Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS), or drug-induced hypersensitivity syndrome, is a severe adverse drug reaction characterized by cutaneous eruption, fever, leukocytosis with eosinophilia or atypical lymphocytosis, lymphadenopathy, and liver or other internal organ involvement.1 DRESS typically presents 2 to 12 weeks after drug exposure.2 Common causative agents include anticonvulsants, allopurinol, antibiotics, and nonsteroidal anti-inflammatory drugs. An association with reactivation of human herpes virus 6 and other herpesviruses has also been found.3 Various long-term outcomes after resolution of DRESS have been reported, few of which are autoimmune sequelae such as autoimmune thyroiditis, sclerodermoid graft-versus-host disease–like skin lesions, and systemic lupus erythematosus.1 Nonautoimmune, fulminant type 1 diabetes mellitus (T1DM), a subtype characterized by rapid onset with absence of diabetes-related autoantibodies, has been reported as a sequela of DRESS.1 In contrast, cases of classic autoimmune T1DM have rarely been presented. Here, we report a case of classic autoimmune T1DM in a patient after resolution of DRESS.
Case report
A 44-year-old Vietnamese man with history of hypertension, congestive heart failure, and prediabetes presented for a rash involving the face, chest, and back. He recently returned from a 2-month stay in Vietnam, and a rash developed 1 week before presentation. After taking hydroxyzine, diphenhydramine, and steroids prescribed at an outside hospital, he had facial swelling, rash extending to extremities and genitals, worsening of pruritus, and scaling with sloughing including mucous membranes. He had stopped taking his prescribed antihypertensive medications for 3 to 4 months and denied taking herbal medications. He reported taking an unspecified antibiotic and pain medications for unclear reasons for several days while in Vietnam a month before presentation. On physical examination, he was afebrile but mildly tachycardic with diffuse confluent erythematous macules on his trunk, palms, upper and lower extremities (Fig 1), and fissuring and hemorrhagic crust on mucosal lips with superficial tongue erosions. Facial edema, increased eye lacrimation, mild conjunctival injection, and cervical lymphadenopathy were noted. There were no pustules, bullae, or Nikolsky sign.
Fig 1.
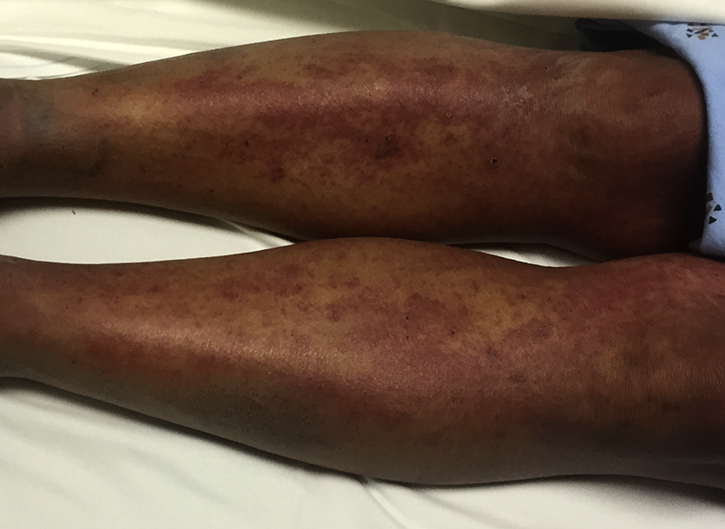
Diffuse confluent erythematous macules of lower extremities.
Laboratory findings were notable for leukocytosis (18600) and eosinophilia (19%), and liver (peak aspartate aminotransferase, 103 U/L; alanine aminotransferase, 276 U/L) and renal dysfunction (creatinine, 2.5 mg/dL; blood urea nitrogen, 59 mg/dL). Skin biopsies on the left clavicle and left forearm found infiltration of lymphocytes in the dermoepidermal junction with apoptotic keratinocytes, parakeratosis, extravasated erythrocytes, and rare dermal eosinophils (Fig 2). Examination and laboratory findings were consistent with DRESS according to the RegiSCAR criteria. Our patient was started on prednisone, 80 mg/d; triamcinolone 0.1% ointment twice a day; and Vaseline 3 times a day. He was discharged 6 days later on prednisone, 60 mg/d after liver, kidney, and hematologic abnormalities normalized.
