Abstract
Patient: Male, 9
Final Diagnosis: Acute promyelocytic leukemia (APL)
Symptoms: Bleeding
Medication: —
Clinical Procedure: —
Specialty: Hematology
Objective:
Rare disease
Background:
Acute promyelocytic leukemia (APL) is a very rare leukemia in children. Extramedullary involvement by APL has been reported in between 3–5% of cases, mainly associated with cases of relapse. A rare case of relapse of APL in a 9-year-old child is presented with skin involvement with myeloid sarcoma.
Case Report:
A 9-year-old male child was admitted to the Oncology Service of the hospital complaining of fever, progressive fatigue, oral petechiae with severe bleeding in the oral cavity. Bone marrow examination showed some promyelocytes. Flow cytometry showed 86% immature myeloid cells with the t(15;17) translocation, and molecular analysis showed expression of the PML/RARα fusion protein, which confirmed the diagnosis of APL. The patient completed a course of daunorubicin, cytarabine, and AII trans-retinoic acid (ATRA) with complete remission. After six months, the patient was re-admitted to hospital with a violaceous lesion on the scalp, with relapse of APL. Histological and immunohistochemistry of the lesion involving the skin of the scalp showed a myeloid sarcoma invading the dermis.
Conclusions:
Myeloid sarcoma, also called granulocytic sarcoma, is an extramedullary tumor of immature myeloid cells, which very rarely presents in children with APL. The mechanisms that lead to myeloid sarcoma in children with APL and the possible association with ATRA therapy remain to be investigated.
MeSH Keywords: Antineoplastic Combined Chemotherapy Protocols; Child; Leukemia, Promyelocytic, Acute; Recurrence
Background
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia that is characterized by a translocation between chromosomes 15 and 17 t(15;17)(q22;q21) and fusion between the PML gene and the retinoic acid receptor alpha gene, RARα. APL is common in adults and extremely rare in children and cytopenia, coagulopathies, and a severe bleeding diathesis are the clinical hallmarks of APL [1,2].
Extramedullary involvement by APL has been reported in between 3–5% of cases, mainly associated with cases of relapse, and the most affected sites for extramedullary involvement are the skin, central nervous system, the gingiva, the lungs, the mediastinum, lymph nodes, the testes and breast [1,2]. Some studies have suggested that there is an association between treatment with AII-trans retinoic acid (ATRA) after induction therapy and complete remission followed by extramedullary relapses [3,4].
In the 1970s, it was demonstrated that malignant cells from patients with APL were sensitive to chemotherapy with anthracyclines (daunorubicin, idarubicin), achieving complete remission in up to 55% of cases, and in 1985, therapy with ATRA was introduced [5,6].
ATRA appeared to revolutionize therapy for APL by modifying the natural history of the disease from being a disease with a high mortality rate to curable disease with rates of complete remission in 90% of cases [5,6].
Here, we report a rare case of APL in a child with skin infiltration with myeloid sarcoma after apparent complete remission following treatment with ATRA.
Case Report
A 9-year-old male child was admitted to the Oncology Service of the Santa Casa de Misericórdia of the Maceió, Brazil, with symptoms of fever, progressive fatigue, and with signs of petechiae and severe bleeding in the oral cavity.
Investigations included a bone marrow aspirate that showed hypercellularity with granular promyelocytes containing Auer rods. Flow cytometry analysis of the bone marrow sample, using labeled antibodies to leukocyte and granulocyte markers showed strongly positive expression of antibodies for CD13, CD33, CD34, CD117, whereas HLA-DR was negative. The cytogenetic and molecular findings showed translocation between chromosomes 15 and 17 t(15;17)(q22;q21) and fusion between the PML gene and the retinoic acid receptor alpha gene, RARα, confirming the diagnosis of APL. Antineoplastic treatment was commenced with intravenous daunorubicin 45 mg/m2/day and continuous oral treatment with AII trans-retinoic acid (ATRA) 45 mg/m2/day, which resulted in initial complete remission.
