Abstract
OBJECTIVE
This study updates previous estimates of the economic burden of diagnosed diabetes and quantifies the increased health resource use and lost productivity associated with diabetes in 2017.
RESEARCH DESIGN AND METHODS
We use a prevalence-based approach that combines the demographics of the U.S. population in 2017 with diabetes prevalence, epidemiological data, health care cost, and economic data into a Cost of Diabetes Model. Health resource use and associated medical costs are analyzed by age, sex, race/ethnicity, insurance coverage, medical condition, and health service category. Data sources include national surveys, Medicare standard analytical files, and one of the largest claims databases for the commercially insured population in the U.S.
RESULTS
The total estimated cost of diagnosed diabetes in 2017 is $327 billion, including $237 billion in direct medical costs and $90 billion in reduced productivity. For the cost categories analyzed, care for people with diagnosed diabetes accounts for 1 in 4 health care dollars in the U.S., and more than half of that expenditure is directly attributable to diabetes. People with diagnosed diabetes incur average medical expenditures of ∼$16,750 per year, of which ∼$9,600 is attributed to diabetes. People with diagnosed diabetes, on average, have medical expenditures ∼2.3 times higher than what expenditures would be in the absence of diabetes. Indirect costs include increased absenteeism ($3.3 billion) and reduced productivity while at work ($26.9 billion) for the employed population, reduced productivity for those not in the labor force ($2.3 billion), inability to work because of disease-related disability ($37.5 billion), and lost productivity due to 277,000 premature deaths attributed to diabetes ($19.9 billion).
CONCLUSIONS
After adjusting for inflation, economic costs of diabetes increased by 26% from 2012 to 2017 due to the increased prevalence of diabetes and the increased cost per person with diabetes. The growth in diabetes prevalence and medical costs is primarily among the population aged 65 years and older, contributing to a growing economic cost to the Medicare program. The estimates in this article highlight the substantial financial burden that diabetes imposes on society, in addition to intangible costs from pain and suffering, resources from care provided by nonpaid caregivers, and costs associated with undiagnosed diabetes.
Introduction
Diabetes imposes a substantial burden on society in the form of higher medical costs, lost productivity, premature mortality, and intangible costs in the form of reduced quality of life. The estimated economic burden associated with diagnosed diabetes in the U.S. in 2012 was $245 billion in the form of higher medical costs ($176 billion) and reduced productivity ($69 billion) (1). The population diagnosed with diabetes has continued to grow, by ∼700,000 people annually between 2012 and 2015, with prevalence projected to continue rising over time as the population grows and ages (2,3). Furthermore, there continue to be changes in the demographics of the population with diabetes, health care use and delivery patterns, technology, medical costs, insurance coverage, and economic conditions that affect the economic burden associated with diabetes. This study updates previous estimates, with the goal to quantify the economic burden of diabetes at the national and state levels in 2017. Such information can help inform and motivate strategies to reduce diabetes prevalence and burden.
Research Design and Methods
The methodology used is similar to that of previous diabetes burden studies sponsored by the American Diabetes Association (1,4), with updated data sources and modifications to refine the analyses where appropriate. Although the primary focus of this analysis is the national economic burden of disease, the national estimates are calculated by summing the state-level estimates that reflect variation across states in demographics, health risk factors and lifestyle choices, prices, and economic outcomes. (State-level estimates of diabetes prevalence and costs are provided in Supplementary Table A-16.) All cost and utilization estimates are extrapolated to the U.S. population in 2017, with cost estimates calculated in 2017 dollars using the hospital services, physician services, and prescription drug components of the medical consumer price index or total consumer price index (5).
Inputs to the study include both state-level and national-level data. Sources for state-level data include the American Community Survey (ACS), Behavioral Risk Factor Surveillance System (BRFSS), Medicare Current Beneficiary Survey (MCBS), and Long Term Care Minimum Data Set (MDS). Sources for national data (which are extrapolated to the state level) include the Current Population Survey (CPS), OptumInsight de-identified Normative Health Information (dNHI) database, Medical Expenditure Panel Survey (MEPS), National Ambulatory Medical Care Survey (NAMCS), National Hospital Ambulatory Medical Care Survey (NHAMCS), National Home and Hospice Care Survey (NHHCS), National Health Interview Survey (NHIS), National (Nationwide) Inpatient Sample (NIS), and Medicare 5% sample Standard Analytical Files (SAFs). We use the most recent year’s data available for each of these data sources, though for certain analyses we combine multiple years of data to increase sample size. Supplementary Table A-1 describes how these data sources are used along with their respective strengths and limitations as pertinent to this study.
Estimating the Size of the Population With Diabetes
For each of the 50 states and the District of Columbia, we estimate the prevalence of diagnosed diabetes for 480 population strata defined by age-group (<18, 18–34, 35–44, 45–54, 55–59, 60–64, 65–69, and ≥70 years), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, non-Hispanic other, and Hispanic), insurance status (commercial; government, including Medicare, Medicaid, Children's Health Insurance Program, Veterans Health Administration, and other government-sponsored coverage; and uninsured), and whether residing in the community, a residential care facility, or a nursing home. (Government employees and military personnel and dependents with insurance are counted under private insurance.) The reason for modeling the large number of strata reflects differences in diabetes prevalence and costs across these strata and that different data sources are used to estimate diabetes prevalence for people residing in the community, in a residential care facility, or in a nursing home.
The population database starts with the 2016 ACS, which contains state-level population estimates by age, sex, race/ethnicity, whether the person has medical insurance, and whether the person resides in a group setting. We use random sampling with replacement to statistically match each person in the 2016 ACS with a similar person in a file containing patient health information and risk factors. ACS individuals residing in the community are matched to a similar individual in the 2015–2016 BRFSS of the same age, sex, race/ethnicity, state, family income level, and insurance type. ACS individuals residing in residential care facilities and nursing homes are matched to a person of similar age, sex, race/ethnicity, and state from the 2015 MDS and 2013 MCBS, respectively.
Diabetes status in the MDS and MCBS is based on clinical diagnosis, whereas diabetes status in the BRFSS is based on respondents answering “yes” to the question, “Have you EVER been told by a doctor or health professional that you have diabetes or sugar diabetes?” The prevalence estimates exclude gestational diabetes mellitus. These sources do not contain diabetes status for children. Therefore, we combined the 2014–2016 NHIS files to estimate national diabetes prevalence rates for children—based on self-report (6) like the BRFSS information—which we then extrapolated to the state population files by age (6–12 and 13–17 years), sex, and race/ethnicity.
To estimate diabetes prevalence in 2017, we scaled the state estimates based on population growth between 2016 and 2017 by demographic group. For validation, when we apply prevalence rates for each strata (demographic, insurance, state) to the 2015 population, our national estimate of diagnosed diabetes is slightly higher than that reported by the Centers for Disease Control and Prevention (CDC) (23.4 million vs. 23.0 million). Our higher estimate possibly reflects that our analysis incorporates data from residential care and nursing facilities, whereas the CDC estimate is based on a representative sample of the noninstitutionalized population.
Estimating the Direct Medical Cost Attributed to Diabetes
We estimate health resource use among the population with diabetes in excess of resource use that would be expected in the absence of diabetes. Diabetes increases the risk of developing neurological, peripheral vascular, cardiovascular, renal, endocrine/metabolic, ophthalmic, and other complications (see Supplementary Appendix 2 for a more comprehensive list of medical conditions and ICD-9 and ICD-10 codes). Diabetes also increases the cost of treating general conditions that are not directly related to diabetes. Therefore, only the relevant portion of health care expenditures for these medical conditions is attributed to diabetes.