Fig 2.
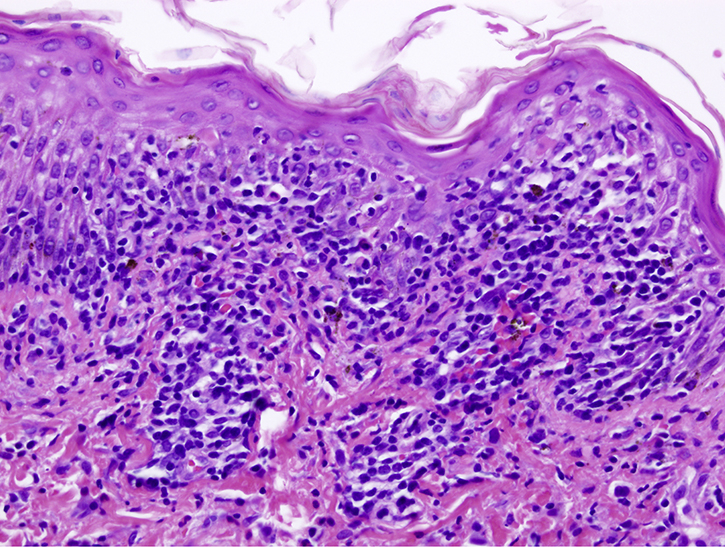
Microscopic examination of skin biopsies shows infiltration of lymphocytes in the dermoepidermal junction with apoptotic keratinocytes, parakeratosis, extravasated erythrocytes, and rare dermal eosinophils. (Hematoxylin-eosin stain; original magnification: ×200.)
Unfortunately, our patient re-presented 9 days later with altered mental status and was admitted for diabetic ketoacidosis with hyperglycemia. Low C-peptide (0.3 ng/mL; normal, 0.9 to 7.1) and glutamic acid decarboxylase (GAD) antibodies (27.1 IU/mL; normal, <5.0) confirmed type 1 diabetes mellitus (T1DM) and indicated an autoimmune pathogenesis. He was monitored closely and provided diabetes education. Because of T1DM, he required a daily regimen of insulin on discharge. He was also discharged on prednisone, 40 mg/d, tapered slowly over 2 months.
Discussion
DRESS is a potentially life-threatening drug reaction characterized by delayed onset, multiorgan involvement, and autoimmune sequelae.1 Pathogenesis of DRESS is not well understood and depends on genetic background, drug metabolism, and virus reactivation. Post-DRESS autoimmune pathogenesis manifestations have not been well defined, but T-regulatory (Treg) cells are believed to play a role by suppressing immune system activation, maintaining self-antigen tolerance, and preventing autoimmune diseases.4 During DRESS resolution, despite being present in normal numbers, Treg cells lose their suppressive ability to inhibit cytokine production and proliferation of effector T cells, contributing to subsequent autoimmune development.
Several studies on sequelae of DRESS have found autoimmune development.1, 5, 6, 7 Rarely reported T1DM cases are predominantly nonautoimmune fulminant type, characterized by rapid onset with absence of islet-related GAD antibodies and near complete destruction of pancreatic β cells, and developing 2 weeks to 10 months after DRESS onset.1 Chen et al5 found 1 of 43 patients who had fulminant T1DM 48 days after DRESS. Kano et al6 reported 5 of 145 patients who had fulminant T1DM within 2 months after DRESS. Chiou et al2 reported 2 of 30 patients who had T1DM of unspecified subtype, 2 and 10 months after DRESS.
In contrast, classic autoimmune T1DM with detection of various autoantibodies including IA2 and anti-GAD is reported less frequently. To date, 3 cases of autoimmune T1DM have been reported (Table I). Ozaki et al8 reported on a 50-year-old man with autoimmune T1DM (GAD antibody 24.1U/mL) diagnosed 4 months after DRESS. Brown et al9 reported on a 15-year-old girl with autoimmune T1DM (elevated GAD, unspecified) and Graves disease 7 months and 5 months, respectively, after DRESS.9 Lan et al10 reported on a 13-year-old girl with autoimmune T1DM (GAD antibody 2 U/mL) development 6 months after DRESS. Our case represents the fourth case of classic autoimmune T1DM after DRESS.
