Abstract
OBJECTIVE
Attaining glycemic targets without severe hypoglycemic events (SHEs) is a challenging treatment goal for patients with type 1 diabetes complicated by impaired awareness of hypoglycemia (IAH). The CIT Consortium Protocol 07 (CIT-07) trial showed islet transplantation to be an effective treatment for subjects with IAH and intractable SHEs. We evaluated health-related quality of life (HRQOL), functional health status, and health utility before and after pancreatic islet transplantation in CIT-07 trial participants.
RESEARCH DESIGN AND METHODS
Four surveys, the Diabetes Distress Scale (DDS), the Hypoglycemic Fear Survey (HFS), the Short Form 36 Health Survey (SF-36), and the EuroQoL 5 Dimensions (EQ-5D), were administered repeatedly before and after islet transplantation. Summary statistics and longitudinal modeling were used to describe changes in survey scores from baseline and to characterize change in relation to a minimally important difference (MID) threshold of half an SD.
RESULTS
Improvements in condition-specific HRQOL met the MID threshold. Reductions from baseline in the DDS total score and its four DDS subscales (all P ≤ 0.0013) and in the HFS total score and its two subscales (all P < 0.0001) were observed across all time points. Improvements were observed after both 1 and 2 years for the EQ-5D visual analog scale (both P < 0.0001).
CONCLUSIONS
In CIT-07, 87.5% of the subjects achieved the primary end point of freedom from SHE along with glycemic control (HbA1c <7% [<53 mmol/mol]) at 1 year post–initial islet transplantation. The same subjects reported consistent, statistically significant, and clinically meaningful improvements in condition-specific HRQOL as well as self-assessments of overall health.
Introduction
Reduction in HbA1c among patients with type 1 diabetes is associated with a reduction in diabetes complications (1). However, iatrogenic hypoglycemia remains a limiting factor in the glycemic management of patients with type 1 diabetes (2,3). Uncontrolled type 1 diabetes adversely affects short- and long-term health-related quality of life (HRQOL), with negative impacts on the physical, emotional, practical, and social well-being of patients with type 1 diabetes (4), and fear of severe hypoglycemic events (SHEs) is recognized as one of the key determinants adversely affecting quality of life (5,6). HRQOL outcomes are critical to our understanding of the benefit of pancreatic islet transplantation (7).
The benefits of pancreatic islet transplantation include the elimination of SHEs in most transplant recipients, improved glycemic control, restoration of hypoglycemia awareness, and, in some cases, insulin independence (8–15). The Clinical Islet Transplantation Consortium Protocol 07 (CIT-07) trial was a phase 3 clinical trial of transplantation of a standardized, well-defined islet product (purified human pancreatic islets) in subjects with type 1 diabetes, impaired awareness of hypoglycemia, and intractable SHEs. The primary end point was the composite of achieving an HbA1c level of <7.0% (53 mmol/mol) at day 365 after the initial islet transplantation and freedom from SHEs from day 28 to day 365 after the initial islet transplantation (16). The primary end point was met by 42 of the 48 subjects (87.5%). This report addresses the impact of pancreatic islet transplantation on the HRQOL, functional health, and health utility of the subjects in the CIT-07.
Research Design and Methods
Overview of CIT-07
Details regarding CIT-07 study results, design, outcome measures, recipient selection, donor selection, islet manufacture, and islet transplantation have been described previously (16,17). Briefly, the CIT-07 trial was a multicenter, phase 3 clinical trial of a standardized, well-defined islet product. All patients in the trial had a history within the past year of SHEs that persisted despite expert medical management. The median (range) age, BMI, and duration of diabetes were 48.4 years (26.2–65.5), 25.1 kg/m2 (18.9–29.8), and 28.5 years (11–57), respectively. Nineteen of the 48 subjects (39.6%) were male. The mean baseline HbA1c level was 7.2% (55 mmol/mol), the mean baseline insulin requirements were 32.6 units/day (0.5 units/kg/day), and the mean baseline Clarke score was 6. The primary end point was the achievement of an HbA1C level of <7.0% (53 mmol/mol) at 1 year and freedom of SHEs from day 28 to day 365 after the initial islet transplant. A SHE was defined as an event with one of the following symptoms: memory loss; confusion; uncontrollable behavior; irrational behavior; unusual difficulty in awakening; suspected seizure; seizure; loss of consciousness; or visual symptoms, in which the subject was unable to treat himself/herself and which was associated with either a blood glucose level of <54 mg/dL (3.0 mmol/L) or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration. The a priori threshold for success was that the proportion of patients meeting the primary end point have an exact one-sided 95% CI that lay entirely above 50%. Overall, 42 of 48 subjects (87.5% [95% one-sided CI 76.8, 100.0]) achieved primary end point success, surpassing the a priori threshold for success.
HRQOL, Functional Health Status, and Health Utility Surveys
The CIT-07 protocol incorporated four surveys at each of the following time points: every 3 months prior to islet transplantation; at days 75, 365, and 730 after the initial islet transplantation; and at days 75, 180, 365, and 730 after the final islet transplantation. Two surveys, the Diabetes Distress Scale (DDS) (18,19) and the Hypoglycemic Fear Survey (HFS) (20,21), measured condition-specific HRQOL. Two other surveys, the Short Form 36 Health Survey (SF-36) version 2 (22–24) and the EuroQoL 5 Dimensions (EQ-5D) (25–27), measured several dimensions of functional health status and health utility.
The 17-item DDS measures overall distress and the following four diabetes-related distress subscales: Emotional Burden, Physician-Related Distress, Regimen-Related Distress, and Interpersonal Distress. Each item in the DDS uses a Likert scale from 1 to 6. The overall distress score and the four distress subscale scores are calculated using the average of their associated items. The HFS is a 23-item survey that measures fear experienced in relation to hypoglycemia. Each item in the HFS uses a Likert scale from 0 to 4. The HFS is reported as a total score and as two subscale scores representing Hypoglycemia Avoidance Behavior and Worry About Hypoglycemia. The overall score and the two subscale scores are calculated by taking the average of their associated items. For both the DDS and the HFS, lower scores indicate lower distress or fear, respectively.