The approach used to quantify the excess health resource use associated with diabetes was influenced by four data limitations: 1) absence of a single data source for all estimates, 2) small sample size in some data sources, 3) correlation of both diabetes and its comorbidities with other factors such as age and obesity, and 4) underreporting of diabetes and its comorbidities in certain data sources such as the NIS, NAMCS, and NHAMCS. Because of these limitations, we estimate diabetes-attributed costs using one of two approaches for each cost component.
For cost components estimated solely from MEPS (ambulance services, home health, podiatry, diabetes supplies, and other equipment and supplies), we use a comparison of annual per capita health resource use for people with and without diabetes controlling for age, sex, and race/ethnicity. For nursing/residential facility use (which is not captured by MEPS) and for cost components that rely on analysis of medical encounter data (hospital inpatient, emergency care, and ambulatory visits), we employ an attributed risk methodology often used in disease burden studies that relies on population etiological fractions (7). Etiological fractions estimate the excess use of health care services among the diabetes population relative to a similar population that does not have diabetes. Both approaches used in this study are equivalent under a reasonable set of assumptions. However, the first approach cannot be used with some national data sources analyzed—e.g., visit/hospital discharge–level files such as NIS, NAMCS, and NHAMCS, which may not identify the patient as having diabetes even if the patient does indeed have diabetes.
The attributable fraction approach combines etiological fractions (ε) with total projected U.S. health service use (U) in 2017 for each age-group (a), sex (s), medical condition (c), and care delivery setting (H), which includes hospital inpatient, emergency department, and ambulatory service (physician office visits and hospital outpatient/clinic visits):
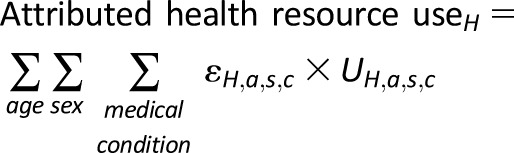 |
The etiological fraction is calculated using the diagnosed diabetes prevalence (P) and the relative rate ratio (R):
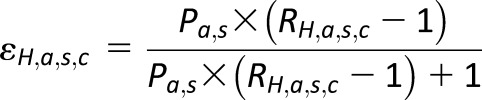 |
The rate ratio for hospital inpatient days, emergency visits, and ambulatory visits represents how annual per capita health service use for the population with diabetes compares to the population without diabetes:
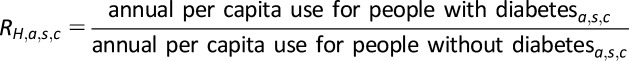 |
Diabetes and its comorbidities are correlated with other patient characteristics such as demographics and body weight. To mitigate bias caused by correlation, we estimate age/sex/setting–specific etiological fractions for each medical condition. The primary data sources for calculating etiological fractions are the 2015 OptumInsight dNHI data and the 2014 Medicare 5% sample SAF. The dNHI data contain a complete set of medical claims for more than 31 million commercially insured beneficiaries in 2015 and allows patient records to be linked during the year and across health delivery settings. This allows us to identify people with a diabetes ICD-9 (250.xx) or ICD-10 diagnosis code in at least one of their inpatient medical claims or in two or more separate noninpatient claims during the year. The Medicare 5% sample SAF contains claims data filed on behalf of Medicare beneficiaries under both Part A and Part B, and as with the dNHI data, we identify people with diabetes based on diabetes ICD-9 diagnosis codes. The large size of these two claims databases enables the generation of age/sex/setting–specific rate ratios for each medical condition that are more stable than the rates estimated using MEPS.
Unlike the MEPS data, the dNHI data and Medicare 5% claims data do not contain race/ethnicity and select patient characteristics that could affect both patient health status and health-seeking behaviors. For the 10 medical conditions that are the largest contributors to the overall cost of diabetes—general medical condition, other chronic ischemic heart disease, myocardial infarction, heart failure, hypertension, conduction disorders and cardiac dysrhythmias, cellulitis, occlusion of cerebral arteries, end-stage renal disease (ESRD), and renal failure and its sequelae—we estimate two multivariate Poisson regressions, using data from 2011–2015 MEPS, to determine the extent to which controlling only for age and sex might bias the rate ratios. First, we estimate a naive model that produces diabetes-related rate ratios for hospital inpatient days, emergency visits, and ambulatory visits controlling for age and sex only. Then, we estimate a full model that includes diabetes status as the main explanatory variable and various known predictors of health service utilization including age, sex, education level, income, marital status, medical insurance status, and race/ethnicity as covariates.
For the full model, our focus is not on the relationship between health care use and the covariates (other than diabetes); instead, these covariates are included to control for patient characteristics not available in medical claims data that could be correlated with both medical conditions and health-seeking behavior. The full model omits indicators for presence of coexisting conditions or complications of diabetes (e.g., hypertension), since including such variables could downward bias the estimated relationship between diabetes and health care use for each of the 10 medical conditions. The rate ratio coefficients for the diabetes flag variable in the naive and full models are then compared. The findings suggest statistically significant overestimates of the rate ratios for eight condition categories for both emergency visits and impatient days when using the naive model. For ambulatory visits, we find significant overestimates in the rate ratios for five condition categories from the MEPS-based naive model compared with the full model.
To remedy the relative risk overestimation for these condition categories, we scaled the rate ratios estimated from dNHI and Medicare 5% sample SAFs using the regression results from the MEPS analysis by applying a scalar (with the scalar calculated as the full model rate ratio divided by the naive model rate ratio). For emergency department visits, claims-based rate ratios are scaled down for other chronic ischemic heart disease (scale = 0.89), myocardial infarction (0.89), heart failure (0.86), hypertension (0.63), cellulitis (0.89), occlusion of cerebral arteries (0.94), chronic renal failure–ESRD (0.73), and renal failure and its sequelae (0.77). For inpatient days, claims-based rate ratios are scaled down for other chronic ischemic heart disease (0.99), myocardial infarction (0.92), heart failure (0.81), hypertension (0.69), cellulitis (0.85), occlusion of cerebral arteries (0.98), chronic renal failure–ESRD (0.72), and renal failure and its sequelae (0.64). Physician office visits are scaled down for myocardial infarction (0.98), heart failure (0.76), hypertension (0.87), occlusion of cerebral arteries (0.93), and renal failure and its sequelae (0.25). We did not find a significant overestimate of the rate ratios for general medical conditions for any of the three health service delivery settings comparing the MEPS-based naive model and the full model. However, a comparison of the claims-based rate ratios with the rate ratios calculated from the MEPS-based naive model finds that the claims-based rate ratios for general conditions are significantly higher than the MEPS-based rate ratios for emergency department visits and inpatient days. Therefore, to be conservative in our cost estimates, we downward adjusted claims-based rate ratios for emergency department visits (0.52) and inpatient days (0.50) for the general condition group by applying a scalar calculated as the MEPS-based naive model rate ratio divided by the claims-based rate ratio.
