Abstract
OBJECTIVE
Understanding how changes in weight over the life course shape risk for diabetes is critical for the prevention of diabetes. Using data from the National Health and Nutrition Examination Survey (NHANES), we investigated the association between self-reported weight change from young adulthood to midlife and incident diabetes.
RESEARCH DESIGN AND METHODS
We categorized individuals into four weight-change groups: those who remained nonobese (stable nonobese), those who moved from an obese BMI to a nonobese BMI (losing), those who moved from a nonobese BMI to an obese BMI (gaining), and those who remained obese (stable obese). Diabetes status was determined by self-report of a prior diagnosis, and age at diagnosis was used to establish time of diabetes onset. Hazard ratios (HRs) relating weight change to incident diabetes over 10 years of follow-up were calculated using Cox models adjusting for covariates.
RESULTS
Those who were obese and lost weight exhibited a significantly lower risk (HR 0.33; 95% CI 0.14, 0.76) of diabetes compared with those with stable obesity. We also observed lower risk among those who were stable nonobese (HR 0.22; 95% CI 0.18, 0.28) and those in the gaining category (HR 0.70; 95% CI 0.57, 0.87). Further, there was evidence of an increased incidence of diabetes among obese individuals who lost weight compared with individuals who were stable nonobese; however, weight loss was rare, and the association was not statistically significant. If those who were obese had become nonobese during the 10-year period, we estimate that 9.1% (95% CI 5.3, 12.8) of observed diabetes cases could have been averted, and if the population had maintained a normal BMI during the period, 64.2% (95% CI 59.4, 68.3) of cases could have been averted.
CONCLUSIONS
The findings from this study underscore the importance of population-level approaches to the prevention and treatment of obesity across the life course of individuals.
Introduction
The incidence and prevalence of diabetes among adults in the U.S. has risen during the past 30 years (1,2). In 1988, 9.8% of the adult population was living with diabetes; by 2014, this prevalence had grown to 12.3% (2). These increases have important implications for U.S. mortality (3), morbidity (4), disability (5), and health care expenditures (6). A recent study found that diabetes was responsible for a higher volume of personal medical expenditures than any other medical condition (6). A 2017 study estimated that nearly one-fifth of deaths among obese adults were attributable to diabetes (3).
A rising trend in the prevalence of obesity can account for most of the increase in diabetes between 1976 and 1980 and between 2007 and 2010 (2,7). The relationship between obesity and diabetes has been established by observational studies of BMI and diabetes status (8–11) and supported further by research into the physiologic link between body fat, insulin resistance, and type 2 diabetes (12). Alongside the effects of prevalent obesity on diabetes, prospective cohort studies have shown weight change over the life course can also have profound effects on the risk of diabetes (11,13–18). A recent study using data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS) examined major health outcomes associated with weight change in adulthood. Of the outcomes considered, type 2 diabetes showed the strongest associations with weight change. A general reduction in risk was observed for weight loss in both cohorts, whereas weight gain was associated with an increased incidence of diabetes commensurate with the absolute change (11). These associations have also been observed in studies outside the U.S. and in studies focused on ethnic differences (17,19–21).
With few exceptions, investigations of the relationship between weight change and the incidence of diabetes have been limited to samples that are not nationally representative. Exceptions include two studies using the National Health and Nutrition Examination Survey (NHANES) I Epidemiologic Follow-Up Study, 1971–1992 (22,23), and two studies that monitored adolescents for one to two decades (24,25). Recent national data on the association between weight change and incident diabetes are not available, largely because the two major national cohorts with ongoing data collection, the NHANES and the National Health Interview Survey (NHIS), have not prospectively monitored individuals for incident disease status.
One opportunity not yet explored is to treat a national probability sample as a retrospective longitudinal study. NHANES has routinely asked questions about weight histories, including weight at age 25 and weight 10 years before the survey, alongside weight at the survey. It has also asked questions about the history of diabetes diagnosis. Leveraging these data provides an opportunity to investigate the relationship between weight histories, in the form of weight at age 25 and weight 10 years before the survey, and the incidence of diabetes during 10 years of follow-up before the survey.
