Abstract
Disorders of the pericardium represent a diverse range of conditions that traditionally may not have received the same level of attention by cardiologists and physicians, owing partly to a lack of research into advanced diagnostic modalities, and limited, evidence-based treatment options. In recent years, there has been a timely resurgence of interest in pericardial diseases, in particular pericarditis. This is attributable to advances in multimodality cardiovascular imaging, in particular cardiac magnetic resonance (CMR), which may help guide treatment decisions for patients with pericardial syndromes. Additionally, increased research and understanding of the pathophysiological basis of pericarditis have shed light on the role of inflammation in pericarditis. This knowledge may help identify potential specific treatment targets. This article aims to provide a practical review of the role of multimodality cardiovascular imaging (echocardiography, multi-detector cardiac computed tomography (MDCT), CMR) in pericardial conditions, focusing on the strengths and potential limitations of each imaging modality.
Keywords: echocardiography, multi-detector cardiac computed tomography, cardiac magnetic resonance imaging, pericardium
Introduction
Disorders of the pericardium include a diverse spectrum of clinical conditions, ranging from common conditions such as acute pericarditis, to rare entities, such as congenital absence of the pericardium (1, 2). Multimodality cardiovascular imaging plays vital roles in the diagnosis and management of these pericardial conditions, providing structural, functional and hemodynamic characteristics (3, 4).
This article aims to provide a practical review of the role of multimodality cardiovascular imaging (echocardiography, multi-detector cardiac computed tomography (MDCT), CMR) in pericardial conditions, focusing on the strengths and potential limitations of each imaging modality.
Echocardiography
Echocardiography is the mainstay first-line imaging investigation for the investigation of patients with suspected pericardial disorders (Table 1). The strengths of transthoracic echocardiography (TTE) include its wide availability, portability and relative low cost. In patients with good imaging windows, TTE can be performed with good spatial resolution. TTE should be the first cardiac imaging investigation for patients with suspected acute pericarditis, although it should be emphasized that the diagnosis of acute pericarditis is mainly based on clinical criteria, including the presence of two or more of the following: pleuritic chest pain, pericardial friction rub and classic electrocardiographic changes including diffuse, concave upward ST-segment elevation and PR depression (3, 4, 5, 6).
Table 1.
The strengths and limitations of the mainstay cardiovascular imaging investigations used for the investigation of patients with suspected pericardial disorders.
| Cardiac imaging modality | Transthoracic echocardiography | Multi-detector cardiac computed tomography | Cardiac magnetic resonance imaging |
|---|---|---|---|
| Strengths | • First-line imaging investigation in the investigation of suspected pericardial conditions • Widely available • Portable with ability to acquire images at the bedside and during an emergency • Relatively low cost • Functional hemodynamic assessment • Respirometry available for respire-phasic assessment • Transesophageal echocardiography can be used as an adjunct imaging modality |
• Assessment of the location and extent of pericardial calcification • Excellent anatomical characterization • Evaluation of extra-cardiac abnormalities • Pre-operative planning • If indicated, respirophasic assessment may be performed with dynamic four-dimensional (4D) imaging |
• Adjunct imaging modality for superior anatomic delineation of the pericardium • Superior tissue characterization • Ability to assess for pericardial inflammation (pericardial edema and delayed gadolinium enhancement) |
| Limitations/weaknesses | • Quality of imaging data is operator dependent • Diagnostic performance can be limited by limited, and suboptimal ultrasound imaging windows (e.g. emphysema, obesity, supine positioning) • Relatively narrow field of view • Low signal-to-noise ratio of the pericardium • Limited tissue characterization ability |
• Ionizing radiation • Iodinated contrast • Functional evaluation only possible with retrospectively gated 4D studies (higher radiation dose) • Imaging acquisition affected by arrhythmias and ectopy • Need for breath-hold • Patients must be hemodynamically stable |
• High cost • Limited availability • Calcification cannot be assessed fully • Delayed-enhancement imaging with gadolinium contrast relatively contraindicated in patients with severe renal impairment (glomerular filtration rate<30 mL/min) • Patients must be hemodynamically stable |
Reprinted from Journal of the American Society of Echocardiography, vol 26, Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, Hung J, Garcia MJ, Kronzon I & Oh JK, American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography, pages 965.e15–1012.e15, Copyright (2013), with permission from Elsevier.
TTE, in the setting of suspected acute pericarditis, provides confirmatory evidence of the diagnosis, when a new or increasing pericardial effusion is detected in the appropriate clinical situation (2, 5). Most commonly, in acute pericarditis, no significant abnormalities are noted on TTE (1, 7). However, for patients presenting with an acute chest pain syndrome, of which acute pericarditis is a possible differential diagnosis, the assessment of regional wall motion can help differentiate and exclude ST-segment elevation myocardial infarction (8). This may translate into a reduction in unnecessary invasive coronary angiography (9). In addition, TTE may play a role in detecting impaired systolic function in cases of perimyocarditis with the involvement of the myocardium (2).
Hemodynamic assessment obtained by TTE can help stratify patients with acute pericarditis into those with, and those without high-risk features, including the presence of tamponade physiology, which has been reported in 3% of patients presenting with acute pericarditis (Table 2) (3). Additionally, there may be clues on TTE that point toward early features of constrictive pathophysiology, such as an abnormal diastolic septal bounce.
Table 2.
The role of cardiac imaging in the diagnosis and management of the different clinical stages of pericarditis.
| Clinical stages of pericarditis with imaging and treatment considerations | |||||
|---|---|---|---|---|---|
| Stages of pericarditis | Acute | First recurrence | Multiple recurrences | Colchicine-resistant or steroid dependent | Constrictive |
Imaging
|
• Echocardiogram for pericardial effusion, myocardial involvement, constriction | • Echocardiogram for constriction • CMR in select cases for pericardial inflammation constriction |
Same as for ‘first recurrence’ | Same as for ‘first recurrence’ | Same as for ‘first recurrence’Plus possible CT for extent of calcification and preoperative planning |
Treatment
|
• NSAIDS (weeks) • Colchicine (3 months) |
• NSAIDS (weeks–months) • Colchicine (≥6 months) |
• NSAIDS • Colchicine • Prednisone (>6 months, taper steroid as tolerated) • Consider steroid sparing agents (warrants further study) |
• NSAIDS • Colchicine • Prednisone • Steroid-sparing agent (>6–12 months taper steroid as tolerated) • Consider pericardiectomy (warrants further study) |
• Intensify medical therapy if inflame • Pericardiectomy if ‘burnt out’ |
NSAIDS, non-steroidal anti-inflammatory drugs.
Reprinted from Journal of the American College of Cardiology, vol 68, Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M & Klein AL, Complicated pericarditis understanding risk factors and pathophysiology to inform imaging and treatment, pages 2311–2328, Copyright (2016), with permission from Elsevier.
Recurrent pericarditis refers to subsequent further episodes of pericarditis, after a documented initial episode of acute pericarditis, with a symptom-free period of 4–6 weeks or longer between the episodes (4). Up to as many as one-third of patients may develop recurrence after an initial episode of acute pericarditis (6, 7, 10, 11). Depending on the clinical context, TTE can be performed in recurrent pericarditis to assess for the presence of a new pericardial effusion or features of constrictive pathophysiology.
Echocardiography is also the first-line imaging investigation for patients with suspected constrictive pericarditis, having a class I recommendation in the European Society of Cardiology guidelines for the diagnosis and management of pericardial diseases (4). Accurate diagnosis can be difficult, although it is very important to make an accurate diagnosis. Patients with end-stage calcific pericardial constriction may be treated with surgical pericardiectomy, while subsets of patients with transient pericardial constriction, as well as early stages of pericardial constriction, can be treated medically. In the developed world, common etiologies for constrictive pericarditis include idiopathic or viral, post cardiac injury or radiation (12, 13). In the developing world, tuberculosis is the most common cause for the constrictive pericarditis (14).
