Abstract
Purpose
Macrophages are known to be important for healing numerous injured tissues depending on their functional phenotypes in response to different stimuli. The objective of this study was to reveal macrophage phenotypic changes involved in exercise-induced skeletal muscle injury and regeneration.
Methods
Adult male Sprague-Dawley rats experienced one session of downhill running (16° decline, 16 m/min) for 90 min. After exercise the blood and soleus muscles were collected at 0 h, 6 h, 12 h, 1 d, 2 d, 3 d, 1 w and 2 w after exercise, separately.
Results
It was showed that CD68+ M1 macrophages mainly infiltrated into muscle necrotic sites at 1–3 d, while CD163+ M2 macrophages were present in muscles from 0 h to 2 weeks after exercise. Using transmission electron microscopy, we observed activated satellite cells 1 d after exercise. Th1-associated transcripts of iNOS and Ccl2 were inhibited post exercise, while COX-2 mRNA was dramatically increased 12 h after running (p < 0.01). M2 phenotype marker Arg-1 increased 12 h and 3 d (p < 0.05, p < 0.01) after exercise, and Clec10a and Mrc2 were up-regulated in muscles 12 h following exercise (p < 0.05, p < 0.05).
Conclusion
The data demonstrate the dynamic patterns of macrophage phenotype in skeletal muscle upon eccentric exercise stimuli, and M1 and M2 phenotypes perform different functions during exercise-induced skeletal muscle injury and recovery.
Keywords: Macrophage phenotype, Muscle injury, Regeneration, Eccentric exercise
Abbreviations: myoglobin, (Mb); inducible NO synthase, (iNOS); CC chemokine ligand 2, (Ccl2); cyclooxygenase-2, (COX-2); arginase-1, (Arg-1); C-type lectin domain family 10 member, (Clec10a); mannose receptor C type 2, (Mrc2)
Introduction
Macrophages are crucial for tissue repair and regeneration, and macrophage deletion or perturbations of macrophage function and/or activation may result in impaired regeneration and fibrosis deposition.1, 2 Macrophages have been operationally defined into the following two separate activation states in vitro: (1) classically activated M1 macrophages and (2) alternatively activated M2 macrophages, which exhibit opposing pro-inflammatory or anti-inflammatory functional roles, respectively, according to their mode of polarization.3, 4 M1 macrophages are associated with the first phases of acute inflammation and mirror Th1 immune response, while M2 macrophages participate in polarized Th2 reactions, parasite clearance, damping of inflammation and facilitating tissue remodeling.3 The diverse functions of macrophages depend on their different phenotypes, which are plastic and determined by the environmental stimuli.
In the context of muscle damage, M1 macrophages are usually found during the early stages of damaged muscle. They infiltrate early to promote the clearance of necrotic debris via the production of large amounts of pro-inflammatory cytokines. During the later stages, M1 macrophages are gradually replaced by the M2 phenotype to facilitate tissue repair or remodeling through the release of growth factors and anti-inflammatory cytokines. This demonstrates that macrophages in the same cell may initially induce pro-inflammatory and cytotoxic reactions and later take part in the resolution of inflammation and tissue remodeling or adaptation.5 Several studies related to muscle damage and regeneration using unloading/reloading model have demonstrated the diverse plasticity of macrophages.6, 7 and this property of macrophages has also been identified in toxin-induced muscle injuries8, 9 or in the mdx mouse model of muscular dystrophy.10
In rats, the former nomenclature ED1+ and ED2+ refers to M1 (CD68+) and M2 (CD163+) macrophage subsets respectively.11, 12 Lapointe et al.13 reported that ED2+ macrophages were elevated in muscles 48 h after lengthening contractions. Yu et al.14 found that CD68+ M1 macrophages infiltrated into the necrotic muscle fibers with highly expressed CD163+ M2 macrophages 2 h after intermittent downhill running in rats. However, Tsivitse failed to find ED2+ macrophage expression in rat soleus muscles during the 72 h of recovery from downhill running; only ED1+ macrophages were elevated at 24 and 48 h after injurious exercise, suggesting a discrepancy in macrophage distribution in rat injurious muscle after eccentric exercise.15 Moreover, normal skeletal muscles have been shown to contain numerous resident macrophages, which have mainly been proven to be ED2+ cells in both endomysial and perimysial sites, with ED1+ cells appearing less frequently.16, 17 In degenerating fibers, ED2+ cells were not present. In contrast, ED1+ cells, were rarely observed within the undamaged regions of the muscles but were abundant within the degenerating muscles.17 Thus, the distribution of macrophage cells, especially resident macrophages or ED2+ (M2) cells, subject to muscle injury and regeneration needs to be further identified.
As a typical eccentric-contraction model, downhill running on a treadmill is commonly used to study exercise-induced muscle damage because the lesions often result from the stresses and strains of normal use, which is more physiological and able to mimic muscle damage that occurs during sports.18, 19 Therefore, the aim of this study was to investigate macrophage phenotypic changes involved in the context of exercise-induced skeletal muscle injury and regeneration process in rats. We supposed that macrophages had the diverse response upon eccentric exercise stimuli, and the activation state of M1 population in the damaged tissues was different from the performance of M2 cell during exercise-induced skeletal muscle injury and recovery.
