Abstract
Case series
Patients: Male, 74 • Female, 69
Final Diagnosis: Hepatobiliary pancreatic cancer
Symptoms: Fever • jaundice • vomiting
Medication: —
Clinical Procedure: —
Specialty: Surgery
Objective:
Unusual setting of medical care
Background:
In cases of celiac axis occlusion requiring pancreaticoduodenectomy for malignancy, both oncologic curability and control of hepatic arterial flow must be considered, but the operative strategy is undeveloped.
Case Reports:
Case 1: A 74-year-old man was diagnosed with hilar cholangiocarcinoma with celiac axis stenosis. The collateral from the superior mesenteric artery ran through the pancreas head but no invasion was observed in pre-operative imaging. Hepatopancreatoduodenectomy with preservation of a collateral was performed.
Case 2: A 69-year-old woman was diagnosed with pancreas head cancer with celiac axis occlusion. The collateral from the superior mesenteric artery ran through pancreas head and tumor invasion was observed. Pancreaticoduodenectomy with bypass revascularization using a vein graft was performed. Both operations were performed safely oncologically under preoperative planning that was based on computed tomographic angiography. The operative procedure was ultimately determined by evaluation of perioperative blood flow under Doppler ultrasonography after clamping the gastroduodenal artery.
Conclusions:
Preoperative simulations of arterial revascularization and perioperative evaluation of blood flow are necessary for the success of this procedure.
MeSH Keywords: Biliary Tract Neoplasms, Celiac Disease, Pancreatic Neoplasms, Pancreaticoduodenectomy
Background
In celiac axis (CA) occlusion due to the median arcuate ligament syndrome, celiac arterial flow is supplied almost entirely by a collateral of the superior mesenteric artery (SMA). In pancreaticoduodenectomy (PD) for malignancy with CA occlusion, in which the collateral artery runs through the pancreaticoduodenal arcade (PA), both oncologic curability and control of the CA system, especially of the hepatic artery, must be considered. Although there have been 17 reports of CA occlusion in PD since 1981 [1–17], the operative strategy for this complex disease is still undeveloped. In the present 2 cases, hepatopancreatoduodenectomy (HPD) for hilar cholangiocarcinoma and PD for pancreatic head carcinoma, both with associated CA occlusion, we used 2 different methods to overcome these problems: preservation of the collateral artery and revascularization with bypass.
Case Reports
Case 1
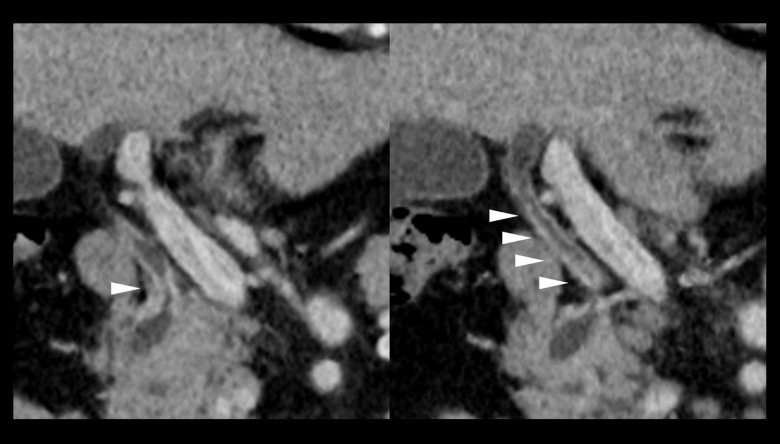
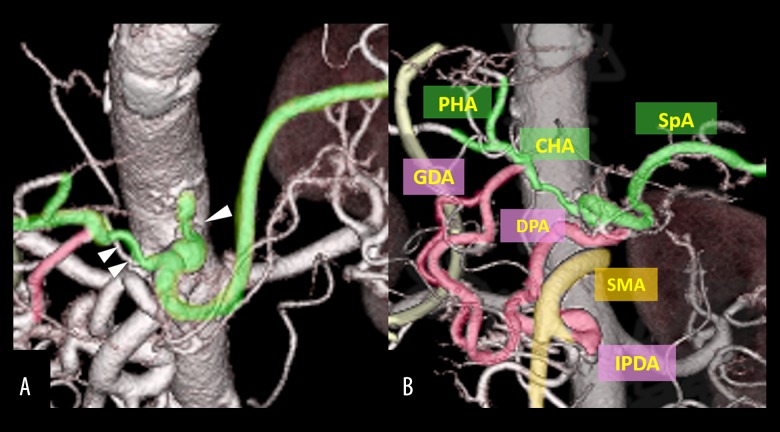