On echocardiography, a restrictive pattern of transmitral filling profile is classic, characterized by very rapid early diastolic filling of the left ventricle, before the pericardial constraint limits further left ventricular filling from mid-diastole onwards (Fig. 1) (1, 2, 15, 16). TTE also plays a vital role in the assessment of ventricular interdependence, a hallmark of constrictive pericarditis, with M-mode, two-dimensional and Doppler imaging, as well as respirometry. Movement of the ventricular septum toward the left ventricle on inspiration, and movement of the ventricular septum toward the right ventricle on expiration can point toward ventricular interdependence on M-mode echocardiography (12). On two-dimensional imaging, diastolic septal bounce and respirophasic septal shift over several respiratory cycles support the diagnosis of ventricular interdependence.
Figure 1.
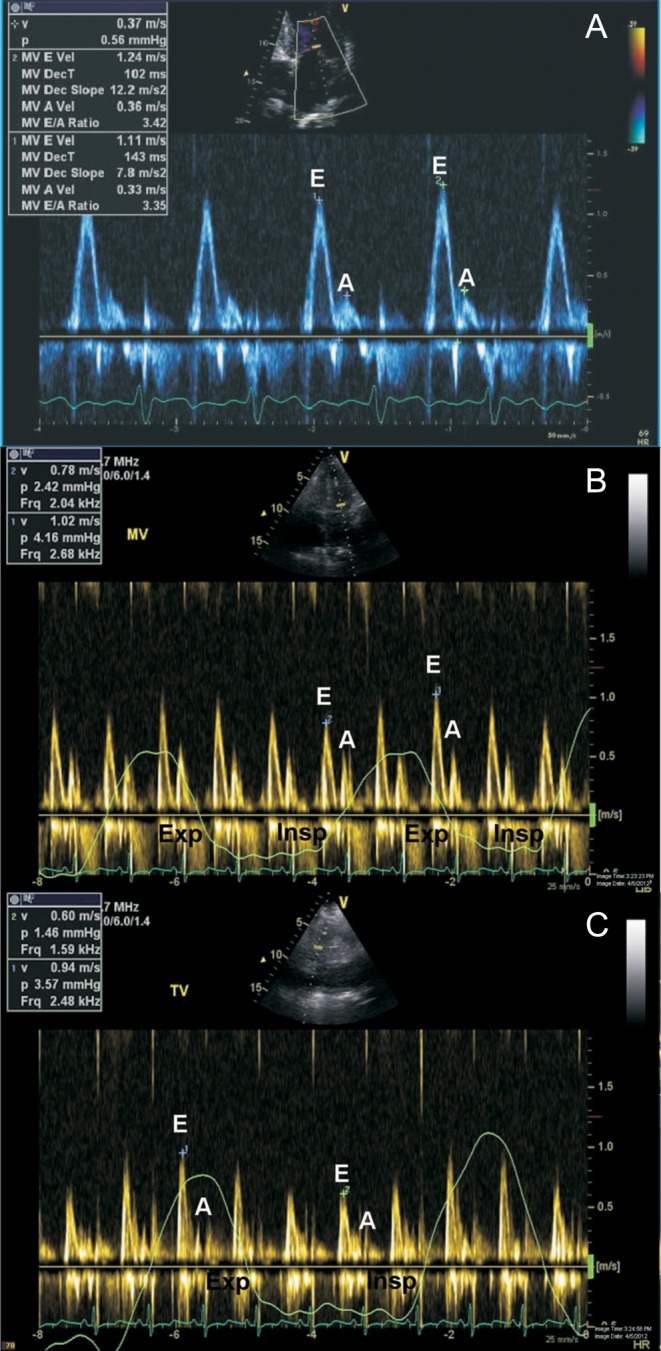
Doppler assessment in constrictive pericarditis. Classically, a restrictive pattern of transmitral inflow profile is seen in constrictive pericarditis (A). Respirophasic variation in transmitral inflow, with a decrease in peak E wave velocity on the first heart beat following inspiration (B). Respirophasic variation in transtricuspid inflow, with an increase in peak E wave velocity on the first heart beat following inspiration (C). Exp, expiration; Insp, inspiration; MV, transmitral inflow; TV, transtricuspid inflow.
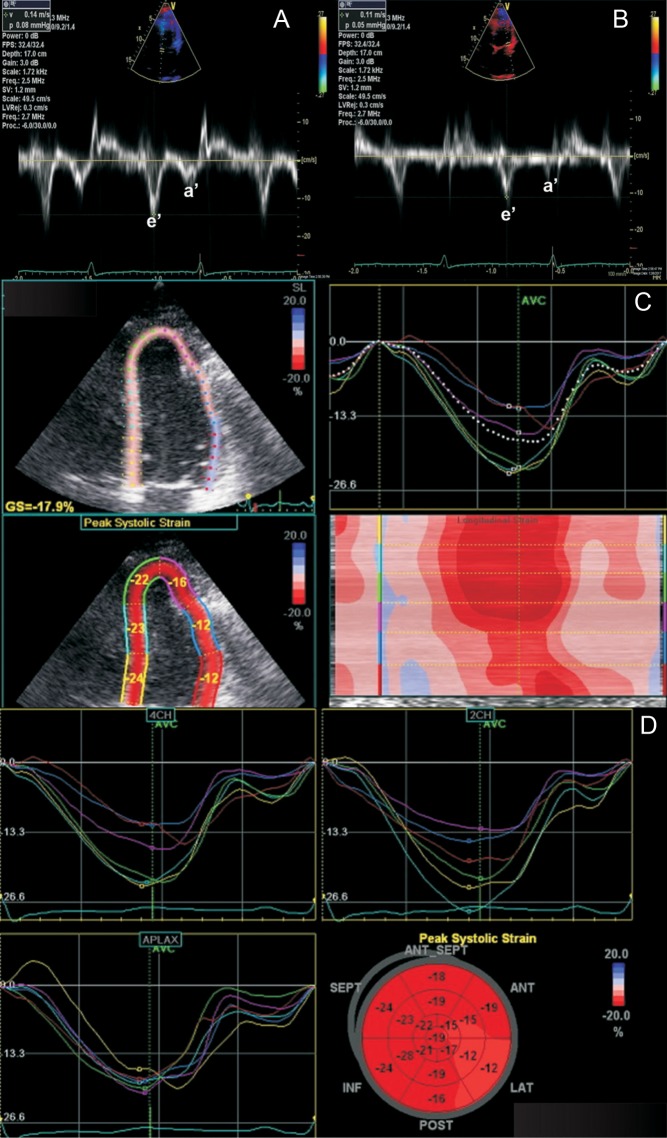
The key strengths of echocardiography in the assessment of suspected constrictive pericarditis lie in its ability to provide detailed hemodynamic assessment. In fact, the echocardiographic laboratory has taken over from cardiac catheterization laboratory for demonstrating the key hemodynamic effects of constriction. In constrictive pericarditis, significant respiratory variation is evident in the inflow profiles across the mitral and tricuspid valves: on inspiration, the peak mitral E wave velocity decreases by ≥25% at the onset of inspiration using pulsed-wave Doppler analysis; at the onset of expiration, the peak tricuspid E wave velocity decreases by ≥40% (17, 18) (Fig. 1). Important clues to underlying constrictive pathophysiology can be obtained by analyzing the pattern of hepatic venous Doppler flow. In constrictive pericarditis, there is prominent expiratory diastolic flow reversal compared to diastolic forward flow. In contrast, in restrictive cardiomyopathy, the exaggerated flow reversal in the hepatic venous Doppler profile occurs on inspiration as well as expiration (3). Tissue Doppler imaging can be helpful in the diagnosis of constrictive pericarditis, with the demonstration of the so-called ‘annulus reversus’, with a normal or elevated medial early mitral annular diastolic velocity (medial e’), which is higher than the lateral mitral annular early diastolic velocity (lateral e’) (Fig. 2) (19, 20). Proposed diagnostic criteria for the diagnosis of constrictive pericarditis have been published (21). The following echocardiographic features were found to be independently associated with constrictive pericarditis: (1) Respirophasic interventricular septal shift; (2) Increased medial early diastolic mitral annular velocity (medial e’) and (3) Increased hepatic venous expiratory diastolic reversal ratio on pulsed-wave Doppler analysis (21). The combination of respirophasic interventricular septal shift and a medial early diastolic mitral annular velocity (medial e’) ≥9 cm/s or a hepatic vein expiratory diastolic reversal to forward velocity ratio of ≥0.79 yielded the highest diagnostic sensitivity (87%) and specificity (91%) (21).