Estimates of health resource use attributed to diabetes are combined with estimates of the average medical cost per unit of health care utilization, in 2017 dollars, to compute total medical costs attributed to diabetes. For hospital inpatient days, office visits, emergency visits, and outpatient visits, we use the average cost per visit/day specific to the medical conditions modeled. We pooled the 2011–2015 MEPS files to estimate average cost per unit of health care utilized. Although MEPS contains both inpatient facility and professional expenditures and NIS contains only facility charges (which are converted to costs using hospital-specific cost-to-charge ratios), the NIS has a much larger sample (n = ∼7 million discharges in 2014) and also contains five-digit diagnosis codes. Therefore, we use the 2014 NIS data to estimate inpatient facility costs and use the pooled 2011–2015 MEPS files to estimate the cost for professional services. Average costs per event or day by medical condition are shown in Supplementary Table A-3.
Utilization of prescription medication (excluding insulin and other antidiabetes agents) for each medical condition is estimated from medications prescribed during physician office, emergency department, and outpatient visits attributed to diabetes. Average number of medications prescribed during a physician office visit for each age/sex/race stratum is estimated using data from the 2013–2015 NAMCS along with 2012–2014 NHAMCS for emergency department visits and 2009–2011 NHAMCS for outpatient visits. We calculate the total number of people with diabetes who use insulin and other antidiabetes agents by combining diabetes prevalence and the rate of use for these antidiabetes agents obtained from the 2013–2015 NHIS. Average cost per prescription filled, yearly average cost per insulin user, and yearly average cost per oral agent and other antidiabetes agent user are obtained from the 2013–2015 MEPS. We combined the utilization of these medications with the average cost per prescription to estimate the cost by age, sex, race/ethnicity, and insurance status. Average per capita cost for diabetes supplies by age/sex/race stratum is calculated from MEPS (excluding over-the-counter medications owing to lack of data on whether diabetes increases use of such medications).
The 2012 cost study estimated prevalence of diagnosed diabetes among the population in nursing homes by demographic using the 2004 National Nursing Home Survey (NNHS) data but scaled the diabetes prevalence estimates to be consistent with an estimated 32.8% prevalence among nursing home residents obtained from the existing literature (8). In this iteration of the study, we use the 2015 Centers for Medicare & Medicaid Services (CMS) MDS data to estimate diabetes prevalence among this population and find that the estimated prevalence of diagnosed diabetes is 25% among the nursing home population in 2017.
Nursing/residential facility use attributed to diabetes is estimated using an attributable risk approach where the prevalence of diabetes among residents is compared with the prevalence of diabetes among the overall population in the same age/sex stratum. The analysis is conducted separately for long-stay and residential facility residents to estimate total days of care. Unlike the 2012 study, due to data unavailability there is no separate analysis done for short stays at nursing/residential facilities. Similar to the previous studies, cost per day per resident is obtained from a geographically representative cost of care survey for 2017 (9).
Hospice days attributed to diabetes represent a combination of length of stay and diabetes prevalence among hospice residents. The 2007 NHHCS is used to calculate the number of hospice residents with diabetes and those that have a primary diagnosis of diabetes along with the average length of stay for each age/sex/race stratum. Based on more recent estimates available from the National Hospice and Palliative Care Organization (NHPCO) on diabetes prevalence among hospice residents (10), the 2007 NHHCS-based prevalence estimates for the various strata are adjusted and updated to impute the 2017 diabetes prevalence. Cost per hospice resident per day is based on the 2017 report from NHPCO (11) and is combined with hospice days attributed to diabetes to estimate total cost of hospice care attributed to diabetes.
The 2011–2015 MEPS files are pooled to increase sample size to analyze use of home health, podiatry, ambulance services, and other equipment and supplies. These cost components are estimated by comparing annual per capita cost for people with and without diabetes, controlling for age. Due to small sample size, sex and race/ethnicity are not included as a stratum when calculating costs per capita.
Estimating the Indirect Cost Attributed to Diabetes
The indirect costs associated with diabetes include work days missed due to health conditions (absenteeism), reduced work productivity while working due to health conditions (presenteeism), reduced workforce participation due to disability, household productivity losses, and lost productivity due to premature mortality (12). The approach mirrors that used in the 2012 study but with more recent data.
Absenteeism is defined as the number of work days missed due to poor health among employed individuals, and prior research finds that people with diabetes have higher rates of absenteeism than the population without diabetes. Estimates from the literature range from no statistically significant diabetes effect on absenteeism to studies reporting 1–6 extra missed work days (and odds ratios of more absences ranging from 1.5 to 3.3) (12–14). Analyzing 2014–2016 NHIS data and using a negative binomial regression to control for overdispersion in self-reported missed work days, we estimate that people with diabetes have statistically higher missed work days—ranging from 1.0 to 4.2 additional days missed per year by demographic group, or 1.7 days on average—after controlling for age-group, sex, race/ethnicity, diagnosed hypertension status (yes/no), and body weight status (normal, overweight, obese, unknown). Diabetes is entered as a dichotomous variable (diagnosed diabetes = 1; otherwise 0) as well as an interaction term with age-group. Controlling for hypertension and body weight produces more conservative estimates of the diabetes impact on absenteeism, as comorbidities of diabetes are correlated with body weight status and a portion of hypertension is attributed to diabetes.
Presenteeism is defined as reduced productivity while at work among employed individuals and is generally measured through worker responses to surveys. These surveys rely on the self-reported inputs on the number of reduced productivity hours incurred over a given time frame. Multiple recent studies report that individuals with diabetes display higher rates of presenteeism than their peers without diabetes (12,15–17). We model productivity loss associated with diabetes-attributed presenteeism using the estimate (6.6%) from the 2012 study—which is toward the lower end of the 1.8–38% range reported in the literature.
Inability to work associated with diabetes is estimated using a conservative approach that focuses on unemployment related to long-term disability. Logistic regression with 2014–2016 NHIS data suggests that people aged 18–65 years with diabetes are significantly less likely to be in the workforce than people without diabetes. It is unclear to what extent people with diabetes voluntarily leave the workforce or do so because of diabetes. Therefore, we use a conservative approach (which likely underestimates the cost associated with inability to work) to estimate the economic burden associated with reduced labor force participation. Using logistic regression, we estimate the relationship between diabetes and receipt of Supplemental Security Income (SSI) payments for disability—controlling for age-group, sex, race/ethnicity, hypertension status, and body weight status (normal, overweight, obese). Diabetes status is included in the regression both as a separate variable and interacted with age-group to provide age-specific impacts. Study results suggest that people with diabetes have a 3.1 percentage point higher rate of being out of the workforce and receiving disability payments compared with their peers without diabetes. The diabetes effect increases with age and varies by demographic—ranging from 2.1 percentage points for non-Hispanic white males aged 60–64 years to 10.6 percentage points for non-Hispanic black females aged 55–59 years. The average daily earnings estimated from the CPS for those in the workforce are used as a proxy for the economic impact of reduced employment due to chronic disability. SSI payments are considered transfer payments and therefore are not included in the cost estimates.
Reduced productivity for those not in the workforce is included in our estimate of the national burden. This population includes all adults aged <65 years who are not employed (including those voluntarily or involuntarily not in the workforce). The contribution of people not in the workforce to national productivity includes time spent providing child care, household activities, and other activities such as volunteering in the community. We use per capita absenteeism estimates for the working population as a proxy for reduced productivity days among the nonemployed population in a similar demographic. Whereas each work day lost due to absenteeism is based on estimated average daily earnings, there is no readily available measure of the value of a day lost for those not in the workforce. Some studies use minimum wage as a proxy for the value of time lost, but this may underestimate the value of time. Using average earnings for their employed counterparts will overestimate the value of time. Similar to the 2012 study, we use 75% of the average earnings for people in the workforce as a productivity proxy for those aged <65 years not in the labor force (which is close to the midpoint between minimum wage and average hourly wage earned by a demographic similar to the unemployed aged <65 years).