Methods
Animals
Adult male Sprague-Dawley rats (body weight 250–290 g) were purchased from Sciple-Kay Laboratory Animal Co. Ltd (Shanghai, China) and maintained in the Shanghai University of Sport Animal Center. All of the animals were kept in an animal room with a 12/12 h light/dark cycle, (23 ± 2)°C and (55 ± 5)% relative humidity. The animals were fed with standard laboratory chow and tap water ad libitum. This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996) and was approved by the Animal Care and Use Committee at Shanghai University of Sport.
Exercise training protocol
The animals were randomly divided into non-exercise (control, C) and exercise groups. The latter was sub-divided into 0 h, 6 h, 12 h, 1 d, 2 d, 3 d, 1 w and 2 w group for sacrifice following exercise, and each group contained eight animals. Rats in the exercise groups conducted one session of downhill running on a motorized treadmill with a 16-degree decline at 16 m/min for 90 min. The animals in the control group were handled similarly to the exercise group, without any form of training.
The animals from the exercise group were sacrificed with chloral hydrate (40 mg/100 g weight) as an anesthetic at 0 h, 6 h, 12 h, 1 d, 2 d, 3 d, 1 w and 2 w after running, which represent the early, intermediate, and late stages of regeneration after exercise-induced injury.18 The animals from the control group were handled similarly with exercise group for sacrifice.
Blood sample and tissue collection
A blood sample was obtained from the inferior vena cava and serum was produced through centrifugation at 3000 g/min for 10 min at 4 °C. After isolation, the serum was immediately frozen and kept in a −80 °C freezer to prepare for myoglobin (Mb) concentration measurement.
To investigate muscle ultrastructural changes, soleus muscles were harvested and placed in 2.5% glutaraldehyde and 1% osmic acid. After fixation, the biopsies were dehydrated in a graded series of ethanol and acetone and embedded in epoxy resin. To evaluate macrophage invasion and histological alteration, soleus muscles were fixed into formalin. Followed by dehydration, the biopsies were embedded in paraffin. All of the unilateral soleus muscles were excised and frozen immediately with liquid nitrogen and then stored at −80 °C for further analysis.
ELISA test and morphological analysis
Serum levels of myoglobin (Mb) before or after exercise in the rats were measured using enzyme-linked immunosorbent assays (ELISAs) according to the instructions in the ELISA kits (ab 157739, Abcam Inc., USA).
For ultrastructural assessment in muscles, 50–60 nm thickness sections were stained with lead citrate for 10 min. The slides were scanned using a transmission electron microscope (PHILIPS CM120, Netherlands) to create digital images (at 11.0 × 103 or 4.80 × 103 magnification).
For histology detection, 5 μm-thick cross sections were stained with hematoxylin and eosin (H&E).20 To evaluate macrophage properties, slides were incubated in 3% H2O2 at room temperature for 10 min and then blocked with 5% goat serum for 20 min. Then, the slices were incubated with mouse antibodies against rat CD68 (AbD Serotec, UK, 1:50) for M1 macrophage cells or CD163 for M2 macrophage cells (AbD Serotec, UK, 1:50) at 4 °C overnight. After incubated with biotin-conjugated secondary antibodies and streptavidin-biotin-peroxidase, separately, 3,3′-diaminobenzidine was used to detect the positive staining for CD68+ or CD163+ expression. All pictures were captured using Olympus DX70 microscope (Olympus, Tokyo, Japan) at 400 × magnification. The amount of stained areas (μm2) for the M1 or M2 cells was calculated with the Image-Pro Plus 4.1 software (Media Cybernetics Inc., USA). Five views (upper left, upper right, lower left and lower right, middle) of each section were measured under microscope.
Real-time quantitative PCR
To reveal the macrophage activation response to exercise stimulation, mRNA expression of the M1 macrophage markers inducible NO synthase (iNOS, also NOS2), CC chemokine ligand 2 (Ccl2), cyclooxygenase-2 (COX-2) and the M2 macrophage markers arginase-1 (Arg-1), C-type lectin domain family 10 member (Clec10a) and mannose receptor C type 2 (Mrc2) were quantified in the muscles at various times after injury using real-time quantitative PCR. Total RNA was isolated with the TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocol and assessed for purity using a NanoDrop system (NanoDrop Technologies, USA). Reverse transcription of the RNA was carried out using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Real-time quantitative PCR reactions with cDNA as the template were performed using SYBR Green Master Mix kits (Bio-Rad, USA). All of the data were represented relative to their expression using the 2−ΔΔCt method21 as a fold change from the control group and normalized to β-actin transcript levels. The PCR primers for each studied gene were shown in Table 1.
Table 1.