Figure 2.
Annulus reversus is a feature of constrictive pathophysiology. On Doppler assessment, in annulus reversus, medial mitral annular early diastolic velocity (14 cm/s; A), is higher than the lateral mitral annular early diastolic velocity (11 cm/s; B). On strain imaging using two-dimensional speckle tracking (C and D), the peak systolic strain in the basal and mid-anterolateral wall segments are reduced, while the peak systolic strain in the other segments are preserved.
Additional diagnostic clues to constrictive pathophysiology can be derived from advanced echocardiographic imaging, including strain imaging. In constrictive pericarditis, global longitudinal strain is generally preserved, whereas circumferential strain, torsion and early diastolic twisting are reduced (22, 23). Pericardial tethering could result in the reduction of left ventricular anterolateral wall and right ventricular free wall strain, while septal strain is relatively preserved (23). This pattern of findings on strain imaging ‘strain reversus’ is analogous to ‘annulus reversus’ on tissue Doppler imaging (Fig. 2) and has been shown to be reversible following pericardiectomy (23). The systolic component of the superior vena caval inflow Doppler profile in constrictive pericarditis is relatively flat, due to impaired cardiac filling in constrictive pathophysiology. Clinically, Kussmaul’s sign, a paradoxical elevation of jugular venous pressure on inspiration, also relates to impaired superior vena caval flow into the right atrium in constrictive pathophysiology.
In contrast to constrictive pericarditis, pericardial effusions generally present more acutely. Due to the potentially life-threatening nature of rapidly accumulating, acute pericardial effusions, early diagnosis is the key. Pericardial effusions result from multiple etiologies, including acute pericarditis, end-stage renal disease, hypothyroidism and malignancy (3, 4). Echocardiographic assessment with TTE imaging is the principal investigation for the assessment of pericardial effusions.
Echocardiography not only provides semi-quantitative assessment of the size of the pericardial effusion, but more importantly, echocardiography assesses the physiological significance of pericardial effusions, including the presence of tamponade physiology. On M-mode echocardiography, separation between the parietal pericardium and epicardium throughout the cardiac cycle has been reported to correlate with pericardial effusions larger than 50 mL (24). A large pericardial effusion is categorized by an end-diastolic measurement greater than 2 cm, while a very large pericardial effusion is categorized by an end-diastolic measurement greater than 2.5 cm on two-dimensional echocardiography (25). Physical characteristics of pericardial effusions, such as the presence of fibrinous stranding, or clots, as well as loculation, may be detected by TTE imaging. Pericardial effusions associated with end-stage kidney disease can also be effectively assessed by TTE imaging (26).
When a hemodynamically significant pericardial effusion significantly restricts cardiac chamber filling, with resultant elevated and equal intra-cardiac diastolic pressures, cardiac tamponade occurs with compromised cardiac output (27). This is a potentially life-threatening situation, and TTE imaging should be performed urgently. The diagnostic accuracy of echocardiography for the presence of a pericardial effusion is excellent (3). Cardiac tamponade is diagnosed clinically (tachycardia, elevated jugular venous pressure, pulsus paradoxus), with important complementary clues provided by echocardiography. In the context of a pericardial effusion, the following TTE imaging lend support to the presence of tamponade physiology: (1) dilated, and fixed inferior vena cava, which does not collapse significantly on inspiration; (2) reduced left ventricular forward stroke volume on Doppler assessment; (3) right ventricular compression, or collapse in severe cases, during diastole and (4) right atrial systolic collapse (28). It must be noted that certain echocardiographic features of tamponade physiology, such as right ventricular compression, may not be present in certain conditions, such as severe pulmonary hypertension and right ventricular hypertrophy (Fig. 3) (29, 30). Therefore, the echocardiographic assessment of tamponade physiology should not be based on the absence of a single imaging feature. Classically, in tamponade physiology, significant respiratory variations in mitral and tricuspid inflows are observed. During the first heart beat on inspiration, the peak mitral E wave velocity decreases >30%, while the peak tricuspid E velocity decreases >60% during the first heart beat on expiration (31).
Figure 3.
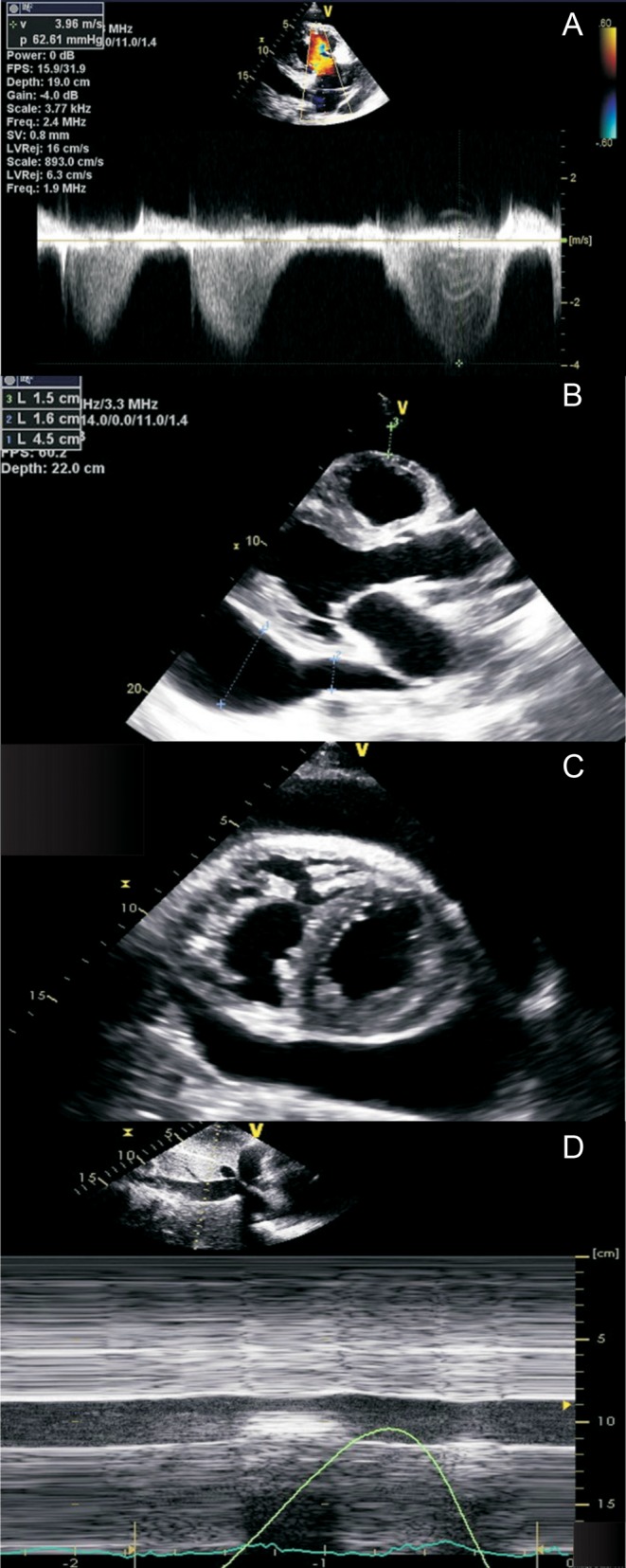
Echocardiographic assessment of pericardial effusion in pulmonary hypertension. Significant elevation of estimated right ventricular systolic pressure is consistent with pulmonary hypertension (A). Transthoracic echocardiographic imaging (parasternal long-axis imaging (B); parasternal short-axis imaging (C)) demonstrates a significant pericardial effusion. However, in the presence of right ventricular hypertrophy and pulmonary hypertension, there are no echocardiographic features of tamponade physiology, such as significant chamber compression (B and C). The inferior vena cava is also normal in caliber, collapsing more than 50% on forced inspiration, suggesting normal estimated right atrial pressure (D).
