Abstract
Background
Percutaneous endoscopic lumbar discectomy (PELD) is a relatively less invasive treatment for lumbar disc herniation (LDH). The present study focused on the transforaminal approach (TFA) and investigated the significance of PELD via this approach for large central LDH.
Methods
LDH that did not show cerebrospinal fluid (CSF) on axial T2-weighted magnetic resonance images was defined as large central LDH. PELD via the TFA was performed in 11 patients with large central LDH. Pre- and post-operative statuses were evaluated using the modified Japanese Orthopedic Association (mJOA) and Numerical Rating Scale (NRS) scores.
Results
The patients’ mean age was 44.1 years; there was single-level involvement, mostly at L4/5 (seven cases). The mean recovery rate of the mJOA score was 48.7%, and mean pre- and post-operative NRS scores were 7.1 and 1.5, respectively. The mean operative time was 38.1 min. Although there were no major complications, the dura was accidentally punctured at the initial operative step for discography in one case. LDH recurred in one case at 5 months after the operation, and the patient was treated by PELD via the TFA on the contralateral side.
Conclusions
The TFA for PELD is a safe, minimally invasive, effective treatment for large central LDH. However, the operator should pay attention to malpositioning of the flat and laterally expanded dural sac.
Keywords: Percutaneous endoscopic lumbar discectomy (PELD), large central lumbar disc herniation (LDH), transforaminal approach (TFA), complication, minimally invasive
Introduction
Percutaneous endoscopic lumbar discectomy (PELD) is one of the most sophisticated operative procedures for the treatment of lumbar disc herniation (LDH) (1-3). The operative outcomes have already been extensively reported, and the results are comparable with those of conventional operative procedures such as open discectomy (4-9). However, PELD has an anatomical limitation for endoscope insertion. Three different operative approaches that complementing each other are available for PELD—interlaminar, transforaminal, and posterolateral—of which the transforaminal approach (TFA) is the most popular.
Large central LDH is a challenge for spinal surgeons. The extent of laminectomy is larger with the conventional posterior approach than that for lateral LDH, and traction of the dural sac tends to be greater with this approach as well. Several surgeons have proposed solutions to these problems (10-14), one of which is use of the trans-dural approach. However, this approach has a potential risk of post-operative cerebrospinal fluid (CSF) leakage (11,12). Further, the bilateral approach has also been proposed as a solution (10,14), but both these procedures are highly invasive and the operative time is consequently prolonged.
From our previous experience with TFA, we concluded that large central LDH is a good indication for PELD via the TFA. Here, we summarize this experience and confirm our conclusions. We also identified the pitfalls of PELD via the TFA in this retrospective analysis. In this article, we present the operative outcomes of PELD via the TFA for large central LDH and provide useful information for prevention of complications.
Methods
Between July 2016 and April 2017, 11 consecutive patients with large central LDH underwent PELD via the TFA conducted using a 7-mm diameter spinal full-endoscopic system (Richard Wolf GmbH, Knittlingen, Germany). All patients had radiculopathy resistant to medical treatment, epidural steroid injection, and/or nerve block. To clarify the surgical benefits of PELD via the TFA, we excluded patients who had previously undergone discectomy at the same vertebral level.
For all patients, PELD via the TFA was conducted at only one vertebral level by a single surgeon (H Koga). Neurological examination, pre-operative computed tomography (CT), and magnetic resonance imaging (MRI) were used to identify the location and size of the LDH. Large central LDH was defined as herniation that did not show CSF on axial T2-weighted MRIs (Figure 1).
Figure 1.
