Abstract
The role of peroxiredoxin-2 (PRDX2) in preventing hydrogen peroxide-induced oxidative stress in the red blood cell was investigated by comparing blood from PRDX2 knockout mice with superoxide dismutase-1 (SOD1) knockout and control mice. Loss of PRDX2 increased basal levels of methemoglobin and heme degradation (a marker for oxidative stress), and reduced red blood cell deformability. In vitro incubation under normoxic conditions, both with and without inhibition of catalase, resulted in a lag phase during which negligible heme degradation occurred followed by a more rapid rate of heme degradation in the absence of PRDX2. The appreciable basal increase in heme degradation for PRDX2 knockout mice, together with the lag during in vitro incubation, implies that PRDX2 neutralizes hydrogen peroxide generated in vivo under the transient hypoxic conditions experienced as the cells pass through the microcirculation.
Keywords: red blood cells, Peroxiredoxin-2, superoxide dismutase, hydrogen peroxide, heme degradation, deformability, oxidative stress
Introduction
Red blood cells (RBCs) are under constant oxidative stress by being exposed to reactive oxygen species (ROS) generated both within and outside the cell. To minimize damage from these ROS, RBCs, contain extensive non-enzymatic (glutathione, Vitamin E, ascorbate, ergothioneine) and enzymatic defense systems including superoxide dismutase (SOD1), which catalyze the dismutation of superoxide to hydrogen peroxide (H2O2), and catalase, glutathione peroxidase (GPx) and peroxiredoxin (PRDX2), which scavenge peroxides [1–3].
The primary source for endogenous ROS in RBCs is the autoxidation of oxyhemoglobin (oxyHb), which generates superoxide [4]. Superoxide is a relatively unreactive radical that rapidly dismutates to H2O2, even without superoxide dismutase. A contribution of superoxide to some forms of oxidative stress is, nevertheless, indicated by studies using SOD1 knockout mice, where the lifetime of superoxide formed by hemoglobin (Hb) autoxidation is extended [5]. While knockout of SOD1 was shown to affect the RBC lifespan, the relative stability of superoxide may explain the observation that many indices of severe oxidative stress were not elevated in RBCs or blood plasma of mice without SOD1. There were, thus, no significant changes in glutathione, plasma thiol groups or carbonyls [6]. These results are consistent with a primary role for H2O2 in RBC oxidative stress that affects oxygen transport and the release of ROS from RBCs [7].
It has been suggested that RBC oxidative stress is due to H2O2 that is not completely neutralized by the cellular antioxidant defense systems. To understand this process, it is necessary to understand the different roles of catalase, GPx and PRDX2 in neutralizing RBC H2O2. Extensive studies have been reported regarding the role of catalase and GPx [8,9] with relatively few studies involving PRDX2 [2,10].
Peroxiredoxins are a family of antioxidant defense enzymes discovered recently. PRDX2 is the third most abundant protein (15 million copies/cell) present in RBCs [2]. PRDX1 is also found in RBCs, but at a much reduced level [11]. Peroxiredoxins decompose H2O2, organic hydroperoxides and peroxynitrite [12,13]. PRDX2 knockout mice develop a mild, chronic hemolytic anemia characterized by increased reticulocyte count, lower Hb and hematocrit, Heinz body formation, fewer free sulfhydryl groups and increased dense cells [2,14]. While it is clear that PRDX2 plays an important role in protecting RBCs from oxidative stress, the relative importance of PRDX2 in scavenging H2O2 in RBC has not been fully elucidated.
Many studies involving catalase and GPx utilize inhibitors of these enzymes [3]. However, inhibiting PRDX2 with a thiol blocking reagent would also inhibit GPx. Therefore, we used PRDX2 knockout mice for these studies. Since we wanted to focus on the role of RBC H2O2, we used SOD1 knockout mice to compare the oxidative stress associated with elevated H2O2 levels to that associated with increased superoxide levels.
As a measure of the pool of non-scavenged H2O2, we used a determination of heme degradation. Heme degradation products are formed during the autoxidation of oxyHb, with a fraction of the H2O2 formed reacting with the heme, generating fluorescent heme degradation products [15–19]. Although removal of H2O2 by enzymatic reactions are faster than the rates for the formation of heme degradation products, heme degradation products were found to form even in vivo with all the antioxidant enzymes present [20–22]. Because these degradation products formed during oxyHb autoxidation have a longer lifetime than H2O2, they reflect the pool of H2O2 not scavenged by the antioxidant enzymes over an extended period of time. We have further shown that the in vivo accumulation of heme degradation products occurs primarily on the membrane where the predominantly cytosolic antioxidant enzymes are not as efficient [22].
In addition to heme degradation which reflects the pool of un–neutralized H2O2, we also measured RBC deformability. This measurement monitors the level of oxidative damage to the RBC membrane constituents or cytoskeleton.
Materials and methods
Mice
All mice were on a C57Bl6/J background with > 10 generations of backcrossing to the parental strain. PRDX2 knockout mice were obtained from the lab of Dr. D.Y. Yu [10]. SOD1 knockout mice were obtained from the lab of Dr. T.T. Huang [23]. All animals, including parental C57Bl6/J were derived from breeding stock housed at The Scripps Research Institute. All animal experiments were reviewed and approved by the TSRI IACUC.
Measurement of heme degradation
Blood was collected from the orbital sinus of mice at the Scripps Research Institute and shipped overnight on ice to the Molecular Dynamics Section. Blood was centrifuged at 1200g, for 10 min. The plasma was removed. 10 µl of RBCs were lysed in water and the spectrum of the Hb from 640 nm to 490 nm was determined. The concentration of the Hb and methemoglobin (metHb) were determined by a least-squares multicomponent fitting program (Perkin-Elmer QuantC v 4.51). The Hb concentration was adjusted to 50 µM by adding water. The degradation of the heme was then determined by measuring the fluorescence at an excitation wavelength of 321 nm and an emission wavelength of 485 nm using a Perkin Elmer LS-50 spectrofluorimeter [22].