We used this approach to examine the relationship between weight change in adulthood and diabetes incidence. We sought to determine whether obese individuals who lost weight were 1) at a reduced risk of diabetes relative to individuals with stable obesity (the “risk reduction” hypothesis), and 2) at an increased risk of diabetes relative to individuals who maintained a nonobese BMI over time (the “residual risk” hypothesis).This latter hypothesis predicts that individuals who have been obese are at higher risk of developing diabetes than those who have never been obese.
Research Design and Methods
Data for this study were drawn from the NHANES, a nationally representative sample of U.S. adults. NHANES was collected periodically from the noninstitutionalized U.S. population before 1999 and continuously thereafter. Interviews were conducted at individuals’ homes, and laboratory and physical examinations were performed by trained technicians using mobile examination centers (26). Institutional Review Board approval was not required for this study because the investigation was based on secondary analyses of publicly available, deidentified data.
We incorporated data on adults aged 40–74 at examination from both the NHANES III (1988–1994) and continuous NHANES (1999–2014). Self-reported weight change was assessed by participant recall of weight at age 25 and 10 years before the NHANES survey. Incident diabetes was determined from respondents affirming that a health care provider had indicated a diagnosis of diabetes. The reported age at diagnosis was used to establish the time of diabetes onset. The study design is visually depicted in Supplementary Fig. 1.
Those who reported a date of onset that was before the initiation of follow-up were considered prevalent diabetes cases and were excluded. An individual who did not report a diagnosis of diabetes but recorded an HbA1c ≥6.5% (48 mmol/mol) at examination was considered to have undiagnosed diabetes and excluded from survival analyses. Diabetes type (type 1 or type 2) was not able to be determined. We also excluded individuals missing observations for BMI, HbA1c, family history of diabetes, or education. After exclusions, a sample size of 21,554 individuals remained for analysis.
Respondents were asked to recall weight at age 25 and weight 10 years before their age at survey. Measured height at the examination was used to calculate BMI, unless the participant was 50 years or older at the time of the survey. In this case, reported height at 25 was used to calculate BMI at 25, and measured height at the examination was used to calculate BMI at 10 years before the examination. Reported height at age 25 was incorporated to account for the possibility of height decline with age. To calculate BMI, weight was converted into kilograms and height was converted to meters. BMI values for both time points were categorized into underweight, normal weight, overweight, obese, and obese II.
BMI change categories were then generated to capture weight change over the life course of an individual. The primary analysis used four BMI change categories based on BMI (kg/m2) at age 25 and on BMI 10 years prior to the survey: stable nonobese (BMIage 25 <30 and BMI10 years prior <30), losing (BMIage 25 ≥30 and BMI10 years prior <30), gaining (BMIage 25 <30 and BMI10 years prior ≥30), and stable obese (BMIage 25 ≥30 and BMI10 years prior ≥30).
Survival Analysis
Two hypotheses, which are referred to as the “risk reduction” hypothesis and the “residual risk” hypothesis, were tested using Cox proportional hazard models predicting the rate of incident diabetes across the four BMI change categories over the time period. A total of 1,877 cases of incident diabetes were recorded during follow-up, with individuals contributing 208,061 person-years, or a rate of 9.02 cases per 1,000 person-years. Models included adjustment for race/ethnicity, sex, educational attainment, family history of diabetes, and age at the start of follow-up. To test the risk reduction hypothesis, the stable obese weight-change category was used as the reference category to which all other weight-change categories were compared. To test the residual risk hypothesis, the same model was used, with the stable nonobese category chosen as the reference category. To determine whether the time period studied had an effect on the results observed, a sensitivity analysis was run excluding NHANES 1988–1994 data (Supplementary Table 2). No meaningful differences were observed, so the primary model with both time periods (1988–1994 and 1999–2014) was preserved.
A secondary analysis was implemented in which those who maintained a normal BMI during the time period were treated as a separate category of BMI change. In doing so, those originally in the stable nonobese category were categorized into two new groups: 1) “stable normal” (BMIage 25 <25 and BMI10 years prior <25), as described; and 2) “max overweight,” comprising those who were never obese but were overweight (BMI ≥25 and BMI <30) at least once at age 25 or 10 years before the survey. Stable normal was used as the reference category for this model.
