Abstract
In the past three decades, rod lens endoscopes had facilitated the development and wide spread applications of video-assisted thoracic surgery (VATS). With the rise of uniportal VATS in recent years, innovations in surgical instruments should once again complement the advancement in surgical technique. While articulated flexible endoscopes have expand the field of view, and can alter viewing direction with minimal maneuvers, they still suffer from problems like trocar crowding and interference with other instruments. Magnetic anchored endoscopes, on the other hand, may provide unique benefits to VATS by replacing the endoscope rigid rod body with magnetic linkage, thus overcoming the challenge of port crowding in single incision surgery. Most magnetic anchored endoscopes reported in literature are not designed for thoracic surgeries. Many of these designs do not allow tilting of endoscopic view, rely on micromotors for actuation, or are ergonomically unfit to be operated within the spatial constraints seen in VATS application. Considering these limitations, we have designed two novel magnetic anchored and steered endoscopes targeted for uniportal VATS. Both designs could be wirelessly actuated by magnetic interaction. One has a silicone rubber formed soft body for compactness, lightweight and safety, while another is a 40 mm long capsule optimized for VATS spatial constraints.
Keywords: Video-assisted thoracic surgery (VATS), magnetic anchored and guided system (MAGS), single-port, endoscope, uniportal
Introduction
Conventional thoracic surgeries often involve creating large wounds, such as thoracotomy or sternotomy incisions, to gain access to the thorax.
In the early 1990s, minimally invasive approach to thoracic surgery had gained interest, as application of cystoscope and rod lens laparoscopes had evolved from diagnostic to surgical (1). Rod lens laparoscopes were used to perform lung biopsy without thoracotomy incision, with patients reporting less postoperative pain (2). Over the following two decades, video-assisted thoracic surgery (VATS) lobectomy has become more popular and is shown to be safe. Furthermore, VATS is associated with lower morbidity and mortality rate, particularly in the elderly and patients with poor pulmonary functions when compared with conventional thoracotomy (3-9).
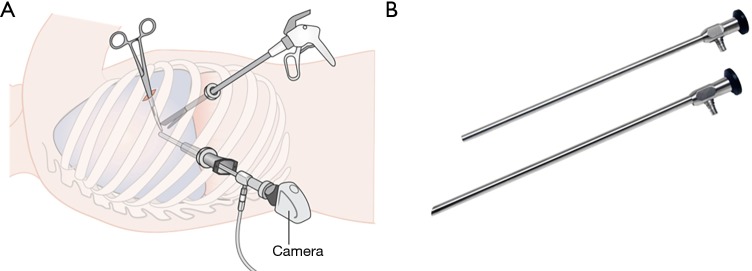
In VATS, multiple small incisions are made to pass thoracoscopic instruments and the rod lens endoscope between the ribs (Figure 1A). The endoscopes usually have a 5–10 mm diameter rigid long shaft and offer fixed viewing angle at 0° or 30° with a beveled tip (Figure 1B). Altering view direction involves steering the endoscope shaft body. This limits the field of vision (FOV) available and increase the risk of colliding with other instruments.
Figure 1.
Typical VATS setup using rigid endoscope. (A) Illustration of a typical 3-port VATS setup; (B) rigid endoscopes with 0° or 30° tip. VATS, video-assisted thoracic surgery.
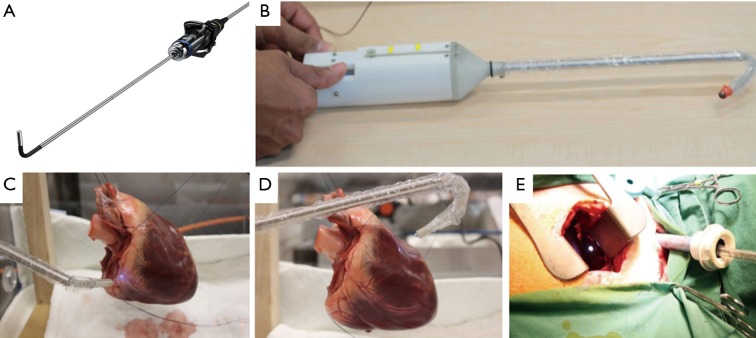
To expand the FOV and allow alternating view with minimal maneuvers, endoscopes with rotatable reflective prism or flexible distal joints were introduced (10). The Olympus Swing Prism Borescope has a rotatable reflective prism near the distal end, and offers 120° change of viewing direction, but prism contamination and post-operative sterilization complicates its surgical application. The Olympus ENDOEYE FLEX has a flexible distal section which can be articulated and bend to ±100° in two orthogonal planes, thus allows distal adjustment of view direction without steering the shaft body, and offers 3D vision in some models (Figure 2A). To widen the bending range further, and to offer more dexterity, the Cardioscope includes an adjustable rigid rod that alters the length of the flexible section, which gives varied workspace under articulation (11,12). This design offers over 180o bending, allowing the surgeon to look at the backside of a target (Figure 2B,C,D,E). Due to limitations of sensors, the Cardioscope has a lower image quality compared to commercial systems.