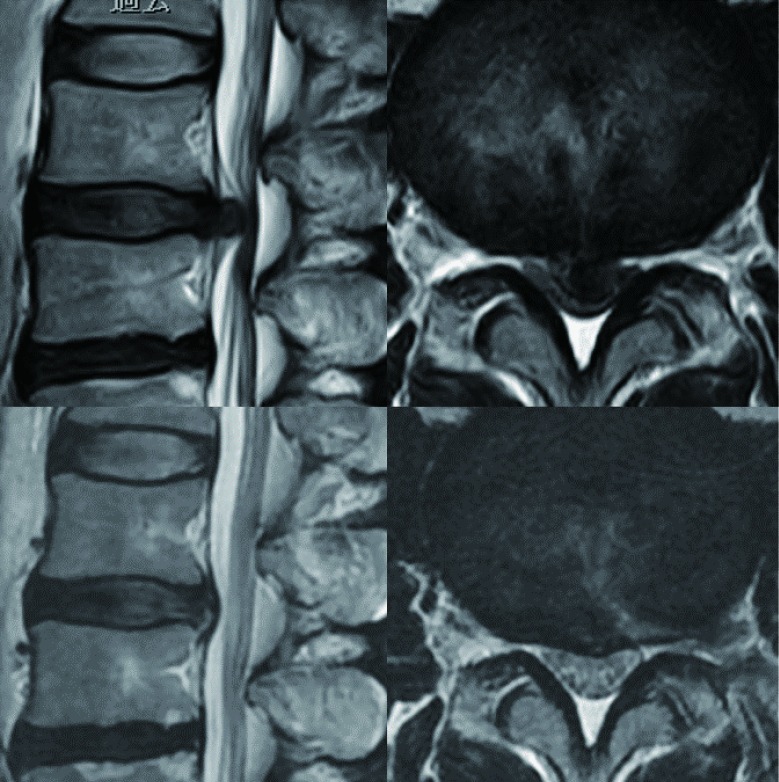
MRI findings of patients with accidental dural puncture during the initial operative step for discography. Pre-operative and post-operative sagittal and axial T2-weighted magnetic resonance images. MRI, magnetic resonance imaging.
Patients were followed up for an average of 8.7 months (range, 5–15 months) postoperatively. Pre- and post-operative neurological statuses were evaluated using the modified Japanese Orthopedic Association (mJOA) score. The recovery rate was determined as follows: recovery rate = post-operative mJOA − pre-operative mJOA/23 (full score) – pre-operative mJOA score × 100. Corresponding leg pain was also evaluated using the Numerical Rating Scale (NRS) score. Statistical analysis was performed using the Student t-test. P values less than 0.05 were considered statistically significant.
Surgical technique
Patients were carefully log-rolled into the prone position. To enlarge the vertebral foramen, a pillow was placed between the operating table and anterior iliac crest. The surgery was performed with the patient under general anesthesia and simultaneous motor evoked potential (MEP) monitoring to avoid intraoperative discomfort and post-operative piriformis syndrome (15). During the operation, a fluoroscope was placed across the center of the operating table to ensure appropriate positioning. An 8-mm skin and fascia incision was made 7–10 cm lateral from the midline at the target disc level under fluoroscopic guidance, after which an 18-gauge spinal needle was inserted into the disc. Discography was performed with indigo carmine and a contrast medium in order to stain the herniated disc material. Following insertion of an obturator, a 45° angled-working sheath of diameter 7 mm was inserted. Then, an endoscope (diameter of working channel: 4.1 mm) was inserted and the annulus fibrosus, lateral part of the yellow ligament, and superior articular process (SAP) were confirmed (Figure 2). Generally, the herniated nucleus is located in the back of the annulus bulge; therefore, the approach side of the annulus was incised and the sequestered nucleus was explored using several types of forceps and dissectors (Figure 2). After complete removal, the compressed ventral surface of the dural sac or the large space between the posterior longitudinal ligament (PLL) and dural sac became visible (Figure 2). Finally, the annular tear and evacuated cavities were electrocoagulated using a bipolar radio-frequency electrode system (Elliquence, Baldwin, NY, USA), followed by careful endoscopic examination for epidural bleeding and hemostasis. After decompression, the working sheath was carefully removed, and the skin was closed with a single suture.
Figure 2.