The eight scales (Physical Function, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health) and two component summary scores of the SF-36 (Physical Component Summary [PCS] and Mental Component Summary [MCS]) can be referenced to both the general population, which is standardized to have a mean of 50 points and an SD of 10 points, and to selected condition-specific norms. The SF-36 version 2 Health Survey, by QualityMetric (now part of Optum) and the Medical Outcomes Trust, was used in CIT-07. The QualityMetric scoring software was used to calculate the eight scales and two summary components, normalized to the 2010 U.S. general population. The EQ-5D addresses five dimensions of health status (Mobility, Self-Care, Usual Activities, Pain/Discomfort, and Anxiety/Depression) and includes a visual analog scale (VAS) of overall health. Dimension responses can be converted to U.S. population-based Health Preference Weights that are useful in cost-utility analyses (28). The five EQ-5D dimensions comprise three-level ordinal categorical responses. These responses were dichotomized as either “No Problems” or “Any Problems,” based on a scoring option referenced in the EQ-5D user guide (29). Higher scores indicate better health status for the SF-36 PCS, SF-36 MCS, EQ-5D Health Preference Weights, and EQ-5D VAS measures.
Statistical Analyses
For all continuous outcomes, summary statistics including means, SDs, medians, and interquartile ranges were calculated. Mann-Whitney U tests were used for comparisons of two independent groups and the Wilcoxon signed rank test was used for comparisons of paired data. For categorical outcomes, frequencies and percentages were calculated. The McNemar test was used for statistical comparisons of paired binary outcomes. In all, 130 hypothesis tests were performed, each resulting in a P value. In order to limit the number of hypothesis tests declared statistically significant in which the null hypothesis is actually true, we used the false discovery rate (FDR) approach to avoid the philosophical challenges that are associated with the Bonferroni correction and other false-positive rate approaches (30). The FDR approach described by Benjamini and Hochberg was applied, and a maximum FDR of 5% was chosen (31). This approach leads to a threshold for statistical significance of P ≤ 0.0143.
The magnitude of within-subject change was calculated as the mean change from baseline divided by the baseline SD, which we refer to as the effect size. A within-subject change (i.e., effect size) greater than or equal to half of an SD (i.e., 0.5) was interpreted to be clinically meaningful. This metric has been described by Norman et al. (32) and is based on the general convergence of statistical effect size–based and anchor-based (e.g., minimally important difference [MID]) methods for interpreting changes in HRQOL. This approach was particularly useful with the CIT-07 survey data because the effect size could be interpreted in a uniform manner for all surveys.
Longitudinal mixed-effects models were developed to assess the impact of SHEs on HRQOL, functional health status, and health utility after islet transplantation. Mean estimates are reported in Supplementary Appendix 5 with their SEs and associated bootstrap 95% CIs. All modeling was performed using the R statistical environment, while summary statistics were created using SAS software version 9.4.
Results
Overall, 47 of 48 subjects provided survey data both before and after islet transplantation (Supplementary Appendix 2). For the longitudinal models, 45 of 48 subjects provided from 205 to 210 post-transplantation survey and metabolic outcome pairs of observations, depending on the survey used. Of the three subjects who were not included in the models, one was a primary end point failure (imputed because of early study termination and the absence of follow-up data), one was a primary end point success with no baseline survey measurements, and one was a primary end point success with no baseline mixed-meal tolerance test results. Three of the 48 subjects experienced at least one post-transplantation SHE. Two of the three subjects with post-transplantation SHE were primary end point failures. One of the three subjects met the primary end point (at day 365), but had an SHE during the 2nd year of follow-up.
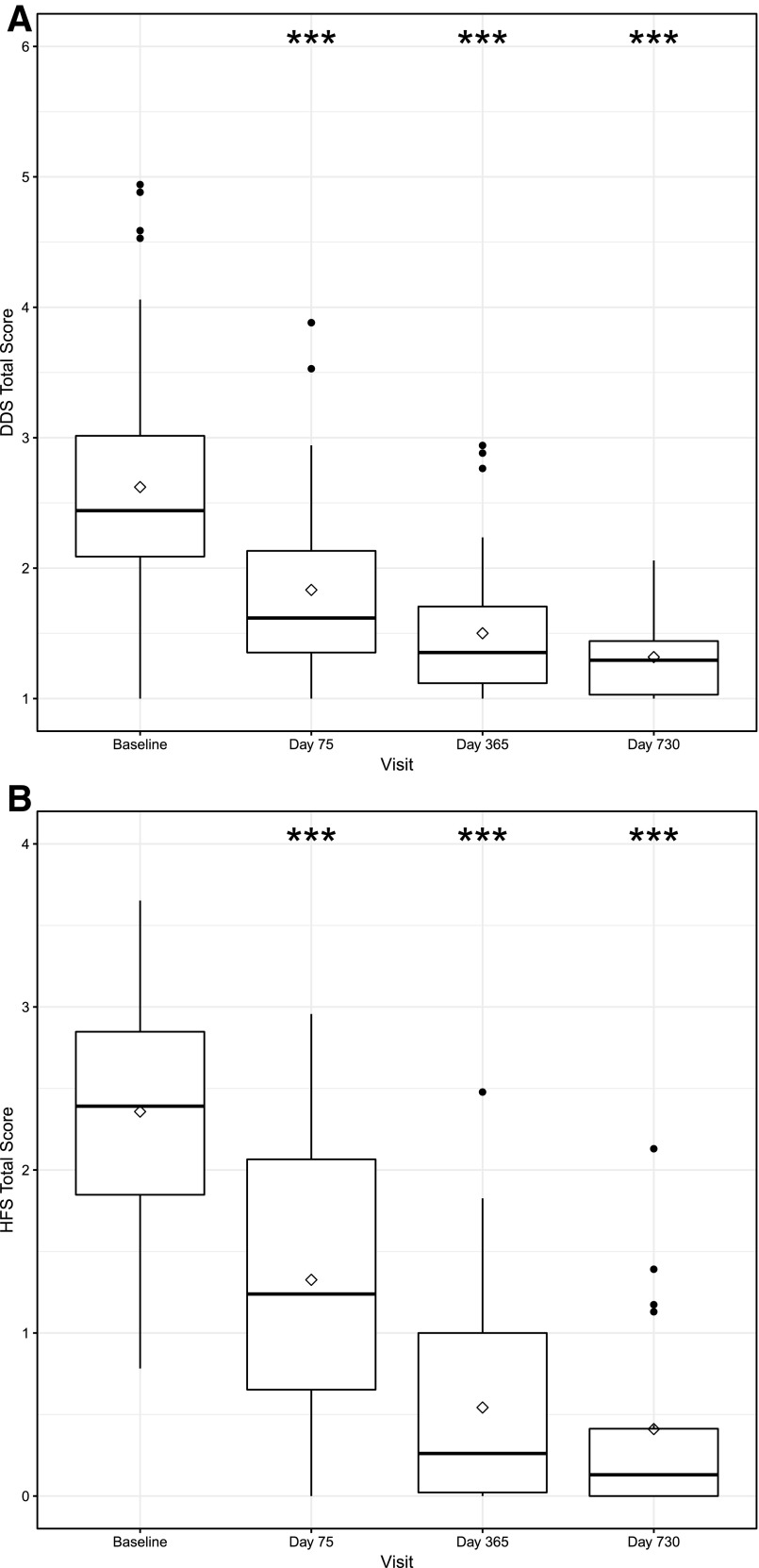