Hypothetical Scenarios
Estimates of the percentage of diabetes cases that could be averted under four hypothetical scenarios were calculated using the following equation for the population-attributable fraction (PAF):
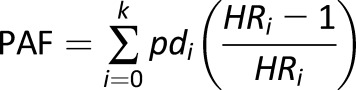 |
where pdi is the proportion of total incident cases in the sample observed in the ith BMI trajectory category, and HRi is the hazard ratio associated with that BMI trajectory (27). To calculate the attributable fractions, we treated individuals with the BMI trajectory exposure of interest as if they were hypothetically part of a different level of exposure based on the respective scenario. In doing so, the PAFs represent the fraction of cases that would be eliminated if people with a particular BMI trajectory were redistributed to another trajectory and experienced the same relative risks as individuals in that new trajectory.
For clarity, we referred to the four scenarios used in PAF calculations with the names 1) “weight loss,” 2) “weight maintenance,” 3) “partial prevention,” and 4) “comprehensive prevention” (Table 3):
Table 3.
Population attributable fractions for population counterfactuals*
| Scenario | PAF (%) | 95% CI | Definition | |
|---|---|---|---|---|
| 1 Weight loss†‡ | 9.1 | 5.3, 12.8 | If those who maintained an obese BMI, instead lost to a nonobese BMI between young adulthood and midlife | |
| 2 Weight maintenance†‡ | 23.5 | 21.8, 25.1 | If those who gained weight, instead remained nonobese between young adulthood and midlife | |
| 3 Partial prevention‡ | 34.5 | 32.4, 36.6 | If the total population had a nonobese BMI from young adulthood that was maintained through midlife | |
| 4 Comprehensive prevention§ | 64.2 | 59.4, 68.3 | If the total population had a normal BMI from young adulthood through midlife | |
*Source: NHANES III (1988–1994) and NHANES (1999–2014).
†Weight loss and weight maintenance represent fraction of cases prevented if one trajectory’s risk were substituted for another, as described.
‡HRi from Table 2.
§HRi from Supplementary Table 1.
The weight loss scenario is a hypothetical scenario in which individuals who were obese in young adulthood lost down to a nonobese BMI at midlife.
The weight maintenance scenario estimates what would have happened if individuals who gained weight—from nonobese in young adulthood to obese by midlife—had not gained this weight, but instead had maintained their nonobese BMI during the time period.
Partial prevention asks what if the total population had a nonobese BMI from young-adulthood that was maintained through midlife.
Comprehensive prevention examines what would happen if the total population had a normal BMI from young adulthood through midlife. The PAF calculation for this scenario used estimates generated for the stable normal category from our secondary analysis, because those who were stable normal approximate the ideal weight-change category for the period from young adulthood to midlife.
Examination sample weights were used for all estimates and analyses. Stata 14 software (StataCorp) was used for statistical analysis and data management. The Stata package punafcc was used to generate PAFs (28). A two-sided P value of <0.05 was used to determine statistical significance.
Results
Table 1 reports characteristics of the sample with weighted estimates and unweighted sample sizes stratified by weight-change category. The mean age of the sample was 43.8 years at baseline, and 50.2% were men. The mean BMI was 23.6 kg/m2 at age 25, 26.6 kg/m2 at 10 years before the survey, and 27.8 kg/m2 at the end of follow-up. The study sample was 78.9% non-Hispanic white, 9.0% non-Hispanic black, and 8.5% Hispanic. Of the individuals in the study, 52.6% reported having more than a high school education. A family history of diabetes was reported by 43.5%, ranging from 54.9% among those who lost weight to 41.1% among those who maintained a stable nonobese BMI.
Table 1.