Deformability
RBC deformability was measured using a microfluidic Rheoscan-D slit-flow ektacytometer (Rheo Meditech, Soul, South Korea) according to previously published methods [24]. Briefly, 500 µl of a mixture of 5.5% Polyvinyl-pyrrolidone in PBS containing the sample (~0.5% hct) was transferred into a microfluidic chamber having a 200 micrometer slit and loaded into the Rheoscan-D. A laser beam is directed through the chamber, while a miniature syringe vacuum pump causes the RBCs to flow through the micro slit with the shear stress decreasing from 20 Pa to 0. Diffraction images are recorded by a CCD camera and are fitted with known images of various diffraction patterns and analyzed by a microcomputer. Based upon the geometry of the elliptical diffraction pattern, an elongation index (EI) is calculated at each shear stress as: EI = (L − W)/(L + W), where L and W are the length and width of the RBC ellipsoid. A decrease in EI at a given shear stress (e.g. ~3Pa) indicates decreased cell deformation and hence lower RBC deformability [25].
Heme degradation during in vitro aging of RBCs
RBCs (10% hct) in PBS pH 7.4 from 4–6 mice were pooled and incubated at 30°C or 37°C in the absence and the presence of sodium azide, a catalase inhibitor. Aliquots of the pooled cells were taken at different time points and heme degradation was determined as described above [22]. The significance of the difference between different groups of mice was determined from the percent change relative to control for the time points where a significant increase in heme degradation was observed over control mice.
Results
Heme degradation in PRDX2 and SOD1 knockout mice
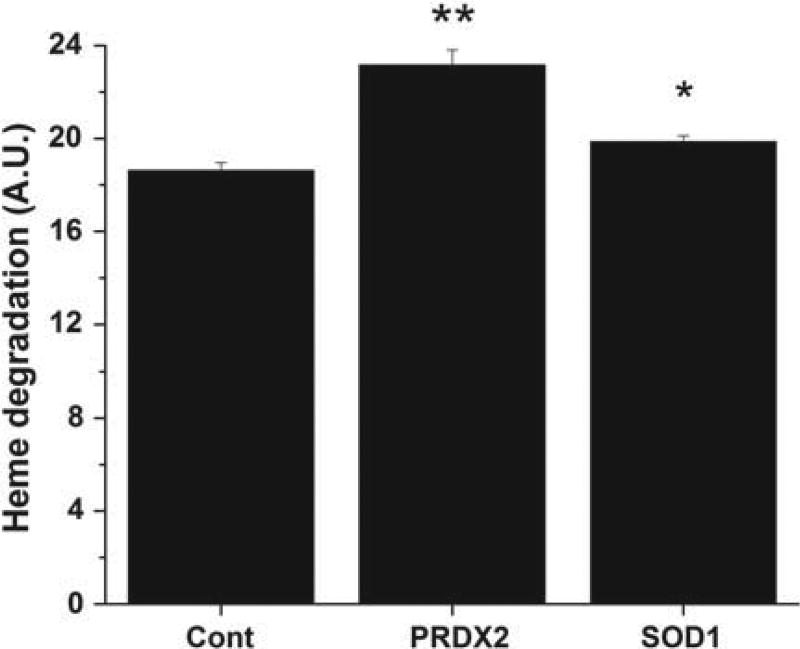
In our previous studies [19,21,22], we have used the formation of fluorescent heme degradation products as a measure of RBC oxidative stress. To assess oxidative stress generated by the elimination of PRDX2 and SOD1, the level of heme degradation products in RBC of PRDX2 and SOD1 knockout mice was measured. As shown in Figure 1, there was a significant (p < 0.001) increase in heme degradation in RBCs of PRDX2 knockout mice relative to control. These results indicate that heme degradation increases in RBCs of PRDX2 knockout mice in spite of the presence of catalase and GPx.
Figure 1.
Heme degradation in RBCs of control, PRDX2 and SOD1 knockout mice. Cells were lysed in water and adjusted to 50 µM heme and the fluorescence was determined as a measure of heme degradation (see Methods section). Values are mean ± SD for 10 to 15 animals in each group. The student t-test was used to determine the significance between different groups. **Significantly different from control group p < 0.001. *Significantly different from control group p < 0.05. A.U. Arbitrary units.
Heme degradation was also significantly higher in SOD1 knockout mice compared to controls, although the difference was smaller than that observed for PRDX2 knockouts. These results are consistent with our earlier observations that the presence of SOD1 does not significantly inhibit or accelerate the formation of heme degradation products during in vitro autoxidation of oxyHb [18]. The small increase in heme degradation in the absence of SOD1 may, however, be attributed to low levels of heme degradation products produced either by the increased levels of superoxide [17] or perhaps the peroxynitrite that forms due to the rapid reaction of superoxide with any NO present.