Premature mortality associated with diabetes reduces future productivity (and not just the current year productivity). Ideally, to model the value of lost productivity in 2017 associated with premature mortality, one would calculate the number and characteristics of all people who would have been alive in 2017 but who died prior to 2017 because of diabetes. Data limitations prevent using this approach. Instead, we estimate the number of premature deaths associated with diabetes in 2017 and calculate the present value of their expected future earnings. To estimate the total number of deaths attributable to diabetes, we analyzed the CDC’s 2015 Mortality Multiple Cause File to obtain mortality data by age, sex, and race/ethnicity for cardiovascular disease, cerebrovascular disease, renal failure, and diabetes. We use the same estimates as our previous study: ∼16% of cardiovascular disease (excluding cerebrovascular disease) deaths can be attributed to diabetes, and ∼28% of deaths listing cerebrovascular disease as the primary cause and ∼55% of deaths listing renal failure as the primary cause can be attributed to diabetes. To generate 2017 estimates, we grow the 2015 CDC mortality data using the annual population growth rate from 2015 to 2017 for each age, sex, and race/ethnicity group.
Productivity loss associated with early mortality is calculated by taking the net present value of future productivity (PVFP) for men and women by age and race/ethnicity using the same discount rate (3%), assumptions, and equation outlined in the 2008 American Diabetes Association report (4). We combined average annual earnings from the CPS, expected mortality rates from the CDC, and employment rates from the CPS by age, sex, and race/ethnicity to calculate the net present value of future earnings of a person who dies prematurely. Employment rates for 2015 are used to calculate PVFP.
We do not count productivity loss for the population aged <18 years. While children constitute a small proportion of the population with diabetes, omitting productivity loss associated with diabetes among children could bias low the cost estimates. For example, the economic cost associated with parents who take time off from work to take their children to the doctor for diabetes-related visits is omitted from these cost estimates.
Results
In 2017, an estimated 24.7 million people in the U.S. are diagnosed with diabetes, representing ∼7.6% of the total population (and 9.7% of the adult population). The estimated national cost of diabetes in 2017 is $327 billion, of which $237 billion (73%) represents direct health care expenditures attributed to diabetes and $90 billion (27%) represents lost productivity from work-related absenteeism, reduced productivity at work and at home, unemployment from chronic disability, and premature mortality. Particularly noteworthy is that excess costs associated with medications constitute 43% of the total direct medical burden. This includes nearly $15 billion for insulin, $15.9 billion for other antidiabetes agents, and $71.2 billion in excess use of other prescription medications attributed to higher disease prevalence associated with diabetes.
Health Resource Use Attributed to Diabetes
Table 1 shows estimates of health resource utilization attributed to diabetes and incurred by people with diabetes as a percentage of total national utilization. For example, of the projected 162 million hospital inpatient days in the U.S. in 2017, an estimated 40.3 million days (24.8%) are incurred by people with diabetes, of which 22.6 million days are attributed to diabetes. About one-fourth of all nursing/residential facility days are incurred by people with diabetes. About half of all physician office visits, emergency department visits, hospital outpatient visits, and medication prescriptions (excluding insulin and other antidiabetes agents) incurred by people with diabetes are attributed to their diabetes.
Table 1.
Health resource use in the U.S., by diabetes status and type of service, 2017 (in millions of units)
| Health resource | Population with diabetes | Incurred by population without diabetes | U.S. total* | |||
|---|---|---|---|---|---|---|
| Attributed to diabetes | Incurred by people with diabetes | |||||
| Units | % of U.S. total | Units | % of U.S. total | |||
| Institutional care | ||||||
| Hospital inpatient days | 22.6 | 13.9 | 40.3 | 24.8 | 122.2 | 162 |
| Nursing/residential facility days | 57.3 | 7.5 | 200.0 | 26.1 | 567.3 | 767 |
| Hospice days | 0.3 | 0.3 | 14.2 | 12.7 | 97.8 | 112 |
| Outpatient care | ||||||
| Physician office visits | 121.6 | 12.5 | 208.6 | 21.5 | 760.4 | 969 |
| Emergency department visits | 7.2 | 5.2 | 16.8 | 12.2 | 121.1 | 138 |
| Hospital outpatient visits | 13.5 | 11.7 | 22.2 | 19.2 | 93.0 | 115 |
| Home health visits | 10.1 | 5.0 | 43.0 | 21.2 | 159.9 | 203 |
| Medication prescriptions | 664.4 | 16.6 | 1,092.8 | 27.4 | 2,898.0 | 3,991 |
Data sources: NIS (2014), CMS MDS (2013), NAMCS (2013–2015), NHAMCS (2012–2014), MEPS (2011–2015), and NHHCS (2007), OptumInsight dNHI (2015), and Medicare 5% SAFs (2014).
Numbers do not necessarily sum to totals because of rounding.
Health Care Expenditures Attributed to Diabetes
Health care expenditures attributed to diabetes reflect the additional expenditures the nation incurs because of diabetes. This equates to the total health care expenditures for people with diabetes minus the projected level of expenditures that would have occurred for those people in the absence of diabetes. Table 2 summarizes national expenditure for the cost components included, accounting for nearly $1.7 trillion in projected expenditure for 2017. Approximately $414 billion of the total is incurred by people with diabetes, reflecting 1 in 4 (24%) of all health care dollars. Costs attributed to diabetes exceed $237 billion, or 57% of total medical costs incurred by people with diabetes. For the cost components included, 1 in every 7 health care dollars (14%) is attributed to diabetes.
Table 2.
Health care expenditures in the U.S., by diabetes status and type of service, 2017 (in millions of dollars)
| Cost component | Population with diabetes | Population without diabetes | Total* | |||
|---|---|---|---|---|---|---|
| Attributed to diabetes | Total incurred by people with diabetes | |||||
| Dollars | % of U.S. total | Dollars | % of U.S. total | |||
| Institutional care | ||||||
| Hospital inpatient | 69,661 | 14 | 122,729 | 25 | 362,855 | 485,584 |
| Nursing/residential facility | 6,439 | 7 | 24,484 | 25 | 71,934 | 96,419 |
| Hospice | 64 | 0.3 | 3,180 | 13 | 21,933 | 25,114 |
| Outpatient care | ||||||
| Physician office | 29,990 | 12 | 51,882 | 21 | 190,024 | 241,906 |
| Emergency department | 7,990 | 5 | 18,651 | 12 | 133,894 | 152,545 |
| Ambulance services | 332 | 8 | 700 | 17 | 3,356 | 4,056 |
| Hospital outpatient | 12,049 | 10 | 21,012 | 18 | 98,872 | 119,884 |
| Home health | 3,388 | 5 | 14,479 | 21 | 53,824 | 68,303 |
| Podiatry | 252 | 10 | 607 | 25 | 1,835 | 2,442 |
| Outpatient medications and supplies | ||||||
| Insulin | 14,981 | 100 | 14,981 | 100 | 0 | 14,981 |
| Diabetes supplies | 3,723 | 100 | 3,723 | 100 | 0 | 3,723 |
| Other antidiabetes agents† | 15,855 | 100 | 15,855 | 100 | 0 | 15,855 |
| Prescription medications | 71,235 | 17 | 117,160 | 27 | 310,697 | 427,856 |
| Other equipment and supplies‡ | 1,310 | 4 | 4,564 | 16 | 24,796 | 29,360 |
| Total | 237,269 | 14 | 414,427 | 24 | 1,277,908 | 1,692,335 |
Data sources: NIS (2014), CMS MDS (2013), NAMCS (2013–2015), NHAMCS (2012–2014), MEPS (2011–2015), NHHCS (2007), NHIS (2014–2016), OptumInsight dNHI (2015), and Medicare 5% SAFs (2014).