Life course obesity progression and onset diabetes in adults ages 40–75*
| Stable nonobeseb | Gainingb | Stable obeseb | Losingb | Total | |
|---|---|---|---|---|---|
| n (%) = 16,454 (79.4) | n (%) = 3,719 (14.6) | n (%) = 1,154 (4.9) | n (%) = 227 (1.1) | N = 21,554 | |
| Age (years), µ (95% CI)a | 43.6 (43.2, 43.9) | 46.2 (45.7, 46.7) | 40.9 (40.1, 41.7) | 43.7 (42.2, 45.1) | 43.8 (43.5, 44.1) |
| Sex, n (%) | |||||
| Male | 8,483 (49.7) | 1,836 (50.8) | 601 (54.8) | 133 (62.6) | 11,053 (50.2) |
| Female | 7,971 (50.3) | 1,883 (49.2) | 553 (45.2) | 94 (37.4) | 10,501 (49.8) |
| Race, n (%) | |||||
| Non-Hispanic | |||||
| White | 8,176 (79.4) | 1,757 (77.5) | 517 (76.5) | 114 (78.3) | 10,554 (78.9) |
| Black | 3,388 (8.3) | 923 (11.3) | 348 (13.9) | 45 (7.8) | 4,704 (9.0) |
| Hispanic | 4,008 (8.4) | 951 (9.0) | 260 (7.7) | 63 (8.4) | 5,282 (8.5) |
| Other | 882 (3.9) | 88 (2.2) | 29 (1.9) | 5 (5.4) | 1,004 (3.5) |
| Education, n (%) | |||||
| Less than high school | 4,687 (18.2) | 1,193 (22.0) | 357 (19.6) | 81 (24.2) | 6,318 (18.9) |
| High school or equivalent | 4,161 (28.0) | 956 (30.0) | 299 (31.4) | 56 (29.4) | 5,472 (28.5) |
| More than high school | 7,606 (53.8) | 1,570 (48.0) | 498 (48.9) | 90 (46.4) | 9,764 (52.6) |
| BMI (kg/m2), µ (95% CI) | |||||
| At age 25c | 22.5 (22.4, 22.6) | 25.2 (25.0, 25.3) | 34.4 (34.1, 34.8) | 33.4 (32.5, 34.2) | 23.6 (23.5, 23.7) |
| 10 years priord | 24.6 (24.5, 24.7) | 33.7 (33.5, 33.9) | 38.0 (37.4, 38.6) | 26.6 (26.0, 27.3) | 26.6 (26.5, 26.7) |
| At surveye | 26.2 (26.1, 26.3) | 33.2 (33.0, 33.4) | 37.3 (36.7, 37.8) | 29.5 (28.4, 30.6) | 27.8 (27.7, 28.0) |
| Family history of diabetesf | 7,058 (41.1) | 1,950 (51.9) | 672 (54.7) | 114 (54.9) | 9,794 (43.5) |
*Source: NHANES III (1988–1994) and NHANES (1999–2014); sample weighted estimates.
aAge at start of follow-up.
bStable nonobese: BMIage 25 <30 and BMI10 years prior <30; losing: BMIage 25 ≥30 and BMI10 years prior <30; gaining: BMIage 25 <30 and BMI10 years prior ≥30; stable obese: BMIage 25 ≥30 and BMI10 years prior ≥30.
cSelf-reported BMI at age 25.
dSelf-reported BMI 10 years before survey.
eSelf-reported BMI at survey.
fSelf-reported family history of diabetes.
Regarding life-course weight change, 79.4% of the population was stable nonobese, 1.1% reported losing from an obese BMI to a nonobese BMI, 14.6% reported gaining weight, and 4.9% remained stable obese between young adulthood and midlife.
