich in potassium ions. Potassium is mainly responsible for the sensory transduction. Its proper recycling is necessary for the hearing mechanism (189). Multiple mutations in these potassium ion transporter genes (gap junction beta-2 protein [GJB2], gap junction beta-3 protein [GJB3], gap junction beta-6 protein [GJB6], potassium voltage-gated channel subfamily E member 1 [KCNE1], potassium voltage-gated channel subfamily Q member 1 [KCNQ1], and potassium voltage-gated channel subfamily Q member 4 [KCNQ4]) lead to both syndromic and nonsyndromic forms of hearing loss (189). Different susceptibility to noise is also associated with SNPs in these genes (145). A deletion in GJB2, also known as Connexin 26, in the cochlear sensory epithelium leads to increased apoptosis. In this loss-of-function model, miR-27 showed an increased expression. Wang et al. observed that the use of miR-27 shRNA inhibited GJB2 knockout-induced apoptosis (201). Moreover, Zhu et al. found that deletion of GJB2 reduced miR-96 expression in the cochlea during postnatal development. This reduction is associated with a cochlear tunnel developmental disorder in these knockout mice (234). In the case of KCNE1 and KCNQ, no evidence for miRNA modulation has been found. However, in the electrical remodeling of atrial fibrillation, miR-1 is responsible for the decreased expression of these genes (85). Thus, the link between miR-1 and KCNE1 and KCNQ in the cochlea needs further investigation.
Monogenic deafness genes
Most of the cases of genetic deafness recognized today are monogenic disorders. The failure of the activity in these genes has also been associated with sensitivity to NIHL. One of the most relevant is related to mutations in cadherin 23 (Cdh23) and protocadherin 15, molecules that link the sensory hair cells in the cochlea. These genes are fundamental for a right mechanoelectrical transduction (42). Mutations in Cdh23 disrupted the stereocilia organization on hair cells, inducing deafness and vestibular dysfunction in the model of the Ames waltzer mouse; the 753A variant of the Cdh23 gene was correlated with increased sensitivity to NIHL. In humans, PCDH15 and CDH23 gene mutations have been described in syndromic and nonsyndromic hearing loss (134).
MYH14 encodes one of the proteins of the myosin superfamily. They are actin-dependent motor proteins regulating cochlear hair cells motility and polarity. Mutation in MYH14 results in autosomal dominant hearing impairment in humans (DFNA4) (98). Nevertheless, no relationship between these genes and miRNAs in the inner ear or other tissues was found. miRNA mutations related to human deafness have been found in three families (123, 175). Mutations in the seed region of miR-96, specifically expressed in the inner and outer hair cells, are associated with hearing loss in human and mice. Studies addressing whether or not a larger number of miRNAs contribute to human deafness have been done, even though some authors propose this is unlikely to be the case (68).
HSP genes
HSPs form a group of conserved proteins assisting in synthesis, folding, assembly, and intracellular transport of many other proteins, whose expression increases under stressful conditions, including noise exposure. Variations in HSP70-1, HSP70-2, and HSP70-hom genes were shown to be associated with susceptibility to NIHL (200). Several publications have shown a correlation between HSP70 and some resident miRNAs in the inner ear such as miR-451 and miR-29 (25, 48, 184). Overexpression of miR-34 and miR-451 in cortical neurons increases HSP70 and vulnerability to apoptosis in the transfected cells (184). This might also be the case in miR-34-induced apoptosis in the cochlea.
Biomarkers
The epidemiological studies directed toward elucidating the potential effects of noise exposure or chronic NIHL on miRNA expression also questioned whether miRNAs can represent biomarkers as indicators of responses to noise exposure or occurrence of NIHL. Extracellular miRNAs in plasma have the potential to serve as stable noninvasive biomarkers in physiological and pathological conditions (165). However, few studies have specifically addressed the problem of miRNAs as biomarkers in NIHL. In a recent publication, Ding et al. studied differences in plasma miRNAs in male textile workers diagnosed with NIHL. They compared a population of 10 noise-exposed individuals and 10 NIHL patients and then attempted to further validate their results in a population of 46 noise-exposed textile workers, which included 23 NIHL patients. More than 73 miRNAs demonstrated significant differential expression in NIHL patients. The sequential validation restricted this group to four significantly upregulated miRNAs (miR-16-5p, miR-24-3p, miR-185-5p, and miR-451). After exclusion analysis, just two of these miRNAs, miR-185-5p and miR-451a, have been identified and validated as biomarkers. The plasma levels of both miRNAs were significantly downregulated in the noise-exposed individuals than in the nonexposed individuals, whereas they were slightly elevated in the NIHL patients than in the noise-exposed individuals (38). miR-451, as mentioned before, has been related to the regulation of oxidative stress in the inner ear. In the case of miR-185, its presence in the cochlea has not been established, but its role in DNA damage responses has been well described (193). In the case of other hearing loss pathologies, miR-34a has been correlated with AHL in mice and humans and its upregulation in plasma has been proposed as a possible biomarker (143). In idiopathic sudden sensorineural hearing loss (SSNHL), the protein Argonaute-2 (AGO2), an essential component of the RISC, was upregulated in SSNHL patients versus healthy control patients (59). However, further studies are necessary to prove the causal association between changes in the expression of miRNAs and noise exposure.
NIHL is a complex disease that results from the interaction of environmental and genetic factors. Recently, miRNAs have emerged as an additional mechanism of noise-induced gene regulation (Fig. 4). Some miRNAs such as miR-96, miR-182, and miR-183 family are highly specific in the inner ear in development and in hearing loss pathology (32). Some other miRNAs such as miR-34a and miR-29, previously involved in apoptosis and oxidative stress in other pathologies, also modulate key targets as SIRT-1 and Nrf2 in the cochlea (221). Other miRNAs have been weakly related to essential proteins in the cochlea function as is the case for miR-27 and miR-96 with the potassium ion transporter GJB2 (234). In addition, some miRNAs such as miR-185-5p and miR-451a have emerged as potential biomarkers in this pathology, leading to an easier and earlier diagnosis. Further studies are needed to complete this puzzle and connect highly regulated miRNAs in cochlea after damage exposure with hearing loss-relevant enzymes. Besides, a comparative analysis in all the different hearing loss pathologies is required to identify potential common causative factors, including hair cell apoptosis (38).
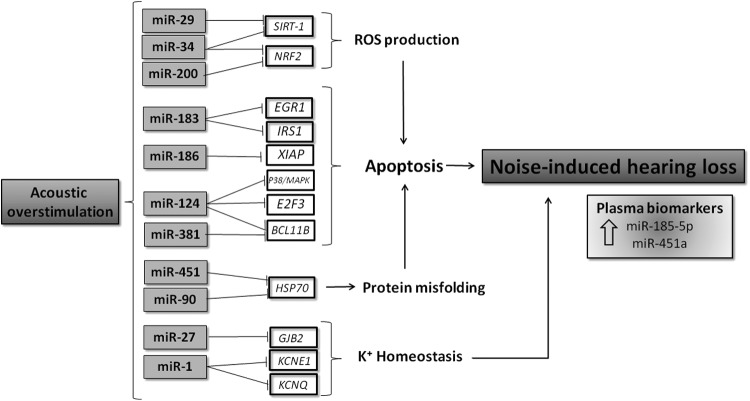
FIG. 4.
miRNAs as regulators of noise-induced hearing loss. Acoustic overstimulation mediates the expression of miRNAs that regulate genes involved in oxidative stress, potassium recycling pathways, monogenic deafness, and heat shock protein genes associated with hearing loss. Some miRNAs such as miR-124, miR-183, and miR-381 regulate apoptosis-related genes such as E2F3, EGR1, and BCL11B, respectively, whereas miR-34a and miR-29 modulate oxidative stress-related targets such as SIRT-1 and Nrf2 in the cochlea, indirectly leading to hair cell apoptosis. Other miRNAs, such as miR-27, regulate the expression of the essential protein for cochlea function, the potassium ion transporter GJB2. In addition, some miRNAs such as miR-185-5p and miR-451a have emerged as potential biomarkers in this pathology. GJB2, gap junction beta-2 protein; SIRT-1, Sirtuin-1.
