Abstract
Polygonum multiflorum Thunb. and its processed products have been used in China for centuries due to their multiple beneficial effects to human body. Currently, liver injuries caused by taking P. multiflorum have been reported worldwide, but the potential toxic components and possible mechanism that caused hepatotoxicity remain unclear. It is worth noting that the processing procedure could significantly decrease the toxicity of raw P. multiflorum and the processed products of P. multiflorum are considered to be relatively safe. However, the processing mechanism is still ambiguous, and there is the lack of a scientific approach to control the quality of P. multiflorum praeparata. This study is the first review that summarizes the recently advances (from 2007 to 2017) in the chemical analysis of P. multiflorum, and provides comprehensive information on the quantitative and qualitative analysis of P. multiflorum as well as its related species. In addition, the processing mechanism and quality evaluation of processed P. multiflorum are discussed. Moreover, the toxicity of P. multiflorum is analyzed from the perspectives of exploration of the proposed toxic ingredients, metabolite identification, metabolomics studies, and exogenous contaminant determination. Furthermore, trends and perspectives for future research of this medicine are discussed.
Keywords: Polygonum multiflorum thunb., chemical analysis, processing mechanism, quality evaluation, hepatotoxicity, stilbene glucosides, anthraquinones, review
Introduction
According to traditional Chinese medicine (TCM) theory, Polygonum multiflorum Thunb. (PM) is one representative drug that possesses different efficacies in its crude and processed forms (Supplementary Figure S1). P. multiflorum praeparata (PMP) is used more frequently in clinical practice mainly because of its tonic and anti-aging effects, whereas PM is commonly applied to resolve toxins, moisten the intestines and free stools (China Pharmacopoeia Committee, 2015). Modern pharmacological studies and clinical practice have indicated these two medicines have various biological activities, including anti-tumor, anti-oxidative, anti-bacterial, anti-hyperlipidemia, anti-atherosclerosis, immunomodulating and hepatoprotective effects (Bounda and Feng, 2015; Lin et al., 2015a; Li et al., 2016a). The chemical profiles demonstrate that stilbenes and anthraquinones are the major characteristic constituents, of which 2,3,5,4′-tetrahydroxystilbene-2-O-β-glucoside (TSG), emodin-8-O-β-D-glucoside (EMG), and physcion-8-O-β-D-glucoside (PG) are found to be dominant in PM, while PMP mainly contains TSG, emodin and physcion (Bounda and Feng, 2015; Lin et al., 2015a; Li et al., 2016a). These compounds are widely believed to be responsible for the bioactivities of PM and PMP. Studies have shown that TSG exhibited many medicinal properties, including delaying the senescence effect, cardiovascular protection, neuroprotective effects, and the promotion of hair growth (Ling and Xu, 2016). On the other hand, anthraquinones also possessed a wide spectrum of pharmacological properties, such as anti-cancer, anti-microbial, anti-inflammatory, anti-oxidant and hepatoprotective activities (Zhou et al., 2015; Dong et al., 2016; Sun et al., 2016). The previous reviews focusing on the botany, phytochemistry, pharmacological effects, toxicology and some other different aspects of PM are listed in Table 1 (Zhang et al., 2009; Shaw, 2010; Sun and Zhang, 2010; Teschke et al., 2014a,b, 2015b, 2016; Wang et al., 2014; Bounda and Feng, 2015; Lee et al., 2015; Lei et al., 2015; Lin et al., 2015a; Teschke and Eickhoff, 2015a; Zhou et al., 2015; Dong et al., 2016; Li et al., 2016a; Ling and Xu, 2016; Sun et al., 2016; Zhang P. et al., 2016).
Table 1.
Overview of PM-related reviews since 2007.
| Topic | References |
|---|---|
| Traditional usages and botany | Bounda and Feng, 2015; Lin et al., 2015a |
| Phytochemistry/Bioactive compounds | Bounda and Feng, 2015; Lin et al., 2015a |
| Pharmacology | Bounda and Feng, 2015; Lin et al., 2015a; Zhou et al., 2015; Dong et al., 2016; Li et al., 2016a; Ling and Xu, 2016; Sun et al., 2016 |
| Clinical studies/Hepatotoxicity case reports | Zhang et al., 2009; Sun and Zhang, 2010; Teschke et al., 2014a,b, 2015b, 2016; Wang et al., 2014; Bounda and Feng, 2015; Lee et al., 2015; Lei et al., 2015; Teschke and Eickhoff, 2015a; Zhang P. et al., 2016 |
| Side effect and safety | Shaw, 2010; Bounda and Feng, 2015; Lin et al., 2015a; Dong et al., 2016; Li et al., 2016a |
| Pharmacokinetics | Bounda and Feng, 2015; Lin et al., 2015a; Dong et al., 2016 |
| TSG | Ling and Xu, 2016 |
| Emodin | Dong et al., 2016 |
| Rhein | Zhou et al., 2015; Sun et al., 2016 |
Nowadays, there are two big problems that seriously hamper the research and development of PM. First, increasing cases related to the hepiatic lesions induced by PM have been reported in China and other countries (But et al., 1996; Park et al., 2001; Mazzanti et al., 2004; Panis et al., 2005; Shaw, 2010; Sun and Zhang, 2010; Jung et al., 2011; Dong et al., 2014; Teschke et al., 2014b; Wang et al., 2014; Lee et al., 2015; Lei et al., 2015; Zhang P. et al., 2016), which draw great attention from scholars. It is worth noting that the initially references were published in Europe. From 2004 to 2010, 10 cases of adverse reactions associated with the intake of Shou-Wu-Pian (is the tablet form of the root tuber of PM) were reported in Italy (Mazzanti et al., 2004; Valente et al., 2010), the Netherlands (Panis et al., 2005), and England (Zhang et al., 2009; Furukawa et al., 2010; Teschke et al., 2014a,b, 2015b, 2016; Stickel and Shouval, 2015; Teschke and Eickhoff, 2015a), respectively. Among these cases, Shou-Wu-Pian was commonly used for hair care, besides one case for the treatment of chronic prostatitis. The age of patients ranged from 5 to 78 years, and 7 of them were female. The duration with ingestion of Shou-Wu-Pian lead to the liver injury ranged from 2 weeks to several months. In the end, all these 10 patients recovered from symptoms of hepatic dysfunction after they discontinued the consumption of Shou-Wu-Pian. With regard to the hepatotoxicity of PM, Shaw (2010) proposed that the incorrect use of PM might be the leading cause, which mainly due to the patients believed herbal medicines were the natural products and harmless, as well as they usually used these preparations without medical supervision. In another hand, anthraquinones and contaminants (mycotoxins, heavy metals, and pesticides) were considered to be the main hepatotoxic components (Ernst, 2002; Mazzanti et al., 2004; Panis et al., 2005; Furukawa et al., 2010), but this issue was still in dispute due to the lack of convincing evidence. In recent years, extensive experiments have been performed both in vivo and in vitro, unfortunately, the potential toxic components and possible mechanism that caused the hepatotoxicity remain unclear (Lin et al., 2015a; Wang J. et al., 2015; Li et al., 2016a; Wang et al., 2016). Second, processing is a very important procedure that played significant roles in the toxicity-attenuating effect as well as the enhancing tonifying efficacy (Lin et al., 2015a; Wang J. et al., 2015; Li et al., 2016a; Wang et al., 2016; Cui et al., 2017), and the quality assurance of PMP is believed to be the foundation of its clinical usage. Nevertheless, the processing mechanism is still ambiguous, and the current pharmacopeia protocols (China Pharmacopoeia Committee, 2015) failed to differentiate PM from PMP mainly due to their poor specificity (Table 2), and as far as we know, there is no appropriate method to evaluate whether PMP is completely processed or not. These two independent and closely implicated questions are the hot spots of PM research in the future.
Table 2.
Quality standards recorded in Chinese pharmacopeia.
| Samples | Analytes | HPLC assay | UV | Limitation |
|---|---|---|---|---|
| PM | TSG | Eluted with acetonitrile: water (25: 75) | 320 nm | TSG not <1.0% |
| Emodin and Physciona | Eluted with methanol: 0.1% formic acid aqueous solution (80: 20) | 254 nm | Combined anthraquiones not <0.10% | |
| PMP | TSG | Eluted with acetonitrile: water (25: 75) | 320 nm | TSG not <0.70% |
| Emodin and Physcionb | Eluted with methanol: 0.1% formic acid aqueous solution (80: 20) | 254 nm | Free anthraquinones not <0.10% | |
| P. multiflorum caulis | TSG | Eluted with acetonitrile: water (26: 74) | 320 nm | TSG not <0.20% |
indirect quantification.
direct quantification.