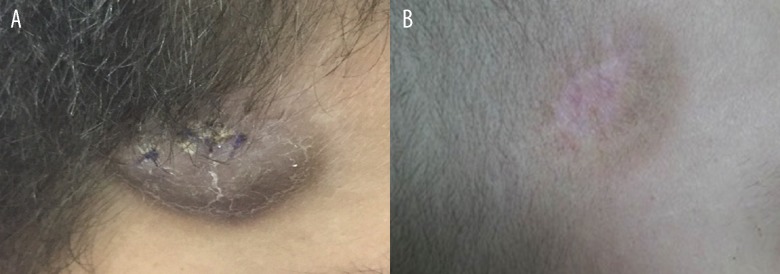
After six months, the patient was re-admitted to hospital with a recurrence of symptoms, including a prominent violaceous lesion on the scalp (Figure 1A), gingival bleeding, fatigue, fever, and pain in the extremities. A blood test showed a hemoglobin level of 5.2 g/dL, a leukocyte count of 5.6×109/L and a platelet count of 14×109/L, with 64% blasts and 9% promyelocytes. Lactate dehydrogenase was measured at 4,942 U/L (normal, <610 U/L). The bone marrow aspirates showed 20% hypergranular promyelocytes with Auer rods. Histopathologic examination of the skin lesion showed a pattern of large cells and a diffuse infiltrate that also involved the dermis. Immunohistochemistry of the skin lesion showed positive immunostaining of the tumor cells with primary antibodies to myeloperoxidase (MPO), CD45, TDT, CD20, and CD79a; immunostaining for PAX, CD2, CD3, CD4, CD5, CD7, and CD8 were negative (Figure 2A–2C). The results from the immunohistochemical panel were consistent with a diagnosis of a myeloid neoplasm, a myeloid sarcoma, in the skin. Cytogenetic analysis showed t(15;17) (q22q21) in 40% of the metaphase preparations analyzed, and polymerase chain reaction (PCR) for the PML-RARα gene showed residual disease, compatible with relapse of APL.
Figure 1.
The scalp lesion in a 9-year-old boy with acute promyelocytic leukemia (APL). (A) Extramedullary infiltration by promyelocytes in the scalp of a 9-year-old child after the first cycle of AII trans-retinoic acid (ATRA). (B) Regression of the tumor mass after relapse following chemotherapy with ATRA.
Figure 2.
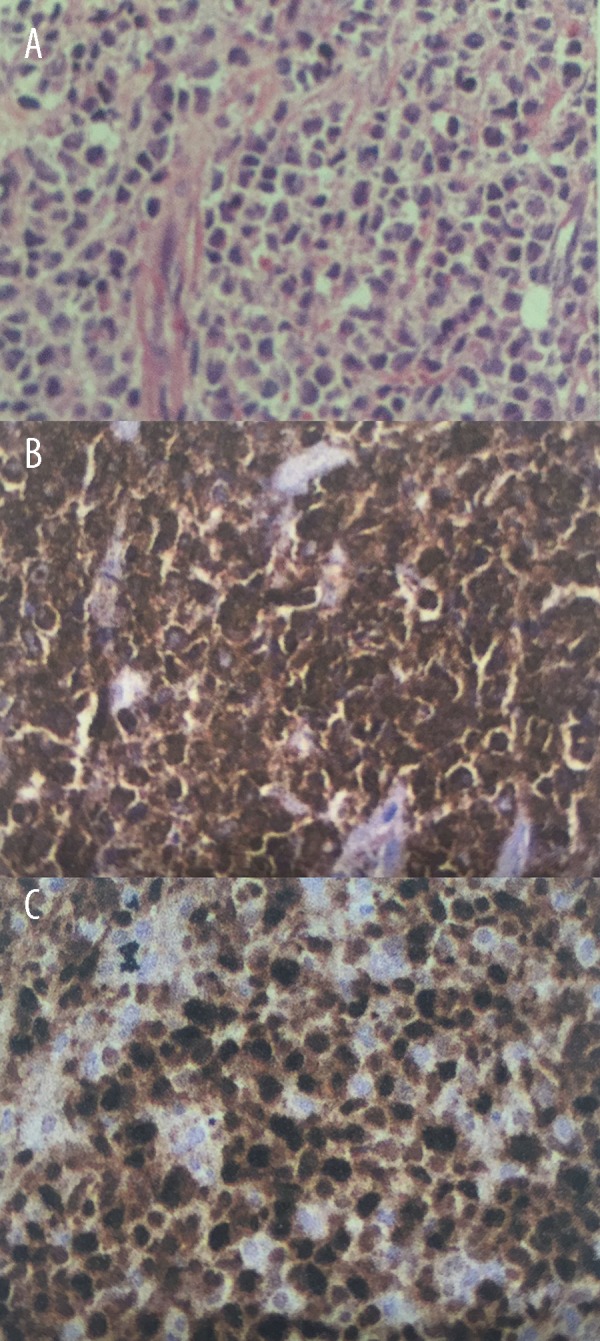
Photomicrographs of the histology and immunohistochemistry of the biopsy tissue from the scalp lesion shows a myeloid sarcoma. (A) Photomicrograph of the histology of the scalp lesion shows a diffuse pattern of intermediate-to-large cells involving the papillary dermis and deep layers of the skin. Hematoxylin and eosin (H&E). (B, C) Photomicrographs of the staining findings from the immunohistochemical panel showing positive expression of myeloperoxidase (MPO), CD45, and TDT. Strong expression of the proliferation marker, KI-67 is seen. Positive immunostaining of the tumor cells is seen with primary antibodies to myeloperoxidase (MPO), CD45, TDT, CD20, and CD79a; immunostaining for PAX, CD2, CD3, CD4, CD5, CD7 and CD8 are negative. The histology and immunohistochemistry confirm a hematopoietic neoplasm of myeloid origin in the skin.