Level of Hb autoxidation as indicated by metHb and its correlation with heme degradation
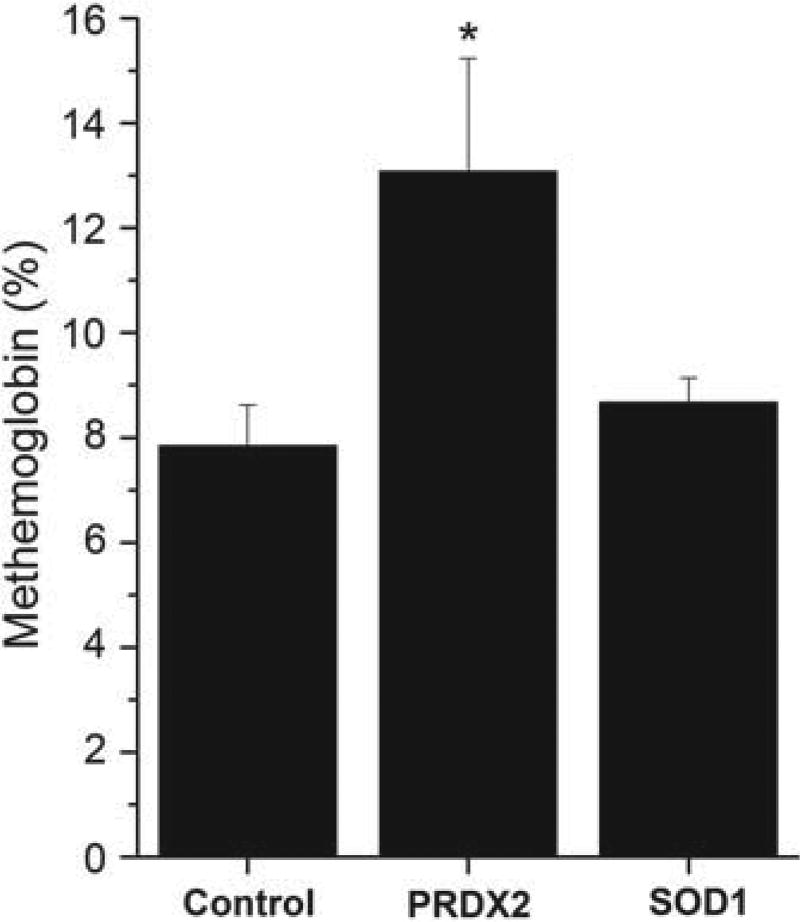
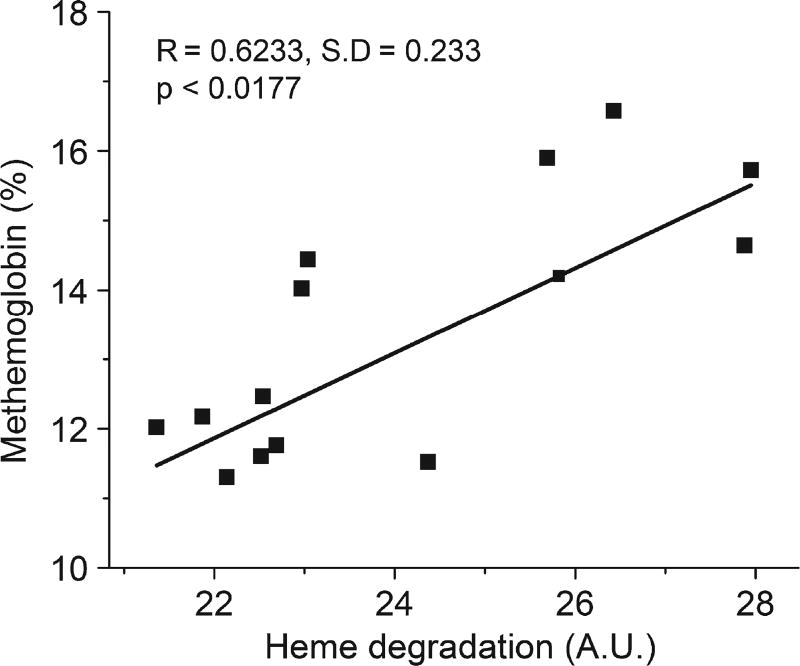
Autoxidation of oxyHb produces an equivalent molar ratio of metHb and superoxide anion. This metHb is reduced back to ferrous Hb by metHb-reductase with low steady state levels maintained under normal physiological conditions. However, metHb levels may increase when there is an increase in Hb autoxidation and the concomitant generation of H2O2 that is not scavenged by antioxidant defense enzymes making it more difficult for metHb-reductase to keep up with the metHb produced. Thus, the measurement of metHb is also an indirect indicator of RBC oxidative stress. As shown in Figure 2, the mean metHb levels were also significantly increased in PRDX2 knockout mice, but not in SOD1 knockout mice compared with control mice. The relationship between the formation of metHb and heme degradation was determined by plotting for each of the PRDX2 knockout mice the value of metHb versus the value of heme degradation. As shown in Figure 3, the level of heme degradation is highly correlated with the level of metHb in RBCs (R = 0.6233, p < 0.0177) supporting the hypothesis that the heme degradation product formed in PRDX2 knockout mice is associated with the un-scavenged H2O2 generated during Hb autoxidation.
Figure 2.
MetHb levels in RBCs of control, PRDX2 and SOD1 knockout mice. MetHb was measured in RBC lysate spectrophotometrically (see Methods section). MetHb values were expressed as percentage of total Hb. Values are mean ± SD for 10 to 14 animals. The student t-test was used to determine the significance between different groups. *Significantly different from control p < 0.05.
Figure 3.
Correlation of heme degradation with metHb in PRDX2 knockout RBCs. Each point represents the heme degradation and metHb values for an individual PRDX2 knockout mouse. The increase in heme degradation is significantly correlated with increase in metHb formation. A.U., Arbitrary units.
