Abstract
Bacterial meningitis is a major diagnostic and therapeutic problem among children and neonates, with severe, rapidly progressing course and potentially life-threatening complications. Early antibacterial treatment is essential for the patient’s favorable prognosis. Cerebral imaging plays an important role in the diagnostic process alongside physical examination and laboratory tests. Magnetic resonance imaging is the gold standard for diagnosing bacterial meningitis. Because of limited availability of magnetic resonance imaging, cranial ultrasound is the first imaging procedure to be performed (if the anterior fontanelle is preserved providing an adequate acoustic window). The safety and reliability of ultrasound examination, possibility to perform the examination at bedside without the need for sedation make cranial ultrasound a useful tool both for preliminary diagnostic investigation and for the monitoring of both treatment and long-term complications. Sonographic findings in patients with bacterial meningitis and possible complications are diverse. Changes can be seen on the surface of the brain, in the extra-axial space, in the ventricular system and in brain tissue. In some cases they can also be visible in the lumbosacral segment of the spinal cord. This paper presents ultrasound characteristics of lesions associated with bacterial meningitis in neonates and infants, based on the authors’ own material and data from the available literature.
Keywords: cranial ultrasound, neuroultrasound, meningitis, pediatrics, neonates
Introduction
Bacterial meningitis (BM) is a serious condition, presenting a major diagnostic and therapeutic challenge in everyday practice. Amongst the pediatric population, neonates (up to 4 weeks of age) and infants are particularly susceptible. The course of infection in this group is severe and usually systemic in nature. The lack of characteristic initial symptoms and rapid progression cause the mortality rate to exceed 50%(1,2).
Early diagnosis and initiation of correct treatment are key aspects for the patient’s prognosis. Apart from laboratory tests, imaging procedures are also important for the diagnostic process. Sonography should be especially appreciated among the available methods as the first modality used in diagnosing neonates and infants with suspected BM. Cranial ultrasound (CU) is also useful for monitoring the therapeutic process and the detection of early and late complications of BM.
This article presents the possibilities of using CU in the preliminary diagnostic investigation of BM and treatment monitoring in neonates and infants based on the authors’ own material and a review of the available literature.
Epidemiology
Neisseria meningitidis is the most common etiological factor for BM in the European countries. The incidence rate for neonates and infants is 20/100,000(3). Data for the Polish population indicate that the most common etiological factor for BM in neonates are Escherichia coli and Streptococcus agalactiae, while in infants Neisseria meningitidis and Streptococcus pneumoniae. The combined prevalence of BM in the analyzed group is 15.71/100,000 (data from 2011)(2).
Pathophysiology
BM is usually blood-borne, and most often associated with a systemic infection. From the clinical standpoint, it is important to confirm or exclude sepsis since it substantially compromises the patient’s prognosis. Initially bacteria accumulate in the lumen of choroid plexus capillaries located in the cerebral ventricles and in the small and medium-sized vessels of the subarachnoid space, where they induce an inflammatory response. Developing vasculitis leads to the inflammation of the arachnoid, dura mater and pia mater.
As a result of disruption to the blood-brain barrier, bacteria and inflammatory mediators (acute-phase proteins, leukotrienes, prostaglandins, among others) penetrate to the cerebrospinal fluid (CSF), thus exacerbating inflammation. In the acute phase cerebral vessels become dilated with increasing blood flow due to the influence of bacterial toxins. In later phases blood flow is reduced, which is associated with impaired vascular autoregulation(4). The cytotoxic activity of oxygen radicals and proteolytic enzymes causes excessive sodium and water accumulation. This leads to impaired recirculation of CSF, dilation of the ventricular system and increased intracranial pressure, leading to brain edema(1,4–9).
Sepsis can damage the endothelium of cerebral capillaries. This results in the formation of parietal thrombi. Severe cases involve diffuse cerebral venous thrombosis (affecting both superficial vessels such as superior sagittal sinus perforator vessels and deep veins such as the vein of Galen) or thrombosis in the confluence of sinuses(1,8).
Vascular occlusion results in the development of infarction foci in deep structures (e.g. in the periventricular area) and in the subcortical regions, which leads to brain tissue damage. This process is accelerated by proteolytic enzymes which are stimulated by an inflammatory response.
Radiological abnormalities caused by encephalopathy are diverse, including focal hypoxic-ischemic lesions, areas of leukomalacia, hydrocephalus as well as focal and diffuse loss of brain tissue(5–10).
Diagnostic imaging
Diagnostic imaging of BM involves the use of cranial ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). CT and MRI are high-resolution modalities allowing full evaluation of cerebral structures, including the assessment of the posterior cranial fossa. Elaborate possibilities of vascular imaging in MRI and CT allow for early detection of thrombosis foci and fresh infarction in the deep structures of the brain, which are often not identifiable in conventional sonographic and color Doppler imaging. In addition, some lesions located in the extra-axial space, particularly in the temporal and parieto-occipital area may not be identifiable in CU. According to the current guidelines, MRI is the reference examination, the gold standard of diagnostic imaging in children(11). Performing CT and MRI requires sedation, which is associated with the risk of additional complications. Moreover, CT uses ionizing radiation, which is another risk factor. It is worth emphasizing that there is often no possibility of performing CT or MRI in some healthcare centers in Poland without transporting the patient to a different hospital or radiology unit. This delays the achievement of a clinical response. In the light of those facts CU seems to be the best alternative since it is easily accessible, can be performed at the point of care and is not associated with any adverse reactions. In the available literature many authors recommend CU as the first-line examination for diagnosing pediatric patients with suspected BM if a preserved anterior fontanelle provides an adequate acoustic window(12).
