Abstract
Background
Exposure to gadolinium-based contrast agents (GBCA) in patients with chronic kidney disease (CKD) has been associated with the development of a potentially fatal disorder, nephrogenic systemic fibrosis (NSF). Although contrast-enhanced computed tomography (CT) is an alternative to magnetic resonance imaging (MRI), it carries the risk of radiation exposure and further reduction of residual renal function. Therefore we sought to assess the feasibility of ferumoxytol as an alternative to GBCA for contrast-enhanced MR angiography (MRA) in a pediatric cohort with CKD. Ferumoxytol is a parenteral iron supplement that contains ultrasmall superparamagnetic iron oxide (USPIO) and is a potent relaxivity agent for MRI.
Methods
We describe the MRI findings in ten pediatric patients who needed detailed vascular mapping. Ferumoxytol (4 mg/kg) was administered intravenously for contrast-enhanced MRA. The patients tolerated the procedure without complications.
Results
Resulting studies were highly diagnostic and were pivotal in guiding patient management. The images were notable for clear delineation of multiple vascular occlusions.
Conclusions
Given the concerns associated with the use of GBCAs in renal failure, ferumoxytol is an excellent alternative contrast agent in pediatric end stage renal disease (ESRD) patients. Future studies are needed in order to further evaluate safety and efficacy of ferumoxytol in this patient population.
Keywords: Children, Chronic kidney disease, Ferumoxytol, Vascular imaging
Introduction
Pediatric patients with chronic kidney disease (CKD) require detailed vascular imaging as a prelude to line placement as well as renal transplantation. Contrast-enhanced magnetic resonance venography and arteriography (ceMRV and ceMRA) are excellent techniques for vascular evaluation in children [1–3]. However, CKD patients are potentially at risk for nephrogenic systemic fibrosis (NSF) if gadolinium-based contrast agents (GBCA) are utilized [4–6].
Originally described over a decade ago for vascular MR imaging by Prince et al. [7], some recent reports have suggested the potential role of ultrasmall, superparamagnetic iron oxide (USPIO) particles as alternatives to GBCAs in adult patients with CKD [1, 8, 9]. Ferumoxytol is a USPIO approved by the US Food and Drug Administration (FDA) for bolus intravenous treatment of iron deficiency anemia in adult patients with CKD [10] and it is marketed in the USA as Feraheme (AMAG Pharmaceuticals, Waltham, MA, USA). As a USPIO, ferumoxytol exerts an enhancing effect on T1 relaxation during magnetic resonance imaging (MRI), which makes blood appear hyperintense on T1-weighted imaging. In addition, the ferumoxytol particle is covered by a carbohydrate (dextran derivative) coat, which prolongs the intravascular residence time and decreases leakage into the extravascular space. These properties suggest ferumoxytol would be a useful vascular imaging contrast agent, especially in patients with CKD, and preliminary studies suggest that it may be superior to GBCA for MRA and perfusion [11–14].
Ferumoxytol has been used in adult patients with CKD for central nervous system and vascular imaging [8, 15]. However, to our knowledge, there are no prior reports describing the use of USPIO in pediatric CKD patients. The purpose of our study, therefore, was to assess the feasibility of using ferumoxytol for high-resolution vascular imaging in a cohort of pediatric patients with CKD who otherwise would not be candidates for contrast-enhanced MRA.
Materials and methods
The Institutional Review Board approved this Health Insurance and Portability and Accountability Act (HIPAA)—compliant retrospective study.