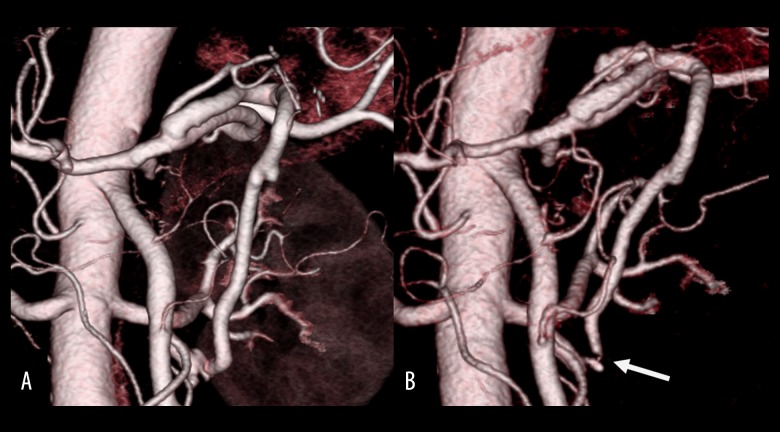
A 74-year-old man visited our hospital complaining of fever and vomiting. Laboratory examinations revealed mild liver dysfunction (aspartate transaminase [AST], 43 IU/L; alanine transaminase [ALT], 45 IU/L). Levels of the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were 2.7 ng/dL and 18.0 U/mL, respectively. Computed tomography (CT) showed enhancement and thickening of the lower biliary tract, without invasion to other organs or lymph nodes, and no distant metastasis (Figure 1). CT angiography (CTA) showed stenosis of the CA and common hepatic artery (Figure 2A) and the development of a collateral artery from the superior mesenteric artery running through the pancreas, the inferior pancreaticoduodenal artery (IPDA) via the PA, and a thickened dorsal pancreatic artery to the gastroduodenal artery (GDA) (Figure 2B). Step biopsy of the common bile duct, B2/3 and B4 showed adenocarcinoma, but the right bile duct was negative. The patient was diagnosed with clinical stage IIIA hilar cholangiocarcinoma (International Union Against Cancer (UICC) 7th edition [18]) with CA stenosis. At that time, the treatment method that carried the greatest possibility of being curative and resulting in the best prognosis was surgical, despite the high degree of invasiveness and the risk of poor postoperative quality of life due to complications [19]. The surgical method was left HPD as per the patient’s wishes after receiving detailed information about his options and providing written informed consent.
Figure 1.
Case 1: CT showing enhancement and thickening of the lower biliary tract (arrowhead) without invasion to other organs or lymph nodes, and no distant metastasis. CT – computed tomography.
Figure 2.
Case 1: CTA showing (A) extensive stenosis at the CA and CHA (arrowhead) and (B) the development of a collateral artery from the SMA via IPDA and PA to GDA. CA – celiac axis; CHA – common hepatic artery; CTA – computed tomography angiography; GDA – gastroduodenal artery; IPDA – inferior pancreaticoduodenal artery; PA – pancreaticoduodenal arcade.
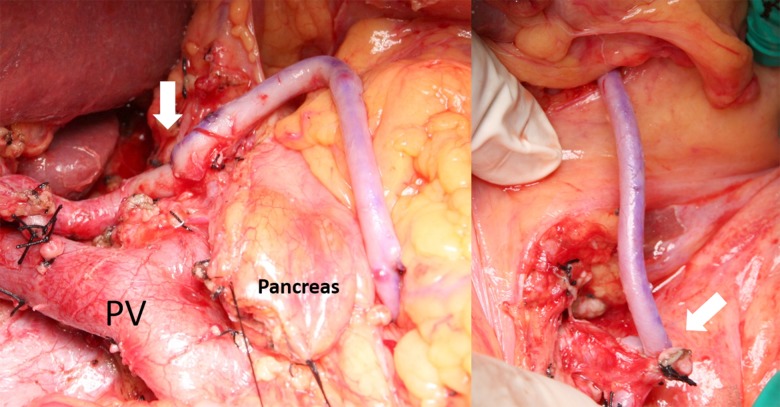
Because no invasion of the PA was seen on preoperative imaging, the operative plan was to preserve the PA. Intraoperatively, we clamped the gastroduodenal artery to decrease hepatic artery flow, which did not recover despite cutting the median arcuate ligament. However, malignant invasion was not observed around the PA preoperatively, and we were able to preserve the GDA and posterior PA oncologically safely (Figure 3). Operation time was 978 minutes and blood loss was 2390 mL.