Primer sequences for real-time quantitative PCR analysis.
| Arg-1 | forward: TGGACTGGACCCAGTATTCA reverse: CCCAAGAGTTGGGTTCACTT |
|---|---|
| Clec10a | forward: GGATTCTGGTGTCTCGGTTT reverse: GAGCTTTACCAGCCTCTTGG |
| Mrc2 | forward: ACCTTTGACCTCTGGATTGG reverse: AGCAGAGGTTCCTGCTCAAT |
| iNOS | forward: GAGACAGGAAAGTCGGAAGC reverse: GTGTTGAAGGCGTAGCTGAA |
| Ccl2 | forward: CAGAAACCAGCCAACTCTCA reverse: AGACAGCACGTGGATGCTAC |
| COX-2 | forward: AATCGCTGTACAAGCAGTGG reverse: GCAGCCATTTCTTTCTCTCC |
Abbreviations: arginase-1 (Arg-1); C-type lectin domain family 10 member (Clec10a); mannose receptor C type 2 (Mrc2); inducible NO synthase (iNOS); CC chemokine ligand 2 (Ccl2); cyclooxygenase-2 (COX-2).
Statistical analysis
Statistical analysis was performed using the SPSS 17.0 software (SPSS, Chicago, IL, USA). All of the results were presented as the mean ± SD. One-way analysis of variance (ANOVA) was conducted to assess significant overall differences, and post hoc test was used to locate differences before and after exercise at various groups. The significance level was set at p < 0.05.
Results
Serum myoglobin (Mb) contents
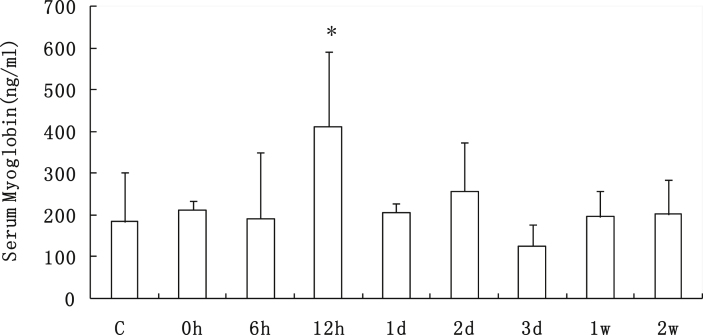
After one session of downhill running, as a damage biomarker of muscle, the concentration of serum myoglobin (Mb) in rats remarkably increased 39.82% (p < 0.05) at 12 h after exercise. Then the value returned to a normal level from 1 d to 2 w after exercise (Fig. 1).
Fig. 1.
Serum Mb content of rats from all of the groups before and after one session of downhill running. *p < 0.05 vs. the control group. Mb: myoglobin. Values are plotted as mean ± SD.
Distribution of M1 and M2 macrophage cells
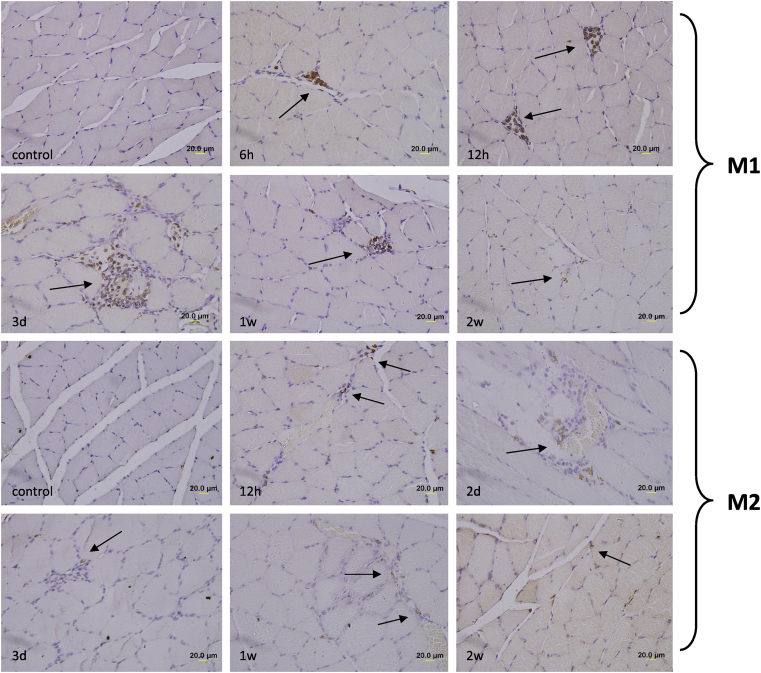
To evaluate the macrophage response to eccentric exercise, immunohistochemical staining for CD68+ (M1 macrophages) and CD163+ (M2 macrophages) was performed in rat soleus muscles before and after one session of downhill running at various time points (Fig. 2).
Fig. 2.
Representative immunohistochemical staining of M1 and M2 macrophages (brown color) in rat soleus section before and after one bout of downhill running, nuclei counterstained with hematoxylin (blue color). Scale bar was 20 μm.