Study population
A total of ten pediatric patients with an average age of 7.5 years (range, 4–18 years) who required detailed vascular mapping underwent non-gadolinium contrast-enhanced MR angiography of the neck, thorax, abdomen, and pelvis using ferumoxytol. Patients were enrolled from June 2013 to March 2014 and informed consent for the use of ferumoxytol was obtained in all cases. Imaging studies were performed to assess vascular patency in all the subjects either for vascular access and/or pre-/post-kidney transplantation. Five of the ten patients were undergoing hemodialysis, two were treated with peritoneal dialysis, and the three remaining patients had a mean estimated glomerular filtration rate (eGFR) 31.07±2.6 ml/min/1.73 m2 estimated within a week of the imaging. The causes of CKD included renal dysplasia (n=5), hypoxic injury (n=2), nephrotoxic medications (n=2), and unknown causes (n=1).
Imaging
MRA for each patient was performed on a 3.0-T, 32-channel, whole-body MRI system (Magnetom TIM Trio, Siemens Medical Solutions, Malvern, PA, USA). Patients less than 8 years of age (n=9) were evaluated under general anesthesia with controlled ventilation. Electrocardiogram, pulse oximetry, end-tidal CO2, and non-invasive blood pressure monitoring was performed throughout the procedure by a pediatric anesthesiologist. A radiologist (JPF) was present routinely to supervise the study and to optimize the MR image acquisition protocol. Ferumoxytol was administered intravenously in a dose of 4 mg/kg and high-resolution 3D CEMRA was acquired during first pass and at a minimum of two time points during the steady-state distribution phase. Partition images were post-processed to generate 3D maximum intensity projection (MIP) and volume-rendered (VR) reconstructions. Raw partition images and processed images were analyzed by two radiologists (TRH, JPF) for image quality and the diagnosis of pathology. Images were graded on a three-point scale for subjective image quality: 1 = poor image quality undermining diagnostic confidence; 2 = good image quality adequate for confident diagnosis; 3 = excellent image quality supporting highly confident diagnosis. The signal-to-noise ratio (SNR) within the inferior vena cava (IVC) and hepatic veins (HV) was measured at two time points during the steady-state distribution phase of ferumoxytol, 2 and 20 min post-injection, to assess the stability of the vascular enhancement. One patient had previously undergone non-contrast-enhanced, “time of flight” MR venography and these images were reviewed in the context of the more recent ferumoxytol enhanced MRA.
Results
All ten patients (Table 1) successfully underwent MR imaging with ferumoxytol without incident. There were no adverse events and vital signs remained stable throughout the procedure and up to 30 min after ferumoxytol injection. Image quality was scored as diagnostic with excellent definition of vascular borders (grade 3.0) in all cases. The signal within the venous and arterial blood pool remained stable from initial steady state distribution for up to 20 min (when the study was completed). In the ferumoxytol-enhanced MRVs, the mean SNRs in the IVC and HV respectively measured 78 (±26) and 78 (±28) and there was no change in the blood signal between 2 and 20 min following injection, confirming stable intravascular residence of the agent.
Table 1.
Demographic characteristics and image scores of the patients
| Patient no. | Age at examination | Weight (kg) | Gender | Indication for study | Image quality score | eGFR (ml/min/1.73 m2) |
|---|---|---|---|---|---|---|
| 1 | 4 | 13.4 | M | Re-transplantation evaluation | 3 | 28.51 |
| 2 | 5 | 26.8 | M | Evaluate biliary/liver dysfunction | 3 | 33.71 |
| 3 | 15 | 55.2 | M | Evaluate cardiac anastomoses | 3 | 31.0 |
| 4 | 8 | 18.2 | M | Evaluation of renal transplant anastomosis | 3 | <15 |
| 5 | 18 | 109.1 | M | Evaluate vascular thromboses | 3 | <15 |
| 6 | 5 | 11.7 | F | Evaluate for access placement | 3 | <15 |
| 7 | 4 | 13.8 | M | Evaluate for access placement | 3 | <15 |
| 8 | 7 | 23.6 | M | Evaluate for access placement | 3 | <15 |
| 9 | 4 | 14.4 | M | Evaluate for access placement | 3 | <15 |
| 10 | 5 | 18.8 | M | Evaluate for access placement | 3 | <15 |
eGFR estimated glomerular filtration rate
In all patients, the entire vascular bed was clearly visualized from the neck to the lower limbs and significant vascular abnormalities were detected in all patients ranging from venous occlusion and stenosis to transplant renal artery occlusion (Figs. 1, 2, and 3). In the patients with prior non-contrast-enhanced (time of flight) MRA, the ferumoxytol study was felt to provide greatly improved vascular definition and detail (Figs. 1a–e and 2a–c). In one patient, MRA/MRV confirmed suspected infarction of a renal graft in a patient with abdominal pain and an absent Doppler signal (Fig. 3). The infarcted graft was immediately removed.