Numbers do not necessarily sum to totals because of rounding.
Includes oral medications and noninsulin injectable antidiabetes agents such as exenatide and pramlintide.
Includes but is not limited to eyewear, orthopedic items, hearing devices, prosthesis, bathroom aids, medical equipment, and disposable supplies.
National health-related expenditures are projected to exceed $3.5 trillion in 2017 (18), but slightly less than half of these expenditures are included in our analysis. These cost estimates omit national expenditures (and any portion of such expenditures that might be attributable to diabetes) for administering government health and private insurance programs, investment in research and infrastructure, over-the-counter medications, disease management and wellness programs, and office visits to nonphysician providers other than podiatrists (e.g., dentists and optometrists).
The largest contributors to the cost of diabetes are higher use of prescription medications beyond antihyperglycemic medications ($71.2 billion), higher use of hospital inpatient services ($69.7 billion), medications and supplies to directly treat diabetes ($34.6 billion), and more office visits to physicians and other health providers ($30.0 billion).
Approximately 61% of all health care expenditures attributed to diabetes are for health resources used by the population aged ≥65 years, much of which is borne by the Medicare program (Table 3). Dividing total attributed health care expenditures by the number of people with diabetes, we estimate the average annual excess expenditures for the population aged <65 years and ≥65 years, respectively, at $6,675 and $13,239. Health care expenditures attributed to diabetes generally increase with age, although among younger people, average costs are slightly higher likely due to a higher proportion of these cases being type 1 versus type 2 diabetes, are slightly higher for men (mainly due to men having higher attributable fractions on several key measures), and are highest for the non-Hispanic black population due to a higher use of emergency care and hospital outpatient care (Table 4).
Table 3.
Health care expenditures attributed to diabetes in the U.S., by age-group and type of service, 2017 (in millions of dollars, with percentages in parentheses)
| Cost component | Age (years) | ||
|---|---|---|---|
| <65 (N = 13.7 million) | ≥65 (N = 11.0 million) | Total* (N = 24.7 million) | |
| Institutional care | |||
| Hospital inpatient | 24,835 (36) | 44,826 (64) | 69,661 |
| Nursing/residential facility | 2,568 (40) | 3,871 (60) | 6,439 |
| Hospice | 6 (9) | 58 (91) | 64 |
| Outpatient care | |||
| Physician office | 9,591 (32) | 20,399 (68) | 29,990 |
| Emergency department | 4,258 (53) | 3,732 (47) | 7,990 |
| Ambulance services | 105 (32) | 227 (68) | 332 |
| Hospital outpatient | 5,322 (44) | 6,728 (56) | 12,049 |
| Home health | 2,588 (76) | 801 (24) | 3,388 |
| Podiatry | 94 (37) | 158 (63) | 252 |
| Outpatient medications and supplies | |||
| Insulin | 8,850 (59) | 6,132 (41) | 14,981 |
| Diabetes supplies | 2,272 (61) | 1,452 (39) | 3,723 |
| Other antidiabetes agents† | 8,456 (53) | 7,399 (47) | 15,855 |
| Prescription medications | 21,702 (30) | 49,534 (70) | 71,235 |
| Other equipment and supplies‡ | 783 (60) | 527 (40) | 1,310 |
| Total* | 91,428 (39) | 145,841 (61) | 237,269 |
| Average cost per person with diabetes (actual dollars) | 6,675 | 13,239 | 9,601 |
Data sources: NIS (2014), CMS MDS (2013), NAMCS (2013–2015), NHAMCS (2012–2014), MEPS (2011–2015), NHHCS (2007), NHIS (2014–2016), OptumInsight dNHI (2015), and Medicare 5% SAFs (2014).
Numbers do not necessarily sum to totals because of rounding.
Includes oral medications and noninsulin injectable antidiabetes agents.
Includes but is not limited to eyewear, orthopedic items, hearing devices, prosthesis, bathroom aids, medical equipment, and disposable supplies.
Table 4.
Health care expenditures attributed to diabetes in the U.S., by demographic
| Characteristics | Diabetes prevalence | Total direct cost ($, millions) | Average cost per person with diabetes ($, actual) |
|---|---|---|---|
| Age (years) | |||
| <18 | 110,000 | 860 | 7,510 |
| 18–34 | 1,020,000 | 6,850 | 6,740 |
| 35–44 | 1,920,000 | 10,510 | 5,480 |
| 45–54 | 4,060,000 | 26,140 | 6,440 |
| 55–59 | 3,050,000 | 22,600 | 7,400 |
| 60–64 | 3,530,000 | 24,460 | 6,920 |
| 65–69 | 3,590,000 | 46,710 | 13,030 |
| ≥70 | 7,430,000 | 99,140 | 13,340 |
| Sex | |||
| Male | 12,810,000 | 128,830 | 10,060 |
| Female | 11,900,000 | 108,450 | 9,110 |
| Race/ethnicity | |||
| White, non-Hispanic | 15,080,000 | 150,260 | 9,800 |
| Black, non-Hispanic | 4,030,000 | 42,240 | 10,470 |
| Other, non-Hispanic | 1,890,000 | 14,880 | 7,890 |
| Hispanic | 3,710,000 | 29,900 | 8,050 |
Data sources: NIS (2014), CMS MDS (2013), NAMCS (2013–2015), NHAMCS (2012–2014), MEPS (2011–2015), NHHCS (2007), NHIS (2014–2016), OptumInsight dNHI (2015), and Medicare 5% SAFs (2014).
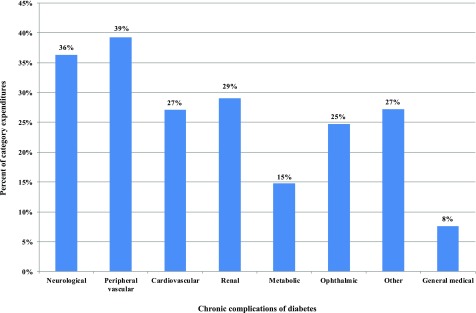
Figure 1 summarizes the proportion of medical expenditures attributed to diabetes for each chronic complication over total U.S. health care expenditure, combining expenditures for hospital inpatient, hospital outpatient, emergency department, and physician and other provider office visits as well as prescription medications. For patients with diabetes who receive care for peripheral vascular conditions, 39% of these expenditures are attributed to diabetes. For the general medical conditions category (which includes all care not included in the other categories), 8% of expenditures incurred by people with diabetes are attributed to their diabetes.
Figure 1.
Percent of medical condition-specific expenditures associated with diabetes. Data sources: NIS (2014), CMS MDS (2013), NAMCS (2013–2015), NHAMCS (2012–2014), MEPS (2011–2015), NHHCS (2007), NHIS (2014–2016), OptumInsight dNHI (2015), and Medicare 5% SAFs (2014). See Supplementary Appendix 2 for diagnosis codes for each category of medical condition.