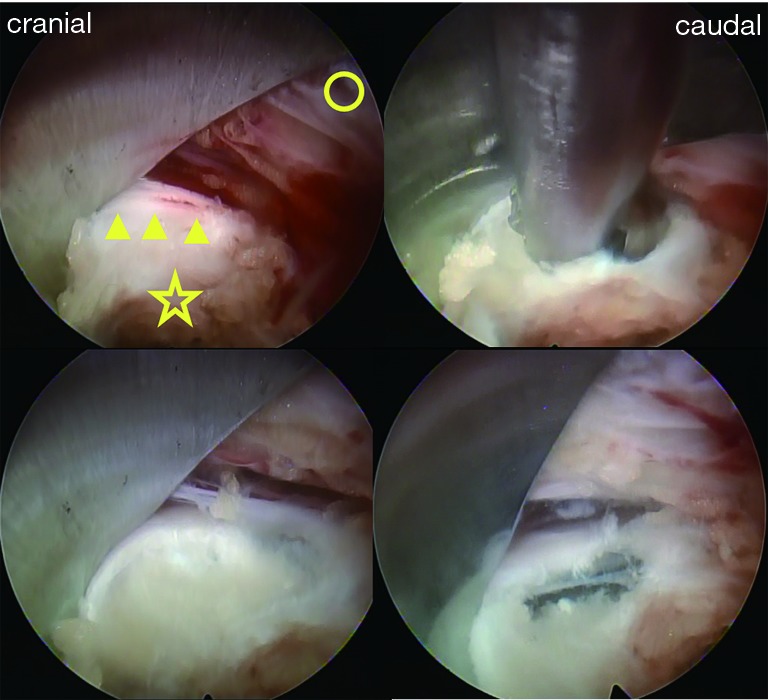
Intraoperative photographs. After reaching the vertebral foramen, we first confirmed the annulus fibrosus (☆), posterior longitudinal ligament (PLL) (arrow heads), and superior articular process (SAP) (○). After puncturing the surface of the annulus fibrosus, we removed the sequestrated nucleus underneath the PLL by using several types of forceps. Subsequently, the PLL gradually shifted to the ventral side, and large spaces were finally visible on both the dorsal and ventral sides of the PLL.
Results
Eleven patients were registered for this study; foraminoplasty was additionally conducted in two cases—an initial case and one in which foraminal stenosis co-occurred with the LDH—although it was not actually necessary for the initial case from the operative finding. Although we assumed that foraminoplasty might help to remove large LDH for the initial case, it was not indicated. Subsequently, we have not generally performed foraminoplasty for the treatment of large LDH.
The mean patient age was 44.1 years (range, 22–72 years), and the male/female ratio was 7/4. The most affected vertebral level was L4/5 (seven cases), followed by L3/4 (four cases). Especially for large LDH, definitive determination of the right or left location is relatively difficult from radiological scans. Therefore, we determined the side on which to conduct PELD via the TFA from the level corresponding to the most severe radiculopathy (Table 1). In one case (No. 7), in which the patient experienced severe bilateral pain and muscle weakness, we performed PELD on the left side, since the axial MRI clearly showed left-sided distribution. The mean operative time was 38.1 min (range, 27–58 min), and the mean volume of the nucleus pulposus removed was 2.1 g (range, 1.1–4.0 g); the mean blood loss was negligible in all patients (Table 1). The mean post-operative hospital stay was 3.5 days (range, 2–13 days); it was particularly long in case No. 7 because of the severe bilateral muscle weakness (Table 1).
Table 1. Summary of the detailed features of the 11 cases of large central LDH.