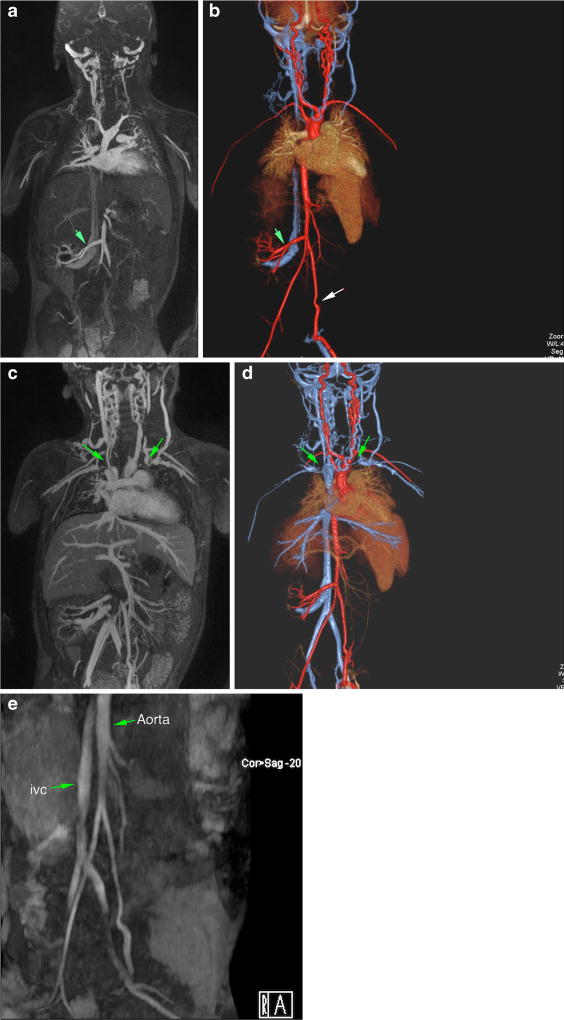
Fig. 1.
a–e Magnetic resonance (MR) angiogram with ferumoxytol in 3-year-old male post-renal transplant. Arterial phase-dominant (a, b) and venous phase-dominant (c, d) images show widely patent graft artery and vein and multiple thrombosed neck/chest veins (green arrows). For comparison (e) is a non-contrast, time-of-flight MR angiogram performed before the transplant, which highlights the superior clarity of the ferumoxytol studies
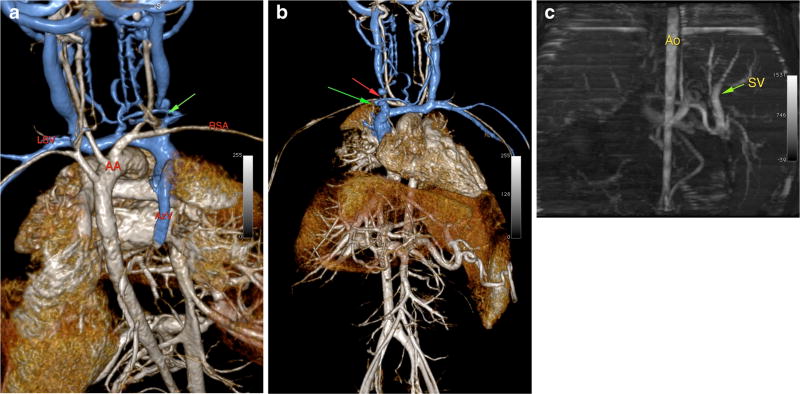
Fig. 2.