Effusive-constrictive pericarditis represents a unique condition, in which a tense pericardial effusion co-exists with pericardial constriction by the visceral pericardium. In a recent single-center study of 205 patients undergoing pericardiocentesis, 33 patients (16%) were found to have effusive-constrictive pericarditis after pericardiocentesis (32). Echocardiography is the first-line imaging modality in this condition, and certain echocardiographic features at baseline, prior to pericardiocentesis, might identify and predict, which patients would be at higher risk of developing effusive-constrictive pericarditis. These features included higher early diastolic septal mitral annular velocity, increased transmitral respiratory variation, increased hepatic venous diastolic flow reversal and increased respirophasic septal shift (32).
Echocardiography is also useful for the assessment of rare pericardial conditions, such as congenital absence of the pericardium (CAP). CAP is often detected as an incidental finding during cardiac imaging. Patients may be asymptomatic, or may have a diverse range of atypical symptoms, such as atypical chest pain and palpitations (3, 50, 51).
TTE imaging should be the first-line imaging investigation for patients with suspected CAP. A proposed systematic algorithm to the diagnosis and management of patients with CAP has been published (33, 34). TTE imaging features that raise the suspicion for CAP include (1) presence of unusual imaging windows; (2) the appearance of right ventricular dilatation and (3) excessive cardiac motion.
Multi-detector cardiac computed tomography
In general, MDCT should not be used as the first imaging investigation for most pericardial conditions. With a high level of use of computed tomography (CT) for investigation of patients presenting to the emergency department, even though investigation of suspected pericardial conditions may not be the primary reason for a CT examination, incidental pericardial findings may be detected at the time of the CT examination, that point toward an underlying pericardial condition. For instance, a thickened pericardium may be occasionally detected on CT, and may be a supportive feature of acute pericarditis (35). Pericardial effusions can also be incidentally detected by CT (36). A small pericardial effusion may lend support to the diagnosis of acute pericarditis, whereas a large pericardial effusion detected by CT should always be investigated further with correlation of the clinical findings, as well as echocardiographic assessment. Data from MDCT reconstructions may help treatment planning for pericardial effusions, including the need for surgical vs percutaneous drainage. A further strength of MDCT in the assessment of pericardial effusion lies in its ability to provide information about the type of pericardial effusion, based on attenuation values. Attenuation values <10 Hounsfield units point to the presence of a transudate; attenuation values between 20 and 60 Hounsfield units point to the presence of exudates; attenuation values >60 Hounsfield units suggest the presence of haemorrhagic pericardial effusion (37, 38).
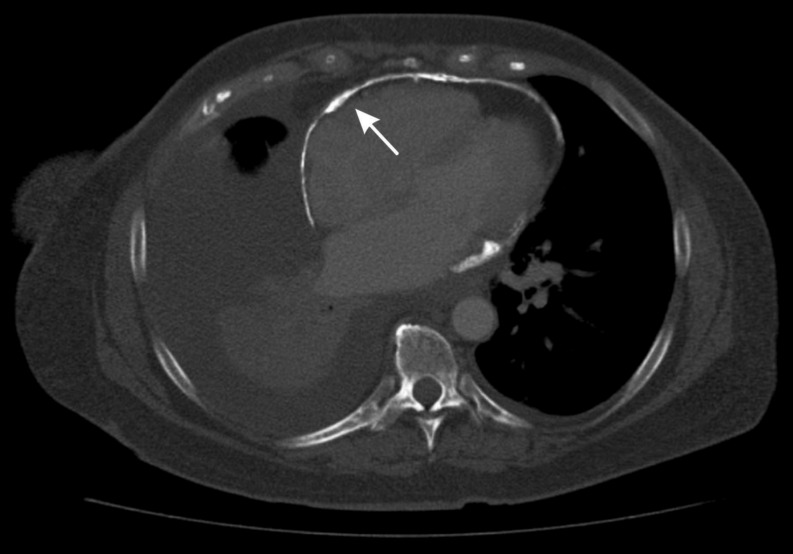
In the context of chronic, end-stage calcific pericardial constriction, MDCT is essential in the evaluation of the location, as well as extent of pericardial calcification (Fig. 4) (39). Pericardial calcification results from chronic, persistent pericardial inflammation with associated irreversible fibrosis and calcific changes (40). MDCT is also critical in the assessment of patients with prior cardiac surgery and radiation heart disease. It not only provides assessment of cardiac structures, but also provides information about parenchymal lung disease, and the proximity of cardiovascular structures to the sternum.
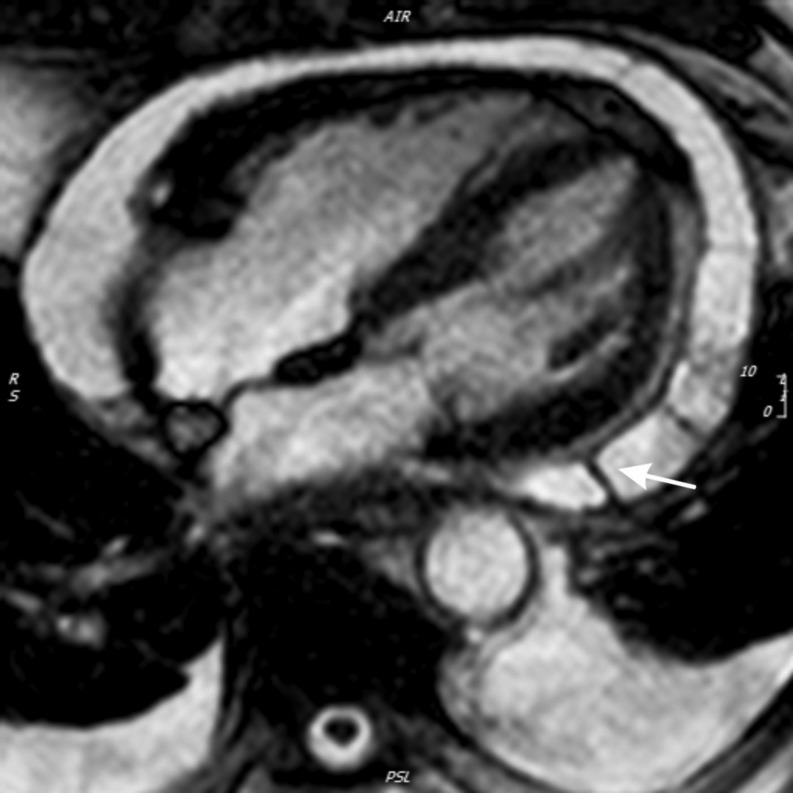
Figure 4.