There were neither necrotic muscle fibers nor CD68+ M1 macrophages in the non-exercised rats (control group), which was consistent with the result from Yu et al.14 CD68+ M1 cells clearly invaded into fibers 6 h after exercise (Fig. 2, M1, 6 h, with arrow), then accumulated within muscular phagocytosis areas at 12 h (Fig. 2, M1, 12 h, with arrows). Around 3 days post exercise, the abundant M1 cells gathered within the vacuolated or necrotic sites (Fig. 2, M1, 3 d, with arrow), consistent with the phagocytic function of M1 macrophages. After that, the M1 macrophage accumulation was attenuated (Fig. 2, M1, 1 w, with arrow) and faintly visualized at 2 w post exercise (Fig. 2, M1, 2 w, with arrow).
Different from the performance of M1 macrophages after exercise, CD163+ M2 macrophage cells were present in fibers before and after exercise, although the expression in the control group was very weak.
They scattered in the intercellular space (Fig. 2, M2, 12 h, with arrows) and had no invasion into the phagotrophic areas, just around the lesion regions in fibers (Fig. 2, M2, 2 d and 3 d, with arrows). From 1 w after exercise, CD163+ M2 cells were visualized mainly on the sarcolemma of muscles (Fig. 2, M2, 1 w, with arrows), rather than the damping of M1 macrophage in muscles at 2 w after exercise (Fig. 2, M2, 2 w, with arrow).
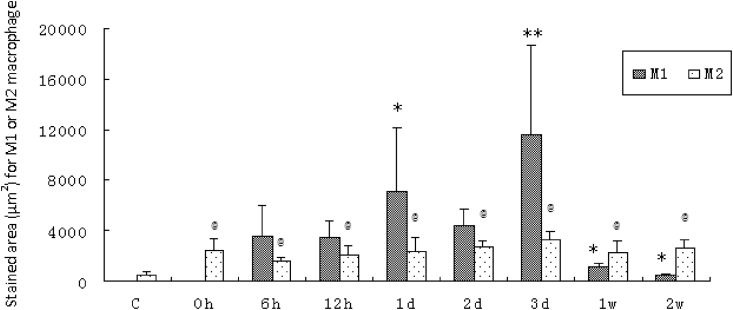
The amount of positive stained area (μm2) for M1 and M2 macrophage expression in muscles showed that M1 and M2 subsets had different response related to downhill running (Fig. 3). The M1 macrophage accumulation reached a plateau at 1 d and 3 d after exercise, increased by 1-fold (p < 0.05) and 2.3-fold (p < 0.01), respectively, above the value of 6 h after exercise. Then the values dramatically attenuated from 1 w (p < 0.05) to 2 w (p < 0.05) compared with 6 h after exercise. Contrary to the absence of M1 macrophage in control group or 0 h after exercise, M2 macrophage cells were faintly visible in normal fibers. Compared with exercise before, the M2 macrophage accumulation was remarkably increased in fibers at the end of downhill running (0 h) and maintained their moderate elevation by 2 w after exercise (Fig. 3).
Fig. 3.
The amount of stained area (μm2) for M1 or M2 macrophages in rat soleus muscle sections before and after one session of downhill running. M1 macrophage: *p < 0.05, **p < 0.01 vs. 6 h after exercise; M2 macrophage: @p < 0.05 vs. the control group. Values are plotted as mean ± SD.
Macrophage activation and muscle regeneration expression markers
To reveal the macrophage cell activation upon exercise stimulation, mRNA expression of the activated M1 phenotype markers iNOS, Ccl2 and COX-2, and three important indicators of M2 phenotype activation, Arg-1, Clec10a and Mrc2 were quantified at various times after exercise using real-time RT-PCR approach.