The role of miRNAs in mental stress and neuropsychiatric disorders
Studies from the past decade have highlighted the role of miRNAs in mental stress, psychiatric disorders, and the response to antipsychotic and antidepressant drugs. miRNAs are involved in a variety of psychiatric disorders such as schizophrenia, anxiety, major depression disorder (MDD), bipolar disorder, or post-traumatic stress disorder (PTSD). Psychiatric disorders are often accompanied by alterations in neuron architecture, function and survival, and synaptic plasticity and are the product of a combination of genetic and environmental factors (140). The molecular mechanisms by which environmental factors contribute to the onset and progression of psychiatric disorders are currently unclear. In this regard, it has been shown that the modulation of epigenetic mechanisms by environmental factors can have an effect on brain plasticity and behavior (159) as well as in the development of mental illness (133).
miRNAs are an important subset of these epigenetic regulators. Recent studies have shown that they can be mediators of the onset and progression of psychiatric disorders as well as modulate the response to treatment with antipsychotic and antidepressant drugs (69). Increasing evidence suggests that psychoactive agents including antidepressants and mood stabilisers utilize miRNAs as downstream effectors. Altering miRNA levels has been shown to alter behavior in a therapeutically desirable manner in preclinical models (135). For instance, miRNAs dysregulation may underlie many of the molecular changes observed in PTSD pathogenesis (56). Thus, Zhou et al. analyzed the role of miRNAs on the immunological dysfunction associated with PTSD. They found 7 upregulated and 64 downregulated miRNAs in combat veterans with PTSD. Specifically, miR-125a downregulation was suggested to be responsible for the increase in interferon gamma (IFNγ) observed in PTSD patients (230). Another study showed that DICER1 levels were reduced in the blood of PTSD patients with comorbid depression, suggesting that this decrease could be responsible for the general downregulation of miRNA levels observed in PTSD patients (210). However, this decrease in blood DICER1 levels was not found in a study of major depression (10). Thus, further human studies must be conducted to discern whether DICER1 downregulation is a specific characteristic of PTSD but is not present in other psychiatric disorders. MDD is the most common psychiatric illness. Recent studies have shown that MDD is associated with alterations in the levels of several miRNAs in whole blood, plasma, or serum. Expression levels of miR-34b-5p and miR-34c-5p are higher in leukocytes of MDD patients than in controls, and the levels of these two miRNAs correlated with the severity of the depression status (179). The miR-34 family is involved in hypothalamic-pituitary-adrenal (HPA) axis that has a prominent role in stress response (69). In fact, it has been reported that miR-34c regulates corticotrophin releasing hormone receptor 1 (CRHR1) mRNA, which modulates anxiety-like behavior and affects regulation (60, 64). According to the role of miR-34 family on depression, it has been shown that miR-34a is downregulated by a combination of lithium and valproic acid, two well-known mood stabilizers, in cultured rat neuronal cells (77).
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that plays essential roles in neuronal development and plasticity. Serum BDNF levels have been proposed to be a good biomarker of depression and it is known that BDNF is associated with the risk of schizophrenia and with aggressiveness and anxiety (120). Serum BDNF levels are lower in patients with depression and, concomitantly, serum levels of the BDNF-targeting miR-132 are higher. Serum miR-132 levels were also positively associated with depression severity (111). Interestingly, BDNF increases the expression of miR-132 in cultured cortical neurons. In contrast, glucocorticoid receptor (GR) activation has been demonstrated to reduce miR-132 expression (90). These authors also showed that miR-132 partly contributes to the BDNF-mediated increase of postsynaptic proteins (90).
Glucocorticoids are important mediators of neuronal function and are associated with behavior, cognition, memory, and emotions (76). Uchida et al. demonstrated that GR protein was lower in the paraventricular nucleus of restrain-stressed F344 rats, although they failed to observe a similar decrease in the hippocampus and prefrontal cortex. They found that miR-18a inhibited translation of the GR mRNA, although this miRNA was not modulated by restrain stress in this model (187). In another study, Fan et al. found that miRNA-26b, miRNA-1972, miRNA-4485, miRNA-4498, and miRNA-4743 were significantly upregulated in the blood of MDD patients. A functional analysis of targets of these miRNAs showed that most of them were involved in axon guidance, glutamatergic synapsis, and long-term potentiation, but they were also involved in mTOR signaling and carbohydrate metabolism (46). Blood miR-26b levels have also been found to be increased after chronic academic stress and decreased once this stress disappears (71). This study also showed academic stress-mediated upregulation in miR-16, miR-20b, miR-126, miR-144, and miR-144*. Moreover, miR-16 levels were positively correlated with anxiety levels. MiR-451a, miR-17-5p, and miR-223-3p have also been found to be upregulated in MDD patients in a cohort from Turkey and miR-451a levels seemed to be correlated with the duration of the depressive episode (19).
Selective serotonin reuptake inhibitors (SSRIs) are the most common antidepressant drugs currently in use. It has been shown that some miRNAs are modified by SSRI treatment and may be used as potential biomarkers of treatment response (41). For instance, escitalopram increased blood levels of 28 miRNAs including those of the let-7 family and miR-29b and decreased levels of miR-34c and miR-770. Escitalopram-modulated miRNAs are involved in fundamental neurological functions, including neuroactive ligand–receptor interaction, axon guidance, long-term potentiation, and depression. However, it is noteworthy that these miRNAs are also involved in metabolism, nutrient sensing (MAPK, insulin, and mTOR signaling), and vascular smooth muscle cell contraction (12). Belzeaux et al. found that miR-589, miR-579, miR-941, miR-133a, miR-494, miR-107, miR-148a, miR-652, and miR-425-3p were upregulated, whereas miR-517b, miR-636, miR-1243, miR-381, and miR-200c were downregulated in patients suffering from a major depressive episode compared with those of normal controls. Furthermore, miR-20b-3p, miR-433, miR-409-3p, miR-410, miR-485-3p, miR-133a, and miR-145 were modified by antidepressant treatment in patients with clinical improvement, suggesting that these miRNAs are potentially accessible biomarkers of treatment response (10). Plasma levels of miR-144-5p and miR-30c-5p are significantly increased after 8 weeks of treatment and SSRIs and plasma levels of miR-144-5p are also inversely correlated with a depressive score (197).
Another study carried out by Lopez et al. measured levels of miRNAs in the ventrolateral prefrontal cortex of depressed individuals compared with those of psychiatrically healthy controls and found that miR-1202 was significantly downregulated. miR-1202 is a primate-specific miRNA that targets metabotropic glutamate receptor 4 (GRM4). Interestingly, brain miR-1202 levels were higher in depressive patients who had been subjected to antidepressant treatment than in those without a previous history of antidepressant treatment. Blood levels of miR-1202 also increased in patients who responded to citalopram treatment, but not in nonresponding patients (114). One of the described mechanisms by which SSRIs reduce depression is the inhibition of serotonin transporter (SERT). A recent study showed that SSRIs increased the expression of miR-135a in the raphe nuclei of a mouse model of depression (81). miR-135 directly targets SERT and, therefore, the authors suggested that the increase in miR-135a levels is a potential mechanism that could explain the antidepressant action of SSRIs. Moreover, authors showed that overexpression of miR-135 resulted in increased resilience in mouse anxiety and depression models. On the contrary, miR-135a knockdown resulted in decreased SSRI treatment efficacy (81). This study also showed that miR-135a levels were lower in the raphe nuclei of depressed patients who had committed suicide. Circulating levels of this miRNA were also found to be lower in depressed patients (81). miR-16 also targets SERT and is increased by fluoxetine, an SSRI, in mouse serotoninergic raphe nuclei (9).
To sum up, these studies suggest that miRNAs play essential roles in the onset and progression of psychiatric disorders. In addition, they show that miRNAs are associated with treatment response and blood and circulating miRNAs are potential biomarkers of both disease progression and treatment response. However, it should be noted that few studies consistently found similar dysregulated miRNAs in brain and blood. This could be because of the small sample size of many of them as well as other factors such as ethnicity and sociodemographic factors.
Environmental factors that modulate neuropsychiatric miRNAs
All these miRNAs are associated with psychiatric disorders and treatment response. Also recent studies have revealed that patients with psychiatric disorders have altered miRNA expression profiles in the circulation and brain and have shown that manipulating the levels of particular miRNAs in the brain can alter behavior (80). Environmental factors can modify miRNA expression, leading to both short- and long-term effects on mood and behavior through the modification of neuronal plasticity or inducing neuronal structural changes (69). The effect of environmental factors on psychiatric miRNAs has been studied mainly in animal models exposed to different types of stressors. It should be borne in mind that depression and other mood disorders are difficult to study in murine models because they are complex disorders with highly heterogeneous symptoms. Moreover, to determine the severity of those disorders, subjective tests are often used that cannot be applied to animal models. Despite these limitations, some animal studies have demonstrated the potential of some environmental factors to contribute to psychiatric disorders through the modulation of related miRNAs. For instance, adult male rats subjected to immobilization stress showed changes in amygdala and hippocampus miRNA profiles. Among the dysregulated miRNAs, miR-134 and miR-183 were both upregulated by acute stress in the central amygdala (121). One of the targets of both miRNAs is Sc35, whose protein accumulates in the prefrontal cortex after acute stress and regulates the stress-induced alternative splicing of acetylcholinesterase mRNA in brain neurons (124). miR-134 has also been involved in synaptic plasticity and long-term memory formation (52).
Environmental pathogens, environmental chemicals, dietary stress, and drugs/alcohol abuse are other environmental factors that can potentially alter psychiatric-related miRNAs. For instance, dietary stress induced by caloric restriction (CR) or high-fat diet (HFD) modifies hypothalamic miRNA profiles in rats. It has been shown that miR-30, miR-200b/c, and let-7 miRNA families were deregulated after persistent nutritional challenge. Specifically, miR-30 and let-7 miRNAs increased by either HFD or CR or both, whereas miR-200 miRNAs were downregulated after CR (164). Let-7 and miR-200 miRNA families were similarly modified in the brain of mice exposed to hexahydro-1,3,5-trinitro-1,3,5-triaxine (the explosive known as RDX), which is a common environmental contaminant. Interestingly, let-7 family was downregulated in the liver of mice exposed to this pollutant (225). Cocaine increased the expression of miR-34b, miR-34c, miR-134, and miR-181, among others, in the hippocampus of addicted rats. However, miR-34b and miR-34c levels decreased after addiction extinction (21). miR-190 levels were higher in hippocampi of rats after cocaine addiction extinction than in control nonaddicted rats (21). miR-190 was also downregulated by fentanyl in mice hippocampi (228).