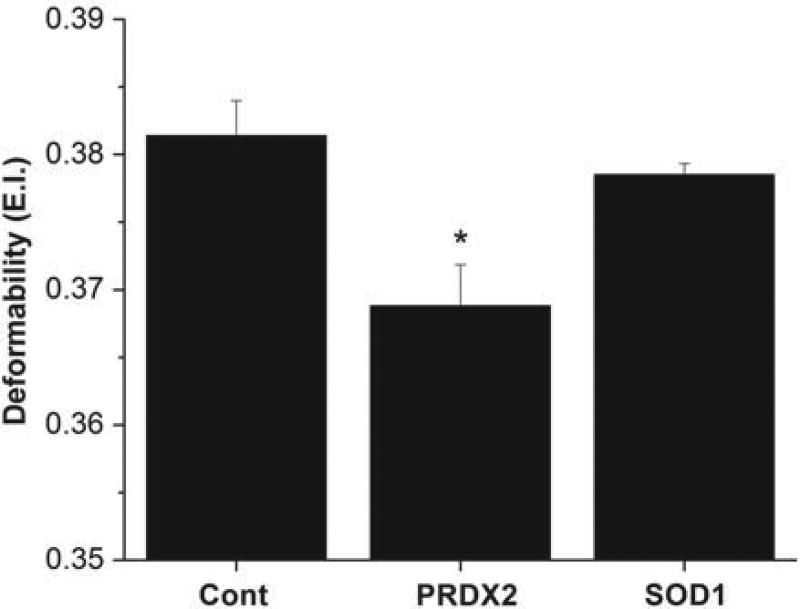
Changes in RBC deformability for PRDX2 and SOD1 knockout mice
In the circulatory system, RBCs have to pass through microcapillaries whose diameter is smaller than their size to deliver oxygen to tissues. They must undergo deformation in order to pass through these capillaries. It has been shown that RBC oxidative stress that damages the membrane reduces the deformability and flexibility of cells. Figure 4 shows a significant decrease in the elongation index, which is a measure of deformability, for the PRDX2 knockout mice. No significant change in the elongation index was found for the SOD1 knockout mice.
Figure 4.
Deformability of RBCs of control, PRDX2 and SOD1 knockout mice. Elongation index of RBCs as a measure of deformability was measured (see Methods section). Values are mean ± SD for 10 to 15 animals in each group. The student t-test was used to determine the significance between different groups. *Significantly different from control p < 0.05.
Changes in heme degradation during in vitro incubation
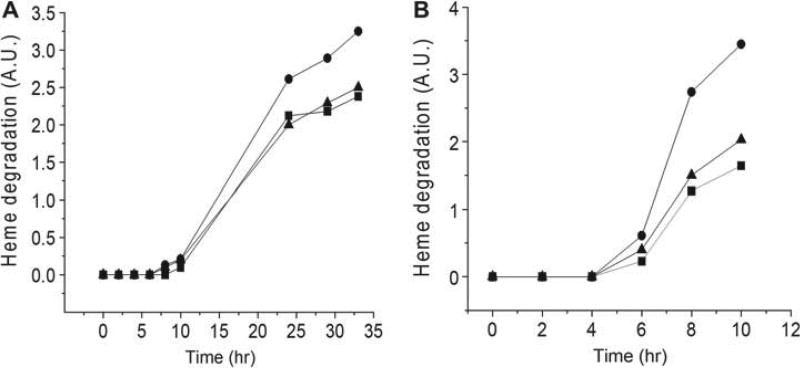
Incubation of RBCs of control and PRDX2 knockout mice in PBS at 30°C results in an increase of fluorescence from the basal level after a lag period of 8 hrs (Figure 5A). The time-dependent increase in heme degradation was greater for RBCs of PRDX2 knockout mice than for RBCs from control mice resulting in a significant (p < 0.01) average percent increase in heme degradation of 30% relative to control for samples incubated > 20 hrs. There was no difference in heme degradation between control and SOD1 knockout mice. PRDX2 deficiency, thus, causes cells to undergo more oxidative stress during in vitro aging, even in the presence of catalase.
Figure 5.
Heme degradation increase during in vitro aging of RBCs. RBCs were incubated and fluorescence intensity, as a measure of heme degradation, was measured at various times. The basal fluorescence intensity at zero time was subtracted from the fluorescence at each time point. A single experiment was performed with red blood cells of 4–6 animals from each group pooled to use for incubation studies. A.U., Arbitrary units intensity. ■, Control; ▲ SOD knockout; ●, PRDX2 knockout. (A) Cells were incubated at 30°C in the absence of azide. (B) Cells were incubated at 37°C in the presence of 1 mM azide to inhibit catalase.
To further delineate the role of PRDX2 in neutralizing H2O2 generated in the RBC, the in vitro incubation study was repeated after adding azide to inhibit catalase (Figure 5B) in control, SOD1 knockout and PRDX2 knockout cells. There was a lag of four hours before any increase in heme degradation was observed in all groups, despite inhibition of catalase. For samples incubated > 4 hrs (6, 8 & 10 hrs) there was a significant (p < 0.01) average percent increase in heme degradation of 130% relative to control. The 39% average increase in heme degradation for SOD1 knockout RBCs was, however, not significant. The appreciable increase in heme degradation for RBCs from PRDX2 knockout mice in the presence of azide indicates that PRDX2 plays a major role in scavenging H2O2 in the absence of catalase, even with GPx present.
Discussion
An extensive antioxidant defense system is required in RBCs, because they are continuously exposed to oxidative stress and lack the capacity to engage in protein synthesis to replace damaged proteins [1,26–29]. The primary source for endogenous ROS in the RBC is the autoxidation of oxyHb, which generates superoxide [4,26]. Superoxide is a relatively unreactive radical that rapidly dismutates to H2O2 even without superoxide dismutase. RBC antioxidant defense includes the enzyme SOD1, which reacts with superoxide, and catalase, GPx and PRDX2 which react with peroxides. Catalase exclusively removes H2O2 [30]. GPx removes both H2O2 and organic hydroperoxides [8,31] whereas PRDX2 removes H2O2 [2], organic hydroperoxides, lipid hydroperoxides, [32,33] peroxynitrite [34] and protein hydroperoxides [35]. The rate constants for the reaction of catalase, GPx and PRDX2, with H2O2 is about ~107 M−1 S−1 [30] ~108 M−1 S−1 [8] and ~107–108 M−1 S−1 [30,34], respectively.