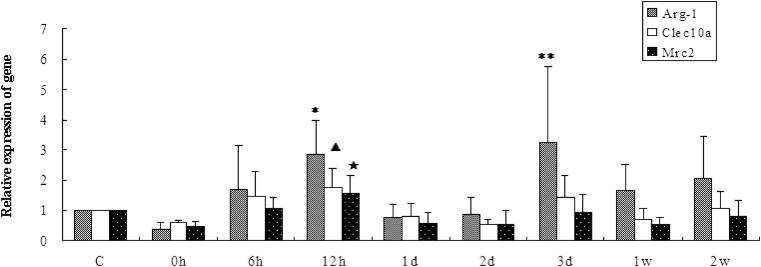
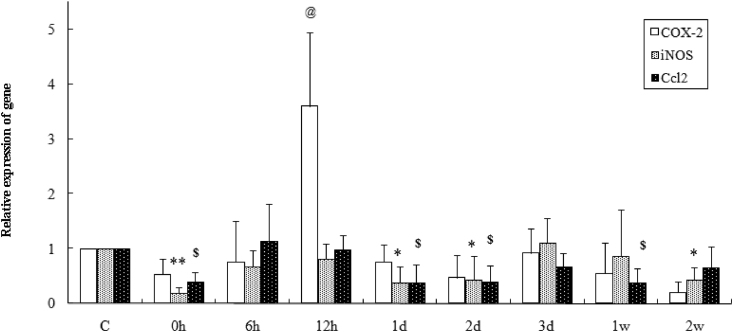
The results showed that Arg-1 gene expression increased 1.83- and 2.26-fold (p < 0.05, p < 0.01, compared with exercise before) in muscles 12 h and 3 d after exercise, separately (Fig. 4), in addition Clec10a and Mrc2 genes were up-regulated (p < 0.05, p < 0.05, compared with exercise before) at 12 h after exercise in fibers (Fig. 4). While as for the M1 phenotype markers, the results showed that iNOS and Ccl2 genes were inhibited at 0 h, 1 d and 2 d after exercise compared with the values of exercise before (Fig. 5, iNOS: p < 0.01, p < 0.05, p < 0.05; Ccl2: p < 0.01, p < 0.01, p < 0.01, respectively), and the iNOS or Ccl2 gene was persistently down-regulated at 2 w or 1 w after exercise in muscles, separately (Fig. 5, iNOS: p < 0.05, Ccl2: p < 0.01, compared with the values of control group). Meanwhile, eccentric exercise caused an approximately 3.6-fold increase in COX-2 (p < 0.01) at 12 h after running, followed by 80% reduction compared with resting value at 2 w after exercise (Fig. 5).
Fig. 4.
Arg-1, Clec10a and Mrc-2 mRNA expression before and after one bout of downhill running in rat soleus muscles using real-time fluorescent quantitation PCR. Arg-1: *p < 0.05, **p < 0.01 vs. control group; Clec10a: ▲p < 0.05 vs. control group; Mrc-2: ★p < 0.05 vs. control group. Values are plotted as mean ± SD.
Fig. 5.
COX-2, iNOS and Ccl2 mRNA expression before and after one bout of downhill running in rat soleus muscles using real-time fluorescent quantitation PCR. COX-2: @p < 0.01 vs. control group; iNOS: *p < 0.05, **p < 0.01 vs. control group; Ccl2: $p < 0.01 vs. control group. Values are plotted as mean ± SD.
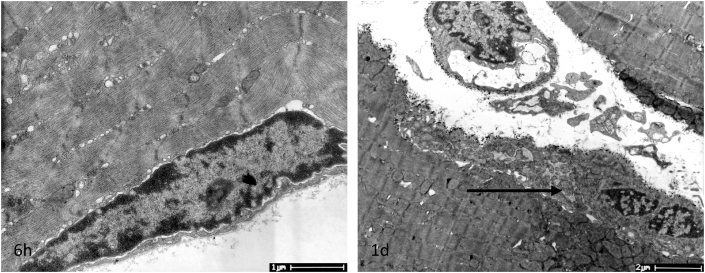
Regarding the ultrastructural changes in muscle subject to eccentric exercise, we found that satellite cells were located under the basal lamina at 6 h post exercise, then activated 1 d after exercise, which was characterized by an increased cytoplasmic volume and apparent cytoplasmic extension22 (Fig. 6, 1 d, as arrow).
Fig. 6.
Satellite cells from soleus muscle were located between the membrane and the basal lamina surrounding the myofiber at 6 h and were activated 1 d after one session of downhill running, as shown in this transmission electron microscopy image. Scale bar: 11.0 × 103 (at 6 h) or 4.80 × 103 (at 1 d).
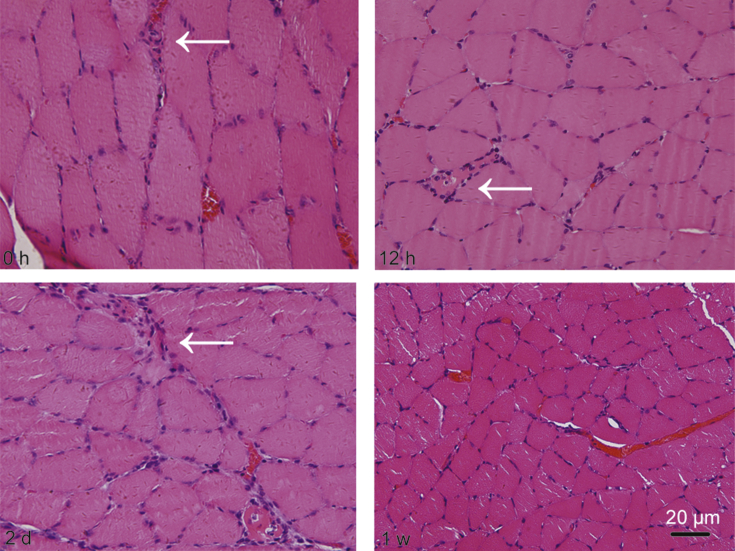
To evaluate the degeneration/regeneration process of muscles after eccentric exercise, we examined the histological alterations of soleus muscle by H&E staining. The results showed that one session of eccentric exercise caused skeletal muscle injury occurred at 0 h, which was characterized with small diameter and angular shaped myofibers accompanied by mononucleated cells infiltrated into interstitial spaces (Fig. 7, 0 h, with arrow). Phagocytosis of necrotic fibers was observed at 12 h (Fig. 7, 12 h, with arrow), accompanied by massive swollen or necrotic cells at 2 d following exercise (Fig. 7, 2 d, with arrow). By 1 week after running, little mononucleated or necrotic cells were present. As for the cell size after exercise, we could find the enlarged fibers from 0 h after exercise (Fig. 7, 0 h) or the rounded cells 2 d post running (Fig. 7 2 d). At 1 w after exercise, the cells restored their normal size, which implied the initial recovery of fibers after injury (Fig. 7, 1 w).