a, b MR venogram with ferumoxytol in a 5-year-old male with CKD, showing multiple upper venous occlusions (red and green arrows). LSV left subclavian vein, RSA right subclavian artery, AA aortic arch, AzV azygos vein vein. c Non-contrast (“time of flight”) MRA done previously in this 5-year-old male patient showing the difference in image quality. Ao aorta
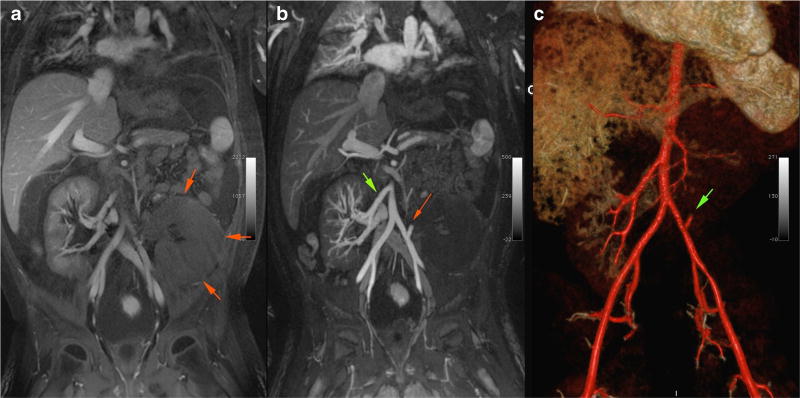
Fig. 3.
Magnetic resonance angiogram with ferumoxytol in an 8-year-old male post-second renal transplant. a Enlarged and edematous graft in the left lower quadrant (as denoted by green arrows). b Patent right renal artery to previous graft (green arrow) and a thrombosed left renal artery (red arrow) to the infarcted second graft. c Volume-rendered 3D image of the same patient, highlighting the thrombosed left renal graft artery (green arrow)
Discussion
The results of our study show that high-resolution, whole-body vascular MR imaging with ferumoxytol is feasible in children with CKD, eliminating concerns about NSF. Our patients had multiple and significant vascular abnormalities, which were shown unequivocally in all patients.
The initial evaluation of pediatric patients presenting with access difficulty often includes non-invasive imaging, such as Doppler ultrasound. Ultrasound has the advantage of not needing sedation in younger children especially under the age of 10 years. However, ultrasound studies may be undermined by poor acoustic windows and patient habitus, and the results may be equivocal and inadequate to guide management decisions. MRI methods using contrast agents are attractive techniques for evaluating vascular patency and planning surgical strategies in that they are practical, reliable, and give three-dimensional anatomic information. However gadolinium-based contrast agents are contraindicated in the pediatric patients in the setting of CKD and are contraindicated in infants due to immature renal function. Also, iodinated contrast agents used in computed tomography (CT) carry the risk of contrast—induced nephropathy and worsening renal function. Children with CKD usually require multiple imaging studies from a very early age, while on dialysis, post-renal transplantation and during subsequent graft failure. The use of CT in the pediatric age group is associated with the risk of radiation-induced cancers, which include leukemias, especially in younger patients and females [16]. Therefore, limiting the use of CT is highly desirable in this patient population.