The population with diabetes is older and sicker than the population without diabetes, and consequently annual medical expenditures are much higher (on average) than for people without diabetes (Table 5). When we compare expenditures for people with diabetes to expenditures for a population of similar age and sex, people with diabetes have health care expenditures that are 2.3 times higher ($16,752 vs. $7,151) than expenditures would be expected for this same population in the absence of diabetes. This suggests that diabetes is responsible for an estimated $9,601 in excess expenditures per year per person with diabetes. This 2.3 multiple is unchanged from the 2007 and 2012 studies.
Table 5.
Annual per capita health care expenditures in the U.S., by diabetes status, 2017 (in actual dollars)
| Cost component | With diabetes ($) | Unadjusted | Adjusted for age and sex | |||
|---|---|---|---|---|---|---|
| Without diabetes ($) | Ratio with to without diabetes | Without diabetes ($) | Ratio with to without diabetes | Attributed to diabetes ($)* | ||
| Institutional care | ||||||
| Hospital inpatient | 4,966 | 1,202 | 4.1 | 2,147 | 2.3 | 2,819 |
| Nursing/residential facility | 991 | 238 | 4.2 | 730 | 1.4 | 261 |
| Hospice | 129 | 73 | 1.8 | 126 | 1.0 | 3 |
| Outpatient care | ||||||
| Physician office | 2,099 | 629 | 3.3 | 886 | 2.4 | 1,213 |
| Emergency | 755 | 443 | 1.7 | 431 | 1.7 | 323 |
| Ambulance services | 28 | 11 | 2.5 | 15 | 1.9 | 13 |
| Hospital outpatient and freestanding ambulatory surgical center | 850 | 327 | 2.6 | 363 | 2.3 | 488 |
| Home health | 586 | 178 | 3.3 | 449 | 1.3 | 137 |
| Podiatry | 25 | 6 | 4.0 | 14 | 1.7 | 10 |
| Outpatient medications and supplies | ||||||
| Insulin | 606 | NA | NA | NA | NA | 606 |
| Diabetes supplies | 151 | NA | NA | NA | NA | 151 |
| Other antidiabetes agents† | 642 | NA | NA | NA | NA | 641 |
| Prescription medications | 4,741 | 1,029 | 4.6 | 1,858 | 2.6 | 2,882 |
| Other equipment and supplies‡ | 185 | 82 | 2.2 | 132 | 1.4 | 53 |
| Total* | 16,752 | 4,220 | 4.0 | 7,151 | 2.3 | 9,601 |
Data sources: NIS (2014), CMS MDS (2013), NAMCS (2013–2015), NHAMCS (2012–2014), MEPS (2011–2015), NHHCS (2007), NHIS (2014–2016), OptumInsight dNHI (2015), Medicare 5% SAFs (2014), and U.S. Census Bureau (2017). NA, not applicable.
Numbers do not necessarily sum to totals because of rounding.
Includes antidiabetes agents such as exenatide and pramlintide.
Includes but is not limited to eyewear, orthopedic items, hearing devices, prosthesis, bathroom aids, medical equipment, and disposable supplies.
After adjusting for inflation, the total cost of insulin and other medications to control blood glucose increased by 45% from 2012 to 2017, to a total of $31 billion. The inflation-adjusted cost of insulin increased by 110% during the same period. These increases are attributable to both an increase in the number of people using these medications and the cost of the medications themselves.
Indirect Costs Attributed to Diabetes
The total indirect cost of diabetes is estimated at $89.9 billion (Table 6). Major contributors to this burden are reduced employment ($37.5 billion), presenteeism ($26.9 billion), and premature mortality ($19.9 billion). Work days absent ($3.3 billion) and reduced productivity for those not in the workforce ($2.3 billion) represent a relatively small portion of the total burden.
Table 6.
Indirect burden of diabetes in the U.S., 2017 (in billions of dollars)
| Cost component | Productivity loss | Total cost attributable to diabetes ($) | Proportion of indirect costs* |
|---|---|---|---|
| Work days absent | 14 million days | 3.3 | 3.7% |
| Reduced performance at work | 114 million days | 26.9 | 29.7% |
| Reduced productivity days for those not in labor force | 14 million days | 2.3 | 2.6% |
| Reduced labor force participation due to disability | 182 million days | 37.5 | 41.7% |
| Mortality | 277,000 deaths | 19.9 | 22.1% |
| Total | 89.9 | 100% |
Data source: analysis of the NHIS (2014–2016), CPS (2016), CDC mortality data, and U.S. Census Bureau population estimates for 2016 and 2017.
Numbers do not necessarily sum to totals because of rounding.
Of the estimated 24.7 million people with diagnosed diabetes, analysis of NHIS data suggests that ∼8.1 million are in the workforce. If people with diabetes participated in the labor force at rates similar to their peers without diabetes, there would be ∼2 million additional people aged 18–64 years in the workforce. However, using a more conservative approach (described previously) where reduced labor force participation is associated with receiving disability payments, we estimate 756,000 fewer working-age adults in the workforce in 2017—equivalent to 182 million lost work days. While disability payments themselves are a cost to the government, from a societal perspective they are considered transfer payments and thus not included in the burden estimates.
The cost of missed work days due to absenteeism is estimated at $3.3 billion, representing 14 million days. If people not in the workforce had similar rates of days where they are unable to work due to poor health as their employed peers, this would equate to 14 million excess sick days with estimated productivity loss valued at $2.3 billion.
Reduced performance at work (presenteeism) accounted for 30% of the indirect cost of diabetes. The estimate of a 6.6% annual decline in productivity attributed to diabetes equates to 114 million lost work days per year.
The estimated number of deaths in 2017 attributable to diabetes is 277,000 (Table 7); for 85,000 deaths, diabetes is listed as the primary cause. Of the 689,000 deaths where cardiovascular disease is listed as the primary cause, ∼111,000 (16%) are attributable to diabetes. Approximately 42,000 cases where cerebrovascular disease is listed as the primary cause of death are attributable to diabetes, and 39,000 cases where renal disease is listed as the primary cause of death are attributable to diabetes. The average cost per premature death declines with age (reflecting fewer remaining expected working years), and across all premature deaths, cost averaged ∼$71,700 per case.
Table 7.
Mortality costs attributed to diabetes, 2017
| Primary cause of death | Total U.S. deaths (thousands)* | Deaths attributed to diabetes | ||
|---|---|---|---|---|
| Deaths (thousands) | % of U.S. deaths in category | Value of lost productivity ($, billions) | ||
| Diabetes | 85 | 85 | 100 | 8.5 |
| Renal disease | 72 | 39 | 54 | 1.9 |
| Cerebrovascular disease | 150 | 42 | 28 | 1.9 |
| Cardiovascular disease | 689 | 111 | 16 | 7.6 |
| Total | NA | 277 | NA | 19.9 |
Data source: CDC National Vital Statistics Reports for total deaths in 2015 by primary cause of death, scaled to 2017 using the annual diabetes population growth rate from 2015 to 2017 for each age, sex, and race/ethnicity group. NA, not applicable.