Exposure to environmental stress during embryonic development can result in altered neurodevelopmental processes that lead to impaired hippocampal development, impaired HPA axis activity and responsiveness, and impaired synaptic plasticity. There is increasing evidence that maternal mental stress induced by anxiety or depression results in neurodevelopmental disorders and higher risk of psychiatric illnesses in the offspring (69). Recently, Zucchi et al. showed that gestational stress induced by restrain of the body or forced swimming in pregnant rats leads to altered miRNA profiles in the hippocampus of newborn offspring. Gestational stress induced upregulation of miR-98, miR-103, and miR-323 and downregulation of miR-145, miR-151, and miR-425 (235). The same authors showed that gestational stress can even have transgenerational effects because they showed that growth retardation and behavioral disorders, while evident in the F1 generation, were even stronger in subsequent F2 and F3 generations. These alterations were accompanied by changes in the level of brain miRNAs in F2, including increases in miR-23b and miR-200c and decreases in miR-200a, miR-200b, miR-96, miR-182, miR-183, miR-141, miR-429, and miR-451 (223).
miRNAs link oxidative stress and mental stress
Proper mitochondrial function is fundamental for neuronal survival. Oxidative stress, especially in astrocytes and microglia, deeply impacts mitochondrial function (105). Mitochondrial dysfunction caused by oxidative stress is associated with psychiatric and mood disorders like bipolar disorder or MDD (26, 100). Recent emerging pieces of evidence suggest that miRNAs play a role in the regulation of oxidative stress-mediated neuronal mitochondrial disease. Six miRNA families have been consistently associated with neuropsychiatric disorders and mental stress and have been shown to be modulated by antipsychotic and antidepressant treatments: miR-29, miR-30, miR-200, miR-34, miR-181, and let-7. These miRNA families also play a role in the cellular response to oxidative stress (Fig. 5).
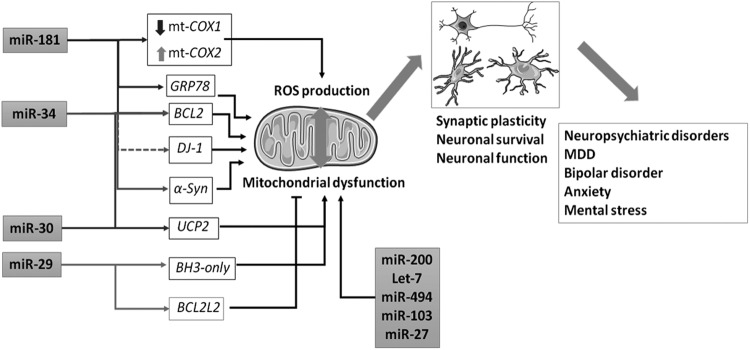
FIG. 5.
miRNAs link oxidative stress and psychiatric disorders. Some families of miRNAs involved in psychiatric disorders have been proven to modulate oxidative stress and ROS production via altered mitochondrial function, all of which are fundamental for neuronal survival. Through modulation of mitochondrial COX1 and COX2, as well as BCL2 and GRP78, the miR-181 family regulates mitochondrial oxidative stress. The miR-34 family affects ROS production and mitochondrial dysfunction through the modulation of BCL2, DJ-1, and α-SYN, although the effect on DJ-1 is indirect (discontinuous arrow). The miR-30 family targets UCP2 and BCL2. The miR-29 family targets BH3-only genes, such as BBC3, protecting neurons from mitochondrial oxidative stress. However, it has been shown that miR-29 also targets BCL2L2, promoting neuronal apoptosis. miRNAs from the let-7 and miR-200 families as well as other miRNAs such as miR-494, miR-103, and miR-27 are also involved in ROS production and mitochondrial function and associated with neuronal survival and, subsequently, with neuropsychiatric disorders. This figure includes images provided by Servier Medical Art under CC By License. ROS, reactive oxygen species.
miR-181c plays a role in mitochondrial function regulating cytochrome c oxidase subunit 1 (mt-COX1) mRNA by producing electron transport chain complex IV remodeling through the increase of mt-COX2 production. This effect of miR-181c on the mitochondria results in higher oxygen consumption and ROS production (33). miR-30e targets uncoupling protein 2 (UCP2) (86). UCP proteins are mitochondrial proton transporters that play key roles in reducing ROS production and have been shown to promote neuroprotection. In particular, UCP2 protects astrocytes and microglia from oxidative stress, contributing to neuronal survival (61). miR-30e, miR-34c (91), and miR-181d (199) target BCL2, which controls mitochondrial outer membrane permeability and Ca++ homeostasis in brain stress conditions (105). In fact, Ouyang et al. showed that all members of the miR-181 family target other members of the BCL2 family. They also showed that miR-181 overexpression induces higher ROS production and lower astrocyte survival after glucose deprivation (137). In another study, these authors showed that, in addition to BCL2, miR-181 also targets GRP78 (138), a fundamental regulator of endoplasmic reticulum stress, which is also involved in ROS production and mitochondrial function (105). The miR-29 family is enriched in astrocytes and its expression increases during neuron maturation. It also enhances neuron resistance to ER stress and death by targeting BH3-only genes that inhibit BCL2 and favor cytochrome c release during apoptosis (96). The role of miR-29 as a promoter of neuron survival was confirmed by Ouyang et al. who showed that miR-29a targets BCL2 binding component 3 (BBC3), a member of the BH3-only-family. They additionally showed that miR-29a overexpression reduces ROS production and increases mitochondrial membrane permeability after glucose deprivation (139). However, it has also been reported that miR-29b promotes neuronal cell death by targeting BCL2L2, a member of the BCL2 family of antiapoptotic mediators (168). Further research is needed to clarify the role of miR-29 in neuronal survival. miR-34b and miR-34c levels are also increased in differentiated SH-SY5Y cells compared with those in undifferentiated cells, suggesting that this miRNA family is also important in neuronal maturation. miR-34b/c downregulation has been shown to be associated with higher ROS production and mitochondrial dysfunction. This effect could be partly because of an indirect effect on DJ-1 (127), which is involved in the maintenance of mitochondrial complex I integrity, and increases neurodegeneration in mice (218). miR-34b/c downregulation also increases alpha-synuclein (α-SYN) expression at both mRNA and protein levels (88). α-SYN is the main component of the neuronal cytoplasmic intrusions called Lewy bodies, whose accumulation is associated with neurodegeneration, partly caused by complex I impairment and ROS production (215). miR-34b/c directly targets α-SYN 3′-UTR (88). In hippocampal HT-22 cells subjected to a cycle of hypoxia/reoxygenation, an increase in ROS production occurs that could be mediated by an increase in miR-200a-3p and miR-200b-3p expression, although the mechanism by which miR-200 miRNAs affect ROS production in neuronal cells is still unclear (205). In B2B human bronchial epithelial cells, it has been shown that members of the let-7 family are downregulated by oxidative stress and, in turn, let-7a overexpression protects cells from the oxidative damage-mediated death by reducing oxidative stress through the direct inhibition of arginase 2 (177). In human Huh-7 hepatocytes, let-7 miRNAs also protect cells from oxidative injury through the direct inhibition of BACH1, a key repressor of the cytoprotective enzyme heme oxygenase 1 (74).
Other miRNAs associated with neuropsychiatric disorders and mental stress are also involved in mitochondrial function and oxidative stress. For instance, miR-494 increases ROS production in neuro2a cells through the direct targeting of DJ-1 gene (218). miR-103 and miR-27a/b become upregulated in SH-SY5Y cells treated with tumor necrosis factor α (TNF-α), whereas miR-23a/b shows downregulation. TNF-α induces mitochondrial oxidative stress in these cells. Among the predicted targets of these miRNAs, there are several mitochondrial complex I subunits, a complex that is compromised by TNF-α treatment (148). In SH-SY5Y cells, it has been demonstrated that miR-27a also directly targets NRF2 (131), a key component of the defensive cellular mechanism against oxidative stress (215).
Taken together, these studies highlight the role of miRNAs in mental, mood, and behavioral disorders and show that many of these neuronal-related miRNAs are involved in the regulation of ROS production, mitochondrial function, neuronal survival, and response to oxidative injury.
Summary and Conclusions
It is evident that the three types of environmental risk factors covered in this review, physicochemical, noise, and mental stress, promote responses involving specific sets of miRNAs. These, in turn, have important regulatory roles upon cellular processes such as apoptosis, signal transduction cascades, and DNA repair mechanisms. Nevertheless, in the overwhelming majority of cases, the available data in the literature have not established definitive or causative roles for miRNAs in the pathological phenotypes associated with the noxious effects of the environmental risk factors covered. Thus, the design of large cohort studies in selected populations exposed to these environmental risk factors is a major unmet need.