Figure 2.
Flexible endoscopes. (A) ENDOEYE FLEX 3D; (B) cardioscope prototype; (C,D) retroflexion of cardioscope, viewing backside of target; (E) cardioscope demo in animal model, notice LED spot in wound from cardioscope under retroflexion.
In the pursue of minimizing invasiveness by reducing the number of access wounds, laparoendoscopic single site surgery (LESS) has seen rising research interest and applications in various surgical disciplines, and thoracic surgery is no exception. Uniportal VATS involves passing multiple instruments through a single port between the ribs. In such crowded environment, the endoscope body increases risk of interfering with other instruments, and hinders their mobility inside the thoracic cavity. In this regard, surgeon’s performance and experience in uniportal VATS may be enhanced if the long shaft body of the endoscope can be eliminated from the incision site thus alleviating the port crowding problem. This can be achieved by replacing the physical connection to the camera with a magnetic linkage (13,14).
While initially designed for abdominal surgery, the magnetic anchored and guidance system (MAGS), proposed by University of Texas (UT) Southwestern Medical Center, offers unique benefits that could improve uniportal VATS. MAGS endoscope includes an external magnet and an internal unit, which is a video camera fitted with permanent magnets. To deploy the endoscope, the internal unit is inserted via a small incision, then it is attracted by the external magnet placed on the patient’s skin, and is anchored on the intracavitary surface against gravity. Once deployed, the surgeon can steer the position of endoscope by moving the external magnet manually. With magnetic linkage replacing the endoscope’s shaft body, the MAGS endoscope can reduce risk of fencing with other instruments, and could provide many views by deploying multiple cameras. While camera translation and panning movements are commonly achieved simply by moving the external magnet, tilting is controlled by various strategies (15). In early MAGS endoscope (16), tilting degree of freedom (DOF) is controlled by a crank mechanism driven by separating two pairs of internal and external permanent magnets (IPM and EPM). However, this design is quite bulky. In 2009, more compact MAGS endoscopes, with fixed 30° view angle, has been demonstrated to be feasible in human trial (17). For fixed angle MAGS endoscopes, tilting is achieved by manually compressing the abdominal wall (18-20). This strategy is unsuitable for applications in thoracic cavity, as the thoracic wall is largely incompressible being supported by rib cage.
Following the initial reports from UT Southwestern Medical Center, various designs of magnetic anchored surgical endoscopes have been proposed by other research groups. Many of these control tilting with on-board micromotors (21-24), adding to the weight and bulk of system, while also complicating fabrication and sterilization. Recently, designs with magnetic actuation of tilting DOF have been proposed (25,26). Although these are more lightweight and compact, they are not targeted for thoracic surgery. For example, the device described by Liu et al. (26) involves anchoring a 68 mm long capsule lengthwise, which would complicate panning on a curved chest wall with low compliance and presence of rib grooves. To make magnetic anchored endoscopes safe and feasible for uniportal VATS, we have designed the following two systems.