Fig. 7.
Representative histological results for soleus muscles stained with hematoxylin and eosin before and after one session of downhill running in rats. Scale bar = 20 μm.
Discussion
Exercise-induced muscle damage is commonly induced by high-force, unaccustomed stretch, and particularly following eccentric muscle contractions, e.g., downhill running, which is known to induce more muscle damage compared to uphill or level running.18 When the damage occurs, ultrastructural skeletal muscle disruption results in the leakage of muscle proteins into the circulation, including creatine kinase (CK), Mb, lactate dehydrogenase, and aldolase, which act as indirect markers of muscle damage.23, 24 Undoubtedly, in our present study we examined the peaked augmentation of serum Mb concentration at 12 h after exercise, demonstrated the muscle injury occurred at early time after exercise, which was consistent with the elevated Mb levels in human blood associated with damage.25 Then the value dropped to normal level, indicating the following recovery at a later stage in muscles.
Accompanied by the occurrence of damage, skeletal muscle regeneration is initiated and comprises several overlapping cellular processes, including inflammation, proliferation, and remodeling. Macrophages are assumed to participate in tissue injury and repair processes. M1 macrophage accumulation is thought to result from the infiltration and differentiation of circulating monocytes recruited to the injury sites to phagocyte necrotic cells and proliferate myogenic cells.8, 26 A significant reduction in the initial monocyte/macrophage influx into injured muscle is associated with moderately impaired repair processes after skeletal muscle injury.27 When phagocytosis is concluded, M1 macrophages start to polarize into M2 phenotypes, inducing myogenic cells to differentiate.8
In our present study, we detected the early invasion of CD 68+ M1 macrophages at 6 h after exercise, which demonstrated the pro-inflammatory action of the M1 subset when injury occurs. However, we also detected the expression of CD163+ M2 macrophage in muscles before 1d after exercise. The simultaneous occurrence of M1 and M2 macrophage cells at early time-points of injury was contrary to the early-M1/late-M2 paradigm. Because according to this criterion, monocytes arriving from the blood may need time to switch from M1 to M2 phenotype, thus M2 macrophage cells should be low or absent during early stages of repair and elevated at later process. In our study, the early accumulation of CD163+ M2 macrophage cells in muscles after injury denied the entire M1 phenotype during the early stages of tissue repair.
Because of the important role of monocyte-derived macrophages during inflammatory environments, the contribution of tissue resident macrophage cells has largely been neglected.28 Tissue macrophages can remain independent from blood monocytes,29 and the existence of resident macrophage cell in tissues suggests a vital role in tissue homeostasis. Côté et al.30 reported that under conditions of monocyte depletion, both infiltrating and resident precursors could contribute to M1 or M2 macrophage accumulation in muscle injury, implying that the activation status of tissue macrophage and monocyte-derived macrophage was influenced by environmental signals. Brigitte et al.31 assessed that recruitment of circulating monocytes into injured muscle was dramatically reduced through intravenous injection of diphtheria toxin (DT) to induce ablation of resident fascia macrophages in a mouse model of notexin-induced myoinjury, demonstrating that resident macrophage cells participated in the recruitment of circulating monocytes into the damaged muscle. In this setting, we inferred the early existence of M2 macrophages after exercise in our present study was related to the activation of resident macrophage in fibers, playing a role to recruit monocytes from blood entry into injury sites at early stage. In contrast to the characteristic of M1 phenotype displayed early-up/later-down in muscles after exercise, CD163+ M2 macrophages were present in muscles throughout the injury-repair time. The existence of M2 macrophage subset during the later stages of inflammation might in fact be the retention or survival of tissue-resident macrophages in muscles. The unconformable emerging time and distribution sites of M1 and M2 populations in fibers indicated the different contribution of M1 and M2 phenotypes upon contraction stimuli. Therefore, the heterogeneity of macrophage population in vivo was not analogous to that in vitro, which including the role of tissue resident macrophage cell should be taken into consideration in the prospective treatment during exercise-induced muscle injury and regeneration.