Survival Analysis
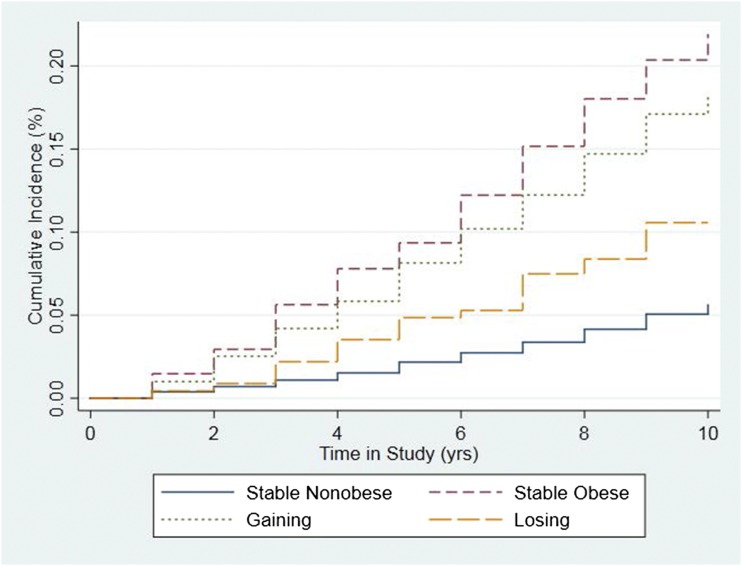
Figure 1 shows cumulative incidence curves by time in study for each weight change group. Compared with stable obese individuals, those who maintained a stable nonobese BMI from young adulthood through midlife had the lowest risk (HR 0.22; 95% CI 0.18, 0.28) of developing diabetes during the 10 years of follow-up (Table 2). Those who reported losing from an obese BMI to a nonobese BMI over their life course had 0.33 (95% CI 0.14, 0.76) times the risk of developing diabetes as those who maintained a stable obese BMI during the time period. Individuals who reported gaining from a nonobese BMI to an obese BMI had 0.70 (95% CI 0.57, 0.87) times the risk of developing incident diabetes as those who were stable obese. When the reference category was changed to the stable nonobese (Table 2), no significant difference (HR 1.47; 95% CI 0.65, 3.36) was observed in the risk of onset diabetes between those reporting losing from an obese BMI to a nonobese BMI and those who maintained a stable nonobese BMI over the time period.
Figure 1.
Cumulative incidence curve for the Cox model. Source: NHANES III (1988–1994) and NHANES (1999–2014). Stable nonobese: BMIage 25 <30 kg/m2 and BMI10 years prior <30; losing: BMIage 25 ≥30 and BMI10 years prior <30; gaining: BMIage 25 <30 and BMI10 years prior ≥30; stable obese: BMIage 25 ≥30 and BMI10 years prior ≥30.
Table 2.
Risk reduction and residual risk: HRs for obesity progression and incident diabetes*
| Hypothesis | BMI change | HR† | 95% CI | P value |
|---|---|---|---|---|
| Risk reduction | Stable nonobese | 0.22 | 0.18, 0 0.28 | <0.001 |
| Losing | 0.33 | 0.14, 0.76 | 0.009 | |
| Gaining | 0.70 | 0.57, 0.87 | 0.002 | |
| Stable obese | 1.00 (Ref) | — | — | |
| Residual risk | Stable obese | 4.45 | 3.59, 5.51 | <0.0001 |
| Gaining | 3.13 | 2.69, 3.63 | <0.0001 | |
| Losing | 1.47 | 0.65, 3.36 | 0.355 | |
| Stable nonobese | 1.00 (Ref) | — | — |
*Source: NHANES III (1988–1994) and NHANES (1999–2014).
†Adjusted for education, race, sex, family history of diabetes, and age at start time.
In the secondary analysis (Supplementary Table 1), we observed that those who gained from a nonobese to an obese BMI during the period had 5.77 (95% CI 4.63, 7.18) times the rate of incident diabetes compared with those who remained in the normal BMI range. Those who remained obese at both times had 8.07 (95% CI 6.28, 10.38) times the rate compared with the stable normal category. Finally, those who were overweight at either time point (max overweight) showed increased incidence of diabetes (HR 2.65; 95% CI 2.12, 3.31) relative to those who were stable normal weight.
Hypothetical Scenarios
PAFs were calculated from the four scenarios that are outlined in the Research Design and Methods and represented in Table 3. In the weight loss scenario, if those who were obese lost to a nonobese BMI, 9.1% (95% CI 5.3, 12.8) of observed diabetes cases could have been averted (Table 3). In the weight maintenance scenario, if those who gained weight during the period had not gained weight, 23.5% (95% CI 21.8, 25.1) of the observed cases would have been averted. In the partial prevention scenario, maintaining a nonobese BMI between young adulthood and midlife would have prevented 34.5% (95% CI 32.4, 36.6) of diabetes cases. In the comprehensive prevention scenario, if the total population had a normal BMI from young adulthood through midlife, 64.2% (95% CI 59.4, 68.3) of cases would have been averted.