Heme degradation is produced by the reaction of a small fraction of this H2O2 reacting with Fe(II) Hb. H2O2 reacts with deoxyhemoglobin and oxyHb to produce ferrylhemoglobin with rate constants of ~103, and 125.0 M−1 S−1 [36], respectively. Ferrylhemoglobin reacts with an additional molecule of H2O2 to produce the fluorescent heme degradation products [17,19]. Despite the very high concentration of Hb (20 mM in terms of heme) in RBCs, the Hb cannot effectively compete with the antioxidant enzymes for H2O2 in the cytosol and a minimal amount of cytosolic hemoglobin will react with H2O2 generated by the autoxidation of cytosolic hemoglobin. However, a more significant but small fraction of the H2O2 generated by the autoxidation of hemoglobin in the region of the membrane, which is relatively inaccessible to cytosolic antioxidant enzymes, has been shown to be able to react with hemoglobin producing heme degradation products, before it diff uses away from the membrane [22].
Despite the spontaneous dismutation of superoxide to H2O2, a contribution of superoxide to some forms of oxidative stress is, nevertheless, indicated by studies using SOD1 knockout mice, where the lifetime of superoxide formed by Hb autoxidation is extended. A subset of these mice develops Heinz bodies and generates autoantibodies resulting in severe hemolytic anemia [6,29,37]. It has also been reported that SOD1 depleted RBCs are more sensitive to peroxidation and hemolysis than normal cells, when exposed to tert-butylhydroperoxide [5]. In addition, a shift in glucose metabolism from glycolysis to the pentose phosphate pathway has been reported [37].
Some of the effects of SOD1 could be attributed to the very rapid reaction between superoxide and nitric oxide, which forms peroxynitrite in the absence of SOD1. However, levels of the highly toxic peroxynitrite remain low, because of the low levels of NO, the reduction of peroxynitrite by PRDX2 [34] and the catalytic isomerization of peroxynitrite to nitrate by oxyHb [38]. Coupled with the relative stability of superoxide, this may explain the observation that many indices of severe oxidative stress were not elevated in RBCs or blood plasma of mice without SOD1 [6]. There were, thus, no significant changes in glutathione, plasma thiol groups or carbonyls.
These results are consistent with a primary role for H2O2 in red cell oxidative stress that affects oxygen transport and the release of ROS from RBCs [7]. Since SOD1 dismutates superoxide to H2O2, it will not inhibit H2O2-induced oxidative stress. This conclusion is confirmed by the data, which show no significant increase in metHb (Figure 2) or decrease in deformability (Figure 3) with the SOD1 knockout mice. For heme degradation, we do obtain a small but significant increase (Figure 1) which can be attributed to direct effects of superoxide [17] and perhaps peroxynitrite on heme degradation.
A fraction of the H2O2/ROS that is not neutralized by the antioxidant enzyme system produces heme degradation products. Therefore, the basal in vivo level of heme degradation products indicates that there is un-neutralized H2O2/ROS formed in vivo. The determination of heme degradation with different enzyme systems turned off provides an evaluation of the role of each enzyme in minimizing the level of H2O2/ROS that is able to bypass the cellular antioxidant system. It is this pool of H2O2/ROS that can damage the RBC and/or be transferred from the RBC to damage other cells and tissues [7].
Our previous in vitro incubation studies [3] under normoxic conditions addressed the relative role of catalase and GPx. A secondary role for catalase was indicated by the finding that iodoacetamide, which reacts with glutathione the substrate for GPx, was able to completely eliminate the lag prior to the formation of increased heme degradation. At the same time, complete inhibition of catalase by azide did not completely eliminate the lag. Although the added iodoacetamide can also react with PRDX1, PRDX2 and thioredoxin, the reducing substrate for PRDX2, the data in Figure 5A &B, which show a lag in the accumulation of heme degradation products even for the PRDX2 knockout mice, indicate that the observed effect of iodoacetamide [3] on the lag is not due to PRDX2, consistent with the reported [34] slow reaction of iodoacetamide with PRDX2. The effect of iodoacetamide can be attributed to a reaction with glutathione, which will inhibit the reaction of GPx, although a contribution of other thiol containing enzymes like PRDX1 cannot be ruled out.
The finding that GPx has a greater role in the inhibition of heme degradation products than catalase [3] is consistent with the primary role of catalase to react with the high concentrations of H2O2 coming from exogenous sources and for GPx to react with the low levels of H2O2 coming from the endogenous autoxidation of Hb [39]. Furthermore, the ability of GPx, but not catalase, to react with organic peroxides suggest that GPx may have greater access to ROS generated in the region of or on the RBC membrane [22].
The in vivo effects that we have observed for PRDX2 knockout mice (Figures 1–4) imply that PRDX2 plays an important role in neutralizing the H2O2 generated in vivo (Figure 6). This finding is consistent with previous reports [3,40] that PRDX plays a major role in eliminating sub-micromolar levels of H2O2 in RBCs. Our in vitro studies (Figure 5) further indicate greater levels of heme degradation after extended periods of time for PRDX2 knockout mice. However, the similar lag observed for control mice with PRDX2 and the PRDX2 knockout mice (Figure 5) in the in vitro experiments indicates that PRDX2, even when catalase is inhibited by axide, is not able to extend the lag phase. Since the reaction with H2O2 under these conditions should extend the lag phase, these results are inconsistent with a dominant role for PRDX2 neutralizing H2O2.