Macrophages expressing pro-inflammatory M1 markers preferentially associate with proliferating satellite cells, whereas at the time of myogenic differentiation, macrophages mainly express anti-inflammatory M2 markers.32 Many groups have demonstrated that monocytes/macrophages produce or express pro-inflammatory cytokines/chemokines, such as Ccl2, iNOS, COX-2, TNF-α, and IL-12, and have an anti-inflammatory profile by their production/expression of cytokine/chemokines, growth factors, and other markers such as IL-10, Arg-1, IGF-1, and platelet-derived growth factor-β (PDGF-β).33, 34 M1 macrophages express iNOS, which is a typical marker of M1 activation that is required to efficiently metabolize l-arginine into NO and citrulline to increase tissue damage.35 M2 macrophages are characterized by expression of enzyme arginase, which hydrolyzes l-arginine to ornithine and urea and then to polyamine and proline, respectively, for cellular proliferation and tissue repair.33 As the common substrate for both enzymes, the competitive utilization of l-arginine for M1/M2 polarization leads to pro- or anti-inflammatory macrophage functions, which influence the course of tissue injury and repair. In our present study, we identified the enhanced expression of the M2 markers Arg-1, Clec10a and Mrc2 at 12 h (P < 0.05, P < 0.05, P < 0.05, respectively) and the further elevation of Arg-1 at 3 d (P < 0.01) after exercise. The first elevation of Arg-1 relative gene expression appeared at 12 h after exercise might be more related to the resident macrophage cells activation upon the damage stimulus, while the second increase of Arg-1 expression at 3 d after exercise might indicate the polarization of M1 into M2 phenotypes at gene level.
Compared with the up-regulation of Arg-1 in fibers, we found the understandable depression of iNOS expression at 2 w after exercise accompanied with the attenuated inflammation in fibers at the same time point. However, iNOS and Ccl2 genes were inhibited at 0 h, 1 d and 2 d after exercise, which did not correlate with the high expression of M1 macrophage in muscles during early stage of injury (Fig. 2). The underlying mechanisms behind this paradox were unclear, partially might be related to the very local inflammatory occurred in this injurious model. Compared with huge inflammatory events, such as trauma, cold injury, heat stress, crushing, or contusion injury in muscle, exercise-induced skeletal muscle damage should have a different molecular pattern responding to this contraction stimulation. The unexpected inhibition of iNOS and Ccl2 gene expression in tissue injury/repair process might be a negative-feedback mechanism initiating a phenotypic switch, which highlighted the complexity of in vivo phenotypic regulation. In addition, iNOS and Ccl2 may be produced by several other cell types in regenerating muscle,36 the detection of iNOS and Ccl2 gene expression in macrophages could be more precise to define the inflammatory status of macrophages than the whole muscle. Further study should be needed to determine the factors that down-regulate iNOS and Ccl2 gene expression in exercise-induced muscle injury and regeneration.
COX-2 is an immediate/early gene whose expression in most tissues is low or absent, but is transiently induced by mitogens and cytokines. It is generally considered to have a pro-inflammatory role37 and the COX-2 pathway may regulate myoblast proliferation because mitogenic activation of satellite cells is associated with up-regulation of COX-2.38 Langenbach et al.39 found that macrophage chemotaxis was impaired in the absence of COX-2 activity; COX-2 null mutants showed less macrophage invasion of injured muscle, which adversely affected myoblast proliferation during regeneration.40 In this sense, COX-2 activity is crucial during the early stages of muscle regeneration, and COX-2 may have a direct effect on muscle cell proliferation and repair.40 In our study, contrast to the down-regulation of iNOS and Ccl2 after exercise, COX-2 mRNA expression was up-regulated 12 h post exercise (P < 0.01) (Fig. 5), indicating the pro-inflammatory role of COX-2 in this model context.
Satellite cells are resident stem cells in skeletal muscle responsible for muscle maintenance and repair.41 In the resting state, satellite cells are quiescent and situated between the plasma membrane and the basal lamina of fiber. Upon monocyte/macrophage recruitment, quiescent satellite cells are activated, which are characterized by an increase in the cytoplasmic volume of the activated cell, while the amount of heterochromatin decreases and organelles, such as Golgi bodies, endoplasmic reticulum, ribosomes, and mitochondria, become apparent.22 All of these features demonstrate the initiated satellite cells activation, followed by a proliferation, self-renewal, differentiation and fusion into new myotubes. In our study, we observed activated satellite cell 1d after exercise, at the early period when the damage occurred in muscle, which was consistent with the time course of M1 macrophages cells infiltrated into fibers at the early stage of injury.
In summary, we observed the distribution of the M1 and M2 phenotypes in rat skeletal muscles after one session of downhill running exercise. We found that M1 macrophages mainly infiltrated into necrotic sites of muscles approximately 1–3 d after exercise, while the M2 phenotype was present in muscles all the time, suggesting the different functions of M1 and M2 macrophage in the injurious muscle after eccentric exercise.