| Case No. | Sex | Age (years) | Chief complaint | Gait disturbance | Muscle weakness | Disc level | Spinal canal stenosis | Approach side | Skin incision** (cm) | Foraminoplasty | Operative time (min) | Removed volume (g) | Hospital stay (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 22 | Left leg pain | (+) | (−) | L3/4 | (−) | L | 7 | (+) | 33 | 1.6 | 3 |
| 2 | F | 31 | Right leg pain | (+) | EHL 4/5 | L4/5 | (−) | R | 7 | (−) | 38 | 4.0 | 2 |
| 3 | F | 36 | Left leg pain | (−) | EHL 4/5 | L4/5 | (−) | L | 7.5 | (−) | 41 | 1.9 | 3 |
| 4 | M | 32 | Right leg pain | (−) | EHL 4/5 | L4/5 | (−) | R | 9 | (−) | 27 | 2.9 | 3 |
| 5 | M | 72 | Bilateral leg pain | (−) | (−) | L3/4 | (+) | R | 7.5 | (−) | 55 | 1.1 | 2 |
| 6 | F | 29 | Right leg numbness | (−) | EHL 1/5 | L4/5 | (−) | R | 9 | (−) | 58 | 2.4 | 2 |
| 7 | M | 66 | Bilateral leg pain | (+) | TA 1/3 | L3/4 | (−) | L | 8 | (−) | 37 | 2.0 | 13 |
| 8 | F | 67 | Right leg pain | (+) | (−) | L4/5 | (+) | R | 8 | (+) | 27 | 1.1 | 3 |
| 9 | M | 47 | Right leg pain | (−) | Gastro 2/2 | L4/5 | (−) | R | 9 | (−) | 29 | 1.5 | 2 |
| 10 | M | 42 | Left leg pain | (+) | (−) | L3/4 | (−) | L | 8 | (−) | 38 | 2.3 | 3 |
| 11 | M | 41 | Left leg pain | (−) | (−) | L4/5 | (−) | L | 10 | (−) | 36 | 2.8 | 2 |
**, the distance from the midline to the skin incision; +, presence; −, absence. EHL, extensor hallucis longus; TA, tibialis anterior; Gastro, gastrocnemius; LDH, lumbar disc herniation; M, male; F, female; L, left; R, right.
The mJOA score improved significantly from 8.3±3.58 to 16.8±2.85, and the recovery rate of this score was 48.7%±29.0%. The NRS scores also improved significantly from 7.1±1.68 to 1.5±1.49 (Table 2). Because of the severe pain and muscle weakness caused by large central LDH, patients experience gait disturbance (16). In the current case series, we also observed muscle weakness and/or gait disturbance in nine cases. Except one patient (No. 7), all the rest were discharged without any walking assistance. Even in case No. 7, the patient showed improved muscle weakness and could visit our out-patient clinic by walking without assistance after 1 month.
Table 2. Surgical outcome of 11 cases.
| Evaluation methods | Average | SD | P value |
|---|---|---|---|
| Follow-up period (months) | 8.7 | 2.8 | − |
| mJOA score | |||
| Pre-operative (months) | 8.3 | 3.58 | <0.01 |
| Post-operative (months) | 16.8 | 2.85 | |
| Recovery rate (%) | 48.7 | 29 | |
| NRS score (months) | |||
| Pre-operative | 7.1 | 1.68 | <0.01 |
| Post-operative | 1.5 | 1.49 | |
Recovery rate = post-operative mJOA − pre-operative mJOA/23 (full score) − pre-operative mJOA score × 100. SD, standard deviation; mJOA, modified Japanese Orthopedic Association; NRS, Numerical Rating Scale.
During the follow-up period (range, 5–15 months; average, 8.7 months), LDH recurred in only one case, that of the oldest patient (case No. 5; 72-year-old patient) who also had spinal canal stenosis (SCS). This patient underwent a second PELD from the contralateral side 5 months after the first operation. We observed no major complications in this case series, although in one case (case No. 10) (Figure 1), the dura was accidentally punctured during the initial operative step for discography. The operation, however, was unremarkable. The post-operative course was normal, and the patient was discharged from hospital 3 days after the operation.
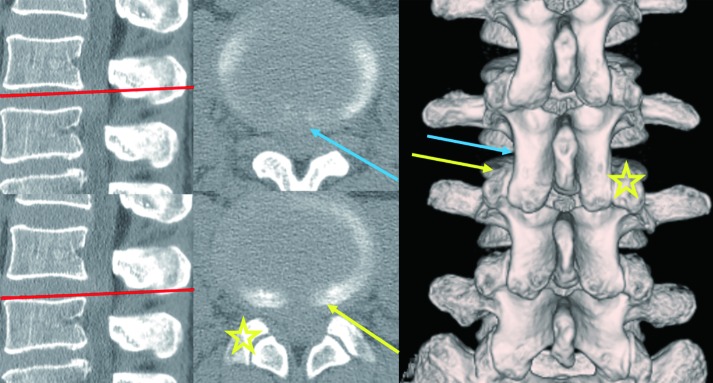
As we perceived the malpositioning of the needle puncture at the initial stage of the operation, we changed the trajectory of the needle toward the caudal side and confirmed that it touched the lateral surface of SAP, after which it was adequately introduced into the vertebral disc (Figure 3). We did not observe dorsal displacement of the dural sac even during the operation (Figure 2). Therefore, we speculated that we had punctured the laterally expanded dural sac underneath the lateral part of the yellow ligament.