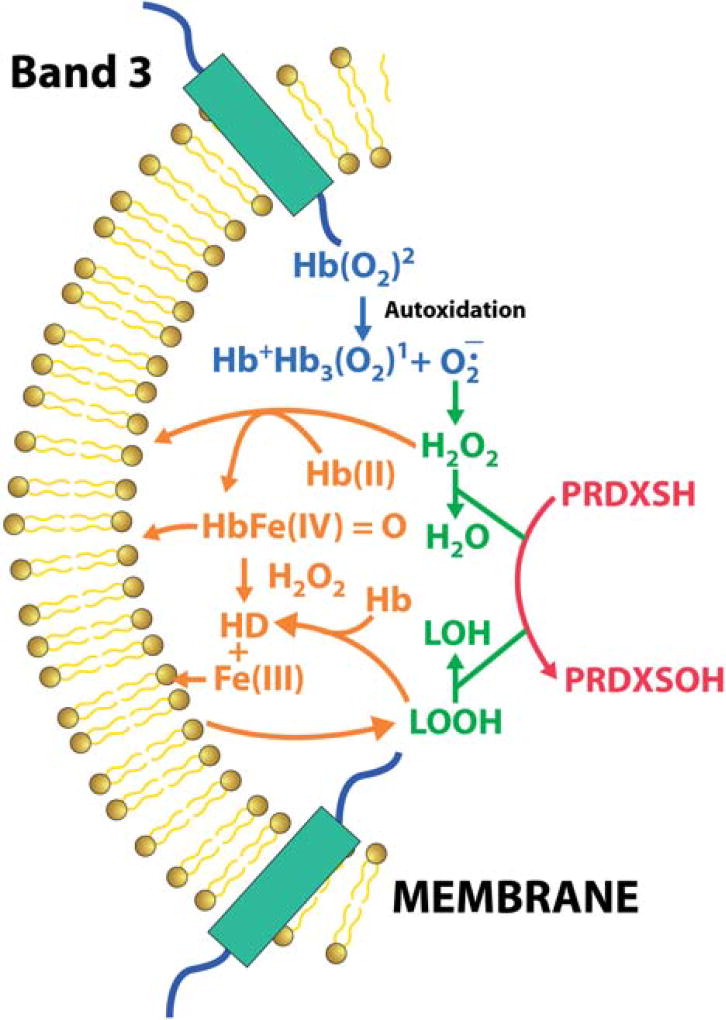
Figure 6.
Schematic diagram of the role of PRDX2 in inhibiting heme degradation formation in RBCs. Superoxide generated by partially deoxygenated hemoglobin bound to membrane band-3 under hypoxic conditions converts to hydrogen peroxide. PRDX2, associated with the RBC membrane, scavenges this hydrogen peroxide preventing the formation of heme degradation products, and the associate release of iron. This inhibition of heme degradation also prevents oxidation of lipids that generate lipid hydroperoxides, which can further degrade the heme to generate fluorescent membrane products. The reaction of PRDX2 with lipid hydroperoxides further prevents any accumulation of lipid hydroperoxides and any associated membrane damage.
The two in vivo factors that need to be considered in explaining the basal effects of PRDX2 (Figures 1–4) in limiting the formation of heme degradation products, which does not affect the in vitro observed lag (Figure 5) are: 1) Cellular Aging: We have previously shown [19] that there are higher levels of heme degradation products in older cells. Since heme degradation measures a cumulative level of the total oxidative stress, older cells have been exposed to oxidative stress for a longer period of time resulting in elevated heme degradation. However, it is also possible that older impaired cells are more susceptible to oxidative stress. PRDX2, which is able to react with low levels of H2O2 even at reduced glutathione levels, may therefore play a role in limiting the increased formation of heme degradation products in older cells. This factor is also expected to play an important role under pathological conditions where increased RBC oxidative stress occurs as found for sickle cell disease, thalassemia and G6PD deficiency [41–43]. (2) Hypoxia: Our in vitro studies were done under normoxic conditions where the rates of autoxidation are slow and the affinity of Hb for the membrane is low. In the circulatory system the RBCs continuously undergo deoxygenation and reoxygenation cycles. We have previously found that for partially oxygenated Hb both the rate of autoxidation [44] and the affinity of Hb for the membrane [45] increase dramatically. Although partial oxygenation is only a transient state in vivo, the detected heme degradation is most likely generated in this state. This is also consistent with the finding that almost all the heme degradation detected is produced by Hb bound to the membrane [22].
Among RBC antioxidant enzymes, PRDX2 is the only enzyme that is at least partially associated with the membrane, which reacts with H2O2. About 5% of the PRDX2 has been shown to be bound to the membrane under normal conditions [46,47]. Considering the high concentration of PRDX2 in RBC, the 5% is not negligible. It has further been shown that the level of PRDX2 association with the membrane is affected by oxidative stress [48,49] as well as hypoxia [50]. Membrane association of PRDX2 thought to involve the C- terminal region of PRDX2,has been shown to react with lipid hydroperoxides [33]. This pool of membrane associated PRDX2 is ideally located to react with H2O2 formed when membrane bound hemoglobin undergoes autoxidation (Figure 6).
The membrane association of PRDX2 and particularly the relationship with oxidative stress and hypoxia [51] suggests that PRDX2 is the preferred enzyme to react with the H2O2 generated by Hb autoxidation under hypoxic conditions (Figure 6). The ability of PRDX2 to preferentially react with H2O2 generated from Hb bound to band 3 of the membrane is further supported by the finding [49]) that the site for membrane binding of PRDX2 is in the same region as hemichromes, which also bind to band 3 of the RBC membrane.
We have used deformability as a measure of oxidative damage to the RBC. ROS are potent modulators of deformability of RBC in vitro and in vivo. We observed a significant reduction in deformability in sickle cell RBCs, which experience increased oxidative stress. The role of PRDX2 in inhibiting impaired deformability can be attributed to both a reduction in ROS as well as a direct reaction of PRDX2 with protein hydroperoxides [52], which will inhibit the damage to cytoskeletal proteins required for impaired deformability.
Conclusion
Each of the antioxidant enzymes plays a different role in protecting from H2O2-induced RBC oxidative stress. SOD1, by increasing the rate for superoxide dismutation, does not inhibit H2O2-induced oxidative stress. Catalase reacts with high levels of H2O2 with its primary role to protect from the H2O2 generated by exogenous oxidants. GPx is able to completely prevent the slow formation of heme degradation produced under normoxic conditions as long as reduced glutathione is available. However, the increased in vivo levels of heme degradation for PRDX2 knockout mice indicate that GPx, which is basically a cytoplasmic protein, does not seem to be able to adequately deal with the H2O2 generated in vivo in the circulatory system. The dominant role of PRDX2 seems to involve its ability to deal with the transiently increased oxidative stress that the RBCs undergo during oxygenation/deoxygenation cycling, which generates H2O2 in the region of the membrane (Figure 6). The role of PRDX2 in limiting RBC oxidative stress may also include its ability to react with H2O2 generated in diseased states and/or during cellular aging when the ability of the RBC to withstand oxidative stress is impaired.