Figure 3.
Case 1: Hepatopancreatoduodenectomy with preservation of the GDA (arrow) and the posterior pancreaticoduodenal artery (arrowhead).
Histopathologic examination revealed negative margins and led to an UICC 7th edition [18] diagnosis of pT2a pN1 cM0, >G3; stage IIIB. The patient was discharged to home on postoperative day 214 despite postoperative bile leakage, cholangitis, and the need for rehabilitation. The cholangitis occurred frequently after discharge, and hence adjuvant therapy was not able to be performed. He had a recurrence of peritoneal carcinomatosis 17 months after HPD and died 1 month later.
Case 2
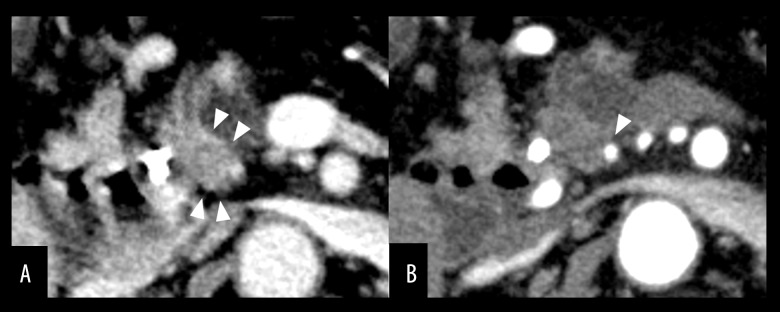
A 69-year-old woman visited her primary care physician complaining of jaundice. Laboratory examinations revealed jaundice and elevated liver and biliary tract enzymes (total serum bili-rubin, 1.6 mg/dL; AST, 39 IU/L; ALT, 70 IU/L; alkaline phosphatase, and 1393 U/L; gamma glutamyl transpeptidase [γ-GTP], 372 U/L). Levels of the tumor markers CEA and CA19-9 were 2.0 ng/dL and 67.9 U/mL, respectively. Dynamic CT showed a hypovascular tumor of the pancreatic head with invasion into the bile duct and surrounding tissue but no nodal or distant metastasis (Figure 4A). The tumor was in contact with the posterior inferior pancreaticoduodenal artery (PIPDA) (Figure 4B), but was 15 mm from the GDA. CTA showed complete occlusion of the CA (Figure 5A), with a collateral artery from the SMA. The CA was supplied by the development of the IPDA via PA to the GDA (Figure 5B). She was diagnosed with cT2 cN0, cM0, cStage IB cancer [18]. Endoscopic biliary drainage was performed via nasal tube, and cytology of the bile revealed adenocarcinoma. The patient was diagnosed with carcinoma of the pancreatic head with CA occlusion and was admitted to our hospital after her jaundice was reduced.
Figure 4.
Case 2: CT showing a hypovascular tumor (arrowhead) of the pancreatic head (A, portal phase) in contact with the PIPDA (arrowhead) (B, arterial phase). CT – computed tomography; PIPDA – posterior inferior pancreaticoduodenal artery.
Figure 5.
Case 2: Preoperative CTA. (A) CA occlusion (arrow) and (B) the collateral from the IPDA via PA to GDA. CA – celiac axis; CTA – computed tomography angiography; GDA – gastroduodenal artery; IPDA – inferior pancreaticoduodenal artery.