Certain GBCAs used in MRI have been associated with nephrogenic systemic fibrosis (NSF), a debilitating and sometimes fatal disease involving fibrosis of the skin, musculoskeletal system, and visceral organs and reported exclusively in patients with renal insufficiency (Cowper et al. [4]). The NSF clinical presentation is characterized by fever with chills, malaise, hypotension, dyspnea, and vomiting in the acute phase. The symptoms are variably present and mimic systemic inflammatory response syndrome and the chronic phase is heralded by the appearance of edema and a plaque-like skin rash with woody induration leading to progressive muscle weakness and contractures [4–6, 17]. A low GFR is a major prerequisite for NSF to develop and there are no optimal treatment options. Hemodialysis is recommended following the administration of gadolinium in patients with advanced CKD to enhance the elimination of the contrast agent but the utility of hemodialysis in the prevention of NSF in unknown [18]. The American College of Radiology (ACR) Manual on Contrast Media and the FDA also recommend that GBCAs be avoided in patients with CKD eGFR<30 ml/min/1.73 m2 [18, 19], and in neonates and infants, independently of renal function status. Thus, the best option to eliminate the risk of NSF is to avoid GBCA exposure.
The recognition of NSF has fueled efforts to avoid gadolinium exposure and has placed increased emphasis on the use of non-contrast MRI techniques in patients with renal impairment. For vascular imaging, the most widely used non-contrast MRA methods have been in the family of “time of flight” (TOF) techniques [20]. TOFMRA has been in clinical use for many years and works well for intracranial arterial imaging and for intracranial MR venography. Like all non-contrast MRA methods, however, TOF techniques are dependent on blood flow to generate vascular signals, and outside of the brain, their application is more demanding and time consuming [21–23]. TOF techniques are generally much slower than CEMRA and are prone to artifacts from motion and complex flow patterns. It is generally accepted that CEMRA is more robust and reliable than non-contrast MRA, and the ability to perform CEMRA without gadolinium is therefore intriguing.
Ferumoxytol is a USPIO particle approved by the FDA as an intravenous iron supplement for patients with anemia secondary to kidney disease. It has an excellent safety profile and has been administered extensively to patients with CKD stages 1–5, although when used therapeutically, the reported rate of serious hypersensitivity reactions is higher (0.2 %) than that associated with gadolinium-based agents such as gadoteridol used for MRI (0.03 %) [19, 24]. This is a 10-fold increase. However, both the dose and rates of injection of ferumoxytol when used for vascular imaging are lower than when used for therapy, but an idiosyncratic reaction could still occur. Ferumoxytol is composed of an iron oxide core surrounded by a poly–glucose sorbitol carboxymethylether envelope with a molecular size of 30 nM and a molecular weight of approximately 750 kD. As a contrast agent, ferumoxytol causes shortening of the T1 relaxation time of its microenvironment. In addition, due to its cardohydrate coating, the agent is not filtered by the kidney, causing it to behave as a blood pool contrast, agent with a long intravascular half-life of 14 to 15 h [14, 25–27].
Ferumoxytol has a number of specific attributes as an MRI contrast agent that merit emphasis. Its half-life in the blood is on the order of 15 h, so that it can continue to influence the MRI signal for weeks after administration. Unless documented and communicated, this could cause confusion among radiologists interpreting a subsequent MRI study. Also, experience with the therapeutic use of ferumoxytol in the pediatric population as an intravenous iron supplement is still relatively limited and in the early phases of CKD.
A limitation of our study is that it was a single–center retrospective review with a relatively small number of cases. Additionally, data on dose optimization for ferumoxytol are in evolution. However, sequential enrollment helped to minimize selection bias and use of anesthesia made it possible to implement a well-defined, high-resolution image acquisition protocol.
Another aspect of our study worth emphasizing is that all of our patients were scanned at 3.0 T. It is well recognized that, for CEMRA, 3.0 T has advantages over 1.5 T in signal-to-noise ratio and in image resolution [28, 29]. Nonetheless, recent work suggests that for all but the smallest children, CEMRA at 1.5 T also produces highly diagnostic studies [30]. It seems likely, therefore, that the results we obtained at 3.0 T could be reproduced at 1.5 T, but this remains to be proven.