Soft bodied MAGS endoscope
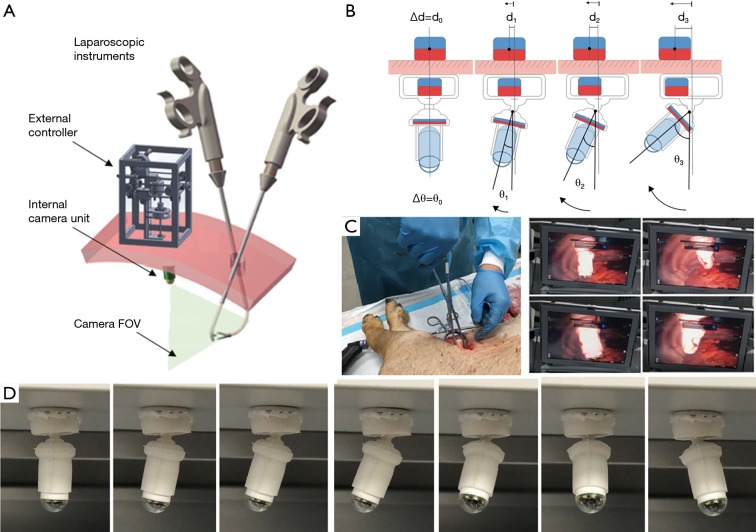
The soft-bodied MAGS endoscope (27) was designed to be safe, compact, and suitable for VATS application. It includes an internal camera unit and an external magnetic controller. The internal camera unit has a silicone rubber body, embedded with a wireless camera module and two IPMs. The external magnetic controller is a motorized box controlling the position of an EPM. The deploying and anchoring process is similar to any typical MAGS endoscope. Tilting and panning, on the other hand, are achieved by magnets interacting with the silicone rubber body. The silicone rubber body is a continuous piece with three functional sections: a top cavity, a middle soft joint, and a bottom camera holder. The top cavity is a 18 mm diameter cylindrical chamber containing a 5 mm diameter upper IPM, the bottom camera holder secures another piece of IPM and a wireless camera module, and the middle soft joint is a bendable section connecting the two. Schematics of the internal unit is shown in Figure 3A. EPM motions parallel to chest wall lead to upper IPM moving inside the top cavity. The upper IPM attracts the IPM in bottom camera holder, bending the middle soft joint, and lifts the camera from pointing vertically downwards to a tilted angle (see Figure 3B). Similarly, panning can be actuated by orbital motion of EPM around an axis normal to chest wall. As such, the view direction can be changed without compressing the chest wall or using on-board motors. The internal unit prototype is compact (42 mm long) and lightweight (16.5 g). It has a compliant body and does not require electric power for actuation, enhancing safety. Compared to articulated system driven by micromotors, the soft joint is simple to fabricate and sterilize. The prototype performs well in both benchtop settings and porcine cadaver (shown in Figure 3C,D, respectively). Similar to the design by Garbin et al. (25), the soft bodied MAGS sweeps a hemispheric workspace, which may not be ideal for operating in the thoracic cavity, as the endoscope may get too close to the operating site. A wireless camera module also limits the design’s video camera framerate and battery life. Considering these limitations, a wired endoscope with camera workspace near the chest wall may be better suited for the thorax and uniportal VATS applications.
Figure 3.
Soft MAGS endoscope. (A) Schematics of soft bodied MAGS endoscopic system; (B) illustration of tilting actuation principle; (C) performance evaluation in porcine cadaver; (D) endoscope tilting and panning sequence in benchtop settings.
Magnetic anchored and steered endoscope
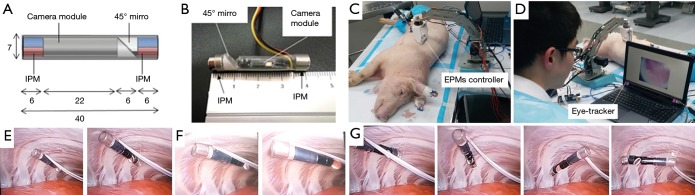
Aiming to improve the design of endoscope for VATS application (28), we seek to combine the benefits described by previous systems (25,26), while addressing their respective limitations. Both the soft MAGS endoscope and Garbin’s design (25) have hemispheric workspace. We adopt a structural design similar to Liu’s magnetic actuated capsule (26), placing an IPM at either ends of the capsule, anchoring the capsule lengthwise and using a 45° mirror to redirect the camera view. However, the previous camera capsule design (26) is 68 mm long, thus its panning motion is unfeasible on the chest wall. Considering this, we adopt the magnetic actuation strategy of Garbin’s camera (25), where relative rotations between diametrically magnetized IPM and EPM pairs control tilting DOF. This strategy eliminates the need of a dedicated actuation magnet, and allows a short capsule (40 mm) to be used (the design schematics are shown in Figure 4A). As such, our new design offers a workspace close to the anchoring surface, and is sufficiently compact to operate on curved chest wall. Figure 4B shows a prototype of the new design. A wired camera is used to improve the framerate, and provide power for unlimited operation duration. Thin wires (0.3 mm in diameter each) were selected for powering and signal transmission, to minimize the effect of tether on endoscope actuation. Compared to soft MAGS endoscope, the new prototype is lightweight (3.6 g), compact (40 mm long, 7 mm in diameter), and has full range of tilting and panning (360°). Using two pieces of smaller EPMs (22 mm diameter, 20 mm long), the anchoring range is 40 mm, sufficient to cover chest wall thickness of an average patient. For obese patients, the anchoring range can reach 80 mm by using two pieces of larger EPMs (30 mm diameter, 60 mm long) instead.
Figure 4.