This study reviews the recently advances in the chemical analysis of PM and PMP (from 2007 to 2017), which provides comprehensive information on the quantitative and qualitative analysis of PM and its related species. In addition, the processing mechanism and quality evaluation of PMP are discussed. Moreover, the toxicity of PM is analyzed from the perspectives of proposed toxic ingredient exploration, metabolite identification, metabolomics studies and exogenous contaminant determination. In addition, trends and perspectives for future research of this TCM are discussed. The Schematic diagram of the review process is shown in Supplementary Figure S2.
Chemical constituents and quality markers
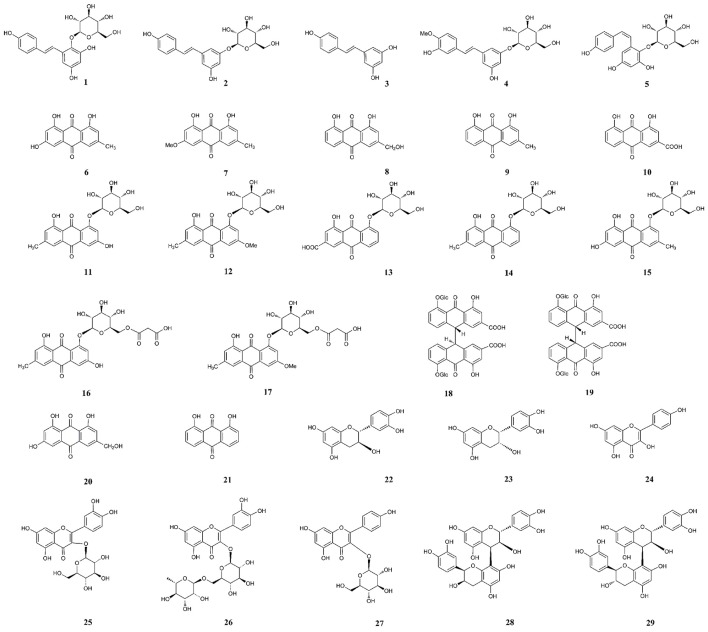
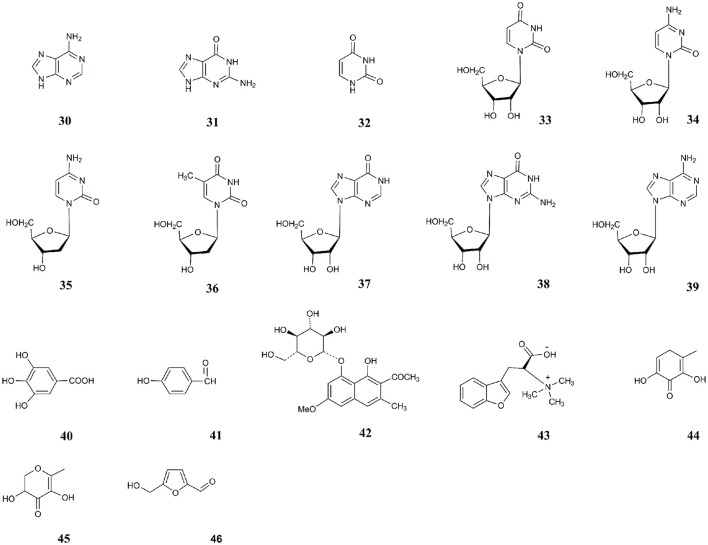
The chemical constituents and pharmacological activities of PM were reviewed in last 2 years (Bounda and Feng, 2015; Lin et al., 2015a), and more than 103 constituents have been isolated and identified, which included stilbenes, quinones, flavonoids, phospholipids, and other compounds. Among these ingredients, stilbene glucosides and anthraquinones are recognized as two major characteristic constituents of PM. Forty six biologically active components or quality markers mentioned in the publications that focused on the topic of “Chemical analysis” of PM were summarized. The chemicals are described as follows (Figures 1, 2): stilbenes: TSG (1), polydatin (2), resveratrol (3), rhaponiticin (4), and cis-TSG (5); anthraquinones: emodin (6), physcion (7), aloe-emodin (8), chrysophanol (9), rhein (10), EMG (11), PG (12), rhein-8-O-β-D-glucoside (RHG) (13), chrysophanol-8-O-β-D-glucoside (CHG) (14), emodin-1-O-β-D-glucoside (EMG1) (15), emodin-8-(6′-O-malonyl)-glucoside (16), physcion-8-(6′-O-malonyl)-glucoside (17), sennoside A (18), sennoside B (19), 6-OH-emodin (20) and danthron (21); flavonoids: catechin (22), epicatechin (23), quercetin (24), hyperin (25), rutin (26), astragalin (27), proanthocyanidin B1 (28), and proanthocyanidin B2 (29); nucleosides: adenine (30), guanine (31), uracil (32), uridine (33), cytidine (34), 2′-deoxycytidine (35), thymidine (36), inosine (37), guanosine (38), and adenosine (39); and phenolic acids and other compounds: gallic acid (40), p-hydraxy benzaldehyde (41), troachrysone-8-O-β-D-glucoside (TOG) (42), hypaphorine (43), hydroxymaltol (44), 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)- pyran-4-one (DDMP) (45), and 5-hydroxymethylfurfural (5-HMF) (46).
Figure 1.
Chemical structures of stilbenes, anthraquinones and flavonoids.
Figure 2.
Chemical structures of nucleosides and other compounds.
Chemical analysis of PM
Qualitative determination
During the past decade, mass spectrometry (MS) and its combination with chromatographic separation techniques have emerged as crucial approaches to describe the chemical profiles of PM, which including high performance liquid chromatography-ion trap-mass spectrometry (HPLC-IT-MS) (Sun et al., 2009a), high performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) (Yi et al., 2007; Zhao et al., 2013), ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF-MS) (Lin et al., 2015c; Wang et al., 2017), and high-performance liquid chromatography/ultra high pressure liquid chromatography-linear ion trap-Orbitrap hybrid mass spectrometry (HPLC/UHPLC-LTQ-Orbitrap-MS) (Xu et al., 2012; Qiu et al., 2013; Wang T. H. et al., 2015), and these results suggest that more than 135 compounds were detected and identified by comparison with the standards or through investigating references. It should be noted that 28 new dianthrone glycosides were characterized for the first time by the means of HPLC-LTQ-Orbitrap-MS (Xu et al., 2012), and their fragmentation behaviors were also proposed. This finding enriched the chemical structure types of PM and offered us more comprehensive information. Actually, several novel dianthrone glycosides from PM were elucidated by the conventional phytochemistry methods 4 years later (Yang J. B. et al., 2016; Yang et al., 2017a,b), which confirmed the existence of dianthrone glycosides in PM. Compared with traditional Q-TOF mass spectrometry, UPLC-LTQ-Orbitrap-MSn provides superior resolution and mass accuracy; meanwhile, with the MSn function, the Orbitrap technology can achieve 10 levels of MS analysis capability (Lin et al., 2015d). In this manner, the phenolic constituents were rapidly screened in the roots of PM, and based on the fragment pattern rules of reference stilbenes and anthraquinone derivatives, 59 constituents were characterized or tentatively identified, of which 22 constituents were the first to be reported in PM and 12 compounds were characterized as potential new compounds (Qiu et al., 2013). Table 3 summarizes the chromatographic approaches for the qualitative analysis of PM.
Table 3.