Because oncologic cure did not allow preservation of the arcade, we planned PD with revascularization. IPDA-splenic artery (SpA) bypass was planned preoperatively. The SpA, rather than the GDA, was chosen as the anastomotic site because the GDA was too close to the tumor; the SpA, because of its location and distance from the IPDA, was relatively easy to anastomose to the IPDA. The graft needed to be at least 10 cm long and of sufficient diameter to supply adequate arterial flow to the CA system; therefore, we planned to use the right greater saphenous vein.
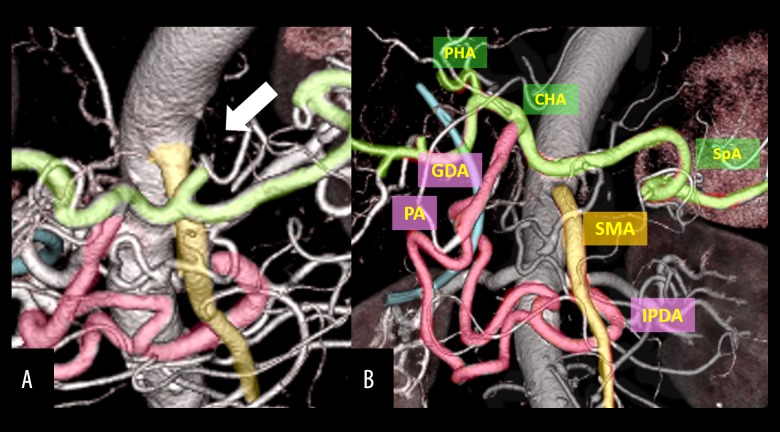
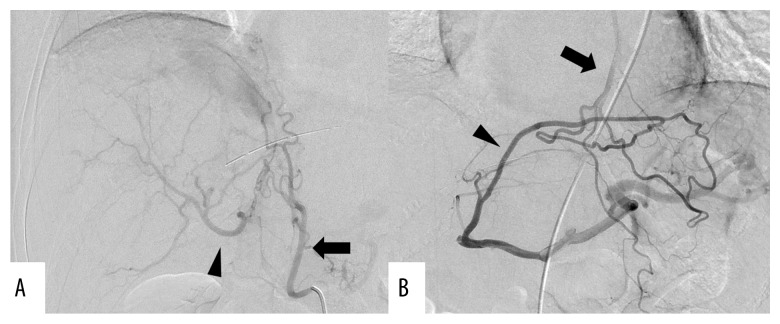
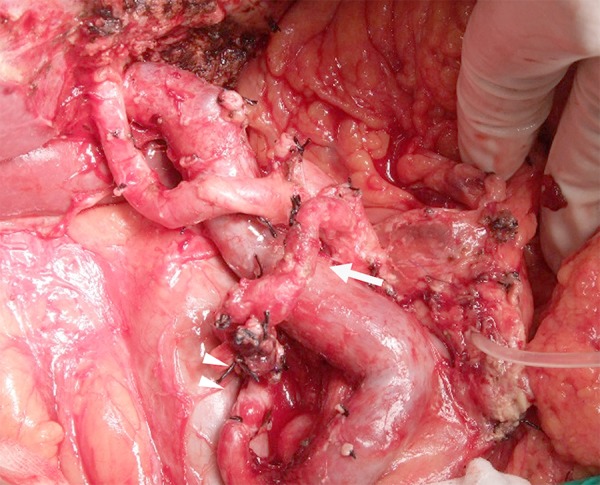
During surgery, clamping of the GDA under Doppler ultrasonography caused disappearance of hepatic artery flow, which was not restored even with release of the median arcuate ligament. Before the resectional stage of the procedure, IPDA-SpA bypass was performed using a right greater saphenous vein graft that was 12 cm in length and was confirmed to have no flexion. After bypass, hepatic artery flow was restored despite GDA clamping, and PD was performed safely, without loss of hepatic artery flow (Figure 6). Operation time was 744 minutes and blood loss was 880 mL. Histopathologic diagnosis was pT3, pN1, cM0, stage IIB [18]. Postoperatively, there was good hepatic artery flow (Figure 7A); however, 2 months after surgery, stenosis was observed at the site of anastomosis of the IPDA and greater saphenous vein (Figure 7B). The stenosis was thought to be caused by thickened vascular intima of the graft; the cause of this thickening was unknown, but may have been due to arterial pressure. Angiography showed that the right and left hepatic arteries were supplied by the right inferior phrenic artery (Figure 8A) and left internal thoracic artery (Figure 8B), respectively. The patient is without recurrence 8 months after PD with adjuvant S-1 chemotherapy.