The DDS results are shown in Fig. 1A and Table 1. There are improvements (decreases) in the DDS total score and each of the four subscales from baseline to days 75, 365, and 730 (all P ≤ 0.0013) after the initial islet transplantation. The change in the DDS total score and in the scores for the subscales of Emotional Burden and Regimen-Related Distress met the MID threshold of a >0.5-SD change from baseline at all follow-up time points, as did the changes in the scores for the Physician-Related Distress and Interpersonal Distress subscales from baseline to days 365 and 730. After the initial transplant, subjects reported having little or no physician-related distress or interpersonal distress (median values are the lowest possible value, 1.00, at all time points after the initial transplant). Similar results for the HFS total score and its two subscales are shown in Fig. 1B and Table 1. Statistically significant improvements (decreases) in each of the three scores from baseline to days 75, 365, and 730 were observed (all P < 0.0001), and all effect sizes met the MID threshold. By day 730, most subjects’ responses indicated having no worries about hypoglycemia (median value is equal to the smallest possible value, 0.00).
Figure 1.
Condition-specific HRQOL score distributions over time. A: DDS total score. The DDS total score ranges from 1 to 6 with lower scores indicating less diabetes-related distress. B: HFS total score. The HFS total score ranges from 0 to 4 with lower scores indicated less fear of hypoglycemia. The thick horizontal line inside each box represents the median value. The diamond represents the mean value. Asterisks represent statistically significant changes from baseline, where *** indicates P < 0.0001.
Table 1.
DDS and HFS
| Measure | Visit | N = 48 | Mean (SD) | Median (IQR) | Effect size | P value | |
|---|---|---|---|---|---|---|---|
| DDS | Total score | Baseline | 44 | 2.62 (0.98) | 2.44 (0.97) | ||
| Day 75 | 44 | 1.83 (0.72) | 1.62 (0.79) | −0.80 | <0.0001 | ||
| Day 365 | 39 | 1.50 (0.51) | 1.35 (0.59) | −1.22 | <0.0001 | ||
| Day 730 | 27 | 1.32 (0.32) | 1.29 (0.47) | −1.33 | <0.0001 | ||
| Emotional Burden | Baseline | 47 | 3.75 (1.31) | 3.80 (2.00) | |||
| Day 75 | 45 | 2.59 (1.23) | 2.20 (1.20) | −0.83 | <0.0001 | ||
| Day 365 | 41 | 1.84 (0.91) | 1.60 (0.80) | −1.48 | <0.0001 | ||
| Day 730 | 29 | 1.43 (0.47) | 1.20 (0.80) | −1.79 | <0.0001 | ||
| Physician-Related Distress | Baseline | 46 | 1.67 (1.02) | 1.13 (1.00) | |||
| Day 75 | 45 | 1.19 (0.45) | 1.00 (0.00) | −0.47 | <0.0001 | ||
| Day 365 | 41 | 1.06 (0.21) | 1.00 (0.00) | −0.58 | <0.0001 | ||
| Day 730 | 31 | 1.10 (0.32) | 1.00 (0.00) | −0.51 | 0.0013 | ||
| Regimen-Related Distress | Baseline | 45 | 2.66 (1.20) | 2.40 (1.60) | |||
| Day 75 | 46 | 1.77 (0.86) | 1.60 (1.00) | −0.78 | <0.0001 | ||
| Day 365 | 40 | 1.61 (0.65) | 1.40 (0.60) | −0.94 | <0.0001 | ||
| Day 730 | 31 | 1.42 (0.43) | 1.20 (0.80) | −1.05 | <0.0001 | ||
| Interpersonal Distress | Baseline | 47 | 2.19 (1.38) | 1.67 (2.00) | |||
| Day 75 | 45 | 1.59 (0.94) | 1.00 (0.67) | −0.42 | 0.0002 | ||
| Day 365 | 40 | 1.47 (0.84) | 1.00 (0.67) | −0.55 | <0.0001 | ||
| Day 730 | 31 | 1.35 (0.69) | 1.00 (0.33) | −0.70 | <0.0001 | ||
| HFS | Total score | Baseline | 39 | 2.36 (0.66) | 2.39 (1.09) | ||
| Day 75 | 40 | 1.33 (0.88) | 1.24 (1.43) | −1.51 | <0.0001 | ||
| Day 365 | 35 | 0.54 (0.66) | 0.26 (1.09) | −2.59 | <0.0001 | ||
| Day 730 | 18 | 0.41 (0.62) | 0.13 (0.43) | −2.85 | <0.0001 | ||
| Hypoglycemic Avoidance Behavior | Baseline | 41 | 2.52 (0.54) | 2.60 (0.80) | |||
| Day 75 | 40 | 1.47 (0.90) | 1.60 (1.60) | −1.86 | <0.0001 | ||
| Day 365 | 35 | 0.64 (0.83) | 0.20 (1.30) | −3.34 | <0.0001 | ||
| Day 730 | 22 | 0.67 (0.82) | 0.25 (1.20) | −3.31 | <0.0001 | ||
| Worry About Hypoglycemia | Baseline | 44 | 2.20 (0.90) | 2.15 (1.23) | |||
| Day 75 | 45 | 1.27 (1.01) | 1.00 (2.00) | −1.13 | <0.0001 | ||
| Day 365 | 40 | 0.53 (0.79) | 0.08 (0.77) | −1.79 | <0.0001 | ||
| Day 730 | 27 | 0.47 (0.81) | 0.00 (0.77) | −1.88 | <0.0001 |
The total DDS score, the four DDS subscales, the total HFS score, and the two HFS subscales stratified by post–initial transplantation study visits. Effect sizes were calculated as the mean individual change from baseline divided by the baseline SD; a value of 0.5 (i.e., half an SD) was determined to be the MID. Smaller DDS and HFS values indicate better HRQOL. Tests for change from baseline were performed using the Wilcoxon signed rank test. IQR, interquartile range.