Ultrasound
Cranial ultrasound imaging can be unaltered in patients with uncomplicated BM. Ultrasound abnormalities are observed in approximately 65% of cases in the acute phase of the infection(12). In neonates and infants with severe neurological symptoms sonographic abnormalities are observed in up to 100% of patients(13).
There is a wide spectrum of possible abnormalities, which can be categorized depending on the location: pathologic changes visible on the surface of the brain, in the ventricular system, in deep structures – brain tissue, and in the lumbosacral segment of the vertebral canal.
Changes on the brain surface
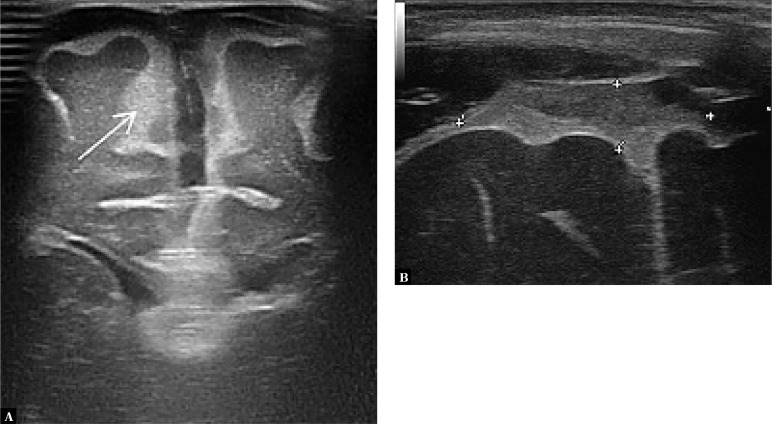
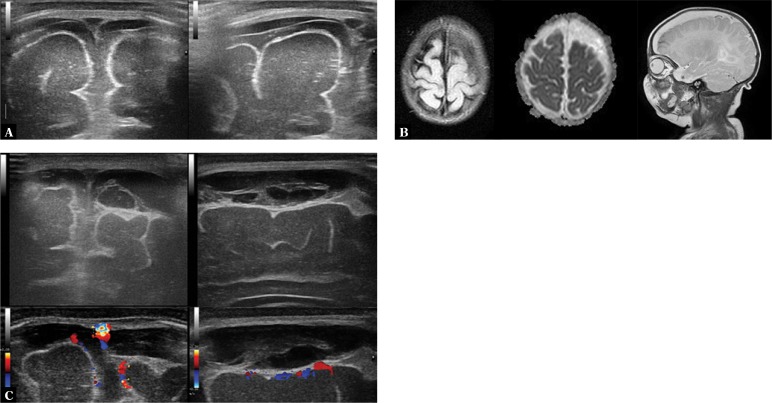
1. Dilated sulci with elevated echogenicity on the brain surface
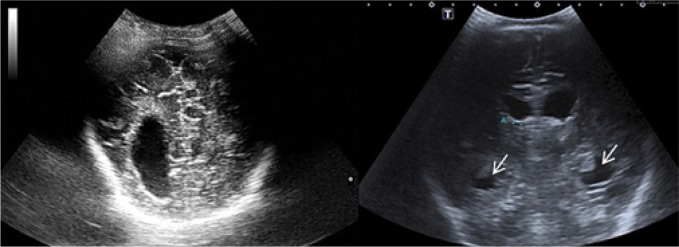
The most common sonographic symptom associated with BM, which is found in approximately 60% of patients. Increased echogenicity is the consequence of the accumulation of inflammatory exudate in this area (Fig. 1).
Fig. 1.
A. Imaging through the anterior fontanelle shows widening of the sulcus pattern of the brain gyri and elevated echogenicity of the space between the sulci (arrow). B. A higher echo is seen in the extra-axial space in the longitudinal plane
2. Meningeal thickening
An enhancement in the form of a hyperechoic line can be visible on the surface of the cerebral gyri, which is formed by the pia mater and the adjacent arachnoid (the leptomeninges); this line becomes thickened in BM. Measurement is taken in the coronal plane through the anterior fontanelle, with the foramina of Monro visualized. The reference value of the meninx thickness is 0.7 mm, whereas thickening is identified with the value of 1.3 mm or higher (Fig. 2). It is also possible to measure sulcus thickness between the frontal gyri; the reference thickness of the sulcus is 1.2 mm and thickening is identified when this value is 2 mm or higher(14).
Fig. 2.