Abbreviations Used
- AHL
age-related hearing loss
- AhR
aryl hydrocarbon receptor
- BC
black carbon
- BDNF
brain-derived neurotrophic factor
- BP
blood pressure
- BPA
bisphenol A
- CAT
catalase
- CFTR
cystic fibrosis transmembrane regulator
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CR
caloric restriction
- CREB
cAMP response element-binding
- EBs
embryoid bodies
- Egr1
early growth response 1
- ER
estrogen receptor
- ESCs
embryonic stem cells
- FGF
fibroblast growth factor
- GDM
gestational diabetes mellitus
- GJB2
gap junction beta-2 protein
- GR
glucocorticoid receptor
- HFD
high-fat diet
- HPA
hypothalamic-pituitary-adrenal
- HSPs
heat-shock proteins
- Irs1
insulin receptor substrate 1
- KCNE1
potassium voltage-gated channel subfamily E member 1
- KCNQ
potassium voltage-gated channel subfamily Q member
- MAPK
mitogen-activated protein kinase
- MDD
major depression disorder
- miRNAs
microRNAs
- mTOR
mammalian target of rapamycin
- NCS
National Children's Study
- NIHL
noise-induced hearing loss
- Nrf2
nuclear factor erythroid 2 like 2
- nt
nucleotides
- PBDEs
polybrominated diphenyl ethers
- PBLs
peripheral blood leukocytes
- PCBs
polychlorinated biphenyls
- PFCAs
perfluorocarboxylic acids
- PFNA
perfluorononanoic acid
- PFOS
perfluorooctanesulfonate
- PGC-1α
proliferator-activated receptor gamma coactivator 1α
- PJVK
pejvakin
- PM
particulate matter
- PTSD
post-traumatic stress disorder
- RISC
RNA-induced silencing complex
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERT
serotonin transporter
- sICAM-1
soluble intercellular adhesion molecule-1
- SIRT-1
Sirtuin-1
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- SREBP
sterol response element binding protein
- SSNHL
sudden sensorineural hearing loss
- SSRI
selective serotonin reuptake inhibitor
- sVCAM-1
soluble vascular adhesion molecule-1
- TNF-α
tumor necrosis factor α
- TSLP
thymic stromal lymphopoietin
- Wnt
wingless-int
Acknowledgments
This work was supported by grants from the Ministerio de Economía, Industria y Competitividad (MINECO) SAF 2015-66107-R; Consolredox Network SAF 2015-71521-REDC; Instituto de Salud Carlos III network REDinREN RD16/0009/0016; and Fundación Renal “Iñigo Alvarez de Toledo,” all from Spain. It also has been supported by European Cooperation in Science and Technology/COST action BM-1203 (EU-ROS). The Centro de Biologia Molecular “Severo Ochoa” (CBMSO) receives institutional support from Fundación “Ramón Areces.” V.M. is supported by the MINECO program of Formación de Personal Investigador (FPI BES-2013-065986). L.D.'s research is supported by a grant from Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias (PI14/01374). IMDEA Food belongs to Madrid Institute for Advanced Studies Network promoted by the Madrid Regional Government (Comunidad de Madrid) and is funded by European Regional Development Funds from the European Union. C.F.-H. is supported, in part, by NIH Grant R35HL135820, Foundation Leducq Transatlantic Network of Excellence in Cardiovascular Research, and AHA Established Investigator Award (16EIA27550004).
References
- 1.U.S. Health in International Perspective: Shorter Lives, Poorer Health, edited by Woolf SH. and Aron L. Washington, DC: The National Academies Press, 2013 [PubMed] [Google Scholar]
- 2.Albina ML, Alonso V, Linares V, Belles M, Sirvent JJ, Domingo JL, and Sanchez DJ. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology 271: 51–56, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Almeida MI, Reis RM, and Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mutat Res 717: 1–8, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Al-Salman F. and Plant N. Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol Appl Pharmacol 263: 7–13, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, Gagne LA, Moen EL, and Marsit CJ. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol 29: 401–406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai W, Chen Y, Yang J, Niu P, Tian L, and Gao A. Aberrant miRNA profiles associated with chronic benzene poisoning. Exp Mol Pathol 96: 426–430, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, and Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329: 1537–1541, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Treziny C, Verrier L, Loundou A, Baumstarck-Barrau K, Boyer L, Gall V, Gabert J, Nguyen C, Azorin JM, Naudin J, and Ibrahim EC. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry 2: e185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleck B, Grunig G, Chiu A, Liu M, Gordon T, Kazeros A, and Reibman J. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol 190: 3757–3763, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, and Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol 23: 602–611, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Bohne BA, Harding GW, and Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res 223: 61–70, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bollati V, Angelici L, Rizzo G, Pergoli L, Rota F, Hoxha M, Nordio F, Bonzini M, Tarantini L, Cantone L, Pesatori AC, Apostoli P, Baccarelli AA, and Bertazzi PA. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J Appl Toxicol 35: 59–67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bollati V, Iodice S, Favero C, Angelici L, Albetti B, Cacace R, Cantone L, Carugno M, Cavalleri T, De Giorgio B, Dioni L, Fustinoni S, Hoxha M, Marinelli B, Motta V, Patrini L, Pergoli L, Riboldi L, Rizzo G, Rota F, Sucato S, Tarantini L, Tirelli AS, Vigna L, Bertazzi P, and Pesatori AC. Susceptibility to particle health effects, miRNA and exosomes: rationale and study protocol of the SPHERE study. BMC Public Health 14: 1137, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M, Pegoraro V, Motta V, Tarantini L, Cantone L, Schwartz J, Bertazzi PA, and Baccarelli A. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect 118: 763–768, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buechner J, Tomte E, Haug BH, Henriksen JR, Lokke C, Flaegstad T, and Einvik C. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer 105: 296–303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calafat AM, Needham LL, Kuklenyik Z, Reidy JA, Tully JS, Aguilar-Villalobos M, and Naeher LP. Perfluorinated chemicals in selected residents of the American continent. Chemosphere 63: 490–496, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Camkurt MA, Acar S, Coskun S, Gunes M, Gunes S, Yilmaz MF, Gorur A, and Tamer L. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatr Res 69: 67–71, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Casarett LJ. and Doull J. Casarett and Doull's Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill Education, 2013, p. c2013 [Google Scholar]
- 21.Chen CL, Liu H, and Guan X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. J Biomed Sci 20: 96, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Xu B, Han X, Mao Z, Talbot P, Chen M, Du G, Chen A, Liu J, Wang X, and Xia Y. Effect of bisphenol A on pluripotency of mouse embryonic stem cells and differentiation capacity in mouse embryoid bodies. Toxicol In Vitro 27: 2249–2255, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Cheng X, Ku CH, and Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med 64: 4–11, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Cho Y, Gong TW, Kanicki A, Altschuler RA, and Lomax MI. Noise overstimulation induces immediate early genes in the rat cochlea. Brain Res Mol Brain Res 130: 134–148, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Choghaei E, Khamisipour G, Falahati M, Naeimi B, Mossahebi-Mohammadi M, Tahmasebi R, Hasanpour M, Shamsian S, and Hashemi ZS. Knockdown of microRNA-29a changes the expression of heat shock proteins in breast carcinoma MCF-7 cells. Oncol Res 23: 69–78, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cikankova T, Sigitova E, Zverova M, Fisar Z, Raboch J, and Hroudova J. Mitochondrial dysfunctions in bipolar disorder: effect of the disease and pharmacotherapy. CNS Neurol Disord Drug Targets 16: 176–186, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Colicino E, Giuliano G, Power MC, Lepeule J, Wilker EH, Vokonas P, Brennan KJ, Fossati S, Hoxha M, Spiro A, 3rd, Weisskopf MG, Schwartz J, and Baccarelli AA. Long-term exposure to black carbon, cognition and single nucleotide polymorphisms in microRNA processing genes in older men. Environ Int 88: 86–93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collotta M, Bertazzi PA, and Bollati V. Epigenetics and pesticides. Toxicology 307: 35–41, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Cosselman KE, Navas-Acien A, and Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol 12: 627–642, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Cruciat CM. and Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5: a015081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, and Münzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol 174: 1591–1619, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dambal S, Shah M, Mihelich B, and Nonn L. The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res 43: 7173–7188, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, and Steenbergen C. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110: 1596–1603, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Felice B, Manfellotto F, Palumbo A, Troisi J, Zullo F, Di Carlo C, Di Spiezio Sardo A, De Stefano N, Ferbo U, Guida M, and Guida M. Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med Genomics 8: 56, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Silva AO. and Mabury SA. Isomer distribution of perfluorocarboxylates in human blood: potential correlation to source. Environ Sci Technol 40: 2903–2909, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Delmaghani S, Defourny J, Aghaie A, Beurg M, Dulon D, Thelen N, Perfettini I, Zelles T, Aller M, Meyer A, Emptoz A, Giraudet F, Leibovici M, Dartevelle S, Soubigou G, Thiry M, Vizi ES, Safieddine S, Hardelin JP, Avan P, and Petit C. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell 163: 894–906, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Ding E, Zhao Q, Bai Y, Xu M, Pan L, Liu Q, Wang B, Song X, Wang J, Chen L, and Zhu B. Plasma microRNAs expression profile in female workers occupationally exposed to mercury. J Thorac Dis 8: 833–841, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding L, Liu J, Shen HX, Pan LP, Liu QD, Zhang HD, Han L, Shuai LG, Ding EM, Zhao QN, Wang BS, and Zhu BL. Analysis of plasma microRNA expression profiles in male textile workers with noise-induced hearing loss. Hear Res 333: 275–282, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Du L, Sun W, Zhang H, and Chen D. BDE-209 inhibits pluripotent genes expression and induces apoptosis in human embryonic stem cells. J Appl Toxicol 36: 659–668, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Du Z, Li S, Liu L, Yang Q, Zhang H, and Gao C. [Corrigendum] NADPH oxidase 3-associated oxidative stress and caspase 3-dependent apoptosis in the cochleae of D-galactose-induced aged rats. Mol Med Rep 13: 1056, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dwivedi Y. Emerging role of microRNAs in major depressive disorder: diagnosis and therapeutic implications. Dialogues Clin Neurosci 16: 43–61, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egilmez OK. and Kalcioglu MT. Genetics of nonsyndromic congenital hearing loss. Scientifica (Cairo) 2016: 7576064, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, and Ho SM. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am J Perinatol 33: 1313–1318, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Fabbri M, Urani C, Sacco MG, Procaccianti C, and Gribaldo L. Whole genome analysis and microRNAs regulation in HepG2 cells exposed to cadmium. ALTEX 29: 173–182, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Falandysz J, Taniyasu S, Yamashita N, Jecek L, Rostkowski P, Gulkowska A, Mostrag A, Walczykiewicz B, Zegarowski L, Falandysz J, and Zalewski K. [Perfluorinated chemicals in the environment, food and human body]. Rocz Panstw Zakl Hig 57: 113–124, 2006 [PubMed] [Google Scholar]