Magnetic anchored and steered endoscope. (A) Schematics of endoscope design; (B) endoscope prototype, with transparent capsule revealing inner components; (C,D) set-up for evaluation in porcine cadaver. EPMs controller shown in (C), and eye-tracker with display screen in (D); (E,F,G) endoscope actuation inside cadaver, including translation (E), tilting (F), and panning (G), as observed by standard laparoscope.
To facilitate operation of the endoscope, a hands-free control using eye-gaze tracking signals and a motorized EPMs controller was implemented. The EPMs controller is assembled by docking a 2 DOF end effector on a robotic arm (DOBOT). For tilting DOF, the end effector rotates EPMs along their length with a pulley mechanism. For panning DOF, the pulley mechanism is docked onto another stepper motor, rotating along an orthogonal axis. This design ensures a minimum of 65 mm separation between the EPMs and the motors, to avoid magnetic interference. An eye-tracking component (SMI REDn Scientific, iMotions, Boston, USA) measures the surgeon’s gaze point on a screen streaming video from the endoscope. When surgeon’s gaze point is close to top or bottom edge of the screen, tilting motion is triggered to place surgeon’s target at center of view. Likewise, when gaze point is near left or right edge, panning motion is initiated. Panning and tilting motions are terminated when surgeon’s gaze falls back to screen center. With this control scheme, a surgeon can control the endoscope view direction intuitively, without communicating to another personnel, and still has both hands to operate other instruments. The system was evaluated in a porcine cadaver, where feasibility of the new endoscope design and the eye-tracking control scheme were demonstrated (Figure 4C,D). Endoscope translation, tilting and panning functions are shown in Figure 4E,F,G respectively.
Conclusions
VATS have seen substantial development in the past two decades. With uniportal VATS becoming more common, innovations in instruments that complement the advancements of surgical techniques is in ever greater demand. The challenges of trocar crowding and instrument interference seen in uniportal VATS are being met by the development of two models of magnetic anchored and steered endoscopes with tailored applications in thoracic surgery. Both designs are motor-free and actuated by magnetic interaction. The soft bodied MAGS endoscope has a wireless camera module, and has a silicone rubber formed body to safely interact with bodily tissues. While its prototype performs well in a porcine cadaver, this design’s shortfall is the low framerate, limited tilting range, and a relatively large vertical workspace footprint that is less compatible with VATS applications. Addressing these issues, we referenced Garbin’s camera and Liu’s magnetic actuated capsule to design an endoscope that combines the strengths of the two (25,26). The new design is lightweight, compact, and has a low workspace profile more compatible with uniportal VATS. The addition of an eye-tracking control scheme allows a solo surgeon to intuitively operate the endoscope. Although the new designs offer many benefits over conventional rod lens and current flexible endoscopic systems, a number of other features need to be added for improved applicability in the clinical setting. Features being considered for incorporation include; a hands-free control of endoscope translation, an in-built smoke evacuation and lens cleaning system, a reliable camera sterilization procedure, and a non-ferromagnetic instrument set for deploying and operating along the magnetic endoscope. Conquering these challenges will be paramount for the future of magnetic anchored and steered endoscopes in VATS.
Acknowledgements
Funding: This work was supported by the Hong Kong Innovative Technology Fund (Project No. ITS/126/16) and, CUHK Direct grant for research (2015.2.011) and Chow Yuk Ho Technology Centre for Innovative Medicine (Project No. TIMSG 15/16-2).
Footnotes
Conflicts of Interest: The authors have no conflicts of interests to declare.