Thickening of the pia mater and the adjacent arachnoid – the leptomeninges layer
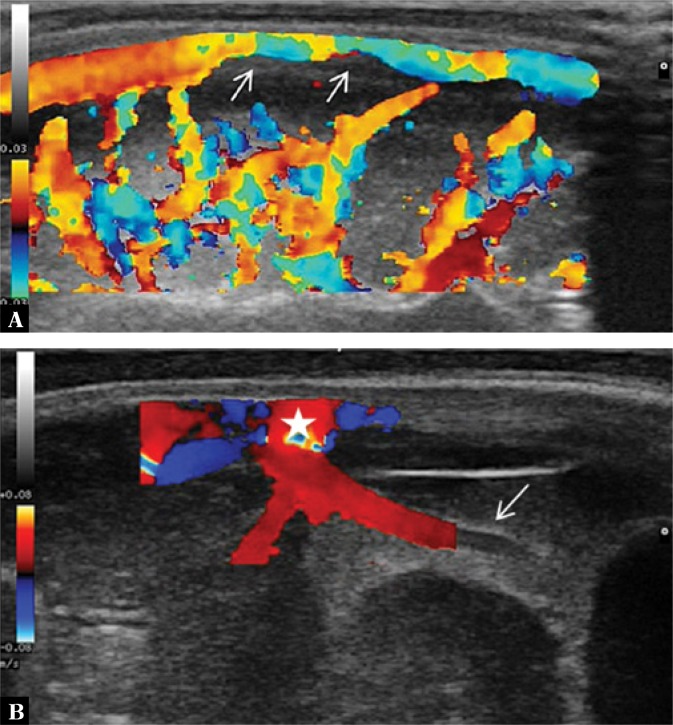
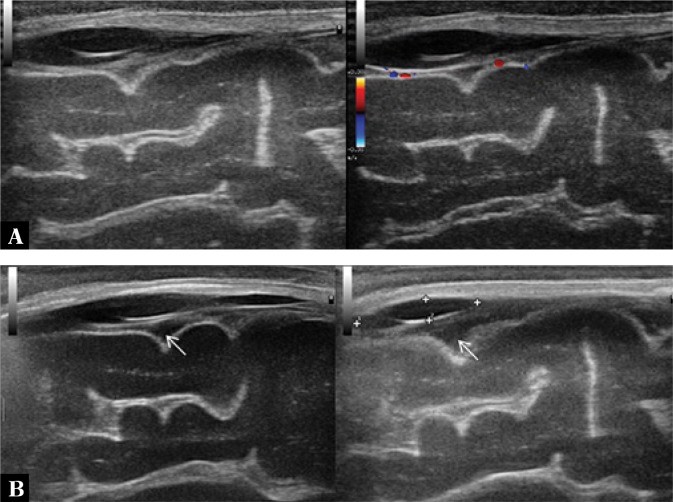
3. Dilation of cerebral vessels in the arachnoid
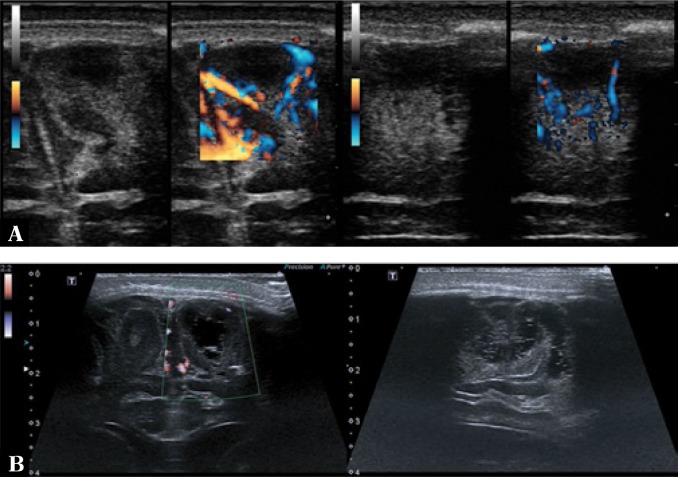
Dilation can be observed in the superficial cerebral veins, perforator vessels and the superior sagittal sinus, among others, which are easily accessible to imaging through the anterior fontanelle (Fig. 3).
Fig. 3.
A. Color Doppler imaging shows increased flow in the superficial cerebral vessels. In the superior sagittal sinus flow constriction associated with the presence of a parietal thrombus (arrows) is seen. B. Imaging through the anterior fontanelle in the longitudinal plane. One of the perforator veins is dilated with a surrounding hyperechoic extra-axial echo. A vessel partly covered by the color Doppler gate with a visible ostium into the superior sagittal sinus (star) is seen
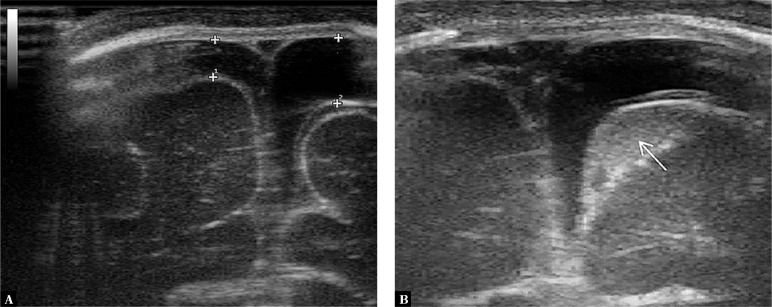
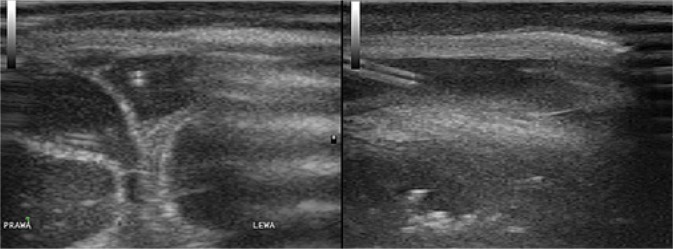
4. Enlargement of extra-axial fluid spaces
The enlargement of the subdural and/or subarachnoid space is caused by the presence of inflammatory exudate (Fig. 4).