Figure 6.
Case 2: PD with IPDA-SpA bypass using GSV graft through the transverse mesocolon (arrow, anastomotic site). GSV – greater saphenous vein; IPDA – inferior pancreaticoduodenal artery; SpA – splenic artery; PV – portal vein.
Figure 7.
Case 2: Postoperative CTA showing (A) no stenosis on postoperative day 15 but (B) extensive stenosis at the IPDA-GSV anastomosis 2 months postoperatively (arrow). CTA – computed tomography angiography; GSV – greater saphenous vein; IPDA – inferior pancreaticoduodenal artery.
Figure 8.
Case 2: Postoperative angiography. Right and left hepatic arteries (arrowhead) supplied by (A) right inferior phrenic artery and (B) left ITA (arrow), respectively. ITA – internal thoracic artery.
Discussion
CA occlusion or stenosis is identified in approximately half of abdominal angiographies [20]. In cases of CA occlusion, the blood flow in the CA system, including the common hepatic artery, SpA, and left gastric artery, is supplied by the collateral pathways that develop from the superior mesenteric artery via the PA, through the GDA, to the liver and other organs. However, in patients with CA occlusion who have developed a collateral through the PA and who require PD for malignancy, we must consider how to secure hepatic artery flow. Radiotherapy and/or chemotherapy can treat the malignant tumor more safely, but surgery is the only method that has the possibility of being curative [19]. The present patients selected surgical treatment after being provided with information regarding the risks of surgery and the possibility of a decline in quality of life postoperatively, and information about other treatment options.
Reports suggest that preservation of the collateral [6,9] or revascularization [3,5,11,17] is useful in overcoming these problems. We have described our pre- and postoperative assessments.
Preoperative evaluation of the abdominal arteries using CTA or angiography should be performed. At our institution, patients who undergo hepatobiliary pancreatic surgery routinely undergo CTA to evaluate the vascular anatomy, and abnormalities are sometimes found incidentally in asymptomatic patients. In cases of CA occlusion, the course of the collateral pathway and the oncologic distance between collateral and tumor must be determined, and the potential for preservation of the collateral should be assessed.
In the patient with bile duct carcinoma in the present study (case 1), the collateral ran through the PA but the tumor was far from the posterior PA. We assessed it to be oncologically safe to preserve the collateral from the IPDA via the posterior PA to the GDA because no invasion was observed on preoperative CT. However, depending on the extent of tumor invasion intraoperatively, a plan for revascularization was needed. In case 2 in the present study (the patient with pancreatic carcinoma), the PIPDA was in contact with the tumor; therefore, we were unable to preserve the collateral, including the PA, and still achieve oncologic cure. Revascularization was therefore needed, and we planned IPDA-SpA bypass using the right greater saphenous vein.
In bypass technique, it is necessary to consider the anastomotic site and which graft to use. In case 2, anastomosing the cut ends of the IPDA and GDA with a graft would have been simple, but we chose an IPDA-SpA bypass because the GDA was too close to the tumor. The length and diameter should be considered when choosing a graft. To anastomose the IPDA and SpA through the transverse mesocolon, no less than 10 cm was needed, and the vessel had to be able to supply enough blood flow to the liver, spleen, and stomach. Considering these factors, we decided to use a greater saphenous vein as a graft.
When addressing CA occlusion intraoperatively, we first clamp the GDA and examine blood flow under Doppler ultrasonography [11]; this will determine whether hepatic artery flow decreases in the absence of GDA flow. If flow through the hepatic artery is maintained while the GDA is clamped, the GDA can be transected without the need to control hepatic artery flow, but in most cases, the flow will decrease or vanish once the GDA is clamped.
Next, releasing the median arcuate ligament may cause hepatic arterial flow to increase, but doing so requires considerable attention. Yamada et al. [21] reported a case of PD with CA occlusion in which hepatic artery flow was increased only by cutting the median arcuate ligament, and flow was maintained during the operation. However, hepatic artery flow was suddenly lost on the night of the operation. This was because of the fragility of the CA, which had been compressed by the median arcuate ligament for a long time. Even if arterial flow is restored only by cutting the median arcuate ligament, this may still be a case requiring revascularization. In addition to examination while clamping the GDA and releasing the median arcuate ligament, the presence of tumor invasion or the possibility of collateral preservation should be confirmed.