Both of the component summary scores and all eight scales of the SF-36 showed improvements (increases) over time (Table 2). With the exception of the General Health scale, baseline scores for the SF-36 PCS and MCS scales averaged within 5 points of the general population mean. There were statistically significant improvements from baseline to days 365 and 730 in SF-36 PCS scores, but only the change from baseline to day 365 met the MID threshold. Of the four SF-36 scales that are most heavily weighted in the computation of the PCS, three (Physical Functioning, Role Physical, and General Health) showed statistically significant improvements during follow-up. However, only the Role Physical and General Health scales at 365 days met the MID threshold. The MCS scores showed a statistically significant change from baseline to day 75 that did not meet the MID threshold. Changes in three of the four scales that are most heavily weighted in the computation of the MCS (Mental Health, Role Emotional, and Social Functioning) generally were not statistically significant and did not meet the MID threshold. The Vitality scale, however, shows statistically significant improvements during follow-up and meets the MID threshold at day 75. The EQ-5D VAS (Table 3) showed significant (P < 0.0001) and meaningful improvements from baseline to days 365 and 730 (SD 0.71 and 0.73, respectively). Neither the EQ-5D Health Preference Weight nor any of the five individual EQ-5D items show consistent, statistically significant changes from baseline, and observed changes did not meet the MID threshold.
Table 2.
SF-36
| Measure | Visit | N = 48 | Mean (SD) | Median (IQR) | Effect size | P value |
|---|---|---|---|---|---|---|
| PCS | Baseline | 47 | 47.52 (8.77) | 49.04 (10.41) | ||
| Day 75 | 45 | 49.97 (8.05) | 50.69 (9.09) | 0.31 | N.S. | |
| Day 365 | 41 | 52.52 (7.69) | 54.39 (8.05) | 0.54 | <0.0001 | |
| Day 730 | 30 | 52.44 (9.06) | 55.84 (9.60) | 0.39 | 0.0004 | |
| MCS | Baseline | 47 | 49.23 (10.48) | 53.60 (18.34) | ||
| Day 75 | 45 | 53.03 (9.65) | 55.70 (8.04) | 0.28 | 0.0081 | |
| Day 365 | 41 | 51.86 (8.94) | 54.61 (12.44) | 0.28 | N.S. | |
| Day 730 | 30 | 54.40 (7.28) | 56.84 (9.88) | 0.30 | N.S. | |
| Physical Functioning scale | Baseline | 47 | 50.62 (8.04) | 53.71 (9.57) | ||
| Day 75 | 46 | 53.30 (6.58) | 55.63 (5.74) | 0.29 | 0.0034 | |
| Day 365 | 42 | 53.67 (7.17) | 57.54 (3.83) | 0.35 | 0.0005 | |
| Day 730 | 32 | 52.29 (10.26) | 57.54 (3.83) | 0.19 | N.S. | |
| Role Physical scale | Baseline | 47 | 45.69 (10.57) | 48.17 (15.72) | ||
| Day 75 | 45 | 49.17 (9.72) | 52.66 (8.99) | 0.31 | 0.0008 | |
| Day 365 | 42 | 51.70 (7.53) | 54.91 (8.99) | 0.57 | <0.0001 | |
| Day 730 | 30 | 52.67 (9.04) | 57.16 (4.50) | 0.48 | <0.0001 | |
| Bodily Pain scale | Baseline | 47 | 50.52 (10.04) | 51.51 (15.32) | ||
| Day 75 | 46 | 49.96 (10.97) | 53.53 (23.39) | −0.05 | N.S. | |
| Day 365 | 41 | 52.72 (8.24) | 51.51 (15.32) | 0.22 | N.S. | |
| Day 730 | 32 | 53.22 (10.19) | 55.55 (17.34) | 0.08 | N.S. | |
| General Health scale | Baseline | 47 | 44.31 (12.42) | 46.05 (21.39) | ||
| Day 75 | 46 | 50.19 (10.45) | 50.81 (16.64) | 0.49 | <0.0001 | |
| Day 365 | 42 | 51.57 (9.92) | 52.00 (15.69) | 0.57 | <0.0001 | |
| Day 730 | 32 | 51.60 (11.57) | 50.81 (18.55) | 0.39 | 0.0064 | |
| Vitality scale | Baseline | 47 | 48.81 (10.94) | 49.63 (14.85) | ||
| Day 75 | 46 | 54.67 (8.92) | 52.60 (11.88) | 0.51 | 0.0001 | |
| Day 365 | 42 | 53.38 (8.53) | 52.60 (8.91) | 0.40 | 0.0029 | |
| Day 730 | 32 | 55.38 (10.62) | 57.06 (11.88) | 0.44 | 0.0015 | |
| Social Functioning scale | Baseline | 47 | 46.99 (10.78) | 47.31 (20.05) | ||
| Day 75 | 46 | 49.38 (9.72) | 52.33 (10.03) | 0.19 | N.S. | |
| Day 365 | 41 | 50.98 (8.24) | 57.34 (10.03) | 0.30 | N.S. | |
| Day 730 | 32 | 51.70 (10.25) | 57.34 (10.03) | 0.28 | N.S. | |
| Role Emotional scale | Baseline | 47 | 48.02 (10.25) | 49.20 (17.41) | ||
| Day 75 | 44 | 51.58 (8.72) | 56.17 (6.97) | 0.20 | N.S. | |
| Day 365 | 41 | 50.82 (8.43) | 56.17 (6.97) | 0.31 | 0.0090 | |
| Day 730 | 30 | 52.11 (7.49) | 56.17 (6.97) | 0.25 | N.S. | |
| Mental Health scale | Baseline | 47 | 51.54 (9.27) | 53.48 (15.70) | ||
| Day 75 | 46 | 53.83 (9.09) | 56.10 (13.08) | 0.21 | N.S. | |
| Day 365 | 42 | 53.69 (8.20) | 55.12 (7.85) | 0.28 | N.S. | |
| Day 730 | 32 | 54.30 (8.26) | 57.41 (13.08) | 0.22 | N.S. |
The PCS score, MCS score, and eight SF-36 scales stratified by post–initial transplantation study visits. Effect sizes were calculated as the mean individual change from baseline divided by the baseline SD; a value of 0.5 (i.e., half an SD) was determined to be the MID. Higher SF-36 values indicate better functional health status. Tests for change from baseline were performed using the Wilcoxon signed rank test. IQR, interquartile range; N.S., not significant.