Axial multi-detector computed tomography, demonstrating significant, near circumferential pericardial calcification (white arrow) in a 62-year-old female patient with end-stage calcific pericardial constriction.
It should be emphasized that generally, the hemodynamic assessment for patients with suspected constrictive pericarditis is primarily performed by echocardiography, complemented by cardiac magnetic resonance (CMR) imaging. Occasionally, in select cases, retrospective four-dimensional (4D) MDCT data set may be acquired for the assessment of respirophasic interventricular septal shift as a marker of constrictive physiology.
Although MDCT can defect thickening of the pericardium, it should also be noted that absence of pericardial thickening does not exclude constrictive pericarditis. Out of 143 patients with constrictive pericarditis confirmed surgically, pericardial thickness was not increased on MDCT for 28% of the study cohort (41).
Cardiac magnetic resonance imaging
CMR imaging has unique niche roles in the assessment of pericardial disorders, beyond conventional imaging with echocardiography and MDCT. CMR is highly sensitive for the diagnosis of active pericarditis, although it is not routinely used for the initial diagnosis of most cases of uncomplicated acute pericarditis. Anatomical findings such as pericardial thickness and pericardial effusion can be evaluated on T1-weighted black-blood imaging (15, 42). T2-weighted short tau inversion recovery (STIR) is a dedicated CMR sequence, which can be used to assess for edema. An increased pericardial signal on T2-STIR imaging suggests active pericardial edema, consistent with an acute/subacute inflammatory process (Fig. 5) (43).
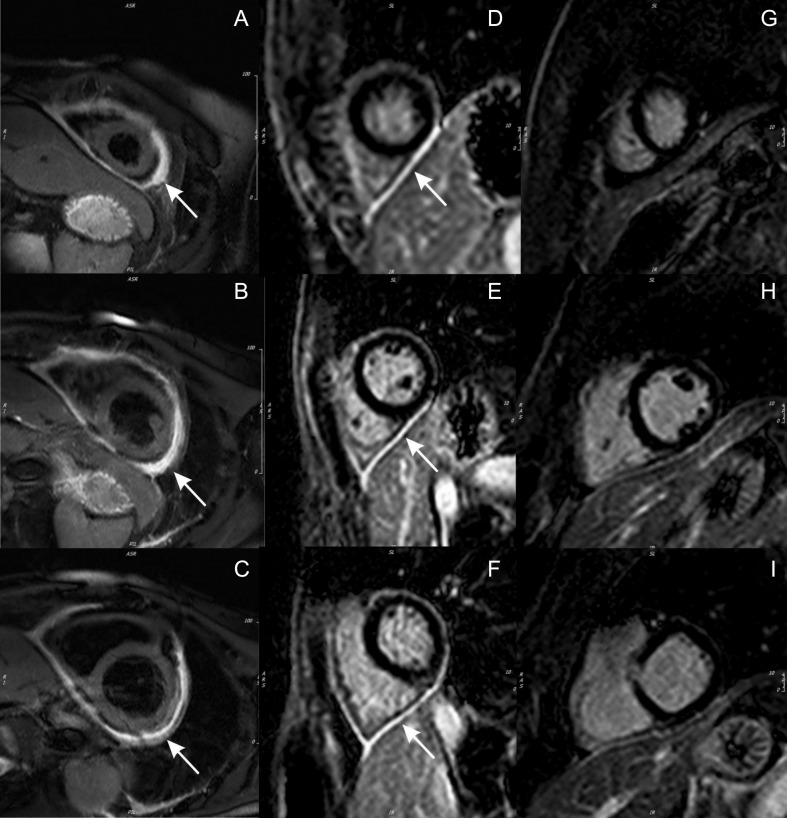
Figure 5.
Cardiac magnetic resonance imaging in pericarditis. T2-weighted short tau inversion recovery (STIR) imaging at the apical (A), mid (B) and basal (C) levels, demonstrating increased pericardial signal (white arrows) consistent with pericardial edema. Delayed enhancement imaging with gadolinium at the apical (D), mid (E) and basal (F) levels, demonstrating increased pericardial signal (white arrows) consistent with pericardial inflammation. Following treatment with anti-inflammatory therapies (colchicine and ibuprofen) and azathioprine, serial follow-up delayed-enhancement imaging with gadolinium at the apical (G), mid (H) and basal (I) levels, demonstrating complete resolution of pericardial delayed enhancement.
For a small proportion of patients, the course of pericarditis may become prolonged, and there may be uncertainties regarding the effectiveness and duration of treatment. In this clinical context, CMR imaging is a very useful imaging investigation for the assessment of ongoing pericardial inflammation. Delayed gadolinium enhancement (DHE) imaging is a unique strength of contrast-enhanced CMR imaging. The histological correlates of pericardial DHE have been characterized: the presence of DHE correlates with increased vascular permeability, neovascularization and fibroblast proliferation in the pericardium (40). The reported sensitivity of pericardial DHE for the detection of pericardial inflammation is excellent (94–100%) (43, 44).
These findings help guide patient management decisions. In a recent retrospective study of 159 patients with recurrent pericarditis, quantitative assessment of pericardial DHE had incremental prognostic value over baseline clinical and laboratory variables (integrated discrimination improvement: 8%; net reclassification improvement: 36%), with patients having a higher quantitative DHE developing recurrence at shorter time intervals (P = 0.012) and a higher recurrence rate at 6 months (P = 0.026) (45). A quantitative approach to evaluation of pericardial delayed enhancement may be superior to visual assessment (46). Patients without pericardial delayed enhancement are unlikely to respond to anti-inflammatory therapy. Although TTE is usually performed in patients with suspected recurrent pericarditis, the most useful diagnostic imaging test is CMR. The presence and extent pericardial DHE could help guide the institution of anti-inflammatory therapy (46) (Fig. 5). It has been shown that patients with significant pericardial DHE were more likely to respond to anti-inflammatory therapy and achieved resolution of constrictive pericarditis (47). CMR is also helpful in excluding recurrent pericarditis by documenting the absence of pericardial inflammation. For instance, if recurrent chest pain for a patient with prior acute pericarditis is associated with increased pericardial DHE, the clinician may integrate this finding, and prolong or intensify the duration of anti-inflammatory therapy. In contrast, if the same patient did not have evidence of pericardial edema or pericardial DHE on CMR imaging, the clinician may be guided toward tapering or discontinuation of medication treatment. In a cohort of 507 patients with recurrent pericarditis, approximately half of these patients were investigated with CMR (48). Patients without significant pericardial enhancement on CMR subsequently received less glucocorticoids (48). Similarly, in patients with constrictive pathophysiology, evidence of pericardial delayed enhancement may guide clinicians toward enhanced and/or increased duration of anti-inflammatory treatment (46).