Remission induction chemotherapy was begun with intravenous cytarabine, 100 mg/m2/day for seven days), continuous treatment with ATRA, 45 mg/m2/day, and with intravenous daunorubicin, 60 mg/m2/day for three days. After 30 days of the second induction treatment, bone marrow analysis was performed, showing normal cell lines; regression of the lesion on the scalp was also seen (Figure 1B) with complete clinical remission. However, the patient died due to severe refractory hypokalemia as a complication of treatment with amphotericin B 50 mg, when he was hospitalized at a later date.
Discussion
Acute promyelocytic leukemia (APL) exhibits genetic alterations involving the retinoic acid alpha-receptor gene, RARα. APL is characterized by a clonal expansion of malignant myeloid cells that are blocked at the promyelocyte stage of development. High doses of AII trans-retinoic acid (ATRA) is used to treat APL by overcoming the deficiency in the retinoic acid protein, resulting in clinical remission in 90% of cases [5,6]. Despite this, 10% of the treated patients undergo relapse, but extramedullary infiltration in children is extremely rare. Myeloid sarcoma is also called granulocytic sarcoma and is an extra-medullary tumor of immature myeloid cells, which very rarely occurs in children with APL.
Willernick et al. studied 26 cases of APL with extramedullary involvement, and only two patients (7.6%) had extramedullary disease that included a pelvic mass and infiltration of the bone marrow and mandible after complete remission following treatment with ATRA [3]. Giralt et al. reported two cases of APL in children with relapse and skin involvement following remission induced by ATRA [7].
The factors associated with extramedullary relapse include age less than 45 years, elevated white blood cell (WBC) count >10.109/L, and the presence of the bcr3 isoform of PML-RARα. Park et al. showed that patients with a high leukocyte count were at greater risk of early mortality, despite ATRA treatment [8]. Botton et al. studied 31 children with ALP, seven children had relapses, with one patient who exhibited extra-medullary disease [9].
Treatment with ATRA has been reported to be increasingly associated with extramedullary relapse following complete remission. There is a possibility that ATRA and anthracycline do not penetrate sites where extramedullary disease usually occurs. Also, the intensity of chemotherapy is often reduced after commencing treatment with ATRA, and its biological characteristics may favor relapse. In addition to inducing differentiation of promyelocytes, ATRA can alter the expression of adhesion molecules, increasing the ability of leukemic cells to migrate to other tissues, and may also promote the proliferation of keratinocytes, increasing the possibility of recurrence in the skin [10,11].