Table 3.
EuroQoL
| Measure | Visit | N = 48 | Mean (SD) or NNo Problems (%) | Median (IQR) or NAny Problems (%) | Effect size | P value |
|---|---|---|---|---|---|---|
| VAS | Baseline | 46 | 74.13 (14.66) | 75.00 (15.00) | ||
| Day 75 | 47 | 79.06 (15.97) | 80.00 (20.00) | 0.29 | N.S. | |
| Day 365 | 42 | 82.86 (14.46) | 85.00 (19.00) | 0.71 | <0.0001 | |
| Day 730 | 31 | 85.74 (15.36) | 91.00 (18.00) | 0.73 | <0.0001 | |
| Health Preference Weight | Baseline | 47 | 0.87 (0.12) | 0.83 (0.22) | ||
| Day 75 | 47 | 0.86 (0.15) | 0.84 (0.17) | −0.06 | N.S. | |
| Day 365 | 41 | 0.86 (0.15) | 0.84 (0.17) | −0.04 | N.S. | |
| Day 730 | 32 | 0.88 (0.18) | 1.00 (0.18) | 0.09 | N.S. | |
| Usual Activities | Baseline | 47 | 32 (68.09%) | 15 (31.91%) | ||
| Day 75 | 47 | 35 (74.47%) | 12 (25.53%) | N.S. | ||
| Day 365 | 42 | 33 (78.57%) | 9 (21.43%) | N.S. | ||
| Day 730 | 32 | 27 (84.38%) | 5 (15.63%) | 0.0143 | ||
| Anxiety/Depression | Baseline | 47 | 32 (68.09%) | 15 (31.91%) | ||
| Day 75 | 47 | 34 (72.34%) | 13 (27.66%) | N.S. | ||
| Day 365 | 42 | 29 (69.05%) | 13 (30.95%) | N.S. | ||
| Day 730 | 32 | 23 (71.88%) | 9 (28.13%) | N.S. | ||
| Mobility | Baseline | 47 | 38 (80.85%) | 9 (19.15%) | ||
| Day 75 | 47 | 40 (85.11%) | 7 (14.89%) | N.S. | ||
| Day 365 | 41 | 34 (82.93%) | 7 (17.07%) | N.S. | ||
| Day 730 | 32 | 26 (81.25%) | 6 (18.75%) | N.S. | ||
| Pain/Discomfort | Baseline | 47 | 25 (53.19%) | 22 (46.81%) | ||
| Day 75 | 47 | 27 (57.45%) | 20 (42.55%) | N.S. | ||
| Day 365 | 42 | 24 (57.14%) | 18 (42.86%) | N.S. | ||
| Day 730 | 32 | 22 (68.75%) | 10 (31.25%) | N.S. | ||
| Self-Care | Baseline | 47 | 45 (95.74%) | 2 (4.26%) | ||
| Day 75 | 47 | 46 (97.87%) | 1 (2.13%) | N.S. | ||
| Day 365 | 42 | 39 (92.86%) | 3 (7.14%) | N.S. | ||
| Day 730 | 32 | 29 (90.63%) | 3 (9.38%) | N.S. |
The EQ-5D VAS, Health Preference Weight, and five dimensions stratified by post–initial transplantation study visits. Effect sizes were calculated as the mean individual change from baseline divided by the baseline SD; a value of 0.5 (i.e., half an SD) was determined to be the MID. No effect size calculations were made for the five categorical dimensions. The five EQ-5D dimensions comprise three-level ordinal categorical responses. These responses were dichotomized as either “No Problems” or “Any Problems” based on a scoring option referenced in the EQ-5D user guide (29). Larger EQ-5D VAS and Health Preference Weight values indicate better health utility. Tests for change from baseline were performed using the Wilcoxon signed rank test for continuous outcomes and the McNemar test for categorical outcomes. IQR, interquartile range; N.S., not significant.
Summary survey scores (DDS total score, HFS total score, SF-36 PCS and MCS, and EQ-5D VAS) at day 365 and day 730 were analyzed as a function of CIT-07 subject primary end point success (Supplementary Appendix 3). Subjects with primary end point failure tended to have worse condition-specific HRQOL post-transplantation, but no statistically significant differences were found. The ability to detect a difference between primary end point successes and failures is limited because of the small number of primary end point failures in the CIT-07 (six primary end point failures). A similar analysis of the above summary scores as a function of insulin independence at day 75, day 365, and day 730 (Supplementary Appendix 4) also revealed no significant differences between those subjects who did or did not achieve insulin independence. For those subjects who did not achieve insulin independence, there is a trend toward worse condition-specific HRQOL, but this trend is smaller than that observed in the comparisons across primary end point success.