Figure 3.
Pre-operative CT finings of patients with accidental dural puncture during the initial operative step for discography. Sagittal, axial, and three-dimensionally reconstructed CT images. The red lines indicate the scanning positions; the blue arrow indicates the first needle trajectory for accidental dural puncture; the yellow arrow indicates the corrected needle trajectory for appropriate PELD via the TFA; the ☆ sign indicates the SAP of the contralateral side. Note that cranial needle puncture is easier to reach the medial site of the annulus fibrosus. CT, computed tomography; PELD, percutaneous endoscopic lumbar discectomy; TFA, transforaminal approach; SAP, superior articular process.
Discussion
Large central LDH at low vertebral levels is the most common cause for cauda equina syndrome (CES), which is characterized by severe lower back pain, sciatica (often bilateral), saddle and/or genital sensory disturbance, bladder, bowel dysfunction, and sexual dysfunction (16). Surgical treatment delayed beyond 48 h leads to persistent bladder, bowel, and sexual dysfunction (17). Fortunately, we did not experience CES in the present series, although case No. 7 was essentially identical to CES. The patient’s bilateral paresis persisted but was completely eliminated after 1 month.
In cases of large central LDH, although the timing of the operation is vital, the operative procedure is also important in order to ensure a good prognosis. Previously used posterior approaches require several types of laminectomy and partial facetectomy for removal of large central LDH (18). Such procedures cause post-operative instability, and fusion operation may occasionally be required. Although some surgeons advocate the trans-dural approach, this approach has the potential disadvantage of post-operative CSF leakage (11,12). Further, these procedures are not only highly invasive but also requiring the prolonged operative time.
The significance of PELD for large central LDH remains controversial. Choi et al. analyzed their experience of 10,228 cases and reported that the frequency of incomplete removal of LDH was high in the case of central LDH (19). On the other hand, Lee et al. reported good operative outcomes for six consecutive patients who presented with back and leg pain and/or weakness due to large central LDH and were treated using percutaneous endoscopic herniotomy via a unilateral intra-annular subligamentous approach (20). Li et al. also reported good operative outcomes in 16 consecutive patients with CES who underwent PELD (18). In the present article, we summarized 11 cases of large central LDH treated by PELD via the TFA. The operative outcomes were not inferior compared to previous outcomes of surgery for LDH (21-23). The surgeon can directly confirm the protruded annulus fibrosus under endoscopic visualization and can confirm the presence of an extruded nucleus under the annulus fibrosus by touching and on the basis of fluoroscopic imaging (Figure 2). Although special training under a skilled supervisor is required for surgeons to perform this surgery, PELD via the TFA is a promising surgical procedure for large central LDH.
We have to carefully consider the operative indication for large central LDH combined with SCS. In general, SCS occurs in elderly patients and is frequently co-occurs with foraminal stenosis and a decrease in vertebral height, which results in a narrow working space at the vertebral foramen and prevents endoscopic manipulation. Case No. 5 was one such case; the removal was insufficient and the symptoms recurred early after the operation. For this patient, we should have beforehand performed sufficient foraminoplasty using a high-speed drill, or we should have combined the wide extent of laminectomy. Recently, several authors have emphasized the significance of foraminoplasty (24,25). Even during the operation, if considered necessary, foraminoplasty should definitely be performed to enlarge the working space.