After these perioperative evaluations, we make a final decision as to revascularization; preservation, or bypass. If there is no change from the preoperative evaluation, the operation can be performed according to the preoperative simulation. In a case of collateral preservation, intraoperative evaluation of margins using rapid pathologic diagnosis should be performed.
However, we believe that bypass may be better than collateral preservation oncologically; except under special circumstances, bypass is our first choice. However, GDA-preserving PD [22] can also be very helpful in special surgical situations (PD in CA occlusion, or PD after prior esophagectomy with gastric tube esophagoplasty) when GDA ligation leads to discontinuation of the blood flow via the right gastroepiploic artery as the sole blood source for the gastric tube. In case 1 in the present study, the patient’s age and preoperative condition made HPD with revascularization too invasive. Ultimately, we chose collateral preservation, not only because of oncologic safety, but also to avoid a highly invasive procedure.
Another method of cutting the GDA safely in PD with CA occlusion is preoperative arterial embolization. This may simplify the operation because perioperative revascularization is rendered unnecessary, but adhesions or bleeding due to embolization-related inflammation can make the operation more challenging. In addition, the collateral artery takes approximately 1 month to develop, which is too long for a patient with biliary pancreatic cancer.
Conclusions
In PD for biliary pancreatic cancer with CA occlusion, we recommend the following points to provide an oncologic cure and to secure hepatic artery flow:
– Preoperative simulation using CTA must be performed to evaluate whether the collateral can be preserved, as well as to evaluate the anastomotic site and select the best graft.
– Intraoperatively, evaluation of the hepatic artery flow under GDA clamping should be performed initially, and also after releasing the median arcuate ligament, to ensure that the operative findings do not differ from the preoperative evaluation.
– When revascularization is completed, the GDA must be cut after ensuring that the liver hepatic artery is secured while the GDA is clamped.
Acknowledgments
We thank all staff members at the Hokkaido University Hospital.
Abbreviations:
- ALT
alanine aminotransferase;
- AST
aspartate aminotransferase;
- CA
celiac axis;
- CA19-9
carbohydrate antigen 19-9;
- CEA
carcinoembryonic antigen;
- CT
computed tomography;
- CTA
CT angiography;
- γGTP
γ-glutamyltranspeptidase;
- GSV
greater saphenous vein;
- HPD
hepatopancreatoduodenectomy;
- PA
pancreaticoduodenal arcade;
- PD
pancreaticoduodenectomy;
- PIPDA
posterior inferior pancreaticoduodenal artery;
- SMA
superior mesenteric artery;
- SpA
splenic artery
Footnotes
Competing interests
None.
References:
- 1.Thompson NW, Eckhauser FE, Talpos G, Cho KJ. Pancreaticoduodenectomy and celiac occlusive disease. Ann Surg. 1981;193:399–406. doi: 10.1097/00000658-198104000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohler TR, Debas H, Crames M, Strandness DE., Jr Pancreaticoduodenectomy and the celiac artery compression syndrome. Ann Vasc Surg. 1990;4(1):77–80. doi: 10.1007/BF02042695. [DOI] [PubMed] [Google Scholar]
- 3.Manabe T, Baba N, Setoyama H, et al. Venous bypass grafting for celiac occlusion in radical pancreaticoduodenectomy. Pancreas. 1991;6(3):368–71. doi: 10.1097/00006676-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Machado MC, Penteado S, Montagnini AL, Machado MA. An alternative technique in the treatment of celiac axis stenosis diagnosed during pancreaticoduodenectomy. HPB Surg. 1998;10(6):371–73. doi: 10.1155/1998/85406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berney T, Pretre R, Chassot G, Morel P. The role of revascularization in celiac occlusion and pancreatoduodenectomy. Am J Surg. 1998;176(4):352–56. doi: 10.1016/s0002-9610(98)00195-0. [DOI] [PubMed] [Google Scholar]