Fig. 4.
A three-month-old infant with purulent BM caused by E. coli. Imaging through the anterior fontanelle in the transverse plane: A. widening of the subdural space on the left side with a normal image of the subarachnoid space; B. a follow-up examination of the patient during fever increase after 2 days. Widening of the subarachnoid space with increased echogenicity due to the accumulation of inflammatory exudate (the subarachnoid space is marked with an arrow in the images)
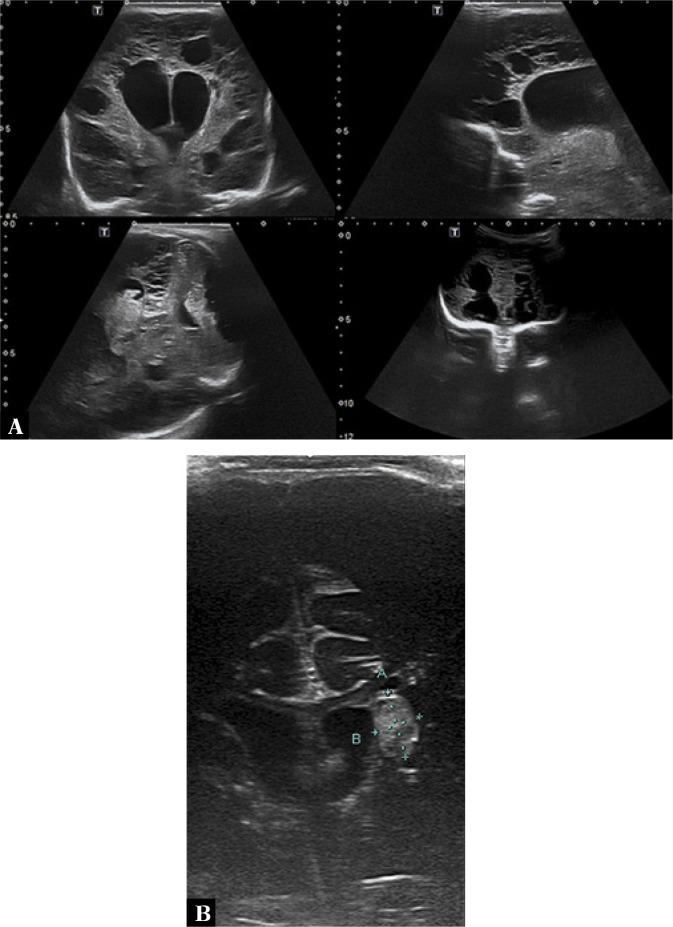
5. Closed fluid cavities
Closed fluid cavities in the extra-axial space (subdural, subarachnoid) take the form of hygromas or empyemas. It is also possible to visualize closed fluid spaces associated with the dissection of the dura mater itself. A hygroma filled with sterile fluid content is visible as a homogeneously anechoic area (Fig. 5). The presence of hygromas usually does not alter the prognosis(15) – fewer than 5% become infected and turn into empyemas. In such cases CU shows higher or mixed echogenicity. Sometimes echogenic debris forms in the lumen of empyemas which by gravitation forms a pattern with fluid levels on the brain surface and on the bottom of gyri. High amount of protein and fibrin in the infected fluid leads to their precipitation, organizing conglomerates of hyperechoic strands with smaller fluid cavities (Fig. 6). From the clinical standpoint, those CU findings are important because the fluid cavities may act as a bacterial reservoir and at the same time be the cause of therapeutic failures due to significantly limited drug penetration. In such cases it is necessary to administer a drug directly into the lesion’s surroundings by subdural puncture (Fig. 7).
Fig. 5.
A three-and-a-half-month-old infant with diagnosed BM caused by E. coli. The evolution of changes during treatment monitoring is presented. A. Imaging through the anterior fontanelle shows subdural hygromas. B. A subdural empyema (longitudinal sections on the left, transverse sections on the right). Echogenic fibrin structures forming separate fluid cavities. Color Doppler imaging shows no vascular flow in the fibrin structures. C. MRI: subdural empyema on the left side, on the surface of the frontal lobes, with signs of impression on brain tissue
Fig. 6.
A three-and-a-half-month-old infant with diagnosed BM caused by E. coli. A. A closed fluid space following subdural empyema treatment. Color Doppler imaging shows no flow in the space. B. Follow-up: gradual regression of the fluid cavity. Notable widening of the subarachnoid space with normal echogenicity (arrows)
Fig. 7.