References
- 1.Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52; discussion 1252-3. 10.1016/0003-4975(93)90661-Z [DOI] [PubMed] [Google Scholar]
- 2.Lewis RJ, Caccavale RJ, Sisler GE. Imaged thoracoscopic lung biopsy. Chest 1992;102:60-2. 10.1378/chest.102.1.60 [DOI] [PubMed] [Google Scholar]
- 3.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. 10.1016/j.athoracsur.2005.07.078 [DOI] [PubMed] [Google Scholar]
- 4.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson SJ, Herndon JE, 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802–a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. 10.1200/JCO.2007.12.6649 [DOI] [PubMed] [Google Scholar]
- 6.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. 10.1016/j.jtcvs.2009.04.026 [DOI] [PubMed] [Google Scholar]
- 7.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg 2009;88:1093-9. 10.1016/j.athoracsur.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 8.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. 10.1016/j.jtcvs.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 9.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons database analysis. Ann Surg 2012;256:487-93. 10.1097/SLA.0b013e318265819c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng CS, Wong RH, Lau RW, et al. Single port video-assisted thoracic surgery: advancing scope technology. Eur J Cardiothorac Surg 2015;47:751. 10.1093/ejcts/ezu236 [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Oo MZ, Nalam V, et al. Design of a novel flexible endoscope—cardioscope. J Mech Robot 2016;8:051014 10.1115/1.4032272 [DOI] [Google Scholar]
- 12.Li Z, Ng CS. Future of uniportal video-assisted thoracoscopic surgery—emerging technology. Ann Cardiothorac Surg 2016;5:127-32. 10.21037/acs.2016.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng CS, Kim HK, Wong RH, et al. Single port video-assisted thoracoscopic major lung resections: experience with 150 consecutive cases. Thorac Cardiovasc Surg 2016;64:348-53. 10.1055/s-0034-1396789 [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Rivas D, Yang Y, Ng C. Advances in uniportal video-assisted thoracoscopic surgery: pushing the envelope. Thorac Surg Clin 2016;26:187-201. 10.1016/j.thorsurg.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 15.Ng CSH, He JX, Rocco G. Innovations and technologies in thoracic surgery. Eur J Cardiothorac Surg 2017;52:203-5. 10.1093/ejcts/ezx192 [DOI] [PubMed] [Google Scholar]
- 16.Park S, Bergs RA, Eberhart R, et al. Trocar-less instrumentation for laparoscopy: magnetic positioning of intraabdominal camera and retractor. Ann Surg 2007;245:379-84. 10.1097/01.sla.0000232518.01447.c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadeddu J, Fernandez R, Desai M, et al. Novel magnetically guided intra-abdominal camera to facilitate laparoendoscopic single-site surgery: initial human experience. Surg Endosc 2009;23:1894-9. 10.1007/s00464-009-0459-6 [DOI] [PubMed] [Google Scholar]
- 18.Raman JD, Bergs RA, Fernandez R, et al. Complete transvaginal NOTES nephrectomy using magnetically anchored instrumentation. J Endourol 2009;23:367-71. 10.1089/end.2008.0220 [DOI] [PubMed] [Google Scholar]
- 19.Best SL, Bergs R, Scott DJ, et al. Solo surgeon laparoendoscopic single site nephrectomy facilitated by new generation magnetically anchored and guided systems cameras. J Endourol 2012;26:214-8. 10.1089/end.2011.0143 [DOI] [PubMed] [Google Scholar]
- 20.Yin G, Han WK, Faddegon S, et al. Laparoendoscopic single site (LESS) in vivo suturing using a magnetic anchoring and guidance system (MAGS) camera in a porcine model: impact on ergonomics and workload. Urology 2013;81:80-4. 10.1016/j.urology.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 21.Terry BS, Mills ZC, Schoen JA, et al. Single-port-access surgery with a novel magnet camera system. IEEE Trans Biomed Eng 2012;59:1187-93. 10.1109/TBME.2012.2187292 [DOI] [PubMed] [Google Scholar]
- 22.Simi M, Pickens R, Menciassi A, et al. Fine tilt tuning of a laparo-scopic camera by local magnetic actuation: two port nephrectomy experience on human cadavers. Surg Innov 2013;20:385-94. 10.1177/1553350612462458 [DOI] [PubMed] [Google Scholar]
- 23.Tortora G, Dario P, Menciassi A. Array of robots augmenting the kinematics of endo-cavitary surgery. IEEE ASME Trans Mechatron 2014;19:1821-9. 10.1109/TMECH.2013.2296531 [DOI] [Google Scholar]
- 24.Garbin N, Di Natali C, Buzzi J, et al. Laparoscopic tissue retractor based on local magnetic actuation. J Med Devices 2015;9:011005 10.1115/1.4028658 [DOI] [Google Scholar]
- 25.Garbin N, Slawinski PR, Aiello G, et al. Laparoscopic camera based on an orthogonal magnet arrangement. IEEE Robot Autom Lett 2016;1:924-9. 10.1109/LRA.2016.2528303 [DOI] [Google Scholar]
- 26.Liu X, Mancini GJ, Tan J. Design and analysis of a magnetic actuated capsule camera robot for single incision laparoscopic surgery. In: Proceedings of 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). 2015: Hamburg, Germany. New York: IEEE, 2015:229-35. [Google Scholar]
- 27.Cheng T, Ng CS, Chiu P, et al. Design and Prototyping of a Soft Magnetic Anchored and Guidance Endoscope System. In Proceedings of 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). 2017: Vancouver, BC, Canada. New York: IEEE, 2017:2903-8. [Google Scholar]
- 28.Cheng T, Zhang X, Ng CS, et al. A novel magnetic anchored and steered camera robot for single port access surgery. In: Proceedings of 2018 IEEE International Conference on Robotics and Automation (ICRA). [In press]. [Google Scholar]