Introduction
The development of biologic treatments has changed the management of severe psoriasis. The adverse effects of such treatments are increasingly well-known and controlled, and they generally occur as infections, which are potentially severe but rarely fatal. We report the first case, to our knowledge, of cutaneous leishmaniasis that developed in a patient who received ustekinumab for cutaneous psoriasis.
Case report
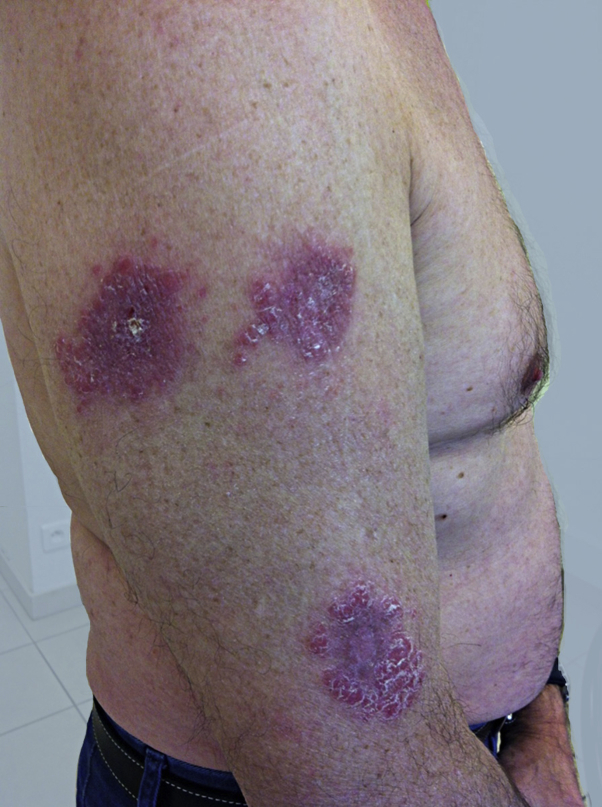
A 62-year-old patient was treated in the dermatology department for severe plaque psoriasis with no rheumatic involvement. After the failure of ultraviolet B TL01 phototherapy and methotrexate for 4 months, ustekinumab was initiated. The pretherapeutic assessment did not reveal any latent infectious disease. The first subcutaneous injections of ustekinumab (45 mg at weeks 0 and 4) were well-tolerated and clinical response was complete at week 5. After the third injection at week 16, the patient traveled to his second home in Ouarzazate, Morocco. He then described the occurrence of inflammatory crusty lesions, first on the right upper limb, which he identified as psoriasis. Ultimately, he consulted after the fourth injection of ustekinumab at week 28, after seeing the lesions persist and increase in number and size (Fig 1).
Fig 1.
Initial skin lesions on the right upper limb.
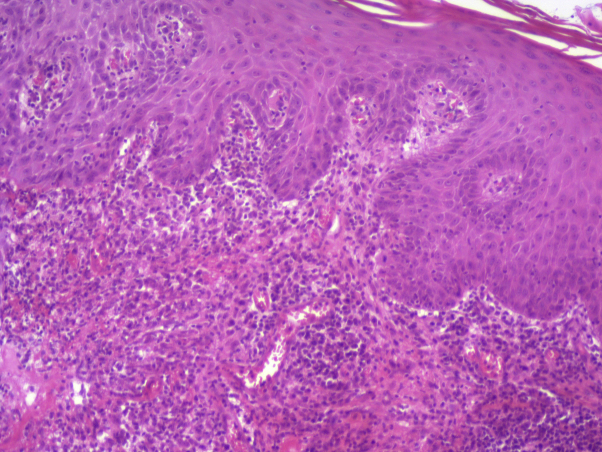
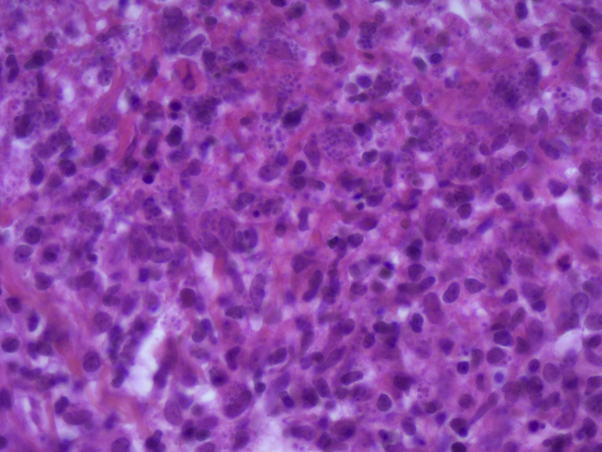
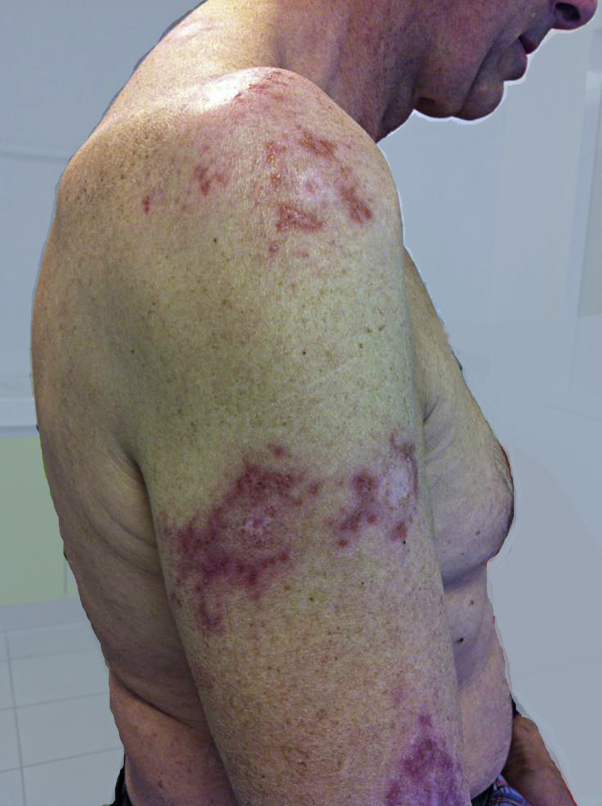
Clinical examination revealed 15 crusted papular lesions on the upper part of the body, including the face. A cutaneous punch biopsy performed on a nodule revealed granulomatous inflammatory lesions with numerous histiocyte-macrophage–containing pathogens evoking Leishman bodies, as confirmed by Giemsa staining (Figs 2 and 3). A cutaneous sample was sent to a leishmaniasis reference center (Hôpital de la Pitié-Salpétrière, Paris), which confirmed the diagnosis of Leishmania major infection. Ustekinumab was then stopped. There was no visceral involvement. A combination treatment with cryotherapy and 3 sets of intralesional injections of meglumine antimoniate was initiated, with no effect; a complementary treatment with oral miltefosine for 28 days was then prescribed, which resulted in an involution of the lesions (Fig 4).