Conclusions
The category with the highest risk of incident diabetes consisted of people who were obese during both young adulthood and midlife. Being nonobese at age 25 or 10 years before the survey was advantageous relative to remaining obese throughout this period. Those who lost from an obese BMI to a nonobese BMI benefited from a reduction in the rate of incident diabetes compared with those who remained obese (HR 0.33; 95% CI 0.14, 0.76). Likewise, those who had been nonobese at age 25 but became obese by a period 10 years before the survey had lower risks than those who were obese throughout the period (HR 0.70; 95% CI 0.57, 0.87) (Table 2). These results are consistent with other evidence based on nonnational sources that duration of obesity predicts the incidence of diabetes (29).
We found some evidence in support of the residual risk hypothesis. Those who were obese at age 25 and became nonobese had an HR of 1.47 (95% CI 0.65, 3.36) relative to those who maintained a nonobese BMI throughout the period. However, the 95% CIs were wide because weight loss from an obese BMI to a nonobese BMI was rare, representing only 1.1% of the total population (Table 1).
We used our estimates of the risks associated with weight change to explore the potential effect of weight loss interventions and prevention initiatives targeting weight gain. The weight loss scenario was designed to approximate a comprehensive weight loss intervention targeting individuals with obesity at age 25. We estimated that if all those who were obese at age 25 lost to a nonobese BMI by midlife, 9.1% (95% CI 5.3, 12.8) of observed incident cases of diabetes could be averted. Preventing weight gain in the population after age 25, represented by the weight maintenance scenario, was associated with a 23.5% (95% CI 21.8, 25.1) reduction in diabetes cases in the population. In total, we found that 64.2% (95% CI 59.4, 68.3) of diabetes cases during this time period could be averted if all individuals in the population maintained a weight in the normal range between early adulthood and midlife.
Our results are consistent with several previous studies that examined weight change in adulthood (11,13,14,16–18). A recent study by Zheng et al. (11) found that increasing levels of weight gain were strongly associated with incident diabetes and that moderate weight loss was associated with reduced risk. Among women in the NHS, those who gained ≥20 kg of weight from early to middle adulthood showed 10.51 times the incidence rate of diabetes compared with those who lost or gained <2.5 kg (11). Similar effects were observed among men in the HPFS. We also found a similar effect from weight gain in our study, with those gaining into the obese category having 5.77 times the incidence rate of diabetes compared with those who maintained a normal BMI (Supplementary Table 2). In an earlier analysis of the HPFS, an elevated BMI of ≥23 at age 21 was associated with diabetes onset later in adulthood, and a weight gain of ≥2.5 kg in adulthood was associated with an elevated risk of diabetes (13). These findings support the results from our study that those who lost weight during the study period did not fully reduce their risk of diabetes relative to those who maintained a nonobese or normal BMI.
Our study had several strengths. Using a retrospective cohort design, we were able to take advantage of a large, nationally representative cohort of U.S. adults to estimate associations between weight change and incident diabetes across the life course. BMI observations at two earlier points in the life course were used to study the incidence of diabetes during a subsequent decade of exposure. Although many other studies have looked at weight change and incident diabetes, the nonrepresentative samples used in previous studies may not be generalizable because covariate distributions can differ, with some of those covariates serving as key effect modifiers of the association between weight change and incident diabetes. As a nationally representative survey, results using NHANES are more broadly generalizable than those from other cohorts like the NHS and the HPFS, which rely on data gathered in disproportionately white populations. Although the focus of NHANES is not on ethnic differences, our findings agree with those from multiethnic studies that more fully consider racial/ethnic differences such the Multiethnic Cohort Study performed in Hawaii and Los Angeles (17,30).
An additional strength of the current study is that by conditioning on survival in the 10-year period between the second weight observation and baseline, any effects of illness-associated weight loss are likely to be substantially mitigated. The prior literature has generally not accounted for potential reverse causal pathways in investigating associations between weight change and incident outcomes (15–18). Some prior studies conditioned on survival for several years subsequent to assessment of weight status; however, there is evidence that illness-induced weight loss can occur with substantial lag times, suggesting that the measures taken in those studies were not sufficient to address the bias (31,32). Finally, by updating height data in our BMI calculations for older adults, we accounted for any age-associated height loss that these individuals might have experienced.