Fund
This work was supported by the funding from Key Laboratory of Exercise and Health Sciences of Ministry of Education, Shanghai University of Sport.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Rigamonti E., Zordan P., Sciorati C. Macrophage plasticity in skeletal muscle repair. Bio Med Res Int. 2014;2014:560629. doi: 10.1155/2014/560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharraz Y., Guerra J., Mann C.J. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediat Inflamm. 2013;2013:491497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A., Biswas S.K., Galdiero M.R. Macrophage plasticity and polarization in tissue repair and remodeling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 4.Wynn T.A., Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A., Sica A., Sozzani S. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Kasper C.E. Sarcolemmal disruption in reloaded atrophic skeletal muscle. J Appl Physiol (1985) 1985;79:607–614. doi: 10.1152/jappl.1995.79.2.607. [DOI] [PubMed] [Google Scholar]
- 7.Krippendorf B.B., Riley D.A. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- 8.Arnold L., Henry A., Poron F. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryer S.C., Fantuzzi G., Van Rooijen N. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J Immunol. 2008;180:1179–1188. doi: 10.4049/jimmunol.180.2.1179. [DOI] [PubMed] [Google Scholar]
- 10.Villalta S.A., Nguyen H.X., Deng B. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLennan I.S. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J Anat. 1996;188:17–28. [PMC free article] [PubMed] [Google Scholar]
- 12.St Pierre B.A., Tidball J.G. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol (1985) 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- 13.Lapointe B.M., Frenette J., Côté C.H. Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion. J Appl Physiol (1985) 2002;92:1995–2004. doi: 10.1152/japplphysiol.00803.2001. [DOI] [PubMed] [Google Scholar]
- 14.Yu S.H., Huang C.Y., Lee S.D. Decreased eccentric exercise-induced macrophage infiltration in skeletal muscle after supplementation with a class of ginseng-derived steroids. PLoS One. 2014;9:e114649. doi: 10.1371/journal.pone.0114649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivitse S.K., McLoughlin T.J., Peterson J.M. Downhill running in rats: influence on neutrophils, macrophages, and MyoD+ cells in skeletal muscle. Eur J Appl Physiol. 2003;90:633–638. doi: 10.1007/s00421-003-0909-0. [DOI] [PubMed] [Google Scholar]
- 16.Honda H., Kimura H., Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990;70:272–277. [PMC free article] [PubMed] [Google Scholar]
- 17.McLennan I.S. Resident macrophages (ED2- and ED3-positive) do not phagocytose degenerating rat skeletal muscle fibres. Cell Tissue Res. 1993;272:193–196. doi: 10.1007/BF00323586. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong R.B., Ogilvie R.W., Schwane J.A. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 19.Kyparos A., Matziari C., Albani M. A decrease in soleus muscle force generation in rats after downhill running. Can J Appl Physiol. 2001;26:323–335. doi: 10.1139/h01-020. [DOI] [PubMed] [Google Scholar]
- 20.Bhagavati S., Ghatpande A., Shafiq S.A. In situ hybridization analysis for expression of myogenic regulatory factors in regenerating muscle of mdx mouse. J Neuropathol Exp Neurol. 1996;55:509–514. doi: 10.1097/00005072-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Schultz E., McCormick K.M. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;23:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- 23.Brancaccio P., Lippi G., Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–767. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 24.Gomes R.V., Santos R.C., Nosaka K. Muscle damage after a tennis match in young players. Biol Sport. 2014;31:27–32. doi: 10.5604/20831862.1083276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda K., Sugama K., Hayashida H. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev. 2013;19:72–85. [PubMed] [Google Scholar]
- 26.Wermuth P.J., Jimenez S.A. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med. 2015;4:2. doi: 10.1186/s40169-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summan M., Warren G.L., Mercer R.R. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;l(6):R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 28.Davies L.C., Jenkins S.J., Allen J.E. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies L.C., Taylor P.R. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Côté C.H., Bouchard P., van Rooijen N. Monocyte depletion increases local proliferation of macrophage subsets after skeletal muscle injury. BMC Musculoskelet Disord. 2013;14:359. doi: 10.1186/1471-2474-14-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brigitte M., Schilte C., Plonquet A. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62:268–279. doi: 10.1002/art.27183. [DOI] [PubMed] [Google Scholar]
- 32.Saclier M., Cuvellier S., Magnan M. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013;280:4118–4130. doi: 10.1111/febs.12166. [DOI] [PubMed] [Google Scholar]
- 33.Rath M., Müller I., Kropf P. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S.L., Castaño A.P., Nowlin B.T. Bone marrow Ly6C high monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 35.Albina J.E., Mills C.D., Henry W.L., Jr. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol. 1990;144:3877–3880. [PubMed] [Google Scholar]
- 36.Novak M.L., Koh T.J. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilroy D.W., Colville-Nash P.R., Willis D. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 38.Iñiguez M.A., Punzón C., Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- 39.Langenbach R., Loftin C., Lee C. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;8:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 40.Bondesen B.A., Mills S.T., Kegley K.M. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 41.Rudnicki M.A., Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]