Fig 2.
Hematoxylin-eosin stain revealed a dense lymphoplasmacytic infiltrate in superficial and medium dermis, featuring histiocytic granulomas. A high-resolution version of this slide for use with the Virtual Microscope is available as eSlide: VM04518.
(Image courtesy of Véronique Duchatelle, Fondation Hôpital Saint-Joseph, Paris.)
Fig 3.
Higher magnification Giemsa-stained image displaying numerous histiocyte-macrophages abundantly filled with Leishman bodies. A high-resolution version of this slide for use with the Virtual Microscope is available as eSlide: VM04519.
(Image courtesy of Véronique Duchatelle, Fondation Hôpital Saint-Joseph, Paris.)
Fig 4.
Involution of lesions of patient with leishmaniasis after treatment with oral miltefosine.
Discussion
Leishmaniasis is an infection found worldwide that is caused by a flagellated protozoan transmitted by the bites of female sandflies. There are approximately 20 different species of Leishmania that can infect humans. The clinical disease spectrum ranges from a cutaneous form that produces scarring to a potentially fatal visceral form.
The biologic diagnosis of leishmaniasis is based on the detection of the Leishmania parasite via microscopic examination of Giemsa-stained tissues. Culture and PCR amplification of parasitic DNA allow for species identification. The serology and intradermal reaction to Leishmania have no indication for cutaneous leishmaniasis.
The treatment depends on the parasitic species involved. In leishmaniasis-endemic countries, a strong clinical presumption and knowledge of the local circulating parasitic species usually allow the curative treatment to begin before the species diagnosis is confirmed.1
Because healing can be spontaneous in cases of limited localization on the skin, therapeutic abstention under surveillance can be considered. In France, treatments for cutaneous leishmaniasis include combination of antimony-derivative intralesional injection with cryotherapy, intravenous liposomal amphotericin B, oral miltefosine (temporarily approved for use by the French medicines agency), and a short course of intramuscular pentamidine. Oral fluconazole, itraconazole, ketoconazole, or topical paromomycin have shown some efficacy in small clinical series.2 Unfortunately, a vaccine has not yet been developed.