ROS are potent modulators of deformability of RBC in vitro and in vivo. The role of PRDX2 in inhibiting impaired deformability can be attributed to both a reduction in ROS as well as a direct reaction of PRDX2 with protein hydroperoxides [52], which will inhibit the damage to cytoskeletal proteins required for impaired deformability.
Acknowledgments
Research Support: This research was supported by intramural research program of National Institutes on Aging, National Institute of Health and to JS Friedman-NIH grant R21 DK075763 and US Army grant W81XWH-10-2-0059.
Footnotes
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Cimen MY. Free radical metabolism in human erythrocytes. Clin Chim Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Low FM, Hampton MB, Peskin AV, Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–2617. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 3.Nagababu E, Chrest FJ, Rifkind JM. Hydrogen-peroxide-induced heme degradation in red blood cells: the protective roles of catalase and glutathione peroxidase. Biochim Biophys Acta. 2003;1620:211–217. doi: 10.1016/s0304-4165(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 4.Misra HP, Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- 5.Bartoli GM, Palozza P, Piccioni E. Enhanced sensitivity to oxidative stress in Cu,ZnSOD depleted rat erythrocytes. Biochim Biophys Acta. 1992;1123:291–295. doi: 10.1016/0005-2760(92)90009-k. [DOI] [PubMed] [Google Scholar]
- 6.Grzelak A, Kruszewski M, Macierzynska E, Piotrowski L, Pulaski L, Rychlik B, Bartosz G. The effects of superoxide dismutase knockout on the oxidative stress parameters and survival of mouse erythrocytes. Cell Mol Biol Lett. 2009;14:23–34. doi: 10.2478/s11658-008-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiefmann R, Rifkind JM, Nagababu E, Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood. 2008;111:5205–5214. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen G, Hochstein P. Glutathione peroxidase: the primary agent for the elimination of hydrogen peroxide in erythrocytes. Biochemistry. 1963;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- 9.Gaetani GF, Kirkman HN, Mangerini R, Ferraris AM. Importance of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1994;84:325–330. [PubMed] [Google Scholar]
- 10.Lee TH, Kim SU, Yu SL, Kim SH, Park DS, Moon HB, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 11.Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 13.Trujillo M, Ferrer-Sueta G, Thomson L, Flohe L, Radi R. Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell Biochem. 2007;44:83–113. doi: 10.1007/978-1-4020-6051-9_5. [DOI] [PubMed] [Google Scholar]
- 14.Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 15.Nagababu E, Rifkind JM. Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem Biophys Res Commun. 1998;247:592–596. doi: 10.1006/bbrc.1998.8846. [DOI] [PubMed] [Google Scholar]
- 16.Nagababu E, Chrest FJ, Rifkind JM. The origin of red cell fluorescence caused by hydrogen peroxide treatment. Free Radic Biol Med. 2000;29:659–663. doi: 10.1016/s0891-5849(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 17.Nagababu E, Rifkind JM. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39:12503–12511. doi: 10.1021/bi992170y. [DOI] [PubMed] [Google Scholar]
- 18.Nagababu E, Rifkind JM. Heme degradation during autoxidation of oxyhemoglobin. Biochem Biophys Res Commun. 2000;273:839–845. doi: 10.1006/bbrc.2000.3025. [DOI] [PubMed] [Google Scholar]
- 19.Nagababu E, Rifkind JM. Heme degradation by reactive oxygen species. Antioxid Redox Signal. 2004;6:967–978. doi: 10.1089/ars.2004.6.967. [DOI] [PubMed] [Google Scholar]
- 20.Nagababu E, Gulyani S, Earley CJ, Cutler RG, Mattson MP, Rifkind JM. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic Res. 2008;42:824–829. doi: 10.1080/10715760802459879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagababu E, Fabry ME, Nagel RL, Rifkind JM. Heme degradation and oxidative stress in murine models for hemoglobinopathies: thalassemia, sickle cell disease and hemoglobin C disease. Blood Cells Mol Dis. 2008;41:60–66. doi: 10.1016/j.bcmd.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagababu E, Mohanty JG, Bhamidipaty S, Ostera GR, Rifkind JM. Role of the membrane in the formation of heme degradation products in red blood cells. Life Sci. 2010;86:133–138. doi: 10.1016/j.lfs.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson RL, Van RH, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 24.Shin S, Hou JX, Suh JS, Singh M. Validation and application of a microfluidic ektacytometer (RheoScan-D) in measuring erythrocyte deformability. Clin Hemorheol Microcirc. 2007;37:319–328. [PubMed] [Google Scholar]
- 25.Baskurt OK, Boynard M, Cokelet GC, Connes P, Cooke BM, Forconi S, et al. New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc. 2009;42:75–97. doi: 10.3233/CH-2009-1202. [DOI] [PubMed] [Google Scholar]
- 26.Balagopalakrishna C, Manoharan PT, Abugo OO, Rifkind JM. Production of superoxide from hemoglobin-bound oxygen under hypoxic conditions. Biochemistry. 1996;35:6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 27.Clark MR. Senescence of red blood cells: progress and problems. Physiol Rev. 1988;68:503–554. doi: 10.1152/physrev.1988.68.2.503. [DOI] [PubMed] [Google Scholar]