A three-and-a-half-month-old infant with diagnosed BM caused by E. coli. Due to the lack of clinical improvement when using triple combination antibiotic therapy (cefotaxime/amikacin/ vancomycin) it was decided to administer a drug directly into the empyema area by anterior fontanelle puncture. The images show the puncture needle end in the transverse and longitudinal planes. Gentamicin was administered into the empyema cavity and approximately 60 ml of pus fluid was drained
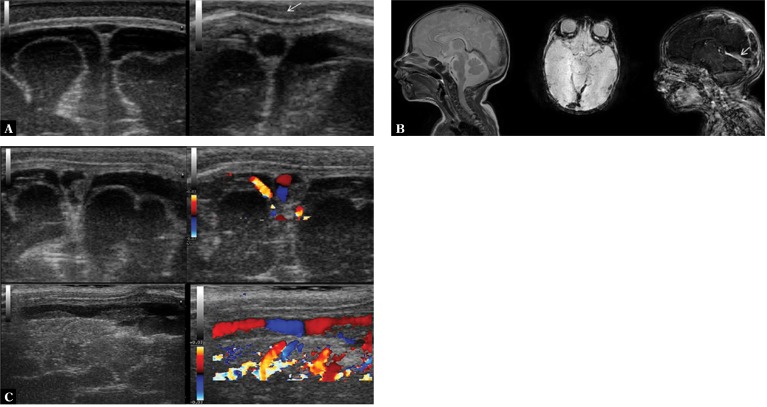
6. Cerebral thrombosis
Echogenic parietal thrombi are usually visualized with cranial ultrasound in the superior sagittal sinus as well as in larger deep veins (e.g. the vein of Galen). Color-coded Doppler imaging shows a characteristic impression of the blood flow shape being influenced by the thrombus. In cases of disseminated intravascular coagulation a substantial portion of the venous system can be affected, including the confluence of sinuses (Fig. 8).
Fig. 8.
A twenty-six-day-old infant with diagnosed BM caused by Streptococcus agalactiae. In the course of the therapeutic process the diagnostic examination of the coagulation system was extended and concomitant protein S deficiency was diagnosed. Low-molecular-weight heparins were used for treatment. A. On the left, imaging through the anterior fontanelle reveals a normal image of the superior sagittal sinus. A susceptible to pressure, triangular lumen of the vessel is notable. On the right, a round section of the superior sagittal sinus which is not susceptible to delicate pressure of the transducer is seen (arrow). If no thrombus is visualized using standard viewing planes, such an image can suggest that thrombosis is present in a segment of the vessel which is not accessible to scanning. B. Parietal thrombus in the superior sagittal sinus visible in transverse and longitudinal planes in B-mode and color Doppler. C. MRI: visible signs of disseminated coagulation in the cerebral venous system including superficial vessels, deep vessels (straight sinus – arrow) and the confluence of sinuses
Abnormalities of the ventricular system
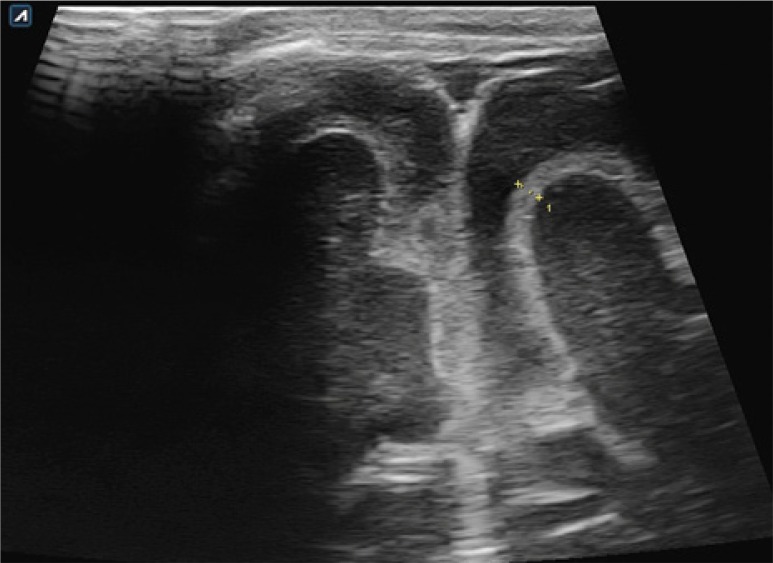
1. Ventricular system dilation
Ventricular system dilation can be found in approximately 30% of patients with BM. Sonographic hallmarks of ventricular dilation are diverse, can be symmetrical, involving the dilation of both ventricles or affect only one side. Changes can be inconspicuous, such as rounded impressions in the anterior horns of the lateral ventricles, or very severe and classified as hydrocephalus. The most sensitive sonographic sign of hydrocephalus is the dilation of the third ventricle or the temporal horns of the lateral ventricles(11) (Fig. 9, 10).
Fig. 9.
On the left, asymmetrical dilation of the ventricular system is seen. On the right: a 7-month-old patient with BM caused by E. coli complicated with Candida infection. Visible signs of hydrocephalus with the dilation of the temporal horns of the lateral ventricles (arrows)
Fig. 10.