- 46.Fan HM, Sun XY, Guo W, Zhong AF, Niu W, Zhao L, Dai YH, Guo ZM, Zhang LY, and Lu J. Differential expression of microRNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients. J Psychiatr Res 59: 45–52, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Farraj AK, Hazari MS, Haykal-Coates N, Lamb C, Winsett DW, Ge Y, Ledbetter AD, Carll AP, Bruno M, Ghio A, and Costa DL. ST depression, arrhythmia, vagal dominance, and reduced cardiac micro-RNA in particulate-exposed rats. Am J Respir Cell Mol Biol 44: 185–196, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, Kim HW, Pasha Z, Wen Z, Rao F, Modi RM, Yu X, and Ashraf M. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 32: 462–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fossati S, Baccarelli A, Zanobetti A, Hoxha M, Vokonas PS, Wright RO, and Schwartz J. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology 25: 68–78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fowles JR, Fairbrother A, Baecher-Steppan L, and Kerkvliet NI. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 86: 49–61, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Frolenkov GI, Belyantseva IA, Friedman TB, and Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet 5: 489–498, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, and Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466: 1105–1109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S, Murinova L, Trnovec T, Loffredo CA, Washington K, Mitra PS, and Dutta SK. Biomarkers linking PCB exposure and obesity. Curr Pharm Biotechnol 15: 1058–1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giandomenico S, Cardellicchio N, Spada L, Annicchiarico C, and Di Leo A. Metals and PCB levels in some edible marine organisms from the Ionian Sea: dietary intake evaluation and risk for consumers. Environ Sci Pollut Res Int 23: 12596–12612, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Giesy JP. and Kannan K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit Rev Toxicol 28: 511–569, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Giridharan VV, Thandavarayan RA, Fries GR, Walss-Bass C, Barichello T, Justice NJ, Reddy MK, and Quevedo J. Newer insights into the role of miRNA a tiny genetic tool in psychiatric disorders: focus on post-traumatic stress disorder. Transl Psychiatry 6: e954, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guida M, Marra ML, Zullo F, Guida M, Trifuoggi M, Biffali E, Borra M, De Mieri G, D'Alessandro R, and De Felice B. Association between exposure to dioxin-like polychlorinated biphenyls and miR-191 expression in human peripheral blood mononuclear cells. Mutat Res 753: 36–41, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Hallgren S, Sinjari T, Hakansson H, and Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol 75: 200–208, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Han SY, Kim S, Shin DH, Cho JH, and Nam SI. The expression of AGO2 and DGCR8 in idiopathic sudden sensorineural hearing loss. Clin Exp Otorhinolaryngol 7: 269–274, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, and Chen A. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci 31: 14191–14203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hass DT. and Barnstable CJ. Uncoupling protein 2 in the glial response to stress: implications for neuroprotection. Neural Regen Res 11: 1197–1200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassan F, Nuovo GJ, Crawford M, Boyaka PN, Kirkby S, Nana-Sinkam SP, and Cormet-Boyaka E. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS One 7: e50837, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayes JD. and Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39: 199–218, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Heinrichs SC. and Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311: 427–440, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Hennig B, Hammock BD, Slim R, Toborek M, Saraswathi V, and Robertson LW. PCB-induced oxidative stress in endothelial cells: modulation by nutrients. Int J Hyg Environ Health 205: 95–102, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Henry TB. Ecotoxicology of polychlorinated biphenyls in fish—a critical review. Crit Rev Toxicol 45: 643–661, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Hertzano R, Elkon R, Kurima K, Morrisson A, Chan SL, Sallin M, Biedlingmaier A, Darling DS, Griffith AJ, Eisenman DJ, and Strome SE. Cell type-specific transcriptome analysis reveals a major role for Zeb1 and miR-200b in mouse inner ear morphogenesis. PLoS Genet 7: e1002309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hildebrand MS, Witmer PD, Xu S, Newton SS, Kahrizi K, Najmabadi H, Valle D, and Smith RJ. miRNA mutations are not a common cause of deafness. Am J Med Genet A 152A: 646–652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hollins SL. and Cairns MJ. MicroRNA: small RNA mediators of the brains genomic response to environmental stress. Prog Neurobiol 143: 61–81, 2016 [DOI] [PubMed] [Google Scholar]
- 70.Holloway JW, Savarimuthu Francis S, Fong KM, and Yang IA. Genomics and the respiratory effects of air pollution exposure. Respirology 17: 590–600, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Honda M, Kuwano Y, Katsuura-Kamano S, Kamezaki Y, Fujita K, Akaike Y, Kano S, Nishida K, Masuda K, and Rokutan K. Chronic academic stress increases a group of microRNAs in peripheral blood. PLoS One 8: e75960, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Honkura Y, Matsuo H, Murakami S, Sakiyama M, Mizutari K, Shiotani A, Yamamoto M, Morita I, Shinomiya N, Kawase T, Katori Y, and Motohashi H. NRF2 is a key target for prevention of noise-induced hearing loss by reducing oxidative damage of cochlea. Sci Rep 6: 19329, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hou L, Barupal J, Zhang W, Zheng Y, Liu L, Zhang X, Dou C, McCracken JP, Diaz A, Motta V, Sanchez-Guerra M, Wolf KR, Bertazzi PA, Schwartz JD, Wang S, and Baccarelli AA. Particulate air pollution exposure and expression of viral and human MicroRNAs in blood: the Beijing Truck Driver Air Pollution Study. Environ Health Perspect 124: 344–350, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou W, Tian Q, Steuerwald NM, Schrum LW, and Bonkovsky HL. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim Biophys Acta 1819: 1113–1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang M, Lou D, Cai Q, Chang X, Wang X, and Zhou Z. Characterization of paraquat-induced miRNA profiling response in hNPCs undergoing proliferation. Int J Mol Sci 15: 18422–18436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hunsberger JG, Austin DR, Chen G, and Manji HK. MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuromolecular Med 11: 173–182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunsberger JG, Fessler EB, Chibane FL, Leng Y, Maric D, Elkahloun AG, and Chuang DM. Mood stabilizer-regulated miRNAs in neuropsychiatric and neurodegenerative diseases: identifying associations and functions. Am J Transl Res 5: 450–464, 2013 [PMC free article] [PubMed] [Google Scholar]
- 78.Huyghe A, Van den Ackerveken P, Sacheli R, Prevot PP, Thelen N, Renauld J, Thiry M, Delacroix L, Nguyen L, and Malgrange B. MicroRNA-124 regulates cell specification in the cochlea through modulation of Sfrp4/5. Cell Rep 13: 31–42, 2015 [DOI] [PubMed] [Google Scholar]
- 79.Isik M, Blackwell TK, and Berezikov E. MicroRNA mir-34 provides robustness to environmental stress response via the DAF-16 network in C. elegans. Sci Rep 6: 36766, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Issler O. and Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16: 201–212, 2015 [DOI] [PubMed] [Google Scholar]
- 81.Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Gil S, Mayberg HS, Dunlop BW, Menke A, Awatramani R, Binder EB, Deneris ES, Lowry CA, and Chen A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 83: 344–360, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Jamesdaniel S, Hu B, Kermany MH, Jiang H, Ding D, Coling D, and Salvi R. Noise induced changes in the expression of p38/MAPK signaling proteins in the sensory epithelium of the inner ear. J Proteomics 75: 410–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jardim MJ. microRNAs: implications for air pollution research. Mutat Res 717: 38–45, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Jeong SC, Song MK, Cho Y, Lee E, and Ryu JC. Integrative analysis of mRNA and microRNA expression of a human alveolar epithelial cell(A549) exposed to water and organic-soluble extract from particulate matter (PM)2.5. Environ Toxicol 32: 302–310, 2017 [DOI] [PubMed] [Google Scholar]