Chromatographic approaches for the qualitative analysis of PM.
| Techniques | Analytes | Samples | Details | References |
|---|---|---|---|---|
| HPLC-IT-MS | 12 glycosides including 3 newly reported | Crude root | Eluted with CH3OH: 10 mM CH3COONH4 (0 min: 0:100; 20 min: 30:70; 50 min: 95:5; 60 min: 95:5) on an Ultimate XB-C18 column | Sun et al., 2009a |
| HPLC-ESI-MS | 11 compounds including 2 unknowns | Crude root | Eluted with H2O: CH3CN (both of them containing 0.5% CH3COOH) (0 min: 90:10; 35 min: 60:40; 50 min: 0:100) on an Alltima C18 column | Yi et al., 2007 |
| HPLC-ESI-MS | 7 compounds | Caulis | Eluted with CH3CN: H2O (containing 0.5% HCOOH) (0 min: 0:100; 22 min: 16:84; 45 min: 34:66; 60 min: 38:62; 70 min: 95:5; 80 min: 95:5) on a Grace Alltima C18 column | Zhao et al., 2013 |
| UPLC-Q-TOF-MS | 29 components including 8 newly reported | Crude root | Eluted with CH3OH: H2O (both of them containing 0.1% CH3COOH) (0 min: 0:100; 25 min: 35:65; 40 min: 70:30; 50 min: 100:0; 53 min: 100:0; 53.1 min: 0:100; 60 min: 0:100) on a Phenomenex Hydro-RP C18 column | Lin et al., 2015c |
| UHPLC-Q-TOF-MS | 131 compounds including 26 unknowns | Crude root | Eluted with CH3CN: H2O (containing 0.1% CH3COOH) (0 min: 97:3; 20 min: 3:97) on a T3 C18 column | Wang et al., 2017 |
| HPLC-LTQ-Orbitrap-MS | 28 new dianthrone glycosides | Crude root | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 5:95; 6 min: 15:85; 12 min: 15:85; 25 min: 38:62; 30 min: 70:30) on a Hypersil Gold C18 column | Xu et al., 2012 |
| UHPLC-LTQ-Orbitrap-MS | 59 phenolic compounds including 12 newly reported | Crude root | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 5:95; 6 min: 15:85; 12 min: 15:85; 25 min: 38:62; 30 min: 70:30; 35 min: 90:10) on a Hypersil Gold C18 column | Qiu et al., 2013 |
| UHPLC-LTQ-Orbitrap-MS | 25 compounds | Crude and processed root | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 13:87; 3.5 min: 35:65; 7.5 min: 90:10; 8.5 min: 95:5; 10 min: 95:5) on an AcQuity UPLC™ BEH C18 column | Wang T. H. et al., 2015 |
Quantitative analysis
Stilbenes are one of the major components in PM. To date, more than 20 stilbenes and analogs have been found, of which TSG is the most representative compound. Previous publications have demonstrated that TSG possessed anti-tumor, anti-aging and liver-protective bioactivities (Bounda and Feng, 2015; Lin et al., 2015a; Ling and Xu, 2016), which matched well with the traditional efficacies of PM. Quinones are the other characteristic components in PM, which had anti-microbial, anti-cancer, anti-oxidant and anti-human cytomegalovirus effects (Bounda and Feng, 2015; Lin et al., 2015a; Zhou et al., 2015; Dong et al., 2016; Sun et al., 2016). Due to the long conjugated system existing in basic structures of stilbenes and anthraquinones, their characteristic ultraviolet absorptions are easily screened. Therefore, HPLC in tandem with an ultraviolet detector (UV) or diode array detector (DAD)/photodiode array detector (PDA) are widely applied in the quantitative evaluation of PM (Yi et al., 2007; Han et al., 2009, 2013; Jiao and Zuo, 2009; Yan et al., 2010; Zhao et al., 2013; Liang et al., 2014; Li et al., 2016b) According to the Chinese pharmacopeia, TSG, emodin and physcion were eluted on a C18 column by the means of HPLC. However, due to the lack of standard references of anthraquinone glycosides, the content of combined anthraquinone was determined by an indirect method, an additional acidic hydrolysis step was needed, and then, the resulting aglycones were assessed. In this way, combined anthraquinones in PM were calculated as the total amount of physcion and emodin. In fact, determination of the authentic composition has always played a vital role the in quality control of herbals, and with the increasing availability of anthraquinone glycoside standards, direct quantification of the combined anthraquinones was performed by some researchers. Their contents of five markers, i.e., TSG, EMG, PG, emodin and physcion, were quantitatively evaluated by HPLC with DAD (Yi et al., 2007). The results indicated that TSG and EMG were the predominant compounds in PM, which account for about 2.6~4.2% and 0.2~0.6%, respectively, of the total dry weight, and the contents of the other three constituents were no more than 0.06%. Another HPLC-DAD approach (Han et al., 2013) was also proposed for the simultaneous determination of 8 hydrophilic bioactive compounds of PM including TSG, EMG, gallic acid, catechin, epicatechin, hypaphorine, and proanthocyanidin B1 as well as B2, which results in similar data. On the other hand, when analytes are present in trace amounts or showed poor separation, combined MS and liquid chromatographic techniques are the preferred alternative, which provided higher sensitivity and selectivity. ESI-MS in the negative mode was most commonly used in the quantitative analysis of PM (Liang et al., 2011; Zhu et al., 2012; Lin et al., 2015b; Wang T. H. et al., 2015; Luo et al., 2016a). An HPLC-MS/MS method was developed for the simultaneous determination of 14 compounds including stilbenes, quinones, flavonoids and phenolic acids, which might be the work that quantified most compounds in PM. Apart from approaches utilizing HPLC in tandem with UV, DAD, and MS, capillary gas chromatography coupled with flame ionization and mass spectrometric detection (GC-FID-MS) (Zuo et al., 2008) and micellar electrokinetic chromatography (MECK) (Lao et al., 2013; Luo et al., 2015a,b, 2016b) were also established for the determination of stilbenes and anthraquinones in PM. However, due to the complicated protocols and additional derivatization step, GC and MECK might not be substitutes for HPLC as a routine test method. Table 4 summarizes the chromatographic methods for the quantitative analysis of PM.
Table 4.
Quantitative analysis methods of PM.
| Techniques | Analytes | Samples | Details | References |
|---|---|---|---|---|
| HPLC-PDA (290, 320 nm) | TSG, emodin, physcion, EMG, PG | Crude root | Eluted with H2O: CH3CN (both of them containing 0.5% CH3COOH) (0 min: 90:10; 35 min: 60:40; 50 min: 0:100) on an Alltima C18 column | Yi et al., 2007 |
| HPLC-PDA (254 nm) | emodin, physcion, aloe-emodin, rhein, chrysophanol | Crude root | Eluted with CH3OH: H2O: H3PO4 (0 min: 600:400:1; 80 min: 600:400:1) on an Agilent C18 reversed-phase column | Jiao and Zuo, 2009 |
| HPLC-UV (254, 320 nm) | TSG, emodin, physcion | Crude root | Eluted with CH3CN: H2O (25:75) on a Diamond C18 analytical column; Eluted with CH3OH: 0.1% H3PO4 (85:15) on a Diamond C18 analytical column | Yan et al., 2010 |
| HPLC-DAD (210, 280, 320 nm) | TSG, EMG, gallic acid, catechin, epicatechin, proanthocyanidin B1 and B2, hypaphorine | Crude root, rhizome, stem | Eluted with CH3CN: H2O (containing 0.05% H3PO4) (0 min: 0:100; 7 min: 6:94; 12 min: 6:94; 20 min: 8:92; 22 min: 12:88; 50 min: 25:75) on a Zorbax SB-AQ column | Han et al., 2013 |
| HPLC-UV (254, 320 nm) | TSG, emodin, physcion | Crude root | Eluted with CH3OH: H2O (containing 0.1% H3PO4) (30:70) and (80:20) on a Waters Nova-Pak C18 column, respectively | Liang et al., 2014 |
| HPLC-UV (254, 320 nm) | TSG, emodin, physcion | Crude root | Eluted with CH3CN: H2O (25:75) and CH3OH: H2O (containing 0.1% H3PO4) (80:20) on a SinoChrom ODS BP C18 RP column, respectively | Li et al., 2016b |
| UPLC-PDA (280, 320 nm) | TSG, EMG, emodin, physcion | Crude and processed root | Eluted with H2O: CH3CN (both of them containing 0.3% CH3COOH) (0 min: 85:15; 2 min: 85:15; 3 min: 75:25; 5 min: 70:30; 6 min: 15:85; 7 min: 0:100) on an Acquity BEH C18 column | Han et al., 2009 |
| HPLC-PDA (290 nm) | TSG, emodin, physcion | Caulis | Eluted with CH3CN: H2O (containing 0.5% HCOOH) (0 min: 0:100; 22 min: 16:84; 45 min: 34:66; 60 min: 38:62; 70 min: 95:5; 80 min: 95:5) on a Grace Alltima C18 column | Zhao et al., 2013 |
| HPLC-MS | TSG, emodin, physcion | Crude root | Eluted with CH3CN: H2O (both of them containing 0.5% CH3COOH) (0 min: 10:90; 45 min: 35:65; 65 min: 100:0) on an Alltima C18 analytical column | Liang et al., 2011 |
| HPLC-MS/MS | TSG, emodin, physcion, gallic acid, resveratrol, polydatin, catechin, epicatechin | Crude and processed root | Eluted with CH3CN: H2O (containing 0.05% HCOOH) (0 min: 10:90; 10 min: 60:40; 15 min: 90:10; 17 min: 10:90; 20 min: 10:90) on an Eclipse Plus C18 column | Zhu et al., 2012 |
| HPLC-MS/MS | TSG, emodin, physcion, EMG, RHG, resveratrol, polydatin, catechin, rutin, epicatechin, gallic acid, rhaponiticin, hyperin, p-hydraxy benzaldehyde | Crude root | Eluted with CH3OH: H2O (both of them containing 0.1% HCOOH) (0 min: 20:80; 2 min: 40:60; 4 min: 50:50; 6 min: 60:40; 8 min: 70:30; 10 min: 80:20; 12 min: 100:0; 15 min: 100:0; 15.1 min: 0:100; 20 min: 20:80) on a Phenomenex Hydro-RP C18 column | Lin et al., 2015b |