Trends in Diabetes Costs, 2007–2017
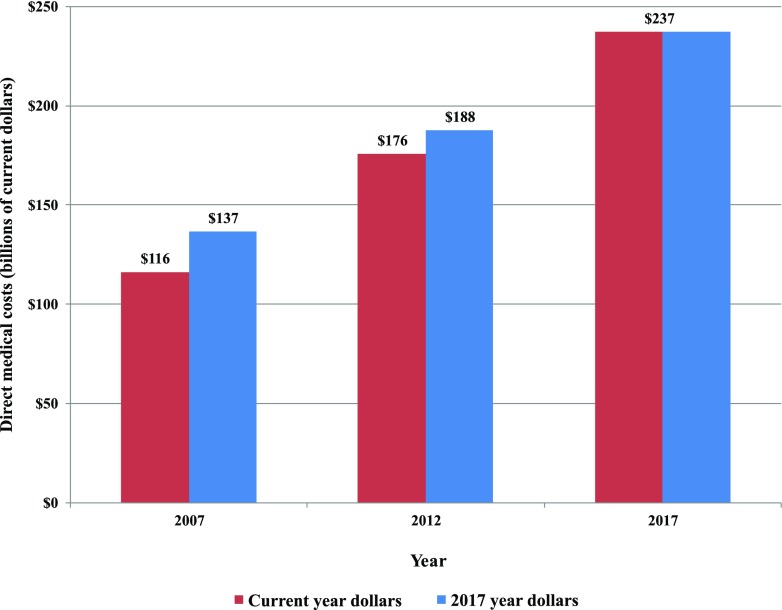
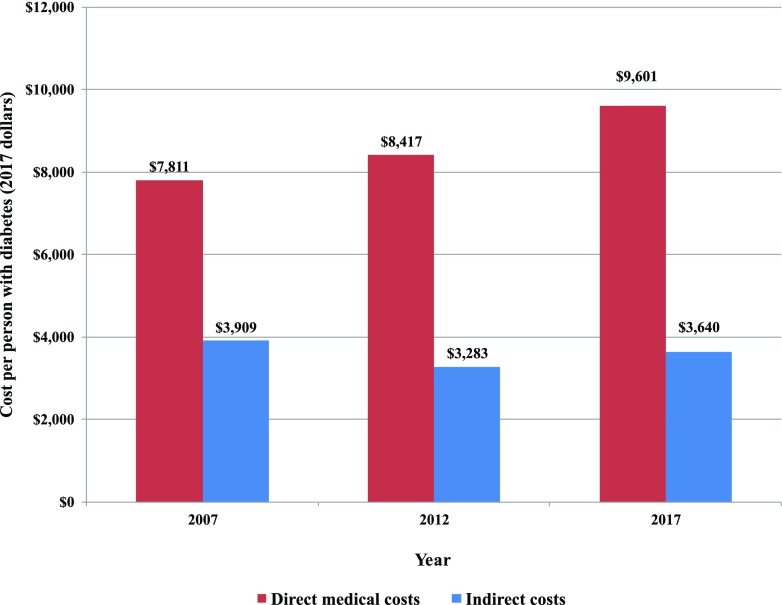
Between 2012 and 2017, we estimate that medical costs associated with diabetes increased by 26% (from $188 billion to $237.3 billion) when adjusted for general inflation (Fig. 2). Adjusting for both inflation and growth in diabetes prevalence, the excess medical cost per person with diabetes grew by 14% (from $8,417 to $9,601 in 2017 dollars) (Fig. 5).
Figure 2.
Total direct costs of diabetes, 2007–2017.
Figure 5.
Average cost of diabetes, 2007–2017 (in 2017 dollars).
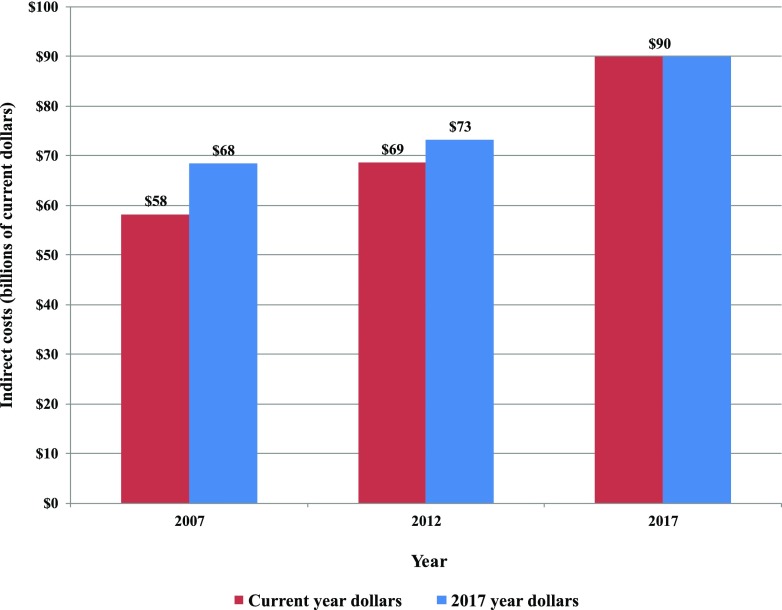
The indirect costs of diabetes grew by 23% when adjusted for general inflation (Fig. 3), which on a per capita basis reflects 11% growth (from $3,283 to $3,640 per person in 2017 dollars) (Fig. 5).
Figure 3.
Total indirect costs of diabetes, 2007–2017.
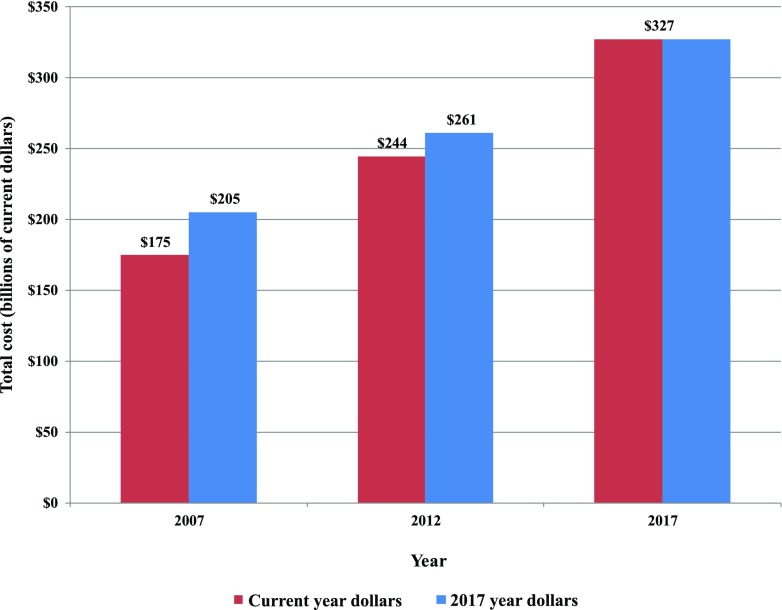
Combined, the inflation adjusted total economic burden of diabetes increased from ∼$261 billion in 2012 to $327.3 billion in 2017 (or 25% growth) (Fig. 4). Adjusted for inflation and growth in diabetes prevalence, the average economic cost associated with diabetes increased from $11,700 to $13,247 (in 2017 dollars), or 13% growth (Fig. 5).
Figure 4.
Total economic cost of diabetes, 2007–2017.
Conclusions
This study estimates ∼24.7 million people (∼9.7% of adults) had diagnosed diabetes in the U.S. in 2017. Diabetes costs the nation ∼$327 billion, which includes $237 billion in direct medical cost and $90 billion in lost productivity. Similar to estimates in 2007 and 2012, after adjusting for age and sex, annual per capita health care expenditure is 2.3 times higher for people with diabetes compared with those without diabetes. A large portion of medical costs associated with diabetes costs is for comorbidities.