A. Acute phase of cortical infarction in the frontal gyri. A hypoechoic area without a visible flow in color Doppler with a surrounding hyperechoic area of reaction (on the left – transverse sections, on the right – longitudinal sections). B. Areas of subcortical leukomalacia. Color Doppler imaging shows no vascular flow in the visualized changes (on the left – transverse section, on the right – longitudinal section)
2. Elevated CSF echogenicity in the ventricles with visible fluid levels
3. Dilation of choroid plexuses with increased echogenicity
4. Ventriculitis
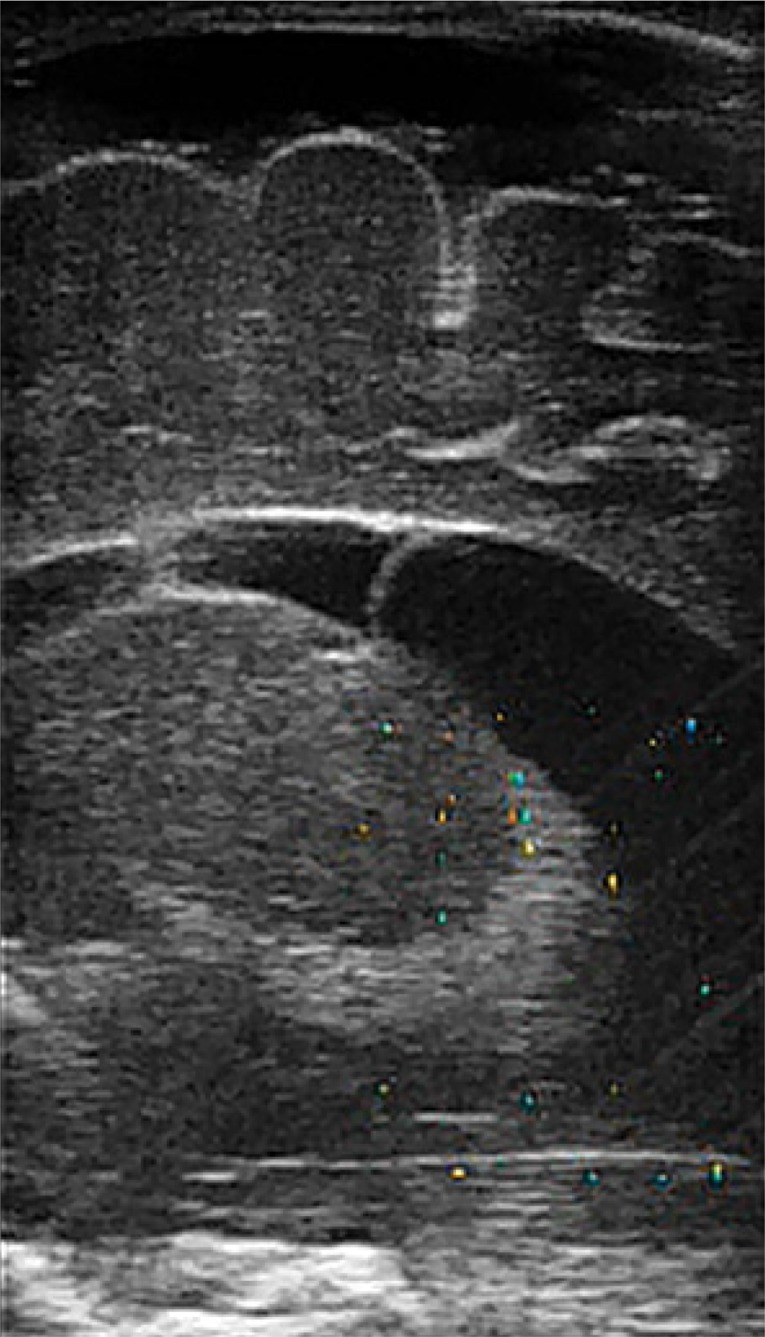
Sonographic signs of ventriculitis include: thickened and irregular contours of the ventricular system, increased ventricular lining echogenicity, increased periventricular white matter echogenicity, irregular contours of choroid plexuses, increased CSF echogenicity inside the ventricles, intraventricular debris and compartmentalized ventricular lumen containing fluid cavities (Fig. 11).
Fig. 11.
Compartmentalization of the ventricles. A visible septum in the central part of the left lateral ventricle
Brain tissue abnormalities
1. Brain edema
One of the most severe complications of BM is brain edema. Its sonographic appearance involves increased echogenicity of sulci and brain tissue. Advanced brain edema presents as obfuscation of the boundaries between gyri and sulci as well as compression and deformation of the ventricular system and the remaining fluid cavities. Color Doppler imaging reveals an increased resistance index (RI) (> 0.8) measured in afferent arteries, e.g. in the anterior cerebral artery(9) (Fig. 12).
Fig. 12.