| UHPLC-LTQ-Orbitrap-MS | TSG, EMG, emodin, gallic acid | Crude and processed root | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 13:87; 3.5 min: 35:65; 7.5 min: 90:10; 8.5 min: 95:5; 10 min: 95:5) on an AcQuity UPLC™ BEH C18 column | Wang T. H. et al., 2015 |
| UPLC-MS/MS | TSG, EMG, aloe-emodin, emodin, rhein, physcion, resveratrol, polydatin, rutin, epicatechin, gallic acid, quercetin, astraglin, hyperoside | Crude root | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 100:0; 1 min: 90:10; 2 min: 10:90; 3 min: 10:90; 4 min: 90:10; 5 min: 90:10) on a Waters BEH C18 column | Luo et al., 2016a |
| Capillary-GC-FID-MS | emodin, physcion, aloe-emodin, rhein, chrysophanol | Crude root | The temperature program was 0 min: 180°C; 1 min: 180°C; 11 min: 300°C; 21min: 300°C on a ECTM-5 capillary column | Zuo et al., 2008 |
| MEKC (210 nm) | TSG, proanthocyanidin B1 and B2, gallic acid, catechin, epicatechin, hypaphorine | Crude root | Optimum separation was obtained within 14 min by using 50 mM phosphate buffer containing 90 mM SDS and 2% (m/v) HP-β-CD (pH 2.5) at 15 kV and 20°C | Lao et al., 2013 |
Besides the stilbenes and quinones, nucleosides and nucleobases also have been determined by MS detection (Luo et al., 2015c,d,e, 2016c,d; Xu et al., 2015).
Comparative analysis of related medicinal plants
According to the Chinese pharmacopeia, PM, Polygonum cuspidatum (PC) and Rheum officinale Baill. (RO) are the most frequently used traditional Chinese medicines in the family polygonaceae, which contribute to a wide range of pharmaceutical properties. Due to the similar types of constituents contained in these similar medicinal plants, several studies focused on the quantification and discrimination have been carried out by means of HPLC in tandem with UV or MS (Avula et al., 2007; Huang et al., 2008; Ma et al., 2012; Li et al., 2014; Feng et al., 2016). In HPLC-MS (Huang et al., 2008; Li et al., 2014; Feng et al., 2016), more than 30 compounds had been identified, which mainly belonged to stilbenes, anthraquinones, phenolic acids, and flavonoids. Among these components, thirteen analyte markers including TSG, EMG, emodin, physcion, aloe-emodin, rhein, chrysophanol, piceid, resveratrol, epicatechin, gallic acid, and sennoside A as well as B were simultaneously determined by an HPLC-variable wavelength detection (VWD) approach (Ma et al., 2012). The vital characteristic components for the quality control of a single herb were found by systematic comparison of the chemical compositions of these three herbs, and the results demonstrated that stilbenes and anthraquinones were the main constituents, while chrysophanol, TSG, and piceid could be used as key markers in the discrimination of RO, PM, and PC, respectively. Besides the routine assays mentioned above, HPLC with fluorescence detection (He et al., 2009) and 1H-NMR approach (Frederich et al., 2011) were also achieved for the quality assessment of these polygonaceous herbs. In addition, the descriptions of macroscopic and microscopic properties also played significant roles in the authentication of the raw materials and their adulteration (Avula et al., 2007; Liang et al., 2011, 2014). Table 5 summarizes the chromatographic methods for the comparative analysis of PM and its related medicinal plants.
Table 5.
Comparative analysis methods of PM-related medicinal plants.
| Techniques | Analytes | Samples | Details | References |
|---|---|---|---|---|
| HPLC-VWD/DAD (254, 280, 320 nm) | TSG, emodin, physcion, aloe-emodin, rhein, chrysophanol, EMG, piceid, resveratrol, epicatechin, gallic acid, sennoside A and B | PM, PC, and RO | Eluted with CH3CN: H2O (containing 0.05% HCOOH) (0 min: 5:95; 2 min: 10:90; 4 min: 15:85; 10 min: 15:85; 11 min: 21:79; 14 min: 21:79; 21 min: 29:71; 23 min: 40:60; 25 min: 50:50; 26 min: 50:50; 28 min: 80:20; 30 min 100:0; 32 min: 100:0) on an Agilent Zorbax Stable Bond-C18 column | Ma et al., 2012 |
| HPLC-PDA (280, 320 nm) | polydatin, resveratrol, aloe-emodin, rhein, emodin, physcion, danthron, chrysophanol | PM, PC, P. aviculare, P. bristorta, and P. vulgare | Eluted with H2O: CH3CN (both containing 0.1% CH3COOH) (0 min: 80:20; 35 min: 0:100) on a Phenomenex Gemini C18 column | Avula et al., 2007 |
| HPLC-DAD-ESI/MS (290 nm) | TSG, rhaponticoside, resveratrol, piceid, aloe-emodin, emodin, physcion, rhein, chrysophanol, EMG, PG, TOG, EMG1, CHG | PM, RO, and P. reynoutria | Eluted with H2O: CH3CN (0 min: 85:15; 10 min: 80:20; 40 min: 47:53; 60 min: 0:100) on an Alltima C18 column | Feng et al., 2016 |
| HPLC-DAD-ESI/MSn (290 nm) | TSG, cis-TSG, resveratrol, piceid, resveratroloside, emodin, physcion, EMG, PG, EMG1 | PM and PC | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min:22:78; 1.5 min: 30:70; 6 min: 65:35; 8 min: 90:10; 9 min: 90:10) on a Kinetex C18 column | Li et al., 2014 |
| HPLC- fluorescence detection (440, 540 nm) | emodin, physcion, rhein, aloe-emodin, chrysophanol | PM and PC | Eluted with CH3OH: H2O (containing 0.1% HCOOH) (0 min: 85:15; 15 min: 85:15) on a Hypersil C18 column | He et al., 2009 |
| NMR | Fingerprint analysis | PM and PC | Performed on a Bruker Avance 500 MHz NMR spectrometer operating at 500.13 MHz | Frederich et al., 2011 |
Chemical analysis of PMP
Investigation of the processing mechanism
On the basis of TCM theory, most of the herbs need to be processed before their clinical usage, and during this procedure, the appearance characteristics and bioactivities of herbs might be changed. Thus, different herbal medicine forms will be selected precisely according to the diagnostics of patients. PM is one typical medicine that has completely different utilities in the crude and processed forms, and especially, the toxicity-attenuating effect of processing has been verified (Yu et al., 2011; Wu et al., 2012; Lin et al., 2015a,e; Wang J. et al., 2015; Li et al., 2016a; Wang et al., 2016; Cui et al., 2017). These fascinating variations have attracted great attention from scientists, and considerable efforts were performed to explore the processing mechanism of PM. Various approaches were applied to monitor the transformation of principal compounds, and the results suggested that the hydrolysis reaction and Maillard reaction were involved in the steaming process of the root of PM (Liu et al., 2009, 2013; Xu et al., 2011; Yu et al., 2011; Chen et al., 2012; Wu et al., 2012; Yang et al., 2015; Zhai et al., 2016; Sun et al., 2017; Zhao et al., 2017). First, the combined anthraquinones were unequivocally hydrolyzed into free ones. Emodin and physcion were the characteristic components in PMP, and the content of emodin was increased by more than 30% after processing, while the content of EMG decreased. Meanwhile, a similar downswing was found regarding the level of TSG, and approximately 60% of TSG was reduced during PMP preparation (Yu et al., 2011; Chen et al., 2012; Wu et al., 2012; Lin et al., 2015e; Yang et al., 2015). It should be noted that when the steaming time was extended, more than 90% of TSG disappeared (Chen et al., 2012). However, the hydrolysis products of TSG could not be detected by most researchers who focused on the rules of PMP processing, and only one publication indicated that a deglycosylated compound had the [TSG+H-Glu+H2O]+ ion at m/z 245.0, which was observed in a direct ionization mass method (Hu et al., 2012). Actually, exploration the products of TSG during the processing is a research project with important scientific significance, which would play a vital role in improving our understanding on the global processing mechanisms of PM. Apart from stilbenes and quinones, the concentration of gallic acid was significantly increased, which might be associated with the hydrolysis of tannin (Chen et al., 2012; Zhai et al., 2016; Zhao et al., 2017). Secondly, the occurrence of a Maillard reaction would likely be responsible for the changes in the contents of 5-HMF, amino acids, sugars, pH and surface color (Liu et al., 2008, 2009). Among these indicators, polysaccharides were closely correlated to the major biological activities of PMP, and an HPLC-evaporative light scattering detection (ELSD) method was proposed for qualification of the sugars in PMP, with the major constituents assigned as glucose, fructose, and sucrose. The contents of D-fructose and sucrose decreased, while the content of D-glucose increased (Liu et al., 2009). In another report, the content of low molecular weight polysaccharides increased after processing (Qiu et al., 2007).