In conclusion, our initial experience suggests that MR imaging with ferumoxytol is feasible as a powerful alternative to GBCAs for high-resolution, detailed vascular mapping in children with CKD. It could also spare children the unnecessary placement of a hemodialysis catheter and acute hemodialysis in an attempt to remove gadolinium and potentially be used in many sick children with acute kidney injury who may need MR imaging. More extensive clinical experience will be required to confirm its efficacy and safety.
Contributor Information
Anjali B. Nayak, Department of Pediatrics, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA 90095, USA Department of Pediatrics, University Oklahoma Health Science Center, 1200N Childeren Avenue Suite 14200, Oklahoma City, OK 73104, USA.
Aarti Luhar, Department of Radiology, David Geffen School of Medicine at University of California, Los Angeles, CA 90095, USA.
Mark Hanudel, Department of Pediatrics, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA 90095, USA.
Barbara Gales, Department of Pediatrics, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA 90095, USA.
Theodore R. Hall, Department of Radiology, David Geffen School of Medicine at University of California, Los Angeles, CA 90095, USA
J. Paul Finn, Department of Radiology, David Geffen School of Medicine at University of California, Los Angeles, CA 90095, USA.
Isidro B. Salusky, Department of Pediatrics, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA 90095, USA
Joshua Zaritsky, Department of Pediatrics, Nemours/Alfred I. DuPont Hospital for Children, Wilmington, DE 19803, USA.
References
- 1.Hadizadeh DR, Kukuk GM, Fahlenkamp UL, Pressacco J, Schafer C, Rabe E, Koscielny A, Verrel F, Schild HH, Willinek WA. Simultaneous MR arteriography and venography with blood pool contrast agent detects deep venous thrombosis in suspected arterial disease. Am J Roentgenol. 2012;198:1188–1195. doi: 10.2214/AJR.11.7306. [DOI] [PubMed] [Google Scholar]
- 2.Huang SY, Kim CY, Miller MJ, Gupta RT, Lessne ML, Horvath JJ, Boll DT, Evans PD, Befera NT, Krishnan P, Chan JL, Merkle EM. Abdominopelvic and lower extremity deep venous thrombosis: evaluation with contrast-enhanced MR venography with a blood-pool agent. Am J Roentgenol. 2013;201:208–214. doi: 10.2214/AJR.12.9611. [DOI] [PubMed] [Google Scholar]
- 3.Pagnan L, Tona G, Belgrano M, Cova M, Pozzi Mucelli R. Direct contrast enhanced MR in the study of central venous accesses in children receiving total parenteral nutrition. Radiol Med. 2005;110:241–248. [PubMed] [Google Scholar]
- 4.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 5.Grobner T. Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 6.Rydahl C, Thomsen HS, Marckmann P. High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol. 2008;43:141–144. doi: 10.1097/RLI.0b013e31815a3407. [DOI] [PubMed] [Google Scholar]
- 7.Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J Xray Sci Technol. 2003;11:231–240. [PubMed] [Google Scholar]
- 8.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M the Chronic Renal Insufficiency Cohort Study I. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2013;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashir MR, Jaffe TA, Brennan TV, Patel UD, Ellis MJ. Renal transplant imaging using magnetic resonance angiography with a nonnephrotoxic contrast agent. Transplantation. 2013;96:91–96. doi: 10.1097/TP.0b013e318295464c. [DOI] [PubMed] [Google Scholar]
- 10.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJ. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gahramanov S, Raslan AM, Muldoon LL, Hamilton BE, Rooney WD, Varallyay CG, Njus JM, Haluska M, Neuwelt EA. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79:514–523. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuwelt EA, Hamilton BE, Varallyay CG, Rooney WR, Edelman RD, Jacobs PM, Watnick SG. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009;75:465–474. doi: 10.1038/ki.2008.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuwelt EA, Varallyay CG, Manninger S, Solymosi D, Haluska M, Hunt MA, Nesbit G, Stevens A, Jerosch-Herold M, Jacobs PM, Hoffman JM. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601–611. doi: 10.1227/01.NEU.0000255350.71700.37. discussion 611–602. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL, Neuwelt EA. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigovan M, Gasper W, Alley HF, Owens CD, Saloner D. USPIO-enhanced MR angiography of arteriovenous fistulas in patients with renal failure. Radiology. 2012;265:584–590. doi: 10.1148/radiol.12112694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, Feigelson HS, Roblin D, Flynn MJ, Vanneman N, Smith-Bindman R. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swaminathan S, Shah SV. New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol. 2007;18:2636–2643. doi: 10.1681/ASN.2007060645. [DOI] [PubMed] [Google Scholar]
- 18.ACR manual on contrast media. American College of Radiology; Reston, VA: 2013. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/Contrast%20Manual/_Contrast_Media.pdf. [Google Scholar]
- 19.Administration USFaD. MedWatch: Feraheme (Ferumoxytol) injection. 2011 http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm235636.htm.