A two-and-a-half-month-old infant with diagnosed BM caused by Streptococcus agalactiae. On the left, a narrow, compressed lumen of the lateral ventricles associated with brain edema is visible. On the right, an elevated RI in the anterior cerebral artery is observed
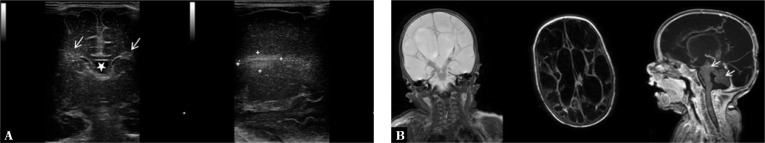
2. Hypoxic-ischemic foci
Pathologic changes in this group are diverse. They may be seen as homogeneous hyperechoic areas or, in advanced disease, as heterogeneous malacia lesions with fluid spaces (Fig. 13, 14).
Fig. 13.
A. Hyperechoic periventricular halo (arrows). A star marks the cavity of the septum pellucidum. B. A well-delimited hypoxic-ischemic lesion in the view of thalamic nuclei on the left side
Fig. 14.
A two-and-a-half-month-old infant with diagnosed BM caused by Streptococcus agalactiae. Infection complicated with aseptic encephalitis. CSF examination revealed signs of protein-cell separation. A. Imaging through the anterior and posterolateral fontanelle shows extensive loss of brain tissue manifesting as malacia cavities and hydrocephalus. B. Follow-up MRI performed directly before discharge from hospital. BM complicated by communicating hydrocephalus with the formation of fluid chambers. Parts of brainstem and cerebellum are visible (arrows)
3. Subcortical infarction foci
Infarction foci develop in brain tissue as a result of thrombosis. In the acute phase they are initially visible as areas of increased echogenicity with evident obfuscation of the echostructure. As a result of ischemia, local necrosis develops and echogenicity decreases in the center of the hyperechoic area with restricted vascular flow, which is visible in color Doppler imaging. After the acute phase recedes, leukomalacia lesions develop in the affected subcortical area (Fig. 10).
Imaging of the lumbosacral spine
Ultrasound imaging of the vertebral canal in the lumbosacral area is used primarily when developmental spinal cord pathology is suspected. It is considered to be the procedure of choice in neonates and infants. The ossification process of vertebral arches and spinous processes lasts until 12 months of age, which provides an adequate acoustic window for ultrasound examination. In older patients the utility of ultrasound examination of this area is limited. Imaging is performed with both convex and linear transducers. The examination is usually used for: the evaluation of spinal cord anatomy with the analysis of potential developmental variants (filar cyst, terminal ventricle), assessment of structural mobility in the vertebral canal (to exclude signs of spinal cord impaction) and examination of soft tissue and osseous structures surrounding the spinal cord in order to exclude potential developmental pathologies (myelomeningocele, spina bifida, skin sinuses/fistulas).
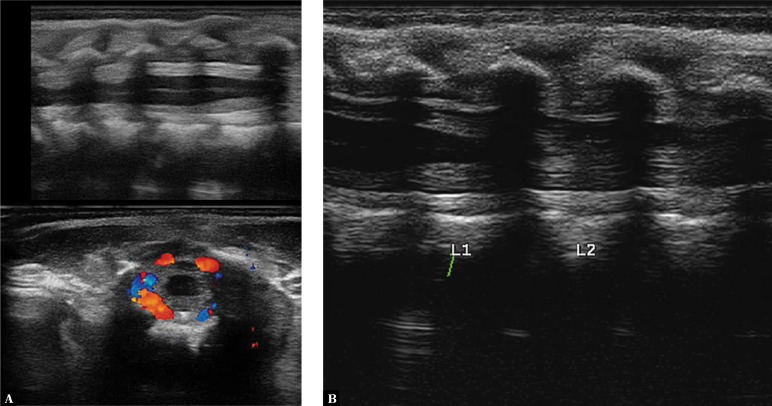
Changes associated with BM observed on the brain surface can also be partially visible in the lumbosacral segment of the spinal cord. This is of high clinical importance for making the diagnosis, planning lumbosacral puncture and predicting long-term complications. The presence of echogenic debris in the subdural space increases the risk of progressive hydrocephalus(16) (Fig. 15).
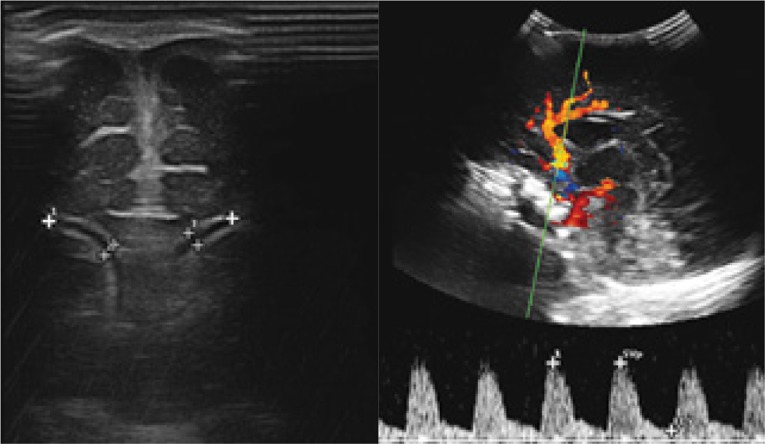
Fig. 15.