The results of fitting longitudinal models to the changes from baseline of the DDS total score and its four subscales, the HFS total score and its two subscales, the SF-36 PCS and MCS, and the EQ-5D VAS are shown in Supplementary Appendix 5. The occurrence of SHE was not selected as an important predictor of the outcome in the DDS total score; the DDS Physician-Related Distress, Regimen-Related Distress, or Interpersonal Distress subscale models; or the HFS total score model. The occurrence of an SHE was identified as an important predictor in the DDS Emotional Burden model; HFS Hypoglycemia Avoidance Behavior and Worry About Hypoglycemia models; the SF-36 PCS and MCS models; and the EQ-5D VAS model. The DDS Emotional Burden (1.08 [95% CI 0.23, 1.92]) and the SF-36 MCS (−7.85 [−14.79, −1.21]) both showed a worsening of HRQOL and functional health status, respectively, after an SHE was experienced. The remaining survey results contain a value of zero in their 95% CIs, suggesting the possibility of no SHE impact. Similar to the comparisons between primary end point successes and failures, few SHEs occurred during the CIT-07 follow-up (only three subjects experienced at least one SHE; thus, our ability to estimate the impact of recurrent SHEs is limited).
Conclusions
The CIT-07 study was successful (87.5% success rate) at providing glycemic control and protection from SHEs in subjects with intractable impaired awareness of hypoglycemia and SHEs. The results discussed here show that the subjects who participated in the CIT-07 reported significant, consistent, and clinically meaningful improvements in HRQOL. In particular, large decreases in both diabetes-related distress and fear of hypoglycemia were observed. Patient self-assessments of personal well-being, as measured by the EQ-5D VAS, also showed meaningful improvements.
Despite the acknowledged burden of immunosuppressive medications, the overall quality of life and functional health status scores after transplantation did not worsen. General measures of functional health status, such as the SF-36 PCS and MCS, and five of the eight SF-36 scale scores showed statistically significant improvements from baseline. The improvements were predominantly among the physical health-related scales and did not generally meet the MID threshold. This is likely due to a ceiling effect wherein pretreatment baseline scores were within general population norms, making it less likely that clinically meaningful improvements would be observed over the course of the study. This phenomenon has been reported previously in pancreatic islet transplant recipients (8,33). Consistent, meaningful change is not a realistic expectation when participants enter a study at or near general population norms. If the patient population starts below the general population norms, however, as has been the case in other islet transplantation studies (9,14), meaningful improvements in general HRQOL, functional health status, and health utility are more likely. For the SF-36 in the CIT-07, both of the summary components and six of the eight scales remained within 1 SD of the general population for the entirety of the trial. Furthermore, a comparison of the CIT-07 SF-36 scores to the published SF-36 1996 diabetes-specific scores (34) found the CIT-07 participants to be within half an SD of the published SF-36 diabetes-specific scores.
A consistent decrease in the fear of hypoglycemia, as assessed by the HFS, has also been observed in other islet transplantation studies (10,33,35,36). In a study of 27 islet recipients, no changes were observed in DDS, but improvements in HFS and EQ-5D VAS were observed (33). Other islet transplantation studies have shown improvements in other disease-specific HRQOL measures such as the Diabetes Quality of Life Questionnaire (DQOL) (37). The DQOL was used in the Diabetes Control and Complications Trial (DCCT) (1) to compare intensive insulin treatment versus conventional treatment (no improvement in DQOL scores was seen with intensive insulin treatment in the DCCT). Improvement in DQOL scores has been observed after islet transplantation (8,9,12,13). Improvements in HRQOL in the CIT-07 subjects were greater than those shown in studies of patients using continuous glucose monitoring (DIAMOND randomized clinical trial) (38) and sensor-augmented pump therapy (Sensor-Augmented Pump Therapy for A1C Reduction 3 [STAR3]) (39). At baseline, the CIT-07 subjects had worse DDS and HFS scores that, at 1 year after the initial transplant, had improved to be comparable to or superior to the continuous glucose monitoring and sensor-augmented pump therapy subjects. The CIT-07 subjects reported 1-year DDS scores similar to those reported in the DIAMOND study at 24 weeks. The CIT-07 subjects also reported better 1-year HFS scores compared with 1-year scores from patients in the sensor-augmented pump therapy group of the STAR3 study. The same relationship holds true for each of the two subscale scores of the HFS, with CIT-07 subjects starting with greater Worry About Hypoglycemia and Hypoglycemia Avoidance Behavior than the STAR3 subjects, but reporting better HFS subscale scores after 1 year of follow-up.
The CIT-07 trial was powered to show a meaningful success rate for the primary end point of HbA1c levels <7.0% (53 mmol/mol) and the prevention of SHEs. Given that there were so few primary end point failures in the CIT-07 (6 of 48 subjects), the ability to detect differences across primary end point success was limited. A little more than half of the CIT-07 subjects (52.1%) achieved insulin independence at day 365, but no statistical differences in HRQOL, functional health status, or health utility were observed between insulin-independent and insulin-dependent subjects. The patient population of the CIT-07 was composed of subjects whose major problem was hypoglycemia but who were otherwise healthy. Therefore, although insulin injections have undoubtedly been a burden, SHEs and the fear associated with those events are likely the largest burden. The eradication of SHEs represents a large improvement in these subjects’ HRQOL that outweighs any concerns about the need to take insulin injections. The burden of exogenous insulin should not be discounted, however, as there is a nonsignificant trend toward insulin independence being associated with better DDS and HFS scores. A similar finding of insulin therapy acting as a significant independent variable in HRQOL has been reported in an earlier study of HRQOL after islet transplantation (8). The impact of exogenous insulin therapy and severe glucose control problems on the HRQOL has also been reviewed in a meta-analysis of patients who have undergone pancreas transplantation alone (40). Furthermore, a study of functioning versus nonfunctional pancreas grafts in patients after pancreas transplant alone, using an investigator-developed survey, showed better glucose control and fewer hypoglycemic events in patients with functioning grafts, which was associated with improved HRQOL (41).
The results of these analyses indicate that the CIT-07 participants feel better and have been relieved of fear of hypoglycemia. Longitudinal models show that, for the few subjects who did experience an SHE after islet transplant, their diabetes-related emotional burden increased, indicated by the scores on the DDS Emotional Burden subscale. Interestingly, SF-36 MCS scores also worsened after subjects experienced an SHE. In prior studies, due to the previously mentioned pretransplantation ceiling effect, the SF-36 MCS (which weights the mental-, social-, and emotional health–oriented scales of the SF-36 more heavily than the physical-, pain-, and general health–oriented scales) often did not show clinically important changes after islet transplantation. The change in SF-36 MCS scores in combination with the change in diabetes-related emotional burden point to the strong emotional, mental, and social burden of SHEs. Post–islet transplantation SHE occurred in only three subjects and was not a significant predictor of change in the HFS, which included the Worry About Hypoglycemia subscale. From these three subjects, a total of 16 follow-up measurements were obtained (8 pre-SHE and 8 post-SHE). Further study is needed to describe the impact of post–islet transplant SHE occurrence on HRQOL.