- 20.Ivancevic MK, Geerts L, Weadock WJ, Chenevert TL. Technical principles of MR angiography methods. Magn Reson Imaging Clin N Am. 2009;17:1–11. doi: 10.1016/j.mric.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Finn JP, Edelman RR, Jenkins RL, Lewis WD, Longmaid HE, Kane RA, Stokes KR, Mattle HP, Clouse ME. Liver transplantation: MR angiography with surgical validation. Radiology. 1991;179:265–269. doi: 10.1148/radiology.179.1.2006289. [DOI] [PubMed] [Google Scholar]
- 22.Finn JP, Zisk JH, Edelman RR, Wallner BK, Hartnell GG, Stokes KR, Longmaid HE. Central venous occlusion: MR angiography. Radiology. 1993;187:245–251. doi: 10.1148/radiology.187.1.8451422. [DOI] [PubMed] [Google Scholar]
- 23.Finn JP, Kane RA, Edelman RR, Jenkins RL, Lewis WD, Muller M, Longmaid HE. Imaging of the portal venous system in patients with cirrhosis: MR angiography vs duplex Doppler sonography. Am J Roentgenol. 1993;161:989–994. doi: 10.2214/ajr.161.5.8273643. [DOI] [PubMed] [Google Scholar]
- 24.Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. Am J Roentgenol. 2011;196:W138–W143. doi: 10.2214/AJR.10.4885. [DOI] [PubMed] [Google Scholar]
- 25.Stabi KLBL. Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann Pharmacother. 2011;45:1571. doi: 10.1345/aph.1Q431. [DOI] [PubMed] [Google Scholar]
- 26.McCullough BJ, Kolokythas O, Maki JH, Green DE. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging. 2013;37:1476–1479. doi: 10.1002/jmri.23879. [DOI] [PubMed] [Google Scholar]
- 27.Bremerich J, Bilecen D, Reimer P. MR angiography with blood pool contrast agents. Eur Radiol. 2007;17:3017–3024. doi: 10.1007/s00330-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 28.Nael K, Michaely HJ, Kramer U, Lee MH, Goldin J, Laub G, Finn JP. Pulmonary circulation: contrast-enhanced 3.0-T MR angiography–initial results. Radiology. 2006;240:858–868. doi: 10.1148/radiol.2403051076. [DOI] [PubMed] [Google Scholar]
- 29.Nael K, Krishnam M, Nael A, Ton A, Ruehm SG, Finn JP. Peripheral contrast-enhanced MR angiography at 3.0 T, improved spatial resolution and low dose contrast: initial clinical experience. Eur Radiol. 2008;18:2893–2900. doi: 10.1007/s00330-008-1074-y. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen KL, Khan SN, Moriarty JM, Mohajer K, Renella P, Satou G, Ayad I, Patel S, Boechat MI, Finn JP. High-field MR imaging in pediatric congenital heart disease: initial results. Pediatr Radiol. 2014 doi: 10.1007/s00247-014-3093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]