- 28.Rifkind JM, Zhang L, Levy A, Manoharan PT. The hypoxic stress on erythrocytes associated with superoxide formation. Free Radic Res Commun. 1991;12–13:645–652. doi: 10.3109/10715769109145842. [DOI] [PubMed] [Google Scholar]
- 29.Winterbourn CC, Hawkins RE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–341. [PubMed] [Google Scholar]
- 30.Mueller S, Riedel HD, Stremmel W. Direct evidence for catalase as the predominant H2O2 -removing enzyme in human erythrocytes. Blood. 1997;90:4973–4978. [PubMed] [Google Scholar]
- 31.Johnson RM, Goyette G, Jr, Ravindranath Y, Ho YS. Oxidation of glutathione peroxidase-deficient red cells by organic peroxides. Blood. 2002;100:1515–1516. doi: 10.1182/blood-2002-04-1124. [DOI] [PubMed] [Google Scholar]
- 32.Cordray P, Doyle K, Edes K, Moos PJ, Fitzpatrick FA. Oxidation of 2-Cys-peroxiredoxins by arachidonic acid peroxide metabolites of lipoxygenases and cyclooxygenase-2. J Biol Chem. 2007;282:32623–32629. doi: 10.1074/jbc.M704369200. [DOI] [PubMed] [Google Scholar]
- 33.Cha MK, Yun CH, Kim IH. Interaction of human thiol-specific antioxidant protein 1 with erythrocyte plasma membrane. Biochemistry. 2000;39:6944–6950. doi: 10.1021/bi000034j. [DOI] [PubMed] [Google Scholar]
- 34.Manta B, Hugo M, Ortiz C, Ferrer-Sueta G, Trujillo M, Denicola A. The peroxidase and peroxynitrite reductase activity of human erythrocyte peroxiredoxin 2. Arch Biochem Biophys. 2009;484:146–154. doi: 10.1016/j.abb.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 36.Eyer P, Hertle H, Kiese M, Klein G. Kinetics of ferrihemoglobin formation by some reducing agents, and the role of hydrogen peroxide. Mol Pharmacol. 1975;11:326–334. [PubMed] [Google Scholar]
- 37.Iuchi Y, Okada F, Takamiya R, Kibe N, Tsunoda S, Nakajima O, et al. Rescue of anaemia and autoimmune responses in SOD1-deficient mice by transgenic expression of human SOD1 in erythrocytes. Biochem J. 2009;422:313–320. doi: 10.1042/BJ20090176. [DOI] [PubMed] [Google Scholar]
- 38.Romero N, Radi R, Linares E, Augusto O, Detweiler CD, Mason RP, Denicola A. Reaction of human hemoglobin with peroxynitrite. Isomerization to nitrate and secondary formation of protein radicals. J Biol Chem. 2003;278:44049–44057. doi: 10.1074/jbc.M305895200. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RM, Ho YS, Yu DY, Kuypers FA, Ravindranath Y, Goyette GW. The effects of disruption of genes for peroxiredoxin-2, glutathione peroxidase-1, and catalase on erythrocyte oxidative metabolism. Free Radic Biol Med. 2010;48:519–525. doi: 10.1016/j.freeradbiomed.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindena J, Wittenberg H, Diederichs F. The decline in catalytic enzyme activity concentration with aging of the rabbit erythrocyte. Enzyme. 1985;34:224–228. doi: 10.1159/000469390. [DOI] [PubMed] [Google Scholar]
- 41.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 42.Hebbel RP. The sickle erythrocyte in double jeopardy: autoxidation and iron decompartmentalization. Semin Hematol. 1990;27:51–69. [PubMed] [Google Scholar]
- 43.Shinar E, Rachmilewitz EA. Oxidative denaturation of red blood cells in thalassemia. Semin Hematol. 1990;27:70–82. [PubMed] [Google Scholar]
- 44.Abugo OO, Rifkind JM. Oxidation of hemoglobin and the enhancement produced by nitroblue tetrazolium. J Biol Chem. 1994;269:24845–24853. [PubMed] [Google Scholar]
- 45.Cao Z, Bell JB, Mohanty JG, Nagababu E, Rifkind JM. Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H1494–H1503. doi: 10.1152/ajpheart.01233.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Low TY, Leow CK, Salto-Tellez M, Chung MC. A proteomic analysis of thioacetamide-induced hepatotoxicity and cirrhosis in rat livers. Proteomics. 2004;4:3960–3974. doi: 10.1002/pmic.200400852. [DOI] [PubMed] [Google Scholar]
- 47.Moore RB, Mankad MV, Shriver SK, Mankad VN, Plishker GA. Reconstitution of Ca(2+)-dependent K+ transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J Biol Chem. 1991;266:18964–18968. [PubMed] [Google Scholar]
- 48.Rocha S, Costa E, Coimbra S, Nascimento H, Catarino C, Rocha-Pereira P, et al. Linkage of cytosolic peroxiredoxin 2 to erythrocyte membrane imposed by hydrogen peroxide-induced oxidative stress. Blood Cells Mol Dis. 2009;43:68–73. doi: 10.1016/j.bcmd.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Matte A, Low PS, Turrini F, Bertoldi M, Campanella ME, Spano D, et al. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic Biol Med. 2010;49:457–466. doi: 10.1016/j.freeradbiomed.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biondani A, Turrini F, Carta F, Matte A, Filippini A, Siciliano A, et al. Heat-shock protein-27, -70 and peroxiredoxin-II show molecular chaperone function in sickle red cells: Evidence from transgenic sickle cell mouse model. Proteomics Clin Appl. 2008;2:706–719. doi: 10.1002/prca.200780058. [DOI] [PubMed] [Google Scholar]
- 51.Rogers SC, Said A, Corcuera D, McLaughlin D, Kell P, Doctor A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009;23:3159–3170. doi: 10.1096/fj.09-130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peskin AV, Cox AG, Nagy P, Morgan PE, Hampton MB, Davies MJ, Winterbourn CC. Removal of amino acid, peptide and protein hydroperoxides by reaction with peroxiredoxins 2 and 3. Biochem J. 2010;432:313–321. doi: 10.1042/BJ20101156. [DOI] [PubMed] [Google Scholar]