Ustekinumab is a humanized monoclonal antibody directed against the shared p40 subunit of interleukins 12 (IL-12) and IL-23. It has been available in France since July 2009 for the treatment of moderate-to-severe plaque psoriasis. In the phase 3 PHOENIX 1 and 2 studies, 67% of patients receiving ustekinumab 45 mg achieved PASI (Psoriasis Area Severity Index) 75 at week 12 and 42% achieved PASI 90; this response was maintained at week 40 in 88% of these patients.3, 4
Nearly 40 cases of leishmaniasis in patients treated with anti–tumor necrosis factor-α (TNF-α) medications have been reported in the literature since 2004.5, 6 All of the patients lived or had lived in a leishmaniasis-endemic area. The most frequently incriminated molecule at the time of diagnosis of leishmaniasis was infliximab, and the only reported fatal case occurred with etanercept; disease control resulted in a cure for all other cases, including 7 in whom anti–TNF-α treatment was not interrupted.5 This increased susceptibility to Leishmania is related to the role that TNF-α plays in macrophage activation and the formation of inflammatory granulomas, which are both essential processes for the control of infections with intracellular pathogens. The inhibition of TNF-α then leads either to a primary infection or the development of a latent infection; the 2 can be distinguished only by determining the date of parasite exposure.
The association between ustekinumab administration and the appearance of cutaneous leishmaniasis is probably not fortuitous because studies seem to demonstrate that IL-12 is essential for controlling Leishmania infection by promoting a TH1 lymphocyte response.7
The eastern part of Morocco, which includes Ouarzazate and borders the Sahara, is a focus of Leishmania major.8 Its characteristic cutaneous lesions are described as wet ulcerative nodular or ulcer-vegetating and localized to the limbs,9 as was the case with our patient. Because he had visited this endemic region several times, precisely dating his exposure to the pathogen was challenging. However, only a few months elapsed between the initiation of biologic therapy and the appearance of cutaneous signs, in contrast with the average of 23 months that has been reported in the literature.5 The clinical lesions of our patient are similar to those seen in immunocompetent travelers. In addition, the potential role of methotrexate as a risk factor for leishmaniasis should not be overlooked.10
The appearance of visceral leishmaniasis and progression of the underlying inflammatory disease might constitute a contraindication to the resumption of biotherapy, limiting the therapeutic arsenal available. However, this contraindication might not be necessary in cases of nonthreatening cutaneous leishmaniasis.
In conclusion, biologic treatments of psoriasis expose patients to the rare but potentially serious risk for leishmaniasis. The disease might be revealed several years after exposure to the parasite. The inhibition of IL-12 appears to be an important risk factor for developing leishmaniasis. It would be of value to assess the benefit of including Leishmania serology in the pretherapeutic assessment of candidates for biologic treatment, especially those who have resided in leishmaniasis-endemic areas, before receiving ustekinumab.
Acknowledgments
We are indebted to Pierre Buffet, MD, PhD, from the Microbiology Department of Groupe Hospitalier Pitié-Salpétrière for his help in the microbiologic diagnosis of the leishmaniasis infection.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Control of the leishmaniases: report of a meeting of WHO Expert Committee on the control of leishmaniases, Geneva 22-26 March 2010. Vol. 949. WHO Technical Report series; 2011. pp. 1–185. [Google Scholar]
- 2.Buffet P.A., Rosenthal E., Gangneux J.P. Traitement des leishmanioses en France: proposition d'un référentiel consensuel. Presse Med. 2011;40(2):173–184. doi: 10.1016/j.lpm.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi C.L., Kimball A.B., Papp K.A. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 4.Papp K.A., Langley R.G., Lebwohl M. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 5.Guedes-Barbosa L.S., Pereira da Costa I., Fernandes V., Henrique da Mota L.M., de Menezes I., Scheinberg M.A. Leishmaniasis during anti-tumor necrosis factor therapy: report of 4 cases and review of the literature (additional 28 cases) Semin Arthritis Rheum. 2013;43:152–157. doi: 10.1016/j.semarthrit.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Guarneri C., Bevelacqua V., Patterson J.W., Tchernev G. Cutaneous and visceral leishmaniasis during anti-TNFα therapy. Wien Med Wochenschr. 2017;167(3-4):78–82. doi: 10.1007/s10354-016-0527-1. [DOI] [PubMed] [Google Scholar]
- 7.Park A.Y., Hondowicz B., Kopf M., Scott P. The role of IL-12 in maintaining resistance to Leishmania major. J Immunol. 2002;168(11):5771–5777. doi: 10.4049/jimmunol.168.11.5771. [DOI] [PubMed] [Google Scholar]
- 8.Tlamçani Z., Er-Rami M. The current status of cutaneous leishmaniasis in Morocco. Turkiye Parazitol Derg. 2014;38(1):5–8. doi: 10.5152/tpd.2014.1401. [DOI] [PubMed] [Google Scholar]
- 9.Aoun K., Bouratbine A. Cutaneous leishmaniasis in North Africa: a review. Parasite. 2014;21:14. doi: 10.1051/parasite/2014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadjipetrou A., Anyfantakis D., Gkogkou A. Visceral leishmaniasis in a psoriatic arthritis patient treated with methotrexate. Infez Med. 2014;22(3):230–235. [PubMed] [Google Scholar]