- 85.Jia X, Zheng S, Xie X, Zhang Y, Wang W, Wang Z, Zhang Y, Wang J, Gao M, and Hou Y. MicroRNA-1 accelerates the shortening of atrial effective refractory period by regulating KCNE1 and KCNB2 expression: an atrial tachypacing rabbit model. PLoS One 8: e85639, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang L, Qiu W, Zhou Y, Wen P, Fang L, Cao H, Zen K, He W, Zhang C, Dai C, and Yang J. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-beta1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int 84: 285–296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jozefczak M, Remans T, Vangronsveld J, and Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13: 3145–3175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kabaria S, Choi DC, Chaudhuri AD, Mouradian MM, and Junn E. Inhibition of miR-34b and miR-34c enhances alpha-synuclein expression in Parkinson's disease. FEBS Lett 589: 319–325, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamimura K, Mishima Y, Obata M, Endo T, Aoyagi Y, and Kominami R. Lack of Bcl11b tumor suppressor results in vulnerability to DNA replication stress and damages. Oncogene 26: 5840–5850, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, Kunugi H, and Hashido K. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165: 1301–1311, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Khanna A, Muthusamy S, Liang R, Sarojini H, and Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany NY) 3: 223–236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kidd Iii AR. and Bao J. Recent advances in the study of age-related hearing loss: a mini-review. Gerontology 58: 490–496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim HJ, Oh GS, Shen A, Lee SB, Choe SK, Kwon KB, Lee S, Seo KS, Kwak TH, Park R, and So HS. Augmentation of NAD(+) by NQO1 attenuates cisplatin-mediated hearing impairment. Cell Death Dis 5: e1292, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klaassen CD, Liu J, and Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol 39: 267–294, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Kodavanti PR, Ward TR, Ludewig G, Robertson LW, and Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol Sci 88: 181–192, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Kole AJ, Swahari V, Hammond SM, and Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev 25: 125–130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kong AP, Xiao K, Choi KC, Wang G, Chan MH, Ho CS, Chan I, Wong CK, Chan JC, and Szeto CC. Associations between microRNA (miR-21, 126, 155 and 221), albuminuria and heavy metals in Hong Kong Chinese adolescents. Clin Chim Acta 413: 1053–1057, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Konings A, Van Laer L, Wiktorek-Smagur A, Rajkowska E, Pawelczyk M, Carlsson PI, Bondeson ML, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J, Borg E, Sliwinska-Kowalska M, and Van Camp G. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Ann Hum Genet 73: 215–224, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O'Neill MS, Herrington DM, Polak JF, and Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol 60: 2158–2166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudlow P, Cha DS, Carvalho AF, and McIntyre RS. Nitric oxide and major depressive disorder: pathophysiology and treatment implications. Curr Mol Med 16: 206–215, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Lee YS, Lewis JA, Ippolito DL, Hussainzada N, Lein PJ, Jackson DA, and Stallings JD. Repeated exposure to neurotoxic levels of chlorpyrifos alters hippocampal expression of neurotrophins and neuropeptides. Toxicology 340: 53–62, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, and Wayman GA. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci 34: 717–725, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levonen AL, Hill BG, Kansanen E, Zhang J, and Darley-Usmar VM. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic Biol Med 71: 196–207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J, Wu Z, Cheng F, Li W, Liu G, and Tang Y. Computational prediction of microRNA networks incorporating environmental toxicity and disease etiology. Sci Rep 4: 5576, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L. and Stary CM. Targeting glial mitochondrial function for protection from cerebral ischemia: relevance, mechanisms, and the role of MicroRNAs. Oxid Med Cell Longev 2016: 6032306, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Q, Kappil MA, Li A, Dassanayake PS, Darrah TH, Friedman AE, Friedman M, Lambertini L, Landrigan P, Stodgell CJ, Xia Y, Nanes JA, Aagaard KM, Schadt EE, Murray JC, Clark EB, Dole N, Culhane J, Swanson J, Varner M, Moye J, Kasten C, Miller RK, and Chen J. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children's Study (NCS). Epigenetics 10: 793–802, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li S, Ran XQ, Xu L, and Wang JF. microRNA and mRNA expression profiling analysis of dichlorvos cytotoxicity in porcine kidney epithelial PK15 cells. DNA Cell Biol 30: 1073–1083, 2011 [DOI] [PubMed] [Google Scholar]
- 108.Li X, Ding Z, Zhang C, Zhang X, Meng Q, Wu S, Wang S, Yin L, Pu Y, and Chen R. MicroRNA-1228(*) inhibit apoptosis in A549 cells exposed to fine particulate matter. Environ Sci Pollut Res Int 23: 10103–10113, 2016 [DOI] [PubMed] [Google Scholar]
- 109.Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R, Wu J, Geng S, and Zhong C. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res 28: 1553–1560, 2014 [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Duan YP, Huang F, Yang J, Xiang N, Meng XZ, and Chen L. Polybrominated diphenyl ethers in e-waste: level and transfer in a typical e-waste recycling site in Shanghai, Eastern China. Waste Manag 34: 1059–1065, 2014 [DOI] [PubMed] [Google Scholar]
- 111.Li YJ, Xu M, Gao ZH, Wang YQ, Yue Z, Zhang YX, Li XX, Zhang C, Xie SY, and Wang PY. Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS One 8: e63648, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lipscomb DM. and Roettger RL. Capillary constriction in cochlear and vestibular tissues during intense noise stimulation. Laryngoscope 83: 259–263, 1973 [DOI] [PubMed] [Google Scholar]
- 113.Longhin E, Gualtieri M, Capasso L, Bengalli R, Mollerup S, Holme JA, Ovrevik J, Casadei S, Di Benedetto C, Parenti P, and Camatini M. Physico-chemical properties and biological effects of diesel and biomass particles. Environ Pollut 215: 366–375, 2016 [DOI] [PubMed] [Google Scholar]
- 114.Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Maussion G, Yang JP, Yerko V, Vigneault E, El Mestikawy S, Mechawar N, Pavlidis P, and Turecki G. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med 20: 764–768, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Louwies T, Vuegen C, Panis LI, Cox B, Vrijens K, Nawrot TS, and De Boever P. miRNA expression profiles and retinal blood vessel calibers are associated with short-term particulate matter air pollution exposure. Environ Res 147: 24–31, 2016 [DOI] [PubMed] [Google Scholar]
- 116.Lyche JL, Rosseland C, Berge G, and Polder A. Human health risk associated with brominated flame-retardants (BFRs). Environ Int 74: 170–180, 2015 [DOI] [PubMed] [Google Scholar]
- 117.Madrigano J, Baccarelli A, Wright RO, Suh H, Sparrow D, Vokonas PS, and Schwartz J. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med 67: 312–317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mahmoudian-Sani MR, Mehri-Ghahfarrokhi A, Ahmadinejad F, Hashemzadeh-Chaleshtori M, Saidijam M, and Jami MS. MicroRNAs: effective elements in ear-related diseases and hearing loss. Eur Arch Otorhinolaryngol 274: 2373–2380, 2017 [DOI] [PubMed] [Google Scholar]
- 119.Marsit CJ, Eddy K, and Kelsey KT. MicroRNA responses to cellular stress. Cancer Res 66: 10843–10848, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Maynard KR, Hill JL, Calcaterra NE, Palko ME, Kardian A, Paredes D, Sukumar M, Adler BD, Jimenez DV, Schloesser RJ, Tessarollo L, Lu B, and Martinowich K. Functional role of BDNF production from unique promoters in aggression and serotonin signaling. Neuropsychopharmacology 41: 1943–1955, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, and Kaufer D. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci 40: 47–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mellstrom B, Savignac M, Gomez-Villafuertes R, and Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev 88: 421–449, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Mencia A, Modamio-Hoybjor S, Redshaw N, Morin M, Mayo-Merino F, Olavarrieta L, Aguirre LA, del Castillo I, Steel KP, Dalmay T, Moreno F, and Moreno-Pelayo MA. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet 41: 609–613, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Meshorer E, Bryk B, Toiber D, Cohen J, Podoly E, Dori A, and Soreq H. SC35 promotes sustainable stress-induced alternative splicing of neuronal acetylcholinesterase mRNA. Mol Psychiatry 10: 985–997, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Mikhed Y, Gorlach A, Knaus UG, and Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol 5: 275–289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miller GW. and Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci 137: 1–2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Minones-Moyano E, Porta S, Escaramis G, Rabionet R, Iraola S, Kagerbauer B, Espinosa-Parrilla Y, Ferrer I, Estivill X, and Marti E. MicroRNA profiling of Parkinson's disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet 20: 3067–3078, 2011 [DOI] [PubMed] [Google Scholar]
- 128.Momi N, Kaur S, Rachagani S, Ganti AK, and Batra SK. Smoking and microRNA dysregulation: a cancerous combination. Trends Mol Med 20: 36–47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Motta V, Favero C, Dioni L, Iodice S, Battaglia C, Angelici L, Vigna L, Pesatori AC, and Bollati V. MicroRNAs are associated with blood-pressure effects of exposure to particulate matter: results from a mediated moderation analysis. Environ Res 146: 274–281, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murillo-Cuesta S, Camarero G, Gonzalez-Rodriguez A, De La Rosa LR, Burks DJ, Avendano C, Valverde AM, and Varela-Nieto I. Insulin receptor substrate 2 (IRS2)-deficient mice show sensorineural hearing loss that is delayed by concomitant protein tyrosine phosphatase 1B (PTP1B) loss of function. Mol Med 18: 260–269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, and Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One 7: e51111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.National Toxicology Program. NTP toxicology and carcinogenesis studies of diglycidyl resorcinol ether (technical grade) (CAS No. 101–190-6) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser 257: 1–222, 1986 [PubMed] [Google Scholar]
- 133.Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, and Akbarian S. Epigenetic basis of mental illness. Neuroscientist 22: 447–463, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noben-Trauth K, Zheng QY, and Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet 35: 21–23, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O'Connor RM, Dinan TG, and Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry 17: 359–376, 2012 [DOI] [PubMed] [Google Scholar]