This study had several limitations. First was our reliance on historic, self-reported weight measures. Although prior studies have shown self-reported weight is a strongly correlated predictor of actual weight (33), our use of historic self-report likely introduced error. However, evidence has demonstrated that historic weight measures can have high agreement with measured weight (34,35).
A second limitation was the use of recalled height to account for age-related height decline. Error could have been introduced when relying on self-reported height at age 25 in adults older than age 50 to calculate BMI at age 25 (36). Evidence suggests a relatively high level of agreement between height recall and historically measured height among older adults (37,38). A validation study in an older population (µage = 61.0 ± 2.9 years) in which recalled historic height was compared with measured historic height at age 45 demonstrated high correlation (r = 0.944) (37). Furthermore, Must et al. (38) found high correlation between measured height in high school and recalled height among an elderly population aged 71–76 years. In light of the evidence, we opted to use recalled height with the older population in an attempt to mitigate underestimation of height due to age-related height decline.
A third limitation is that we could not adjust for physical activity or diet because recall data on these variables were not collected. The results may thus partly reflect the effects of physical activity and dietary factors over the life course.
Another limitation is that members of the relevant cohorts who had died before the survey were not represented in the retrospective data set. Their experience might have differed from that of survivors in ways that affected the estimated relationship between obesity and diabetes. Furthermore, our report relied on self-reported data on diabetes status, which may have missed people who had not been diagnosed with the condition. However, recent evidence from the NHANES indicates that the proportion of people with undiagnosed diabetes is relatively low in the U.S.; thus, relying on self-reported diabetes status is unlikely to represent a major source of bias in estimates (39). We further accounted for undiagnosed cases in the analysis by excluding individuals with HbA1c values that exceeded the relevant threshold. As a result, we expect any error introduced from the use of self-report diagnosis to be small. Finally, the study did not account for weight cycling between reported weight measures or distinguish subtypes of diabetes.
Conclusion
In testing the risk reduction hypothesis, we found those who lost weight between young adulthood and midlife showed statistically significant reductions in risk for diabetes onset compared with those who remained obese. When considering the residual risk hypothesis, those who had been obese at age 25 but had subsequently become nonobese had a higher risk of developing diabetes than those who remained nonobese throughout their life course, but the difference was not statistically significant. A large percentage of the observed diabetes cases could have been averted with effective intervention and prevention efforts in young adulthood.
This study used a novel application of NHANES survey data to explore the associations and implications of weight change from young adulthood through midlife and demonstrated the viability of using historic self-reported weight data for longitudinal analyses. We show that remaining or becoming obese raises the risk of incident diabetes relative to remaining nonobese. The findings from a national sample underscore the importance of developing policies and programs that reduce the prevalence of obesity.
Supplementary Material
Article Information
Funding. Research reported in this publication was supported by the National Institutes of Health, National Institute on Aging under award number R03-AG-055724-01, and by Johnson & Johnson.