Quality control of PMP
PMP is one representative processed drug that needs repeated steaming. According to the record of ancient writings (Cui et al., 2017), nine cycles of steaming and solarization were required, but in current practice, this traditional method was commonly replaced with steaming once within a few hours. Moreover, PM and its adulterants stained with black dye to simulate PMP without processing were found in the market. It was noted that the pharmacopeia protocols failed to differentiate PM from PMP mainly due to the poor specificity, as the same targets including TSG, emodin and physcion were determined in both of these two medicines. All of these factors would pose a serious health risk. Therefore, the critical markers should be screened and identified in order to distinguish PM and PMP as well as evaluate the quality of PMP. On this occasion, comparative studies of chemical constituents were carried out based on the platform of HPLC-DAD and mass spectrometry (Liang et al., 2010; Liu et al., 2011). 5-HMF was first proposed as a key ingredient to authenticate PMP and PM on account of it being recognized as a product of the Maillard reaction and being newly formed during processing (Liu et al., 2009). However, some scientists found contrary results that 5-HMF could not be observed in PMP in their HPLC analyses (Wu et al., 2012). Furthermore, 5-HMF was a controversial agent due to its toxicity (Severin et al., 2010; Bauer-Marinovic et al., 2012; Islam et al., 2014), as studies had shown that 5-HMF exhibited cytotoxic, genotoxic and tumoral effects. On the other hand, six ingredients, namely catechin, flavanol gallate dimer, polygoninmitin B, emodin-1-O-glucoside, emodin-8-O-(6′-O-malonyl)-glucoside, and physcion-8-O-(6′-O-malonyl)-glucoside, disappeared or decreased significantly after processing, which were assigned as chemical markers for differentiating PM from PMP (Liang et al., 2010; Liu et al., 2011). Nevertheless, the repeatability and reliability of this strategy needs to be validated in further research, which is mainly due to that the amounts of these markers were relatively minor compared to other components in PM before steaming, and the batches of samples investigated in the studies were limited.
As mentioned above, it was difficult to screen the markers only from the perspective of chemical compounds because variations in the plant origins and processing technologies of PM would result in significant differences of index components. In order to assess the quality of PMP, some new approaches were performed based on chemical profiling combined with a bioactivity assay. Chang et al. (2016) conducted an activity-based integrated UHPLC/Q-TOF-MS-FC method to clarify the effect of the processing time on the lipase inhibitory activity of PMP. Chen et al. (2016) developed an online HPLC-DAD-CL assay based on the three reactive oxygen species to evaluate the quality of PMP. In addition, in some other methods, the chemical compositions were analyzed and toxicity monitoring was established to evaluate the processing technologies and quality of PMP (Pang et al., 2014; Ma Z. J. et al., 2015).
Chemical analysis of hepatotoxic components
Analysis of the proposed toxic ingredients
Currently, the hepatotoxicity of PM has attracted great concern, and a considerable number of experiments related to PM-induced liver injury were carried out, which offered us comprehensive information to understand the mechanisms. In general, the extracts of PMP are considered to be relatively safe, while hepatotoxicity is found in the PM extracts. Several publications have focused on comparatively studying the toxicities of various extraction solvents from PM and PMP, and the results suggested that the ethanol extract could induce hepatic lesions more easily than that of water decocta (Lv et al., 2013, 2015; Lin et al., 2015e). The order of toxicity was described as PM ethanol extract > PM water extract > PMP ethanol extract > PMP water extract, and in another manuscript (Wu et al., 2012), the toxicity order was proposed as follows: PM water extract > PM acetone extract > PMP acetone extract. Although the hepatotoxic chemicals attributing to the hepatic lesions of PM remain in dispute, emodin and its derivates were believed to be the most likely hepatotoxic components (Yu et al., 2011; Ma J. et al., 2015), and they have also gained much attention from researchers. Recent studies indicated that emodin showed severe cytotoxicity against the human liver cell line L-02 in a concentration- and time-dependent manner. Furthermore, a time-dependent intracellular accumulation of emodin was found in cellular toxicokinetic research by using an HPLC-MS method (Li et al., 2012). Subsequently, an experiment emphasizing the multicomponent interactions of PM was conducted by the same research group, and the results suggested that TSG could delay the elimination of emodin, with the mechanism possibly associated with the inhibition of UGT1A8 mRNA expression (Ma et al., 2013). Lv et al. (2015) explored the toxic components of PM based on biospecific hepatocyte extraction, and the results demonstrated that emodin, physcion, EG and PG were proposed as hepatotoxic components. Lin et al. (2015e) established an UPLC-Q-TOF/MS approach coupled with Progenesis QI and Makerlynx XS software to screen the toxic components from extractions of PM, in which the suspected targets were recognized as emodin-O-(malonyl)-hex, emodin-O-glc, emodin, emodin-8-O-glc, emodin-O-(acetyl)-hex, and emodin-O-hex-sulfate. It was noteworthy that some reports speculated that the toxicity of PM might not be correlated with the content of emodin derivatives but depended on the contents of TSG or the relative content of TSG and emodin (Wu et al., 2012; Yang M. et al., 2016), which is mainly because the amount of emodin was relatively small in PM. Actually, the idiosyncratic hepatotoxicity induced by the isomerization of TSG (cis-TSG) in LPS-treated rats was found in the latest publication (Li et al., 2017), which provided us a new perspective on liver injury by PM. In addition, tannin is another major component in PM, which accounts for approximately 15% of the total dry weight. It was hypothesized that tannin is one of the reasons for the induced liver damage mainly because the content of tannin decreased by 9% after processing (Liu et al., 2005), which meanwhile attenuated the toxicity. In a preliminary investigation, the significant changes of liver biochemical indices were observed after the oral administration of tannin extracts of PM (Hu et al., 2010, 2011). However, in current practice, the chemical analysis approaches regarding tannin are still in the beginning stages, and no individual reference is available due to its complicated structure. Furthermore, the influence of some other factors, i.e., specification (Li Y. M. et al., 2016) and geographical areas (Lin et al., 2017), on the hepatocyte toxicity of PM were discussed. Table 6 summarizes the chromatographic methods for the hepatotoxic analysis of PM in vitro and in vivo.
Table 6.