- 136.Olsen GW, Huang HY, Helzlsouer KJ, Hansen KJ, Butenhoff JL, and Mandel JH. Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ Health Perspect 113: 539–545, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ouyang YB, Lu Y, Yue S, and Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 12: 213–219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, and Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis 45: 555–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, and Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 61: 1784–1794, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Padmanabhan JL, Shah JL, Tandon N, and Keshavan MS. The “polyenviromic risk score”: aggregating environmental risk factors predicts conversion to psychosis in familial high-risk subjects. Schizophr Res 181: 17–22, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pallocca G, Fabbri M, Sacco MG, Gribaldo L, Pamies D, Laurenza I, and Bal-Price A. miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol Toxicol 29: 239–257, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Pan HL, Wen ZS, Huang YC, Cheng X, Wang GZ, Zhou YC, Wang ZY, Guo YQ, Cao Y, and Zhou GB. Down-regulation of microRNA-144 in air pollution-related lung cancer. Sci Rep 5: 14331, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pang J, Xiong H, Yang H, Ou Y, Xu Y, Huang Q, Lai L, Chen S, Zhang Z, Cai Y, and Zheng Y. Circulating miR-34a levels correlate with age-related hearing loss in mice and humans. Exp Gerontol 76: 58–67, 2016 [DOI] [PubMed] [Google Scholar]
- 144.Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, and Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn 227: 203–215, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pawelczyk M, Van Laer L, Fransen E, Rajkowska E, Konings A, Carlsson PI, Borg E, Van Camp G, and Sliwinska-Kowalska M. Analysis of gene polymorphisms associated with K ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann Hum Genet 73: 411–421, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Perdomo C, Spira A, and Schembri F. MiRNAs as regulators of the response to inhaled environmental toxins and airway carcinogenesis. Mutat Res 717: 32–37, 2011 [DOI] [PubMed] [Google Scholar]
- 147.Poulose N. and Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta 1852: 2442–2455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Prajapati P, Sripada L, Singh K, Bhatelia K, and Singh R. TNF-alpha regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim Biophys Acta 1852: 451–461, 2015 [DOI] [PubMed] [Google Scholar]
- 149.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, and van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155: 805–817, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prusinski L, Al-Hendy A, and Yang Q. Developmental exposure to endocrine disrupting chemicals alters the epigenome: identification of reprogrammed targets. Gynecol Obstet Res 3: 1–6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rabinowitz PM. Noise-induced hearing loss. Am Fam Physician 61: 2749–2756, 2759–2760, 2000 [PubMed] [Google Scholar]
- 152.Rabinowitz PM, Pierce Wise J, Sr., Hur Mobo B, Antonucci PG, Powell C, and Slade M. Antioxidant status and hearing function in noise-exposed workers. Hear Res 173: 164–171, 2002 [DOI] [PubMed] [Google Scholar]
- 153.Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobna Z, Currier J, Douillet C, Olshan AF, Rubio-Andrade M, Styblo M, Garcia-Vargas G, and Fry RC. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen 55: 196–208, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rand AA, Rooney JP, Butt CM, Meyer JN, and Mabury SA. Cellular toxicity associated with exposure to perfluorinated carboxylates (PFCAs) and their metabolic precursors. Chem Res Toxicol 27: 42–50, 2014 [DOI] [PubMed] [Google Scholar]
- 155.Rappaport SM. Discovering environmental causes of disease. J Epidemiol Community Health 66: 99–102, 2012 [DOI] [PubMed] [Google Scholar]
- 156.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, and Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328: 1570–1573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rodosthenous RS, Coull BA, Lu Q, Vokonas PS, Schwartz JD, and Baccarelli AA. Ambient particulate matter and microRNAs in extracellular vesicles: a pilot study of older individuals. Part Fibre Toxicol 13: 13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ross JA, Blackman CF, Thai SF, Li Z, Kohan M, Jones CP, and Chen T. A potential microRNA signature for tumorigenic conazoles in mouse liver. Mol Carcinog 49: 320–323, 2010 [DOI] [PubMed] [Google Scholar]
- 159.Roth TL. and Sweatt JD. Annual research review: epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiatry 52: 398–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rousset F, Carnesecchi S, Senn P, and Krause KH. Nox3-targeted therapies for inner ear pathologies. Curr Pharm Des 21: 5977–5987, 2015 [DOI] [PubMed] [Google Scholar]
- 161.Rudnicki A. and Avraham KB. microRNAs: the art of silencing in the ear. EMBO Mol Med 4: 849–859, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rudnicki A, Isakov O, Ushakov K, Shivatzki S, Weiss I, Friedman LM, Shomron N, and Avraham KB. Next-generation sequencing of small RNAs from inner ear sensory epithelium identifies microRNAs and defines regulatory pathways. BMC Genomics 15: 484, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sanders AP, Burris HH, Just AC, Motta V, Amarasiriwardena C, Svensson K, Oken E, Solano-Gonzalez M, Mercado-Garcia A, Pantic I, Schwartz J, Tellez-Rojo MM, Baccarelli AA, and Wright RO. Altered miRNA expression in the cervix during pregnancy associated with lead and mercury exposure. Epigenomics 7: 885–896, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sangiao-Alvarellos S, Pena-Bello L, Manfredi-Lozano M, Tena-Sempere M, and Cordido F. Perturbation of hypothalamic microRNA expression patterns in male rats after metabolic distress: impact of obesity and conditions of negative energy balance. Endocrinology 155: 1838–1850, 2014 [DOI] [PubMed] [Google Scholar]
- 165.Sayed AS, Xia K, Salma U, Yang T, and Peng J. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ 23: 503–510, 2014 [DOI] [PubMed] [Google Scholar]
- 166.Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol 18: 217–227; discussion 229–276, 1996 [DOI] [PubMed] [Google Scholar]
- 167.Schantz SL, Widholm JJ, and Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111: 357–576, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, and Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2 L2 after ischemic brain injury. Exp Brain Res 216: 225–230, 2012 [DOI] [PubMed] [Google Scholar]
- 169.Siddiqi MA, Laessig RH, and Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res 1: 281–290, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sinclair E. and Kannan K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ Sci Technol 40: 1408–1414, 2006 [DOI] [PubMed] [Google Scholar]
- 171.Singh S. and Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci 13: 10143–10153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sivakumaran TA, Resendes BL, Robertson NG, Giersch AB, and Morton CC. Characterization of an abundant COL9A1 transcript in the cochlea with a novel 3′ UTR: expression studies and detection of miRNA target sequence. J Assoc Res Otolaryngol 7: 160–172, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sliwinska-Kowalska M. and Pawelczyk M. Contribution of genetic factors to noise-induced hearing loss: a human studies review. Mutat Res 752: 61–65, 2013 [DOI] [PubMed] [Google Scholar]
- 174.Smithwick M, Muir DC, Mabury SA, Solomon KR, Martin JW, Sonne C, Born EW, Letcher RJ, and Dietz R. Perflouroalkyl contaminants in liver tissue from East Greenland polar bears (Ursus maritimus). Environ Toxicol Chem 24: 981–986, 2005 [DOI] [PubMed] [Google Scholar]
- 175.Solda G, Robusto M, Primignani P, Castorina P, Benzoni E, Cesarani A, Ambrosetti U, Asselta R, and Duga S. A novel mutation within the MIR96 gene causes non-syndromic inherited hearing loss in an Italian family by altering pre-miRNA processing. Hum Mol Genet 21: 577–585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sollome J, Martin E, Sethupathy P, and Fry RC. Environmental contaminants and microRNA regulation: transcription factors as regulators of toxicant-altered microRNA expression. Toxicol Appl Pharmacol 312: 61–66, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Song L, Li D, Gu Y, Li X, and Peng L. Let-7a modulates particulate matter (≤2.5 μm)-induced oxidative stress and injury in human airway epithelial cells by targeting arginase 2. J Appl Toxicol 36: 1302–1310, 2016 [DOI] [PubMed] [Google Scholar]
- 177a.Song MK, and Ryu JC. Blood miRNAs as sensitive and specific biological indicators of environmental and occupational exposure to volatile organic compound (VOC). Int J Hyg Environ Health 218: 590–602, 2015 [DOI] [PubMed] [Google Scholar]
- 178.Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, and Harfe BD. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol 328: 328–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun N, Lei L, Wang Y, Yang C, Liu Z, Li X, and Zhang K. Preliminary comparison of plasma notch-associated microRNA-34b and −34c levels in drug naive, first episode depressed patients and healthy controls. J Affect Disord 194: 109–114, 2016 [DOI] [PubMed] [Google Scholar]
- 180.Tadros SF, D'Souza M, Zhu X, and Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis 13: 1303–1321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tavanai E. and Mohammadkhani G. Role of antioxidants in prevention of age-related hearing loss: a review of literature. Eur Arch Otorhinolaryngol 274: 1821–1834, 2017 [DOI] [PubMed] [Google Scholar]
- 182.Tilghman SL, Bratton MR, Segar HC, Martin EC, Rhodes LV, Li M, McLachlan JA, Wiese TE, Nephew KP, and Burow ME. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS One 7: e32754, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Toft G. Persistent organochlorine pollutants and human reproductive health. Dan Med J 61: B4967, 2014 [PubMed] [Google Scholar]
- 184.Truettner JS, Motti D, and Dietrich WD. MicroRNA overexpression increases cortical neuronal vulnerability to injury. Brain Res 1533: 122–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tsamou M, Vrijens K, Madhloum N, Lefebvre W, Vanpoucke C, and Nawrot TS. Air pollution-induced placental epigenetic alterations in early life: a candidate miRNA approach. Epigenetics 2016. [Epub ahead of print]; DOI: 10.1080/15592294.2016.1155012 [DOI] [PMC free article] [PubMed]