Table I.
Reported cases of autoimmune type 1 diabetes mellitus after DRESS/drug-induced hypersensitivity syndrome
| Reference | Age (y)/gender/race | Culprit drug | Time to T1DM development | Relevant T1DM values | Other sequelae |
|---|---|---|---|---|---|
| 2006 Ozaki et al.8 | 50/male/Japanese | Methimazole | 4 mo | C-peptide, 0.35 ng/mL; GAD antibody, 24.1 U/mL | None |
| 2009 Brown et al.9 | 15/female/— | Minocycline | 7 mo | Unspecified elevated GAD antibody level, detected IA2 antibody titer, HLA DRB1-DQA1-DQB1 haplotypes | Autoimmune thyroiditis, Graves disease; elevated ANA, anti-Smith, and anti-SS-A/Ro antibody titers |
| 2016 Lan et al.10 | 13/female/— | Minocycline | 6 mo | GAD antibody 2 U/mL, negative islet cell antibody | Positive antithyroid peroxidase and antithyroglobulin antibodies |
ANA, Antinuclear antibodies; HLA, human leukocyte antigen.
Our case is distinct from previous cases of autoimmune T1DM with earlier onset of 3 weeks after DRESS, compared with 4 to 7 months in other studies. In our case, an unknown antibiotic that our patient took abroad was thought to be the culprit medication. The cause of autoimmune T1DM was unclear in our patient but presumably involved decreased functional Treg cells during DRESS resolution. Given that T1DM frequently presents as initial diabetic ketoacidosis, it is vital to monitor patients for 7 months or longer after DRESS resolution for early detection and intervention of this and other autoimmune sequelae. Future studies differentiating genetic associations, viral reactivation, and causative drugs in fulminant and autoimmune diabetes will help elucidate a mechanism of development and identify at-risk patients who require long-term monitoring of autoimmune markers after DRESS.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Kano Y., Ishida T., Hirahara K., Shiohara T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med Clin North Am. 2010;94:743–759. doi: 10.1016/j.mcna.2010.03.004. xi. [DOI] [PubMed] [Google Scholar]
- 2.Chiou C.C., Yang L.C., Hung S.I. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. 2008;22:1044–1049. doi: 10.1111/j.1468-3083.2008.02585.x. [DOI] [PubMed] [Google Scholar]
- 3.Shiohara T., Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007;33:124–133. doi: 10.1007/s12016-007-8010-9. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi R., Kano Y., Yamazaki Y., Kimishima M., Mizukawa Y., Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182:8071–8079. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y.C., Chang C.Y., Cho Y.T., Chiu H.C., Chu C.Y. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol. 2013;68:459–465. doi: 10.1016/j.jaad.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Kano Y., Tohyama M., Aihara M. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR) J Dermatol. 2015;42:276–282. doi: 10.1111/1346-8138.12770. [DOI] [PubMed] [Google Scholar]
- 7.Ushigome Y., Kano Y., Ishida T., Hirahara K., Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721–728. doi: 10.1016/j.jaad.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki N., Miura Y., Oiso Y. A case of type 1 diabetes followed by methimazole-induced hypersensitivity syndrome. Diabetes Care. 2006;29:1179–1180. doi: 10.2337/diacare.2951179. [DOI] [PubMed] [Google Scholar]
- 9.Brown R.J., Rother K.I., Artman H. Minocycline-induced drug hypersensitivity syndrome followed by multiple autoimmune sequelae. Arch Dermatol. 2009;145:63–66. doi: 10.1001/archdermatol.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan J., Lahoti A., Lew D.B. A severe case of minocycline-induced DRESS resulting in liver transplantation and autoimmune sequelae. Ann Allergy Asthma Immunol. 2016;116:367–368. doi: 10.1016/j.anai.2015.12.010. [DOI] [PubMed] [Google Scholar]