- 6.Pelloni A, Gertsch P. [Cephalic duodenopancreatectomy with preservation of pancreaticoduodenal arcades in coeliac trunk occlusion] Ann Chir. 2000;125(7):660–64. doi: 10.1016/s0003-3944(00)00259-5. [in French] [DOI] [PubMed] [Google Scholar]
- 7.Okamoto H, Suminaga Y, Toyama N, et al. Autogenous vein graft from iliac artery to splenic artery for celiac occlusion in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2003;10(1):109–12. doi: 10.1007/s10534-002-0831-7. [DOI] [PubMed] [Google Scholar]
- 8.Kurosaki I, Hatakeyama K, Nihei KE, Oyamatsu M. Celiac axis stenosis in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2004;11(2):119–24. doi: 10.1007/s00534-003-0871-6. [DOI] [PubMed] [Google Scholar]
- 9.Otani T, Kurosaki I. Preservation of collateral pathways during pancreaticoduodenectomy in case of celiac axis occlusion. J Hepatobiliary Pancreat Surg. 2005;12(2):173–74. doi: 10.1007/s00534-004-0955-y. [DOI] [PubMed] [Google Scholar]
- 10.Hayashibe A, Sakamoto K, Shinbo M, et al. A resected case of advanced duodenal carcinoma with occlusion of the celiac artery. J Surg Oncol. 2005;91(4):270–72. doi: 10.1002/jso.20319. [DOI] [PubMed] [Google Scholar]
- 11.Nara S, Sakamoto Y, Shimada K, et al. Arterial reconstruction during pancreatoduodenectomy in patients with celiac axis stenosis – utility of Doppler ultrasonography. World J Surg. 2005;29(7):885–89. doi: 10.1007/s00268-005-7878-x. [DOI] [PubMed] [Google Scholar]
- 12.Soonawalla Z, Ganeshan A, Friend P. Celiac artery occlusion encountered during pancreatic resection: A case report. Ann R Coll Surg Engl. 2007;89(1):W15–17. doi: 10.1308/147870807X160344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farma JM, Hoffman JP. Nonneoplastic celiac axis occlusion in patients undergoing pancreaticoduodenectomy. Am J Surg. 2007;193(3):341–44. doi: 10.1016/j.amjsurg.2006.09.027. discussion 344. [DOI] [PubMed] [Google Scholar]
- 14.Nakano H, Yamamura T, Yamaguchi S, Otsubo T. Celiac axis occlusion of a patient undergoing pancreaticoduodenectomy after distal gastrectomy. Hepatogastroenterology. 2007;54(74):595–98. [PubMed] [Google Scholar]
- 15.Berselli M, Sperti C, Ballotta E, et al. Pancreaticoduodenectomy with unusual artery reconstruction in a patient with celiac axis occlusion: Report of a case. Updates Surg. 2010;62(2):117–20. doi: 10.1007/s13304-010-0015-x. [DOI] [PubMed] [Google Scholar]
- 16.Whistance RN, Shah V, Grist ER, et al. Management of median arcuate ligament syndrome in patients who require pancreaticoduodenectomy. Ann R Coll Surg Engl. 2011;93(4):e11–14. doi: 10.1308/003588411X13008915740787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celik S, Ringe KI, Boru CE, et al. A case of pancreatic cancer with concomitant median arcuate ligament syndrome treated successfully using an allograft arterial transposition. J Surg Case Rep. 2015;2015(12) doi: 10.1093/jscr/rjv161. pii: rjv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobin LH, Gospodarowicz MK, Wittekind C, editors. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. 7th ed. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 19.Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: Expert consensus statement. HPB (Oxford) 2015;17(8):691–99. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colapinto RF, McLaughlin MJ, Weisbrod GL. The routine lateral aortogram and the celiac compression syndrome. Radiology. 1972;103:557–63. doi: 10.1148/103.3.557. [DOI] [PubMed] [Google Scholar]
- 21.Yamada D, Ohtsuka T, Nakajima H, et al. [The strategy of pancreatoduodenectomy for celiac axis occlusion] J Biliary T Panc. 2015;36:281–86. [in Japanese] [Google Scholar]
- 22.Nagai H, Ohki J, Kondo Y, et al. Pancreatoduodenectomy with preservation of the pylorus and gastroduodenal artery. Ann Surg. 1996;223:194–98. doi: 10.1097/00000658-199602000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]