In conclusion, the CIT-07 provided an opportunity to prospectively assess condition-specific HRQOL, functional health status, and health utility outcomes among islet transplant recipients in a phase 3 study. Among the CIT-07 transplant recipients, there was meaningful, consistent, and statistically significant improvement in the condition-specific HRQOL scores (DDS and HFS) as well as in the EQ-5D VAS. There was also statistically significant improvement in functional health status (SF-36 PCS and MCS and several individual scales), but these changes did not consistently meet the MID threshold, most likely because of a pretransplantation ceiling effect. Longitudinal models suggest that the occurrence of SHEs has an adverse impact on the DDS, specifically the diabetes-related Emotional Burden subscale, and the MCS scores of the SF-36. These patient-reported outcomes corroborate the clinical importance of the objective benefits of islet transplantation already documented in the CIT-07 (16).
Supplementary Material
Article Information
Acknowledgments. All members of the CIT Consortium thank the study participants and the referring endocrinologists and diabetologists. The authors also thank the following industry sponsors for contributing their products to the trial: Astellas Pharma US; Sanofi Genzyme; Pfizer/Wyeth Pharmaceuticals, Inc.; and LifeScan, Inc./Johnson & Johnson.
Funding. This research was funded by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), ClinicalTrials.gov no. NCT00434811. This research was supported by the following grants from the NIAID and the NIDDK: at Emory University, NIAID U01-AI-089317; at Northwestern University, NIAID U01-AI-089316; at the University of Alberta, Edmonton, NIAID U01-AI-065191; at the University of California, San Francisco, NIDDK U01-DK-085531; at the University of Illinois, Chicago, NIDDK 5U01-DK-070431-10; at the University of Iowa, NIDDK U01-DK-070431; at the University of Miami, NIDDK U01-DK-070460; at the University of Minnesota, NIAID U01-AI-065193; at the University of Pennsylvania, NIDDK U01-DK-070430; and at Uppsala University, NIAID U01-AI-065192. In addition, the study was supported by the following General Clinical Research Center grants and Clinical and Translational Science Awards: at Emory University, UL1-TR-000454; at Northwestern University, 5UL1-RR-025741 and 8UL1-TR-000150; at the University of California, San Francisco, UL1-TR-000004; at the University of Illinois, Chicago, UL1-TR-000050; at the University of Miami, 1UL1-TR-000460; at the University of Minnesota, 5M01-RR-000400 and UL1-TR-000114; and at the University of Pennsylvania, M01-RR00040 and UL1-TR-000003.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.D.F., N.D.B., and I.D.F. made substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work. E.D.F., N.B.D., I.D.F., T.L.E., L.G.H., and R.A. contributed to the drafting of the work or revising it critically for important intellectual content. E.D.F., N.B.D., I.D.F., T.L.E., L.G.H., and R.A. gave final approval of the version of the manuscript to be published. E.D.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00434811, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1779/-/DC1.
Additional members of the Clinical Islet Transplantation Consortium who contributed to this study are listed in Supplementary Appendix 1.
Contributor Information
Collaborators: Clinical Islet Transplantation Consortium, Beth Begley, Jose Cano, Sallie Carpentier, Jennifer Hutchinson, Christian P. Larsen, Johanna Moreno, Marti Sears, Nicole A. Turgeon, Dasia Webster, James F. Markmann, Patrice Al-saden, Xioajuan Chen, Angela Hecyk, Xunrong Luo, Mark Molitch, Natalie Monson, Elyse Stuart, Amisha Wallia, Lingjia Wang, Shusen Wang, Xiaomin Zhang, Christine W. Czarniecki, Julia S. Goldstein, Allison Priore, Mark A. Robien, Elizabeth Schneider, Guillermo Arreaza-Rubin, Neal Green, David L. Bigam, Patricia Campbell, Parastoo Dinyari, Tatsuya Kin, Norman M. Kneteman, James Lyon, Andrew Malcolm, Doug O’Gorman, Chris Onderka, Richard Owen, Rena Pawlick, Brad Richer, Shawn Rosichuk, Donna Sarman, Adam Schroeder, Peter A. Senior, A. M. James Shapiro, Lana Toth, Vali Toth, Wendy Zhai, Kristina Johnson, Joan McElroy, Andrew M. Posselt, Marissa Ramos, Tara Rojas, Peter G. Stock, Gregory Szot, Barbara Barbaro, Joan Martellotto, Jose Oberholzer, Meirigeng Qi, Yong Wang, Levent Bayman, Kathryn Chaloner, William R. Clarke, Joseph S. Dillon, Cynthia Diltz, Gregory C. Doelle, Dixie Ecklund, Deb Feddersen, Carol Jasperson, David-Erick Lafontant, Tina Neill-Hudson, Deb Nollen, Julie Qidwai, Holly Riss, Traci Schwieger, Jamie Willits, Jon Yankey, Andrea Curry Corrales, Raquel Faradji, Tatiana Froud, Ana Alvarez Gil, Eva Herrada, Luca Inverardi, Norma Kenyon, Aisha Khan, Elina Linetsky, Eduardo Peixoto, Camillo Ricordi, Muhamad H. Abdulla, A. N. Balamurugan, Melena D. Bellin, Mary Brandenburg, James V. Harmon, Bernhard J. Hering, Raja Kandaswamy, Gopal Loganathan, Kate Mueller, Klearchos K. Papas, Jayne Pedersen, Joshua J. Wilhelm, Jean Witson, Cornelia Dalton-Bakes, Hongxing Fu, Malek Kamoun, Jane Kearns, Yanjing Li, Chengyang Liu, Eline Luning-Prak, Yanping Luo, Eileen Markmann, Zaw Min, Ali Naji, Maral Palanjian, Michael R. Rickels, Richard Shlansky-Goldberg, Kumar Vivek, Amin Sam Ziaie, Dixon B. Kaufman, and Olle Korsgren
References
- 1.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the diabetes control and complications trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 3.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 4.Vanstone M, Rewegan A, Brundisini F, Dejean D, Giacomini M. Patient perspectives on quality of life with uncontrolled type 1 diabetes mellitus: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser 2015;15:1–29 [PMC free article] [PubMed] [Google Scholar]
- 5.Böhme P, Bertin E, Cosson E, Chevalier N; GEODE Group . Fear of hypoglycaemia in patients with type 1 diabetes: do patients and diabetologists feel the same way? Diabetes Metab 2013;39:63–70 [DOI] [PubMed] [Google Scholar]