For costs that include hospital and office-based services as well as prescription medications and supplies, the costs to directly treat diabetes are estimated at $29.3 billion. An estimated $37.3 billion in cardiovascular-related spending is associated with diabetes (with the presence of diabetes contributing to higher medical expenditures among patients seeking cardiovascular-related care). Outside of the chronic complication categories modeled, the presence of diabetes is associated with greater use of health care services in general—including longer stays in the hospital regardless of primary reason for hospitalization. This underscores that simply aggregating all costs associated only with diabetes diagnosis codes grossly underestimates the medical costs directly attributable to diabetes.
While much of the cost of diabetes appears to fall on insurers (especially Medicare) and employers (in the form of reduced productivity at work, missed work days, and higher employer expenditures for health care), in reality such costs are passed along to all of society in the form of higher insurance premiums and taxes, reduced earnings, and reduced standard of living.
Comparing the 2017 estimates with those produced for 2012, the overall cost of diabetes appears to have increased by ∼25% after adjusting for inflation, reflecting an 11% increase in national prevalence of diagnosed diabetes and a 13% increase in the average annual diabetes-attributed cost per person with diabetes.
Study limitations include the following:
Due to data limitations, we omitted from this analysis potential increase in the use of over-the-counter medications and optometry and dental services. Diabetes increases the risk of periodontal disease, so one would expect dental costs to be higher for people with diabetes. Small sample size in MEPS data prevented meaningful analysis of these cost components. We also omitted expenditures for prevention programs targeted to people with diabetes, research activities, and health administration costs. These omissions underestimate the full medical costs associated with diabetes.
The study omits lost productivity associated with care for diabetes of family members (e.g., time off from work to care for a child or an elderly parent with diabetes). The value of informal care and personal aides is excluded from our cost estimate. Time and costs associated with traveling to doctor visits and other medical emergencies are omitted. These omissions underestimate the indirect costs associated with diabetes.
Also omitted from the cost estimates are the intangible costs of diabetes such as pain, suffering, and reduced quality of life.
A complicating factor in estimating costs attributed to diabetes is that health behavior that affects both the presence of diabetes and the presence of other comorbidities, unless controlled for, could result in an overestimate of the link between diabetes and use of health resources. Controlling for demographics helps to control for this correlation. In addition, for the top 10 cost drivers we conducted additional analysis controlling for other important explanatory variables using MEPS data, and based on the results we reduced the etiological fractions for several diabetes complications and for the general medical conditions group—depending on care delivery setting. This potential limitation also applies to the estimates of indirect costs attributed to diabetes, especially the estimated productivity loss due to presenteeism, potentially biasing these estimates high.
Other study limitations discussed previously include small sample size for some data sources used, the use of a data source (dNHI) that overrepresents the commercially insured population for the population younger than age 65 years, and the need to use different approaches to model different cost components because of data limitations. Another limitation common to claims-based analysis is the possibility of inaccurate diagnosis codes. Claims data tend to be less accurate than medical records in identifying patients with specific conditions due to reasons such as rule-out diagnosis, coding error, etc. The direction of such bias on our risk ratio calculations is unknown, although it is anticipated to be small as there is no reason to believe that the coding of comorbidities would be significantly different for people with and without diabetes.
Using a methodology that is largely consistent with our previous studies conducted in 2007 and 2012, with updated national survey and claims data from previous data sources, we estimate the total burden of diabetes in 2017. The estimates presented here show that diabetes places an enormous burden on society and has increased over time—both in the economic terms presented here and in reduced quality of life.
Supplementary Material
Article Information
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
This report was prepared under the direction of the American Diabetes Association by Wenya Yang (The Lewin Group, Inc., Falls Church, VA), Timothy M. Dall (IHS Markit, Washington, DC), Kaleigh Beronjia (The Lewin Group, Inc.), Janice Lin (The Lewin Group, Inc.), April P. Semilla (IHS Markit), Ritashree Chakrabarti (IHS Markit), and Paul F. Hogan (The Lewin Group, Inc.).
This ADA statement was reviewed and approved by the American Diabetes Association Professional Practice Committee in March 2018 and ratified by the American Diabetes Association Board of Directors in March 2018.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dci18-0007/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. National Diabetes Statistics Report [Internet], 2017. Available from https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 30 November 2017.
- 3.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 5. U.S. Bureau of Labor Statistics. Consumer Price Index (CPI) databases [Internet]. Available from https://www.bls.gov/cpi/data.htm. Accessed 30 November 2017.
- 6.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 7.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res 2001;10:195–216 [DOI] [PubMed] [Google Scholar]
- 8.Dybicz SB, Thompson S, Molotsky S, Stuart B. Prevalence of diabetes and the burden of comorbid conditions among elderly nursing home residents. Am J Geriatr Pharmacother 2011;9:212–223 [DOI] [PubMed] [Google Scholar]
- 9.Genworth Financial, Inc Compare long term care costs across the United States [Internet], 2017. Available from https://www.genworth.com/about-us/industry-expertise/cost-of-care.html. Accessed 4 December 2017.
- 10.National Hospice and Palliative Care Organization Hospice policy compendium [Internet], 2016. Available from https://www.nhpco.org/sites/default/files/public/public_policy/Hospice_Policy_Compendium.pdf. Accessed 5 December 2017.
- 11.National Hospice and Palliative Care Organization Facts and figures: Hospice care in America [Internet], 2016. Available from https://www.nhpco.org/sites/default/files/public/Statistics_Research/2016_Facts_Figures.pdf. Accessed 5 December 2017.
- 12.Breton M-C, Guénette L, Amiche MA, Kayibanda J-F, Grégoire J-P, Moisan J. Burden of diabetes on the ability to work: a systematic review. Diabetes Care 2013;36:740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asay GRB, Roy K, Lang JE, Payne RL, Howard DH. Absenteeism and employer costs associated with chronic diseases and health risk factors in the US workforce. Prev Chronic Dis 2016;13:E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gallup-Sharecare Well-Being Index. State of American well-being: The cost of diabetes in the U.S.: Economic and well-being impact [Internet], 2017. Available from https://info.healthways.com/hubfs/Gallup-Sharecare%20State%20of%20American%20Well-Being_Cost%20of%20Diabetes%20vFINAL.pdf?t=1510870191218&__hstc=56314740.d054ffd56b854b6aed2c4302f7119e90.1512068793130.1512068793130.1512068793130.1&__hssc=56314740.5.1512068793131&__hsfp=436238590. Accessed 30 November 2017.
- 15.DiBonaventura M, Link C, Pollack MF, Wagner JS, Williams SA. The relationship between patient-reported tolerability issues with oral antidiabetic agents and work productivity among patients having type 2 diabetes. J Occup Environ Med 2011;53:204–210 [DOI] [PubMed] [Google Scholar]
- 16.Loeppke R, Taitel M, Haufle V, Parry T, Kessler RC, Jinnett K. Health and productivity as a business strategy: a multiemployer study. J Occup Environ Med 2009;51:411–428 [DOI] [PubMed] [Google Scholar]
- 17.Rodbard HW, Fox KM, Grandy S; Shield Study Group . Impact of obesity on work productivity and role disability in individuals with and at risk for diabetes mellitus. Am J Health Promot 2009;23:353–360 [DOI] [PubMed] [Google Scholar]
- 18. Centers for Medicare & Medicaid Services. National health expenditure data: projected [Internet], 2017. Available from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsProjected.html. Accessed 30 November 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.