Hepatotoxic analysis of PM in vivo and in vitro.
| Techniques | Analytes | Species, administration and biological sample | Details | References |
|---|---|---|---|---|
| HPLC-DAD (290 nm) | TSG, EG, PG, emodin, physcion, chrysophanol | Mice, p.o., repeated 28 days (5, 10 and 20 g/kg/day of water and acetone extracts of PM and PMP, respectively), blood samples, histopathologic examination and biochemical analysis | Eluted with CH3CN: H2O (0 min: 10:90; 35 min: 40:60; 60 min: 100:0) on a hypersil C18 column | Wu et al., 2012 |
| HPLC-MS | TSG, emodin, EG | Rats, p.o., repeated 21 days (1 and 20 g/kg/day of 80% ethanol extractions of PM), blood samples and tissues, histopathologic examination and biochemical analysis | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 20:80; 3.5 min: 35:65; 4.5 min: 35:65; 6 min: 40:60; 7 min: 40:60; 8 min: 100:0; 11 min: 100:0) On an Agilent Extend-C18 column | Ma J. et al., 2015 |
| HPLC-MS | TSG, emodin | Rats, p.o. repeated 7 days (TSG, 117 mg/kg), on the 8th day, p.o. (emodin 82.4 mg/kg), blood samples | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 70:30; 2 min:70:30) On an Agilent Extend-C18 column | Ma et al., 2013 |
| HPLC-DAD (254 nm) | TSG, emodin, physcion | Human hepatocytes cell L-02, treated with serial concentrations of water, 50% and 95% ethanol extracts of PM and PMP (20~100 μg/mL, respectively), MTT assay | Eluted with CH3OH: H2O (containing 0.1% H3PO4) (0 min: 40:60; 5 min: 70:30; 10 min: 80:20; 15min: 85:15; 20min: 90:10; 25 min: 90:10) On a Zorbax SB-C18 analytical column. | Yu et al., 2011 |
| HPLC-DAD (210, 280, 320 nm) | 15 components | Human hepatocytes cell L-02, treated with serial concentrations of water and ethanol extracts of PM (0, 0.5, 1, 2.5, 5 mg/mL, respectively), MTT assay | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 6:94; 7 min: 6:94; 12 min: 6:94; 20 min: 8:92; 22 min: 12:88; 50 min: 25:75; 55 min: 0:100) on a Zorbax SB-AQ C18 column | Lv et al., 2015 |
| UPLC-Q-TOF-MS | Non-targeted | Human hepatocytes cell L-02, treated with serial concentrations of water and ethanol extracts of PM and PMP (7.81~1,000.0 μg/mL, respectively), MTT assay | Eluted with CH3CN: H2O (both of them containing 0.1% HCOOH) (0 min: 0:100; 3 min: 10:90; 10 min: 20:80; 20 min: 70:30; 21 min: 100:0; 21.1 min: 0:100; 25 min: 0:100) on an ACQUITY UPLC HSS T3 column | Lin et al., 2015e |
| HPLC-MS | emodin | Human hepatocytes cell L-02, treated with serial concentrations of emodin (0, 10, 20, 40, 60, 120 μM), Cell Counting Kit (CCK)-8 assay | Eluted with CH3CN: H2O (containing 0.2% HCOOH) (0 min: 45:55; 15 min: 30:70) On a C18 column | Li et al., 2012 |
| UPLC-MS | emodin | Human hepatocytes cell L-02, treated with serial concentrations of emodin (10~120 μM), MTT assay | Eluted with CH3CN: H2O (containing 0.1% HCOOH) (0 min: 5:95; 3 min: 50:50; 15 min: 100:0; 20 min: 100:0; 21 min: 5:95; 26 min: 5:95) On a Zorbax Eclipse plus C18 column | Liu et al., 2015 |
Identification of the metabolites
Exploration of the metabolites plays an important role in clarifying the possible mechanism associated with the liver damage resulting from PM. Lin et al. (2015c) established an U-HPLC-Q-TOF/MS method to describe the absorption and metabolism of PM extract in rat plasm after oral administration, and 16 of 23 compounds were indicated as prototype components of PM, while seven compounds were predicted to be metabolites, which included three isomers of stilbene glucoside glucuronidation, two isomers of emodin glucuronidation, torachrysone glucuronidation, and torachrysone. Through an in vitro study, the metabolism of active compounds of PM was investigated in human normal liver cells (L-02) by means of LC-MS (Liu et al., 2015) and HPLC (Lin et al., 2015), and the results suggested that eight phase II metabolites of TSG and emodin were detected. Their chemical structures were elucidated based on their characteristic fragments, including three isomers of the glucuronidation of TSG, three isomers of the glucuronidation of emodin, one sulfation of emodin, and one emodin-cysteine adduct. In addition, the formation of emodin metabolites mediated by cytochrome P450 was investigated (Qin et al., 2016), and three hydroxylation metabolites named 2-hydroxyemodin, 5-hydroxyemodin, and ω-hydroxyemodin as well as three N-acetyl cysteine conjugates and two emodin-derived GSH conjugates were identified. Among these metabolites, emodin-cysteine was suspected to be associated with liver injury due to the formation of an adduct disturbing GSH and fatty acid metabolism in human liver cells. However, further validation should be carried out to confirm this hypothesis. Due to the multiple phenolic hydroxyl groups in TSG and emodin as well as the limitation of the mass spectrum, the combined positions of glucuronide/ sulfatide/cysteine were still uncertain. In addition, the bioactivities and toxicities of these metabolites need to be evaluated.
Metabolomics studies
In last few years, studies used metabolomics methods integrated with pattern recognition to investigate the potential hepatotoxicity of PM have been constantly reported, which provided preliminary information on the mechanisms of liver injury induced by PM (Dong et al., 2015; Zhang et al., 2015; Li et al., 2016; Zhang C. E. et al., 2016; Ma et al., 2017; Xia et al., 2017). It should be noted that in these experiments, the rats were usually administrated a high dose of PM orally for more than 28 days. In a targeted metabolomics study, the perturbation of nine bile acids (BAs) associated with PM-induced liver injury were evaluated. The glycodeoxycholic acid (GDCA) in bile and hyodeoxycholic acid (HDCA) in serum significantly decreased and were assigned as potential biomarkers for PM-induced liver injury in rats (Dong et al., 2015). In untargeted metabolomics research, 16 possible endogenous metabolites in serum along with 10 metabolites in liver tissue samples were identified by means of GC-MS, and these markers were involved in amino acid, fatty acid, and energy metabolism pathways (Zhang et al., 2015; Xia et al., 2017). Additionally, 16 significantly disturbed biomarkers in urine samples were authenticated by the LC-MS assay, and the pathway analysis showed that vitamin B6 metabolism, tryptophan metabolism and the citrate cycle might be the most important pathways involved in the PM-induced hepatotoxicity (Zhang C. E. et al., 2016). Furthermore, 21 potential metabolomics biomarkers related to the idiosyncratic hepatotoxicity of PM were detected by an UHPLC-MS approach, which is mainly associated with the tricarboxylic acid cycle and sphingolipid metabolism pathways (Li et al., 2016). Table 7 summarizes the chromatographic methods for the metabolomics analysis of PM.
Table 7.
Metabolomics analysis of PM.