- 6.Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, Nemeth J, Quinn L. Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention - a review. J Diabetes Complications 2016;30:167–177 [DOI] [PubMed] [Google Scholar]
- 7.Speight J, Reaney MD, Woodcock AJ, Smith RM, Shaw JA. Patient-reported outcomes following islet cell or pancreas transplantation (alone or after kidney) in type 1 diabetes: a systematic review. Diabet Med 2010;27:812–822 [DOI] [PubMed] [Google Scholar]
- 8.Poggioli R, Faradji RN, Ponte G, et al. . Quality of life after islet transplantation. Am J Transplant 2006;6:371–378 [DOI] [PubMed] [Google Scholar]
- 9.Benhamou PY, Milliat-Guittard L, Wojtusciszyn A, et al.; GRAGIL Group . Quality of life after islet transplantation: data from the GRAGIL 1 and 2 trials. Diabet Med 2009;26:617–621 [DOI] [PubMed] [Google Scholar]
- 10.Johnson JA, Kotovych M, Ryan EA, Shapiro AM. Reduced fear of hypoglycemia in successful islet transplantation. Diabetes Care 2004;27:624–625 [DOI] [PubMed] [Google Scholar]
- 11.Ryan EA, Paty BW, Senior PA, et al. . Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 12.Tharavanij T, Betancourt A, Messinger S, et al. . Improved long-term health-related quality of life after islet transplantation. Transplantation 2008;86:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cure P, Pileggi A, Froud T, et al. . Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation 2008;85:801–812 [DOI] [PubMed] [Google Scholar]
- 14.Bellin MD, Freeman ML, Schwarzenberg SJ, et al. . Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol 2011;9:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinnakotla S, Radosevich DM, Dunn TB, et al. . Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg 2014;218:530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hering BJ, Clarke WR, Bridges ND, et al.; Clinical Islet Transplantation Consortium . Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricordi C, Goldstein JS, Balamurugan AN, et al. . National Institutes of Health-sponsored clinical islet transplantation consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes 2016;65:3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polonsky WH, Fisher L, Earles J, et al. . Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 19.Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 21.Irvine AA, Cox D, Gonder-Frederick L. Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychol 1992;11:135–138 [DOI] [PubMed] [Google Scholar]
- 22.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994;17:267–274 [DOI] [PubMed] [Google Scholar]
- 23.Ahroni JH, Boyko EJ. Responsiveness of the SF-36 among veterans with diabetes mellitus. J Diabetes Complications 2000;14:31–39 [DOI] [PubMed] [Google Scholar]
- 24.Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health 2013;16:993–1000 [DOI] [PubMed] [Google Scholar]
- 25.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208 [DOI] [PubMed] [Google Scholar]
- 26.Hart HE, Bilo HJ, Redekop WK, Stolk RP, Assink JH, Meyboom-de Jong B. Quality of life of patients with type I diabetes mellitus. Qual Life Res 2003;12:1089–1097 [DOI] [PubMed] [Google Scholar]
- 27.Janssen MF, Lubetkin EI, Sekhobo JP, Pickard AS. The use of the EQ-5D preference-based health status measure in adults with type 2 diabetes mellitus. Diabet Med 2011;28:395–413 [DOI] [PubMed] [Google Scholar]
- 28.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220 [DOI] [PubMed] [Google Scholar]
- 29.Reenen MV, Oppe M. EQ-5D-3L user guide, version 5.1 [Internet], 2015. EuroQoL Research Foundation. Available from http://www.euroqol.org/. Accessed 22 February 2017
- 30.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014;67:850–857 [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300 [Google Scholar]
- 32.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–592 [DOI] [PubMed] [Google Scholar]
- 33.Radosevich DM, Jevne R, Bellin M, Kandaswamy R, Sutherland DER, Hering BJ. Comprehensive health assessment and five-yr follow-up of allogeneic islet transplant recipients. Clin Transplant 2013;27:E715–E724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware JE Jr., Kosinski M, Gandek B. SF-36 Health Survey Manual & Interpretation Guide. Vol. 10 Lincoln, RI, QualityMetric Incorporated, 2000, p. 24 [Google Scholar]
- 35.Barshes NR, Vanatta JM, Mote A, et al. . Health-related quality of life after pancreatic islet transplantation: a longitudinal study. Transplantation 2005;79:1727–1730 [DOI] [PubMed] [Google Scholar]
- 36.Toso C, Shapiro AMJ, Bowker S, et al. . Quality of life after islet transplant: impact of the number of islet infusions and metabolic outcome. Transplantation 2007;84:664–666 [DOI] [PubMed] [Google Scholar]
- 37.The DCCT Research Group Reliability and validity of a diabetes quality-of-life measure for the Diabetes Control and Complications Trial (DCCT). Diabetes Care 1988;11:725–732 [DOI] [PubMed] [Google Scholar]
- 38.Polonsky WH, Hessler D, Ruedy KJ, Beck RW; The DIAMOND Study Group . The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736–741 [DOI] [PubMed] [Google Scholar]
- 39.Rubin RR, Peyrot M; STAR 3 Study Group . Health-related quality of life and treatment satisfaction in the Sensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) trial. Diabetes Technol Ther 2012;14:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehrabi A, Golriz M, Adili-Aghdam F, et al. . Expanding the indications of pancreas transplantation alone. Pancreas 2014;43:1190–1193 [DOI] [PubMed] [Google Scholar]
- 41.Scalea JR, Pettinato L, Fiscella B, et al. . Successful pancreas transplantation alone is associated with excellent self-identified health score and glucose control: a retrospective study from a high-volume center in the United States. Clin Transplant. 1 January 2018 [Epub ahead of print]. DOI: 10.1111/ctr.13177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.