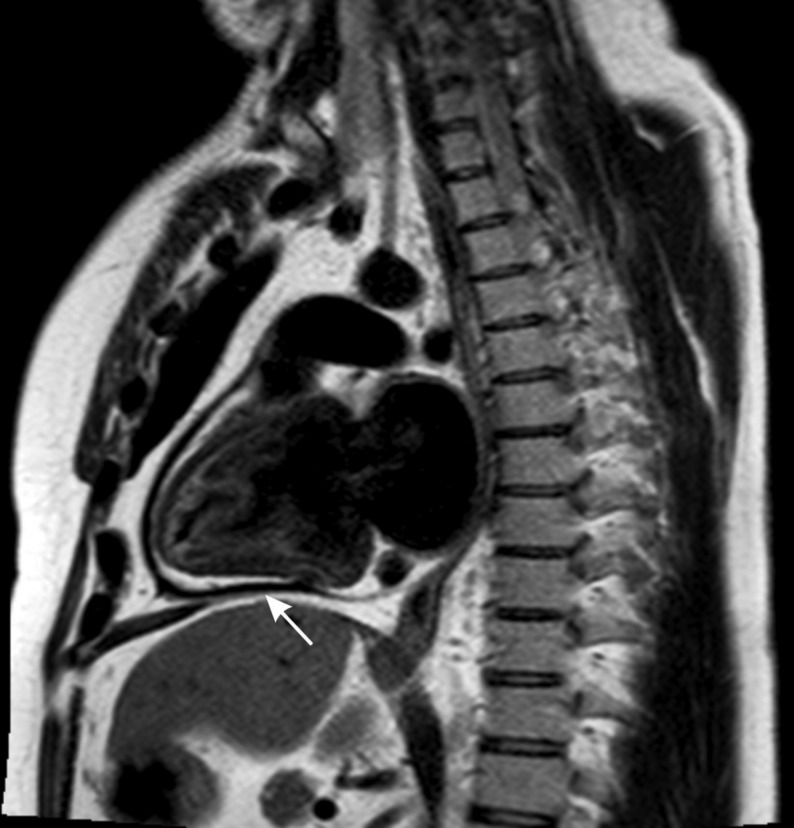
In cases of ambiguity about the diagnosis of constrictive pathophysiology after thorough echocardiographic assessment, CMR may provide useful complementary assessment. Early diastolic septal flattening is a feature of constrictive pathophysiology, which could be effectively assessed by CMR (49). During real-time cine imaging with free-breathing, an increased relative septal excursion is also a specific finding for constrictive pathophysiology (49, 50, 51). An additional supportive feature of pericardial constriction on CMR is markedly thickened pericardium (43, 51) (Fig. 6). Although less frequently performed, CMR utilizing real-time phase contrast imaging, has been previously reported, in a similar fashion to echocardiographic hemodynamic assessment, to be valuable for the diagnosis of constrictive pathophysiology, when ≥25% variation in transmitral flow was demonstrated during respiration (52).
Figure 6.
Demonstration of thickened pericardium (white arrow) on black-blood cardiac magnetic resonance imaging (sagittal projection).
For patients with end-stage, calcific constrictive pericarditis, or recalcitrant treatment-resistant cases of recurrent pericarditis, published observational data support pericardiectomy as a treatment option. In a retrospective study of 58 patients undergoing pericardiectomy after failed medical therapy for recurrent pericarditis, patients in the surgical group had a decreased relapse rate, compared to patients who were medically treated (53).
In the investigation of pericardial effusions, CMR can provide helpful additional information for the evaluation of complex, loculated pericardial effusions (Fig. 7). For the rare condition of CAP, advanced cardiovascular imaging with CMR and/or MDCT could be considered for symptomatic patients. These imaging investigations may help guide potential surgical planning, evaluation of high-risk anatomy features, which may predispose to strangulation, and further risk stratification. Displacement of the heart into the left hemithorax (levorotation), with posterolateral displacement of the left ventricular apex, is a key diagnostic feature of CAP on CMR and MDCT.
Figure 7.
Evaluation of pericardial effusion on cardiac magnetic resonance imaging (axial projection on steady-state free precession), demonstrating the presence of septations within the pericardial effusion (white arrow).
Transient constrictive pericarditis
A unique entity known as transient constrictive pericarditis affects an important subset of patients with constrictive pathophysiology. Accurate identification of this entity is important. The predominant pathophysiological process involved in transient constrictive pericarditis is ongoing pericardial inflammation. Therefore, transient constrictive pericarditis is usually post pericarditis, and could be viewed as a ‘temporary’ form of pericardial constriction, which can resolve with anti-inflammatory therapy (4, 54).
TTE and CMR imaging can be used to assess for anatomical features of pericardial thickening and pericardial effusion, as well as hemodynamic features of constrictive pathophysiology, in a similar fashion to the workup of patients with constrictive pericarditis.
Positron emission tomography
The novel application of [18F] fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for transient constriction deserves special mention (55). Recently, it was demonstrated in patients with tuberculosis constrictive pericarditis that [18F] FDG PET/CT could successfully predict the response to steroid therapy with a high accuracy (55). Data are limited regarding the utility of PET/CT imaging in constrictive pericarditis, although results of the early study suggest that this imaging modality may become more widely used in the future, adding to the existing armamentarium of multimodality cardiovascular imaging for cardiologists in the investigation of patients with suspected pericardial disorders. Additionally, [18F] Fluorodeoxyglucose (FDG) positron emission tomography/cardiac magnetic resonance (PET/CMR) has been described in the diagnosis of active pericarditis (56).
Conclusion
While disorders of the pericardium are heterogeneous, with a good understanding of the strengths and relative limitations of existing mainstay cardiovascular imaging investigations (echocardiography, MDCT, CMR), appropriate selection and interpretation of the results of these imaging investigations can help clinicians achieve timely and accurate diagnosis, and appropriate management of most patients with these diverse conditions. Echocardiography is the first-line imaging investigation for patients with most pericardial conditions, due to its proven safety, availability, portability, excellent temporal resolution and ability to provide anatomical as well as hemodynamic assessment. MDCT provides excellent anatomical assessment of the pericardium, as well as extra-cardiac structures. CMR is a powerful adjuvant imaging modality, which provides valuable information about the presence of active pericardial inflammation and edema, allowing clinicians to integrate results of imaging investigations into treatment decisions for patients.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Khandaker MH, Espinosa RE, Nishimura RA, Sinak LJ, Hayes SN, Melduni RM, Oh JK. Pericardial disease: diagnosis and management. Mayo Clinic Proceedings 2010. 85 572–593. ( 10.4065/mcp.2010.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imazio M, Spodick DH, Brucato A, Trinchero R, Adler Y. Controversial issues in the management of pericardial diseases. Circulation 2010. 121 916–928. ( 10.1161/CIRCULATIONAHA.108.844753) [DOI] [PubMed] [Google Scholar]
- 3.Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, Hung J, Garcia MJ, Kronzon I, Oh JK. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. Journal of the American Society of Echocardiography 2013. 26 965.e15–1012.e15. ( 10.1016/j.echo.2013.06.023) [DOI] [PubMed] [Google Scholar]
- 4.Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C. ESC guidelines for the diagnosis and management of pericardial diseases. European Heart Journal 2015. 36 2921–2964.26320112 [Google Scholar]
- 5.Motte G. Acute pericarditis. New England Journal of Medicine 2014. 371 2410–2416. [DOI] [PubMed] [Google Scholar]
- 6.Imazio M, Adler Y, Charron P. Recurrent pericarditis: modern approach in 2016. Current Cardiology Reports 2016. 18 1–8. ( 10.1007/s11886-015-0682-9) [DOI] [PubMed] [Google Scholar]