Duality of Interest. A.S. has received research funding from Johnson & Johnson. B.F.G., R.F.S., and C.-W.H. are employees of Ethicon (a Johnson & Johnson Company); S.S.J. and E.M.A. are employees of Johnson & Johnson. B.F.G., R.F.S., C.-W.H., and S.S.J. are stockholders of Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S. designed the study, performed analyses, interpreted the data, and wrote the manuscript. J.M.C. performed analyses and contributed to interpreting the data and writing the manuscript. B.F.G., R.F.S., C.-W.H., and S.S.J. contributed to study conception, study design, interpretation of the data, and reviewed and edited the manuscript. E.M.A. contributed to the interpretation of the data and reviewed and edited the manuscript. J.E.M. reviewed and edited the manuscript and contributed to the discussion. S.H.P. reviewed and edited the manuscript and contributed to the introduction and discussion. A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2336/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Cheng YJ, Imperatore G, Geiss LS, et al. Secular changes in the age-specific prevalence of diabetes among U.S. adults: 1988-2010. Diabetes Care 2013;36:2690–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 3.Stokes A, Preston SH. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS One 2017;12:e0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelgau M, Geiss L, Saadine J. The evolving diabetes burden in the United States. Ann Intern Med 2004;140:945–950 [DOI] [PubMed] [Google Scholar]
- 5.Fishman EI. Incident diabetes and mobility limitations: reducing bias through risk-set matching. J Gerontol A Biol Sci Med Sci 2014;70:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA 2016;316:2627–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stokes A, Preston SH. The contribution of rising adiposity to the increasing prevalence of diabetes in the United States. Prev Med 2017;101:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg 2011;21:351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays HE, Chapman RH, Grandy S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract 2007;61:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janiszewski P, Janssen I, Ross R. Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care 2007;30:3105–3109 [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 13.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol 2014;179:1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs-van der Bruggen MA, Spijkerman A, van Baal PH, et al. Weight change and incident diabetes: addressing an unresolved issue. Am J Epidemiol 2010;172:263–270 [DOI] [PubMed] [Google Scholar]
- 15.Nanri A, Mizoue T, Takahashi Y, et al. Association of weight change in different periods of adulthood with risk of type 2 diabetes in Japanese men and women: the Japan Public Health Center-Based Prospective Study. J Epidemiol Community Health 2011;65:1104–1110 [DOI] [PubMed] [Google Scholar]
- 16.Waring ME, Eaton CB, Lasater TM, Lapane KL. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol 2010;171:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto Y, Schembre SM, Steinbrecher A, et al. Ethnic differences in weight gain and diabetes risk: the Multiethnic Cohort Study. Diabetes Metab 2011;37:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brancati F, Wang N, Mead L, Liang KY, Klag MJ. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus: the Johns Hopkins Precursors Study. Arch Intern Med 1999;159:957–963 [DOI] [PubMed] [Google Scholar]
- 19.Verhaeghe N, De Greve O, Annemans L. The potential health and economic effect of a body mass index decrease in the overweight and obese population in Belgium. Public Health 2016;134:26–33 [DOI] [PubMed] [Google Scholar]
- 20.Langenberg C, Sharp SJ, Schulze MB, et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS Med 2012;9:e1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84:427–433 [DOI] [PubMed] [Google Scholar]
- 22.Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health 2000;54:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214–222 [DOI] [PubMed] [Google Scholar]
- 24.The NS; Richardson AS, Gordon-Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care 2013;36:865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, Gebremariam A, Vijan S, Gurney JG. Excess body mass index-years, a measure of degree and duration of excess weight, and risk for incident diabetes. Arch Pediatr Adolesc Med 2012;166:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Available from https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 16 March 2017
- 27.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newson R. Roger Newson Stata packages. punafcc. 2013. Available from http://www.rogernewsonresources.org.uk/stata.htm. Accessed 30 March 2017
- 29.Abdullah A, Stoelwinder J, Shortreed S, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr 2011;14:119–126 [DOI] [PubMed] [Google Scholar]
- 30.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes 2009;58:1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alley DE, Metter EJ, Griswold ME, et al. Changes in weight at the end of life: characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol 2010;172:558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu E, Ley SH, Manson JE, et al. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med 2017;166:613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stokes A, Ni Y. Validating a summary measure of weight history for modeling the health consequences of obesity. Ann Epidemiol 2016;26:821–826.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casey V, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr 1991;53:1493–1498 [DOI] [PubMed] [Google Scholar]
- 35.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology 1995;6:61–66 [DOI] [PubMed] [Google Scholar]
- 36.Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index: the Baltimore Longitudinal Study of aging. Am J Epidemiol 1999;150:969–977 [DOI] [PubMed] [Google Scholar]
- 37.Heaney RP, Ryan R. Relation between measured and recalled body height. N Engl J Med 1988;312:2016. [DOI] [PubMed] [Google Scholar]
- 38.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993;138:56–64 [DOI] [PubMed] [Google Scholar]
- 39.Selvin E, Wang D, Lee AK, Bergenstal RM, Coresh J. Identifying trends in undiagnosed diabetes in U.S. adults by using a confirmatory definition: a cross-sectional study. Ann Intern Med 2017;167:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.