The advantage of the TFA is that it ensures safety in “Kambin’s triangle” (26). This triangle is not completely safe in the case of large central LDH, because the dural sac becomes flat and laterally expanded because of the extreme compression caused by the LDH. If the needle is introduced more cranially, the trajectory of the needle puncture draws along with the lateral aspect of the inferior articular process (IAP). Thus, the dural sac may get punctured. We also encountered one such case (case No. 10). Further, since the lateral aspect of the SAP is not hypertrophic and smoothly transfers to the lateral aspect of the IAP, the trajectory of the needle puncture can easily be drawn more medially even along the lateral aspect of the SAP. To avoid injuries to the dura or the underlying cauda equina, the “walking technique” is very important, in which the operator confirms the anatomical structures (lateral surfaces of the IAP and SAP, the caudal pedicle, and annulus fibrosus) in a stepwise manner using the tip of the needle (1). Since the initial operative step of the needle puncture involves blind manipulation, fluoroscopic imagining in both lateral and anteroposterior views can help confirm the needle’s position. Especially for higher vertebral levels, it must be kept in mind that the lateral aspect of the IAP draws nearer to the dural sac.
PELD is a recently developed surgical technique that has the advantages of requiring a small incision size (8 mm), rapid recovery, short hospital stay, limited blood loss, less destruction of the surrounding tissues, and less post-operative pain. Nevertheless, some PELD-specific problems do exist. Especially for large central LDH, the co-occurrence of SCS or foraminal stenosis is one of the reasons for incomplete removal. The possibility of inappropriate puncture of the dural sac in the initial stage of PELD is another pitfall of this procedure. After overcoming these problems, PELD should be the first line of treatment for large central LDH.
Conclusions
The preliminary results obtained during our short follow-up period show that PELD via the TFA is feasible for the treatment of large central LDH. However, the operator should pay attention to malpositioning of the flat and laterally expanded dural sac in order to avoid dural and cauda equina injuries.
Acknowledgements
We would like to thank all the operating room staff for their technical assistance, and the medical records clerks who helped collect patient data. We would also like to thank all radiological department staff for recording CT and MRI data. This work was partly supported by a grant from the Iwai Medical Foundation.
Ethical Statement: This study was approved by ethics committee of the Iwai Medical Foundation, and informed consent was obtained from the patients for publication of this study and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Sairyo K, Egawa H, Matsuura T, et al. State of the art: Transforaminal approach for percutaneous endoscopic lumbar discectomy under local anesthesia. J Med Invest 2014;61:217-25. 10.2152/jmi.61.217 [DOI] [PubMed] [Google Scholar]
- 2.Choi G, Lee SH, Deshpande K, et al. Working channel endoscope in lumbar spine surgery. J Neurosurg Sci 2014;58:77-85. [PubMed] [Google Scholar]
- 3.Yeung AT. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon’s Experience Incorporating Adjunctive Techologies. SAS J 2007;1:108-17. 10.1016/S1935-9810(07)70055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn SS, Kim SH, Kim DW, et al. Comparison of Outcomes of Percutaneous Endoscopic Lumbar Discectomy and Open Lumbar Microdiscectomy for Young Adults: A Retrospective Matched Cohort Study. World Neurosurg 2016;86:250-8. 10.1016/j.wneu.2015.09.047 [DOI] [PubMed] [Google Scholar]
- 5.Chen HC, Lee CH, Wei L, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar surgery for adjacent segment degeneration and recurrent disc herniation. Neurol Res Int 2015;2015:791943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi KC, Park CK. Percutaneous Endoscopic Lumbar Discectomy for L5-S1 Disc Herniation: Consideration of the Relation between the Iliac Crest and L5-S1 Disc. Pain Physician 2016;19:E301-8. [PubMed] [Google Scholar]