A three-month-old infant with diagnosed BM caused by Streptococcus agalactiae. A. Imaging of the lumbar spine following lumbar puncture. A hyperechoic area of reaction around the spinal cord. Color Doppler imaging shows a rich vascular flow. B. A scan after 5 days of treatment before a planned follow-up puncture: a normal image of neural structures with the perimedullary space is seen
Conclusion
Cranial ultrasound is a useful diagnostic method when bacterial meningitis is suspected in neonates and infants. The spectrum of characteristic signs visualized in BM using conventional imaging and Doppler imaging allows for quick preliminary diagnosis and initiation of treatment, which has a significant impact on the patient’s prognosis. The possibility of brain imaging without any adverse reactions and performing the procedure at the point of care makes cranial ultrasound an ideal tool not only for the preliminary diagnosis of patients with suspected BM, but also for treatment monitoring and identification of long-term complications of the disease.
Conflict of interest
The authors do not report any financial or personal affiliations to persons or organizations that could negatively affect the content of or claim to have rights to this publication.
References
- 1.Albrecht P, Hryniewicz W, Kuch A, Przyjałkowski W, Skoczyńska A, Szenborn L. Rekomendacje postępowania w zakażeniach bakteryjnych ośrodkowego układu nerwowego. Warszawa: Narodowy Instytut Leków; 2011. [Google Scholar]
- 2.Skoczyńska A, Waśko I, Kuch A, Kadłubowski M, Gołębiewska A, Foryś M, et al. A decade of invasive meningococcal disease surveillance in Poland. PLoS One. 2013;8:e71943. doi: 10.1371/journal.pone.0071943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl. 2):B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 4.Ashwal S, Stringer W, Tomasi L, Schneider S, Thompson J, Perkin R. Cerebral blood flow and carbon dioxide reactivity in children with bacterial meningitis. J Pediatr. 1990;117:523–530. doi: 10.1016/s0022-3476(05)80683-3. [DOI] [PubMed] [Google Scholar]
- 5.Berman PH, Banker BQ. Neonatal meningitis. A clinical and pathological study of 29 cases. Pediatrics. 1966;38:6–24. [PubMed] [Google Scholar]
- 6.Tunkel AR, Scheld WM. Pathogenesis and pathophysiology of bacterial meningitis. Annu Rev Med. 1993;44:103–120. doi: 10.1146/annurev.me.44.020193.000535. [DOI] [PubMed] [Google Scholar]
- 7.Pfister HW, Fontana A, Täuber MG, Tomasz A, Scheld WM. Mechanisms of brain injury in bacterial meningitis: Workshop summary. Clin Infect Dis. 1994;19:463–479. doi: 10.1093/clinids/19.3.463. [DOI] [PubMed] [Google Scholar]
- 8.DiNubile MJ, Boom WH, Southwick FS. Septic cortical thrombophlebitis. J Infect Dis. 1990;161:1216–1220. doi: 10.1093/infdis/161.6.1216. [DOI] [PubMed] [Google Scholar]
- 9.Tunkel AR, Scheld WM. Alterations of the blood-brain barrier in bacterial meningitis: in vivo and in vitro models. Pediatr Infect Dis J. 1989;8:911–913. doi: 10.1097/00006454-198912000-00040. [DOI] [PubMed] [Google Scholar]
- 10.Babcock DS, Han BK. Sonographic recognition of gyral infarction in meningitis. AJR Am J Roentgenol. 1985;144:833–836. doi: 10.2214/ajr.144.4.833. [DOI] [PubMed] [Google Scholar]
- 11.Hughes DC, Raghavan A, Mordekar SR, Griffiths PD, Connolly DJ. Role of imaging in the diagnosis of acute bacterial meningitis and its complications. Postgrad Med J. 2010;86:478–485. doi: 10.1136/pgmj.2010.097022. [DOI] [PubMed] [Google Scholar]
- 12.Yikilmaz A, Taylor GA. Sonographic findings in bacterial meningitis in neonates and young infants. Pediatric Radiol. 2008;38:129–137. doi: 10.1007/s00247-007-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan R, Lodha A, Anand R, Patwari AK, Anand VK, Garg DP. Cranial sonography in bacterial meningitis. Indian Pediatr. 1995;32:989–993. [PubMed] [Google Scholar]
- 14.Jéquier S, Jéquier JC. Sonographic nomogram of the leptomeninges (pia-glial plate) and its usefulness for evaluating bacterial meningitis in infants. AJNR Am J Neuroradiol. 1999;20:1359–1364. [PMC free article] [PubMed] [Google Scholar]
- 15.Barkovich AJ. Infections of Central Nervous System. In: Barkovich AJ, editor. Pediatric Neuroimaging. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 801–868. [Google Scholar]
- 16.Rudas G, Almássy Z, Papp B, Varga E, Méder U, Taylor GA. Echodense spinal subarachnoid space in neonates with progressive ventricular dilatation: a marker of noncommunicating hydrocephalus. AJR Am J Roentgenol. 1998;171:1119–1121. doi: 10.2214/ajr.171.4.9763007. [DOI] [PubMed] [Google Scholar]