The differential diagnosis of skin manifestations of APL includes specific primary skin lesions resulting from direct infiltration of skin and subcutaneous tissue by leukemic cells. Papulonodular lesions of cutaneous leukemia appear as hard papules, plaques, or dermal nodules that are reddish-brown to violet (violaceous) in color. The initial lesions may be macular. Other clinical presentations of leukemic involvement of the skin include blisters, ulcers, and erythroderma. Myeloid, or granulocytic, sarcomas are extramedullary masses of leukemic cells that can be found in various regions of the body, with common sites being the skin of the face, the skin of the breast, the orbit, the paravertebral area, the long bones and lymph nodes. The extramedullary tumor masses are neoplastic infiltrates that are histologically are found only in an acute leukemia environment. The presence of cutaneous lesions is a marker of poor prognosis and can precede the relapse of systemic leukemia. Cutaneous lesions infiltration may be the first or the only sign of progression, and it is important that physicians are familiar with the clinical manifestations of myeloid, or granulocytic, sarcoma involving the skin, a condition that is also known as a form of ‘leukemia cutis’ [12].
This report has described a rare case of childhood APL with cutaneous infiltration of leukemic cells following treatment with ATRA. The diagnosis of APL was confirmed by the use of polymerase chain reaction (PCR) for the detection of PML/RARα gene expression. This case report reinforces the possibility that treatment with ATRA may be associated with a higher incidence of extramedullary APL at the time of relapse.
Conclusions
Myeloid sarcoma, also called granulocytic sarcoma, is an extra-medullary tumor of immature myeloid cells, which very rarely presents in children with APL. Treatment for APL with AII transretinoic acid (ATRA) may be associated with an increased incidence of extramedullary disease, including cutaneous lesions. Further studies are important to elucidate the effects of chemotherapy with ATRA for the early prevention of extramedullary complications in children with APL.
Footnotes
Conflict of interest
None.
References:
- 1.Stein EM, Tallman MS. Acute promyelocytic leukemia in children and adolescents. Acta Haematologica. 2014;132:307–12. doi: 10.1159/000365117. [DOI] [PubMed] [Google Scholar]
- 2.Vega-Ruiz A, Faderl S, Estrov Z, et al. Incidence of extramedullary disease in patients with promyelocytic leukemia: A single institution experience. Int J Hematol. 2009;89:489–96. doi: 10.1007/s12185-009-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willernick PH, Bellis R, Muxi P, et al. Extramedullary acute promyelocytic leukemia. Cancer. 1996;15:2510–14. doi: 10.1002/(sici)1097-0142(19961215)78:12<2510::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Botton S, Sanz MA, Chevret S, et al. Extramedullary relapse in acute promyelocytic leukemia treated with alltrans retinoic acid and chemotherapy. Leukemia. 2006;20:35–41. doi: 10.1038/sj.leu.2404006. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Tallman MS. Treatment of acute promyelocytic leukemia without cytotoxic chemotherapy. Oncology. 2011;25:733–41. [PubMed] [Google Scholar]
- 6.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 7.Giralt S, O’Brien S, Weeks E, et al. Leukemia cutis in acute promyelocytic leukemia: Report of three cases after treatment with AII-trans retinoic acid. Leuk Lymphoma. 1994;14:453–56. doi: 10.3109/10428199409049703. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22:1404–12. doi: 10.1200/JCO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106:447–53. doi: 10.1182/blood-2004-05-1971. [DOI] [PubMed] [Google Scholar]
- 11.Bakst RL, Tallman MD, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785–93. doi: 10.1182/blood-2011-04-347229. [DOI] [PubMed] [Google Scholar]
- 12.Peña-Romero AG, Domínguez-Cherit J, Méndez-Flores S. Leukemia cutis: Clinical features of 27 Mexican patients and a review of the literature. Gac Med Mex. 2016;152(5):439–43. [PubMed] [Google Scholar]