- 7.Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004. 363 717–27. ( 10.1016/S0140-6736(04)15648-1) [DOI] [PubMed] [Google Scholar]
- 8.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. Journal of the American College of Cardiology 2011. 57 1126–1166. ( 10.1016/j.jacc.2010.11.002) [DOI] [PubMed] [Google Scholar]
- 9.Salisbury AC, Olalla-Gómez C, Rihal CS, Bell MR, Ting HH, Casaclang-Verzosa G, Oh JK. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clinic Proceedings 2009. 84 11–15. ( 10.4065/84.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, Trinchero R, Spodick DH, Adler Y. & CORP (COlchicine for Recurrent Pericarditis) Investigators. Colchicine for Recurrent Pericarditis (CORP) a randomized trial. Annals of Internal Medicine 2011. 155 409–414. ( 10.7326/0003-4819-155-7-201110040-00359) [DOI] [PubMed] [Google Scholar]
- 11.Shabetai R. Recurrent pericarditis: recent advances and remaining questions. Circulation 2005. 112 1921–1923. ( 10.1161/CIRCULATIONAHA.105.569244) [DOI] [PubMed] [Google Scholar]
- 12.Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, Lytle BW, Blackstone EH, Lauer MS, Klein AL. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. Journal of the American College of Cardiology 2004. 43 1445–1452. ( 10.1016/j.jacc.2003.11.048) [DOI] [PubMed] [Google Scholar]
- 13.Ling LH, Oh JK, Schaff HV, Danielson GK, Douglas W, Seward JB, Tajik AJ. Constrictive pericarditis in the modern era. Circulation 1999. 100 1380–1386. ( 10.1161/01.CIR.100.13.1380) [DOI] [PubMed] [Google Scholar]
- 14.Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 2005. 112 3608–3616. ( 10.1161/CIRCULATIONAHA.105.543066) [DOI] [PubMed] [Google Scholar]
- 15.Gentry J, Klein AL, Jellis C. Transient constrictive pericarditis: current diagnostic and therapeutic strategies. Current Cardiology Reports 2016. 18 41 ( 10.1007/s11886-016-0720-2) [DOI] [PubMed] [Google Scholar]
- 16.Adler Y, Charron P. The 2015 ESC guidelines on the diagnosis and management of pericardial diseases. European Heart Journal 2015. 36 2873–2874. ( 10.1093/eurheartj/ehv479) [DOI] [PubMed] [Google Scholar]
- 17.Hatle LK, Appleton CP, Popp RL. Differentiation of constrictive pericarditis and restrictive cardiomyopathy by Doppler echocardiography. Circulation 1989. 79 357–370. ( 10.1161/01.CIR.79.2.357) [DOI] [PubMed] [Google Scholar]
- 18.Oh JK, Hatle LK, Seward JB, Danielson GK, Schaff HV, Reeder GS, Tajik AJ. Diagnostic role of Doppler echocardiography in constrictive pericarditis. Journal of the American College of Cardiology 1994. 23 154–162. ( 10.1016/0735-1097(94)90514-2) [DOI] [PubMed] [Google Scholar]
- 19.Choi JH, Choi JO, Ryu DR, Lee SC, Park SW, Choe YH, Oh JK. Mitral and tricuspid annular velocities in constrictive pericarditis and restrictive cardiomyopathy: correlation with pericardial thickness on computed tomography. JACC Cardiovascular Imaging 2011. 4 567–575. ( 10.1016/j.jcmg.2011.01.018) [DOI] [PubMed] [Google Scholar]
- 20.Reuss CS, Wilansky SM, Lester SJ, Lusk JL, Grill DE, Oh JK, Tajik AJ. Using mitral “annulus reversus” to diagnose constrictive pericarditis. European Journal of Echocardiography 2009. 10 372–375. ( 10.1093/ejechocard/jen258) [DOI] [PubMed] [Google Scholar]
- 21.Welch TD, Ling LH, Espinosa RE, Anavekar NS, Wiste HJ, Lahr BD, Schaff HV, Oh JK. Echocardiographic diagnosis of constrictive pericarditis Mayo Clinic criteria. Circulation: Cardiovascular Imaging 2014. 7 526–534. ( 10.1161/CIRCIMAGING.113.001613) [DOI] [PubMed] [Google Scholar]
- 22.Sengupta PP, Krishnamoorthy VK, Abhayaratna WP, Korinek J, Belohlavek M, Sundt TM, Chandrasekaran K, Mookadam F, Seward JB, Tajik AJ, et al Disparate patterns of left ventricular mechanics differentiate constrictive pericarditis from restrictive cardiomyopathy. JACC Cardiovascular Imaging 2008. 1 29–38. ( 10.1016/j.jcmg.2007.10.006) [DOI] [PubMed] [Google Scholar]
- 23.Kusunose K, Dahiya A, Popović ZB, Motoki H, Alraies MC, Zurick AO, Bolen MA, Kwon DH, Flamm SD, Klein AL. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circulation: Cardiovascular Imaging 2013. 6 399–406. ( 10.1161/CIRCIMAGING.112.000078) [DOI] [PubMed] [Google Scholar]
- 24.Galve E, Garcia-Del-Castillo H, Evangelista A., Batlle J, Permanyer-Miralda G, Soler-Soler J. Pericardial effusion in the course of myocardial infarction: incidence, natural history, and clinical relevance. Circulation 1986. 73 294–299. ( 10.1161/01.CIR.73.2.294) [DOI] [PubMed] [Google Scholar]
- 25.Weitzman LB. The incidence and natural history of pericardial elfusion after cardiac surgery – an echocardiographic study. Circulation 1984. 69 506–511. ( 10.1161/01.CIR.69.3.506) [DOI] [PubMed] [Google Scholar]
- 26.Rehman KA, Betancor J, Xu B, Kumar A, Rivas CG, Sato K, Wong LP, Asher CR, Klein AL. Uremic pericarditis, pericardial effusion, and constrictive pericarditis in end-stage renal disease: insights and pathophysiology. Clinical Cardiology 2017. 40 839–846. ( 10.1002/clc.22770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spodick DH. Acute cardiac tamponade. New England Journal of Medicine 2003. 349 684–690. ( 10.1056/NEJMra022643) [DOI] [PubMed] [Google Scholar]
- 28.Gillam LD, Guyer DE, Gibson TC, King ME, Marshall JE, Weyman AE. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation 1983. 68 294–301. ( 10.1161/01.CIR.68.2.294) [DOI] [PubMed] [Google Scholar]
- 29.Hoit BD, Gabel M, Fowler NO. Cardiac tamponade in left ventricular dysfunction. Circulation 1990. 82 1370–1376. ( 10.1161/01.CIR.82.4.1370) [DOI] [PubMed] [Google Scholar]
- 30.Hoit BD, Fowler NO. Influence of acute right ventricular dysfunction on cardiac tamponade. Journal of the American College of Cardiology 1991. 18 1787–1793. ( 10.1016/0735-1097(91)90522-B) [DOI] [PubMed] [Google Scholar]
- 31.Appleton CP, Hatle LK, Popp RL. Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. Journal of the American College of Cardiology 1988. 11 1020–1030. ( 10.1016/S0735-1097(98)90060-2) [DOI] [PubMed] [Google Scholar]
- 32.Kim KH, Miranda WR, Sinak LJ, Syed FF, Melduni RM, Espinosa RE, Kane GC, Oh JK. Effusive-constrictive pericarditis after pericardiocentesis: incidence, associated findings, and natural history. JACC Cardiovascular Imaging 2018. 11 534–541. ( 10.1016/j.jcmg.2017.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatzoulis MA, Munk MD, Merchant N, Van Arsdell GS, McCrindle BW, Webb GD. Isolated congenital absence of the pericardium: clinical presentation, diagnosis, and management. Annals of Thoracic Surgery 2000. 69 1209–1215. ( 10.1016/S0003-4975(99)01552-0) [DOI] [PubMed] [Google Scholar]