- 186.Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, and Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect 116: 1635–1641, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen BS, and Watanabe Y. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci 27: 2250–2261, 2008 [DOI] [PubMed] [Google Scholar]
- 188.Ushakov K, Rudnicki A, and Avraham KB. MicroRNAs in sensorineural diseases of the ear. Front Mol Neurosci 6: 52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Van Laer L, Carlsson PI, Ottschytsch N, Bondeson ML, Konings A, Vandevelde A, Dieltjens N, Fransen E, Snyders D, Borg E, Raes A, and Van Camp G. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum Mutat 27: 786–795, 2006 [DOI] [PubMed] [Google Scholar]
- 190.Veiga-Lopez A, Luense LJ, Christenson LK, and Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154: 1873–1884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wahlang B, Petriello MC, Perkins JT, Shen S, and Hennig B. Polychlorinated biphenyl exposure alters the expression profile of microRNAs associated with vascular diseases. Toxicol In Vitro 35: 180–187, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wan C, Han R, Liu L, Zhang F, Li F, Xiang M, and Ding W. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicol Appl Pharmacol 295: 85–93, 2016 [DOI] [PubMed] [Google Scholar]
- 193.Wang J, He J, Su F, Ding N, Hu W, Yao B, Wang W, and Zhou G. Repression of ATR pathway by miR-185 enhances radiation-induced apoptosis and proliferation inhibition. Cell Death Dis 4: e699, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang J, Tymczyszyn N, Yu Z, Yin S, Bance M, and Robertson GS. Overexpression of X-linked inhibitor of apoptosis protein protects against noise-induced hearing loss in mice. Gene Ther 18: 560–568, 2011 [DOI] [PubMed] [Google Scholar]
- 195.Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, and Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci 23: 8596–8607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang J, Yan S, Zhang W, Zhang H, and Dai J. Integrated proteomic and miRNA transcriptional analysis reveals the hepatotoxicity mechanism of PFNA exposure in mice. J Proteome Res 14: 330–341, 2015 [DOI] [PubMed] [Google Scholar]
- 197.Wang X, Sundquist K, Hedelius A, Palmer K, Memon AA, and Sundquist J. Circulating microRNA-144-5p is associated with depressive disorders. Clin Epigenetics 7: 69, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang X, Zhou S, Ding X, Zhu G, and Guo J. Effect of triazophos, fipronil and their mixture on miRNA expression in adult zebrafish. J Environ Sci Health B 45: 648–657, 2010 [DOI] [PubMed] [Google Scholar]
- 199.Wang XF, Shi ZM, Wang XR, Cao L, Wang YY, Zhang JX, Yin Y, Luo H, Kang CS, Liu N, Jiang T, and You YP. MiR-181d acts as a tumor suppressor in glioma by targeting K-ras and Bcl-2. J Cancer Res Clin Oncol 138: 573–584, 2012 [DOI] [PubMed] [Google Scholar]
- 200.Wang XW, Wang XJ, Song JS, Chen HX, and Man YH. Influence of evoked HSP70 expression on hearing function of the cochlea in guinea pigs. Di Yi Jun Yi Da Xue Xue Bao 22: 922–924, 2002 [PubMed] [Google Scholar]
- 201.Wang Y, Lin C, He Y, Li A, Ni W, Sun S, Gu X, Li J, and Li H. Mir-27a promotes apoptosis of cochlear sensory epithelium in Cx26 knockout mice. Front Biosci (Landmark Ed) 21: 364–373, 2016 [DOI] [PubMed] [Google Scholar]
- 202.Wang Y, Vogelsang M, Schafer G, Matejcic M, and Parker MI. MicroRNA polymorphisms and environmental smoke exposure as risk factors for oesophageal squamous cell carcinoma. PLoS One 8: e78520, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang W, and Zou F. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res 1346: 14–25, 2010 [DOI] [PubMed] [Google Scholar]
- 204.Wei J, Li F, Yang J, Liu X, and Cho WC. MicroRNAs as regulators of airborne pollution-induced lung inflammation and carcinogenesis. Arch Toxicol 89: 677–685, 2015 [DOI] [PubMed] [Google Scholar]
- 205.Wei R, Zhang R, Xie Y, Shen L, and Chen F. Hydrogen suppresses hypoxia/reoxygenation-induced cell death in hippocampal neurons through reducing oxidative stress. Cell Physiol Biochem 36: 585–598, 2015 [DOI] [PubMed] [Google Scholar]
- 206.Weldon BA, Shubin SP, Smith MN, Workman T, Artemenko A, Griffith WC, Thompson B, and Faustman EM. Urinary microRNAs as potential biomarkers of pesticide exposure. Toxicol Appl Pharmacol 312: 19–25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, and Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res 1111: 95–104, 2006 [DOI] [PubMed] [Google Scholar]
- 208.Wilker EH, Alexeeff SE, Suh H, Vokonas PS, Baccarelli A, and Schwartz J. Ambient pollutants, polymorphisms associated with microRNA processing and adhesion molecules: the Normative Aging Study. Environ Health 10: 45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wilker EH, Baccarelli A, Suh H, Vokonas P, Wright RO, and Schwartz J. Black carbon exposures, blood pressure, and interactions with single nucleotide polymorphisms in MicroRNA processing genes. Environ Health Perspect 118: 943–948, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wingo AP, Almli LM, Stevens JS, Klengel T, Uddin M, Li Y, Bustamante AC, Lori A, Koen N, Stein DJ, Smith AK, Aiello AE, Koenen KC, Wildman DE, Galea S, Bradley B, Binder EB, Jin P, Gibson G, and Ressler KJ. DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat Commun 6: 10106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Winneke G, Walkowiak J, and Lilienthal H. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology 181–182: 161–165, 2002 [DOI] [PubMed] [Google Scholar]
- 212.Wong F, Cousins IT, and Macleod M. Bounding uncertainties in intrinsic human elimination half-lives and intake of polybrominated diphenyl ethers in the North American population. Environ Int 59: 168–174, 2013 [DOI] [PubMed] [Google Scholar]
- 213.Xiao R, Noel A, Perveen Z, and Penn AL. In utero exposure to second-hand smoke activates pro-asthmatic and oncogenic miRNAs in adult asthmatic mice. Environ Mol Mutagen 57: 190–199, 2016 [DOI] [PubMed] [Google Scholar]
- 214.Xie X, Song J, and Li G. MiR-21a-5p suppresses bisphenol A-induced pre-adipocyte differentiation by targeting map2k3 through MKK3/p38/MAPK. Biochem Biophys Res Commun 473: 140–146, 2016 [DOI] [PubMed] [Google Scholar]
- 215.Xie Y. and Chen Y. microRNAs: emerging targets regulating oxidative stress in the models of Parkinson's disease. Front Neurosci 10: 298, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xiong H, Dai M, Ou Y, Pang J, Yang H, Huang Q, Chen S, Zhang Z, Xu Y, Cai Y, Liang M, Zhang X, Lai L, and Zheng Y. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp Gerontol 51: 8–14, 2014 [DOI] [PubMed] [Google Scholar]
- 217.Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, Huang Q, Chen S, Zhang Z, Xu Y, Lai L, and Zheng Y. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging 36: 1692–1701, 2015 [DOI] [PubMed] [Google Scholar]
- 218.Xiong R, Wang Z, Zhao Z, Li H, Chen W, Zhang B, Wang L, Wu L, Li W, Ding J, and Chen S. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol Aging 35: 705–714, 2014 [DOI] [PubMed] [Google Scholar]
- 219.Xu J, Shimpi P, Armstrong L, Salter D, and Slitt AL. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol 290: 21–30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xue T, Wei L, Zha DJ, Qiu JH, Chen FQ, Qiao L, and Qiu Y. miR-29b overexpression induces cochlear hair cell apoptosis through the regulation of SIRT1/PGC-1alpha signaling: implications for age-related hearing loss. Int J Mol Med 38: 1387–1394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yamakuchi M. MicroRNA regulation of SIRT1. Front Physiol 3: 68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang JS. and Lai EC. Dicer-independent, Ago2-mediated microRNA biogenesis in vertebrates. Cell Cycle 9: 4455–4460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yao Y, Robinson AM, Zucchi FC, Robbins JC, Babenko O, Kovalchuk O, Kovalchuk I, Olson DM, and Metz GA. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med 12: 121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, Dore LC, Yao Y, D'Souza J, Zhang Z, Ghaffari S, Choi J, Friend S, Tong W, Orange JS, Paw BH, and Weiss MJ. miR-451 protects against erythroid oxidant stress by repressing 14–3-3zeta. Genes Dev 24: 1620–1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224a.Yuan W, Yang N, and Li X. Advances in understanding how heavy metal pollution triggers gastric cancer. Biomed Res Int 2016: 1–10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang B. and Pan X. RDX induces aberrant expression of microRNAs in mouse brain and liver. Environ Health Perspect 117: 231–240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, and He DZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One 8: e62786, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang ZF, Zhang YQ, Fan SH, Zhuang J, Zheng YL, Lu J, Wu DM, Shan Q, and Hu B. Troxerutin protects against 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD(+)-depletion. J Hazard Mater 283: 98–109, 2015 [DOI] [PubMed] [Google Scholar]
- 228.Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, and Law PY. mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol 77: 102–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhou F, Li S, Jia W, Lv G, Song C, Kang C, and Zhang Q. Effects of diesel exhaust particles on microRNA-21 in human bronchial epithelial cells and potential carcinogenic mechanisms. Mol Med Rep 12: 2329–2335, 2015 [DOI] [PubMed] [Google Scholar]
- 230.Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, and Nagarkatti M. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One 9: e94075, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhou L, Xu DY, Sha WG, Shen L, Lu GY, Yin X, and Wang MJ. High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J Transl Med 13: 352, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhou T, Ross DG, DeVito MJ, and Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci 61: 76–82, 2001 [DOI] [PubMed] [Google Scholar]
- 233.Zhou T, Taylor MM, DeVito MJ, and Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci 66: 105–116, 2002 [DOI] [PubMed] [Google Scholar]
- 234.Zhu Y, Zong L, Mei L, and Zhao HB. Connexin26 gap junction mediates miRNA intercellular genetic communication in the cochlea and is required for inner ear development. Sci Rep 5: 15647, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, Kovalchuk I, Kovalchuk O, and Metz GA. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS One 8: e56967, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]