- 7.Sinkemani A, Hong X, Gao ZX, et al. Outcomes of Microendoscopic Discectomy and Percutaneous Transforaminal Endoscopic Discectomy for the Treatment of Lumbar Disc Herniation: A Comparative Retrospective Study. Asian Spine J 2015;9:833-40. 10.4184/asj.2015.9.6.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadjradj PS, van Tulder MW, Dirven CM, et al. Clinical outcomes after percutaneous transforaminal endoscopic discectomy for lumbar disc herniation: a prospective case series. Neurosurg Focus 2016;40:E3. 10.3171/2015.10.FOCUS15484 [DOI] [PubMed] [Google Scholar]
- 9.Sencer A, Yorukoglu AG, Akcakaya MO, et al. Fully endoscopic interlaminar and transforaminal lumbar discectomy: short-term clinical results of 163 surgically treated patients. World Neurosurg 2014;82:884-90. 10.1016/j.wneu.2014.05.032 [DOI] [PubMed] [Google Scholar]
- 10.Bärlocher CB, Krauss JK, Seiler RW. Central lumbar disc herniation. Acta Neurochir (Wien) 2000;142:1369-74; discussion 1374-5. 10.1007/s007010070007 [DOI] [PubMed] [Google Scholar]
- 11.Choi JW, Lee JK, Moon KS, et al. Transdural approach for calcified central disc herniations of the upper lumbar spine. J Neurosurg Spine 2007;7:370-4. 10.3171/SPI-07/09/370 [DOI] [PubMed] [Google Scholar]
- 12.Kim DS, Lee JK, Jang JW, et al. Clinical Features and Treatments of Upper Lumbar Disc Herniations. J Korean Neurosurg Soc 2010;48:119-24. 10.3340/jkns.2010.48.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon KH, Lee SH, Kong BJ, et al. An Oblique Paraspinal Approach for Intracanalicular Disc Herniations of the Upper Lumbar Spine: Technical Case Report. Operative Neurosurgery 2006;59:ONSE487-8; discussion ONSE488. [DOI] [PubMed]
- 14.Choi KC, Kim JS, Park CK. Percutaneous Endoscopic Lumbar Discectomy as an Alternative to Open Lumbar Microdiscectomy for Large Lumbar Disc Herniation. Pain Physician 2016;19:E291-300. [PubMed] [Google Scholar]
- 15.Kim JE, Kim KH. Piriformis syndrome after percutaneous endoscopic lumbar discectomy via the posterolateral approach. Eur Spine J 2011;20:1663-8. 10.1007/s00586-011-1764-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner A, Gardner E, Morley T. Cauda equina syndrome: a review of the current clinical and medico-legal position. Eur Spine J 2011;20:690-7. 10.1007/s00586-010-1668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleave JR, Macfarlane R. Cauda equina syndrome: what is the relationship between timing of surgery and outcome? Br J Neurosurg 2002;16:325-8. 10.1080/0268869021000032887 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Dou Q, Hu S, et al. Treatment of cauda equina syndrome caused by lumbar disc herniation with percutaneous endoscopic lumbar discectomy. Acta Neurol Belg 2016;116:185-90. 10.1007/s13760-015-0530-0 [DOI] [PubMed] [Google Scholar]
- 19.Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery 2015;76:372-80; discussion 380-1; quiz 381. 10.1227/NEU.0000000000000628 [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Choi KC, Baek OK, et al. Percutaneous Endoscopic Intra-annular Subligamentous Herniotomy for Large Central Disc Herniation. Spine (Phila Pa 1976) 2014;39:E473-9. 10.1097/BRS.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 21.Kang Q, Li X, Cheng Z, et al. Effects of release and decompression techniques on nerve roots through percutaneous transforaminal endoscopic discectomy on patients with central lumbar disc herniation. Exp Ther Med 2017;13:2927-33. 10.3892/etm.2017.4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan K, Xu J, Schultz K, et al. Full-endoscopic versus micro-endoscopic and open discectomy: A systematic review and meta-analysis of outcomes and complications. Clin Neurol Neurosurg 2017;154:1-12. 10.1016/j.clineuro.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Ran J, Hu Y, Zheng Z, et al. Comparison of Discectomy versus Sequestrectomy in Lumbar Disc Herniation: A Meta-Analysis of Comparative Studies. PLoS One 2015;10:e0121816. 10.1371/journal.pone.0121816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KC, Shim HK, Park CJ, et al. Usefulness of Percutaneous Endoscopic Lumbar Foraminoplasty for Lumbar Disc Herniation. World Neurosurg 2017;106:484-92. 10.1016/j.wneu.2017.07.035 [DOI] [PubMed] [Google Scholar]
- 25.Li ZZ, Hou SX, Shang WL, et al. Modified Percutaneous Lumbar Foraminoplasty and Percutaneous Endoscopic Lumbar Discectomy: Instrument Design, Technique Notes, and 5 Years Follow-up. Pain Physician 2017;20:E85-98. [PubMed] [Google Scholar]
- 26.Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res 1986;(207):37-43. [PubMed] [Google Scholar]