| Techniques | Biomarkers | Pathway analysis | Species, administration, and biological sample | Details | References |
|---|---|---|---|---|---|
| LC-MS | GDCA, HDCA | bile acids metabolism | Rats, p.o., repeated 42 days (50 g/kg/day of 75% ethanol extracts of PM and PMP, respectively), blood and bile samples | Eluted with CH3OH (containing 0.1% HCOOH): H2O (containing 0.1% HCOOH and 1mM CH3COONH4) (0 min: 70:30; 3 min: 80:20; 8 min: 90:10; 8.5 min: 95:5; 14.4 min: 100:0; 14.5 min: 70:30; 20 min: 70:30) On a Ulimate C18 column | Dong et al., 2015 |
| GC-MS | 18 biomarkers | amino acid, lipid and energy metabolism | Rats, p.o., repeated 28 days (30 g/kg/day of water and 80% ethanol extracts of PM, respectively), blood and liver tissues | The temperature program was 0–5 min: 80°C, 6–23 min: 80–260°C, 24–34 min: 260°C, on a HP-5MS column | Zhang et al., 2015 |
| GC-MS | 10 biomarkers | amino acid, fatty acid, and energy metabolism | Rats, p.o., repeated 28 days (19.2, 192, and 1920 mg/kg/day of 95% ethanol extracts of PM), serum samples | The temperature program was 0–25 min: 80–280°C, 25–29 min: 280°C, on a DB-5MS column | Xia et al., 2017 |
| LC-MS | 16 biomarkers | vitamin and tryptophan metabolism, citrate cycle | Rats, p.o., repeated 28 days (20 g/kg/day of 75% ethanol extractions of PM and PMP, respectively), urine samples | Eluted with CH3OH : H2O (0 min: 30:70; 5 min: 90:10; 5.1 min: 90:10; 40 min: 30:70) On an Agilent ZORBAX SB-C18 column | Zhang C. E. et al., 2016 |
| UHPLC-MS | 21 biomarkers | sphingolipid metabolim and tricarboxylic acid cycle | Rats. p.o., different extracts of PM, blood samples | Eluted with H2O: CH3CN (both of them containing 0.1% HCOOH(0 min: 95:5; 1 min: 95:5; 9 min: 60:40; 19 min: 10:90; 21 min: 0:100; 25min: 0:100) On a ZORBOX RRHD C18 analytical column | Li et al., 2016 |
Exogenous contaminants
Exogenous contaminants (e.g., mycotoxins, heavy metals, and pesticides) are also considered to be the main reasons for the cause of herbal (Drug-Induced Liver Injury) DILI, especially, the process of steaming would make aflatoxins easily appear if the solarization of PMP was not in time, which usually is known as its acute hepatotoxicity and carcinogenic feature. Several publications focused on the determination of these mycotoxins by means of UHPLC-MS, including aflatoxins B1, B2, G1, G2, M1, and M2 (Han et al., 2010a), ochratoxins A and B (Han et al., 2010b), fumonisins B1, B2, and B3 (Han et al., 2010c), five type B trichothecenes, which contained deoxynivalenol (DON), 3-acetyldeoxynivalenol (3-ADON), 15-ADON, nivalenol and fusarenon X (Han et al., 2010d), zearalenone (ZEN) and its derivatives (Han et al., 2011). It should be noted that the isotope dilution method was employed in all of the experiments mentioned above, which is attributed to the advantage that the isotopic IS had similar behavior to the target during the sample pretreatment and ionization process. Thus, in this way the matrix effects were minimized, and the recoveries were calibrated. The result revealed positive findings of ZEN (1.1 μg/kg) as well as fumonisins B1 (1.25 μg/kg) and B2 (0.82 μg/kg) in randomly selected PM samples, respectively. On the other hand, the heavy metals and inorganic elements were evaluated by using inductively coupled plasma mass spectrometry (ICP-MS) (Luo et al., 2014, 2015f,g) and atomic absorption spectrometry (AAS) (Shi et al., 2011), and the results showed that parts of samples with detected Hg, As and Pb exceeded the safety limits specified by the Green Trade Standards of Importing and Exporting Medicinal Plants and Preparations of China (Pb ≦ 5 μg/g, Cd ≦ 0.3 μg/g, Hg ≦ 0.2 μg/g, Cu ≦ 20 μg/g, and As ≦ 2 μg/g), which should raise significant concerns regarding this issue. In another translational medicine study, five batches of identified PM or PMP were collected from patients with suspected PM DILI, and hazardous materials of these samples, which are comprised of heavy metals, mycotoxins, and pesticides, were determined according to the Chinese pharmacopeia or European Union standards. The laboratory reports demonstrated that there were no targets exceeding the safety limits (Wang J. et al., 2015).
Conclusions and future perspectives
As one of the most widely used traditional medicines in China, PM and its processed products have been widely used for the clinical treatment of fatty liver disease, hyperlipidemia, cirrhosis, hepatitis B, learning and memory obstructions, Alzheimer's disease and Parkinson's disease (Lin et al., 2015a; Li et al., 2016a; Ling and Xu, 2016). In recent years, the hepatotoxicity of PM has been well-documented, but the mechanisms of the toxicity remain unknown. Moreover, the quality evaluation of PMP has attracted great concern due to that the processing procedure could significantly decrease the toxicity. However, the processing mechanism was still unclear, and a scientific quality standard to control the quality of PMP was lacking. In the current review, we summarize the existing studies on the chemical analysis of PM and PMP, and a considerable amount of experimental works were carried out that focused on the difficult points mentioned above. Nevertheless, the following aspects still require investigation.
First, systemic chemical constituent studies could lay the foundation for the deeper understanding of the pharmacological efficacies, adverse effects, qualitative determination as well as quantitative analysis of PM and PMP. Besides Lin et al. (2015a) summarized 103 chemical compounds of PM, one new type agents were discovered by our group (Yang J. B. et al., 2016; Yang et al., 2017a,b), more than 30 novel dianthrone glycosides were elucidated unambiguously by spectroscopic analysis. In addition, the toxicities of parts compounds were evaluated against L-02 cell lines and KB tumor cell lines, and the results indicated that these constituents showed moderate hepatotoxicities. These findings provided us a new perspective on liver injury by PM. Apart from these dianthrone glycosides, novel dimeric stilbene glucosides along with polysaccharides were found in the last 2 years (Yan et al., 2014; Park et al., 2016; Zhang and Cui, 2016; Zhao et al., 2016; Zhu et al., 2016, 2017), and their anti-neuroinflammatory effects, antioxidant and antitumor properties were evaluated. In this way, careful chemical exploration should be performed involving the conventional phytochemistry methods as well as MS techniques which depend on the standards and fragmentation pattern rules of references.
Second, the detailed transformation of major compounds in PM during the processing procedure could allow us to better understand the mechanisms of preparation, which would facilitate the establishment of a quality control method and the normalization of the processing technology. As we discussed in this review, combined anthraquinones were unequivocally hydrolyzed into free ones, but the compounds from the degradation of TSG were difficult to detect. Additionally, the concentration of TSG decreased drastically after processing, and the most predominant constituents in PM-TSG seemed to disappear. In fact, the basic TSG structure that consists of two aromatic rings, which are linked through one alkene double bond, is a focus of reactivity. TSG was speculated to be easily degraded or isomerized under different conditions (Figueiras et al., 2011). Several studies focusing on the stability of TSG have been carried out, and the results demonstrated that the degradation of TSG was pH-, temperature-, irradiation- and metal ion-dependent. Two degradants together with one isomerized product were observed in acidic, alkaline and irradiation conditions (Sun et al., 2009b; Ren et al., 2011; Wang et al., 2011), respectively. In addition, the products of the TSG dimer with a water molecule were found in water containing Fe3+ (Li R. Y. et al., 2016) and with H2O2 (Lv et al., 2008) (Supplementary Figure S3). It is hypothesized that there are two pathways associated with the transformation mechanisms of TSG. On the one hand, due to the alkene double bond is a focus reactivity of TSG, the degradation reaction was occurred, and the transformation products might be the small molecules as phenolic acids (Lv et al., 2008; Li R. Y. et al., 2016). On the other hand, the polymerisation was happened, the transformation products might be the dimers of TSG, and this speculation is based on the results of TSG in Fe3+ solutions (Li R. Y. et al., 2016) or H2O2 (Lv et al., 2008), but also the stilbene glucoside dimers were isolated from processed roots of P. multiflorum (Yan et al., 2014). Furthermore, concerning the transformation products of TSG were newly formed after preparation and also their contents changed with the variation of processing time, these targets were proposed as the critical markers in assessing the quality of P. multiflorum praeparata.
Author contributions
SM and SL conceived the review; YL wrote the manuscript; XG, JY, and WL collected the literatures; and QW edited the manuscript. All the authors read and approved the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by the National Natural Science Foundation of China (Nos. 81503347, 81773874, and 81703665) and the 12th 5 Year National significant new drugs creation feature subjects-traditional Chinese medicine quality safety evaluation and risk control technology platform (No. 2014ZX09304307-002).