- 34.Xu B, Betancor J, Asher C, Rosario A, Klein A. Congenital absence of the pericardium: a systematic approach to diagnosis and management. Cardiology 2017. 136 270–278. ( 10.1159/000452441) [DOI] [PubMed] [Google Scholar]
- 35.Rivera MP, Parker LA, Carson SS, Salem W, Carolina N. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Archives of Internal Medicine 2009. 169 1961–1965. ( 10.1001/archinternmed.2009.360) [DOI] [PubMed] [Google Scholar]
- 36.Bogaert J, Centonze M, Vanneste R. Cardiac and pericardial abnormalities on chest computed tomography: what can we see? La Radiologia Medica 2010. 115 175–190. ( 10.1007/s11547-010-0514-3) [DOI] [PubMed] [Google Scholar]
- 37.Oyama N, Oyama N, Komuro K, Nambu T, Manning W, Miyasaka K. Computed tomography and magnetic resonance imaging of the pericardium: anatomy and pathology. Magnetic Resonance in Medical Sciences 2004. 3 145–152. ( 10.2463/mrms.3.145) [DOI] [PubMed] [Google Scholar]
- 38.Verhaert D, Gabrie RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circulation: Cardiovascular Imaging 2010. 3 333–343. ( 10.1161/CIRCIMAGING.109.921791) [DOI] [PubMed] [Google Scholar]
- 39.Kamdar AR, Meadows TA, Roselli EE, Gorodeski EZ, Curtin RJ, Sabik JF, Schoenhagen P, White RD, Lytle BW, Flamm SD, et al. Multidetector computed tomographic angiography in planning of reoperative cardiothoracic surgery. Annals of Thoracic Surgery 2008. 85 1239–1245. ( 10.1016/j.athoracsur.2007.11.075) [DOI] [PubMed] [Google Scholar]
- 40.Zurick AO, Bolen MA, Kwon DH, Tan CD, Popovic ZB, Rajeswaran J, Rodriguez ER, Flamm SD, Klein AL. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. JACC Cardiovascular Imaging 2011. 4 1180–1191. ( 10.1016/j.jcmg.2011.08.011) [DOI] [PubMed] [Google Scholar]
- 41.Talreja DR, Edwards WD, Danielson GK, Schaff HV, Tajik AJ, Tazelaar HD, Breen JF, Oh JK. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 2003. 108 1852–1857. ( 10.1161/01.CIR.0000087606.18453.FD) [DOI] [PubMed] [Google Scholar]
- 42.Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J. Multimodality imaging of pericardial diseases. JACC Cardiovascular Imaging 2010. 3 650–660. ( 10.1016/j.jcmg.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 43.Young PM, Glockner JF, Williamson EE, Morris MF, Araoz PA, Julsrud PR, Schaff HV, Edwards WD, Oh JK, Breen JF. MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. International Journal of Cardiovascular Imaging 2012. 28 1099–1109. ( 10.1007/s10554-011-9916-0) [DOI] [PubMed] [Google Scholar]
- 44.Taylor AM, Dymarkowski S, Verbeken EK, Bogaert J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. European Radiology 2006. 16 569–574. ( 10.1007/s00330-005-0025-0) [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Sato K, Yzeiraj E, Betancor J, Lin L, Tamarappoo BK, Kwon DH, Hachamovitch R, Klein AL. Quantitative pericardial delayed hyperenhancement informs clinical course in recurrent pericarditis. JACC Cardiovascular Imaging 2017. 10 1337–1346. ( 10.1016/j.jcmg.2016.10.020) [DOI] [PubMed] [Google Scholar]
- 46.Cremer PC, Tariq MU, Karwa A, Alraies MC, Benatti R, Schuster A, Agarwal S, Flamm SD, Kwon DH, Klein AL. Quantitative assessment of pericardial delayed hyperenhancement predicts clinical improvement in patients with constrictive pericarditis treated with anti-inflammatory therapy. Circulation: Cardiovascular Imaging 2015. 8 1–8. ( 10.1007/s12410-014-9318-5) [DOI] [PubMed] [Google Scholar]
- 47.Feng D, Glockner J, Kim K, Martinez M, Syed IS, Araoz P, Breen J, Espinosa RE, Sundt T, Schaff HV, et al Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: a pilot study. Circulation 2011. 124 1830–1837. ( 10.1161/CIRCULATIONAHA.111.026070) [DOI] [PubMed] [Google Scholar]
- 48.Alraies MC, AlJaroudi W, Yarmohammadi H, Yingchoncharoen T, Schuster A, Senapati A, Tariq M, Kwon D, Griffin BP, Klein AL. Usefulness of cardiac magnetic resonance-guided management in patients with recurrent pericarditis. American Journal of Cardiology 2015. 115 542–547. ( 10.1016/j.amjcard.2014.11.041) [DOI] [PubMed] [Google Scholar]
- 49.Francone M, Dymarkowski S, Kalantzi M, Rademakers FE, Bogaert J. Assessment of ventricular coupling with real-time cine MRI and its value to differentiate constrictive pericarditis from restrictive cardiomyopathy. European Radiology 2006. 16 944–951. ( 10.1007/s00330-005-0009-0) [DOI] [PubMed] [Google Scholar]
- 50.Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, Klein AL. Complicated pericarditis understanding risk factors and pathophysiology to inform imaging and treatment. Journal of the American College of Cardiology 2016. 68 2311–2328. ( 10.1016/j.jacc.2016.07.785) [DOI] [PubMed] [Google Scholar]
- 51.Bolen MA, Rajiah P, Kusunose K, Collier P, Klein A, Popović ZB, Flamm SD. Cardiac MR imaging in constrictive pericarditis: multiparametric assessment in patients with surgically proven constriction. International Journal of Cardiovascular Imaging 2015. 31 859–866. ( 10.1007/s10554-015-0616-z) [DOI] [PubMed] [Google Scholar]
- 52.Thavendiranathan P, Verhaert D, Walls MC, Bender JA, Rajagopalan S, Chung YC, Simonetti OP, Raman SV. Simultaneous right and left heart real-time, free-breathing CMR flow quantification identifies constrictive physiology. JACC Cardiovascular Imaging 2012. 5 15–24. ( 10.1016/j.jcmg.2011.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khandaker MH, Schaff HV, Greason KL, Anavekar NS, Espinosa RE, Hayes SN, Nishimura RA, Oh JK. Pericardiectomy vs medical management in patients with relapsing pericarditis. Mayo Clinic Proceedings 2017. 87 1062–1070. ( 10.1016/j.mayocp.2012.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK. Transient constrictive pericarditis: causes and natural history. Journal of the American College of Cardiology 2004. 43 271–275. ( 10.1016/j.jacc.2003.08.032) [DOI] [PubMed] [Google Scholar]
- 55.Chang SA, Choi JY, Kim E, Hyun S, Jang S, Choi J, Park S, Lee S, Park SW, Oh JK. [18F]Fluorodeoxyglucose PET/CT predicts response to steroid therapy in constrictive pericarditis. Journal of the American College of Cardiology 2017. 69 750–752. ( 10.1016/j.jacc.2016.11.059) [DOI] [PubMed] [Google Scholar]
- 56.Xu B, Huang SS, Jellis C, Flamm SD. Diagnosis of active pericarditis by positron emission tomography (PET)/cardiac magnetic resonance (CMR) imaging. European Heart Journal 2018. 39 179 ( 10.1093/eurheartj/ehv127) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a