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00364/full#supplementary-material
References
- Avula B., Joshi V. C., Wang Y. H., Khan K. A. (2007). Simultaneous identification and quantification of anthraquinones, polydatin, and resveratrol in Polygonum multiflorum, various Polygonum species, and dietary supplements by liquid chromatography and microscopic study of Polygonum species. J. AOAC. Int. 90, 1532–1538. [PubMed] [Google Scholar]
- Bauer-Marinovic M., Taugner F., Florian S., Glatt H. (2012). Toxicity studies with 5-hydroxymethylfurfural and its metabolite 5-sulphooxy-methylfurfural in wild-type mice and transgenic mice expressing human sulphotransferases 1A1 and 1A2. Arch. Toxicol. 86, 701–711. 10.1007/s00204-012-0807-5 [DOI] [PubMed] [Google Scholar]
- Bounda G. A., Feng Y. (2015). Review of clinical studies of Polygonum multiflorum thunb. and its isolated bioactive compounds. Pharmacognosy Res. 7, 225–236. 10.4103/0974-8490.157957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- But P. P., Tomlinson B., Lee K. L. (1996). Hepatitis related to the Chinese medicine Shou-Wu-Pian manufactured from Polygonum multiflorum. Vet. Hum. Toxicol. 38, 280–282. [PubMed] [Google Scholar]
- Chang Y. X., Ge A. H., Jiang Y., Azietaku J. T., Li J., Gao X. M. (2016). A bioactivity-based method for screening, identification of lipase inhibitors, and clarifying the effects of processing time on lipase inhibitory activity of Polygonum multiflorum. Evid. Based Complement. Alternat. Med. 2016:5965067. 10.1155/2016/5965067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. F., Chen Y. H., Liu C. H., Wang L., Chen X., Yu B. Y., et al. (2016). Integrated chemometric fingerprints of antioxidant activities and HPLC-DAD-CL for assessing the quality of the processed roots of Polygonum multiflorum thunb. Chin. Med. 11, 18. 10.1186/s13020-016-0087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. T., Zhou L. H., Xu W., Huang Z. H., Qiu X. H. (2012). Content changes of 5 components in Polygohum muhiflorum during processing. Chin. J. Exp. Tradit. Med. Form. 18, 66–71. 10.13422/j.cnki.syfjx.2012.05.028 [DOI] [Google Scholar]
- China Pharmacopoeia Committee (2015). Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science and Technology Press. [Google Scholar]
- Cui H. R., Bai Z. F., Song H. B., Jia T. Z., Wang J. B., Xiao X. H. (2017). Investigation of potential toxic factors for fleece-flower root: from perspective of processing methods evolution. Chin. J. Chin. Mater. Med. 41, 333–339. 10.4268/cjcmm20160227 [DOI] [PubMed] [Google Scholar]
- Dong H., Slain D., Cheng J., Ma W., Liang W. (2014). Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement. Ther. Med. 22, 70–74. 10.1016/j.ctim.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Dong Q., Li N., Li Q., Zhang C. E., Feng W. W., Li G. Q., et al. (2015). Screening for biomarkers of liver injury induced by Polygonum multiflorum: a targeted metabolomic study. Front. Pharmacol. 6:217. 10.3389/fphar.2015.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Fu J., Yin X., Cao S., Li X., Lin L., et al. (2016). Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 30, 1207–1218. 10.1002/ptr.5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E. (2002). Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol. Sci. 23, 136–139. 10.1016/S0165-6147(00)01972-6 [DOI] [PubMed] [Google Scholar]
- Feng J. F., Ren H. Z., Gou Q. F., Zhu L., Ji H., Yi T. (2016). Comparative analysis of the major constituents in three related polygonaceous medicinal plants using pressurized liquid extraction and HPLC-ESI/MS. Anal. Methods 8, 1557–1564. 10.1039/C5AY02941D [DOI] [Google Scholar]
- Figueiras T. S., Neves-Petersen M. T., Petersen S. B. (2011). Activation energy of light induced isomerization of resveratrol. J. Fluoresc. 21, 1897–1906. 10.1007/s10895-011-0886-3 [DOI] [PubMed] [Google Scholar]
- Frederich M., Wauters J. N., Tits M., Jason C., de Tullio P., Van der Heyden Y., et al. (2011). Quality assessment of Polygonum cuspidatum and Polygonum multiflorum by 1H NMR metabolite fingerprinting and profiling analysis. Planta Med. 77, 81–86. 10.1055/s-0030-1250132 [DOI] [PubMed] [Google Scholar]
- Furukawa M., Kasajima S., Nakamura Y., Shouzushima M., Nagatani N., Takinishi A., et al. (2010). Toxic hepatitis induced by Show-Wu-Pian, a Chinese herbal preparation. Intern. Med. 49, 1537–1540. 10.2169/internalmedicine.49.3509 [DOI] [PubMed] [Google Scholar]
- Han D. Q., Zhao J., Xu J., Peng H. S., Chen X. J., Li S. P. (2013). Quality evaluation of Polygonum multiflorum in China based on HPLC analysis of hydrophilic bioactive compounds and chemometrics. J. Pharm. Biomed. Anal. 72, 223–230. 10.1016/j.jpba.2012.08.026 [DOI] [PubMed] [Google Scholar]
- Han L. F., Wu B., Pan G. X., Wang Y. F., Song X. B., Gao X. M. (2009). UPLC-PDA analysis for simultaneous quantification of four active compounds in crude and processed rhizome of Polygonum multiflorum thunb. Chromatographia 70, 657–659. 10.1365/s10337-009-1180-2 [DOI] [Google Scholar]
- Han Z., Liu X., Ren Y., Luan L., Wu Y. (2010d). A rapid method with ultra-high-performance liquid chromatography-tandem mass spectrometry for simultaneous determination of five type B trichothecenes in traditional Chinese medicines. J. Sep. Sci. 33, 1923–1932. 10.1002/jssc.201000094 [DOI] [PubMed] [Google Scholar]
- Han Z., Ren Y., Liu X., Luan L., Wu Y. (2010c). A reliable isotope dilution method for simultaneous determination of fumonisins B1, B2 and B3 in traditional Chinese medicines by ultra-high-performance liquid chromatography -tandem mass spectrometry. J. Sep. Sci. 33, 2723–2733. 10.1002/jssc.201000423 [DOI] [PubMed] [Google Scholar]
- Han Z., Ren Y., Zhou H., Luan L., Cai Z., Wu Y. (2011). A rapid method for simultaneous determination of zearalenone, alpha-zearalenol, beta-zearalenol, zearalanone, alpha-zearalanol and beta-zearalanol in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 879, 411–420. 10.1016/j.jchromb.2010.12.028 [DOI] [PubMed] [Google Scholar]
- Han Z., Zheng Y., Luan L., Cai Z., Ren Y., Wu Y. (2010a). An ultra-high-performance liquid chromatography-tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal. Chim. Acta 664, 165–171. 10.1016/j.aca.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Han Z., Zheng Y. L., Luan L. J., Ren Y. P., Wu Y. J. (2010b). Analysis of ochratoxin A and ochratoxin B in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry using [13C20]-ochratoxin A as an internal standard. J. Chromatogr. A 1217, 4365–4374. 10.1016/j.chroma.2010.04.052 [DOI] [PubMed] [Google Scholar]
- He D., Chen B., Tian Q., Yao S. (2009). Simultaneous determination of five anthraquinones in medicinal plants and pharmaceutical preparations by HPLC with fluorescence detection. J. Pharm. Biomed. Anal. 49, 1123–1127. 10.1016/j.jpba.2009.02.014 [DOI] [PubMed] [Google Scholar]
- Hu B., Lai Y. H., So P. K., Chen H., Yao Z. P. (2012). Direct ionization of biological tissue for mass spectrometric analysis. Analyst 137, 3613–3619. 10.1039/c2an16223g [DOI] [PubMed] [Google Scholar]
- Hu X. Q., Li M., Yang H. L., Wang L., Li D., Li Y. L., et al. (2011). Effects of different ratios of tannin and stilbene glucoside from Polygonum multiflorum on liver biochemical indexes in rats. Shanghai Zhongyiyao Zazhi 45, 56–59. 10.16305/j.1007-1334.2011.04.025 [DOI] [Google Scholar]
- Hu X. Q., Li Y. L., Wang L. (2010). Effect of tannin in Polygonum multiflorum on liver biochemical indexes of rats. Yaowu Pingjia Yanjiu 33, 63–65. [Google Scholar]
- Huang W. Y., Cai Y. Z., Xing J., Corke H., Sun M. (2008). Comparative analysis of bioactivities of four Polygonum species. Planta Med. 74, 43–49. 10.1055/s-2007-993759 [DOI] [PubMed] [Google Scholar]
- Islam M. N., Khalil M. I., Islam M. A., Gan S. H. (2014). Toxic compounds in honey. J. Appl. Toxicol. 34, 733–742. 10.1002/jat.2952 [DOI] [PubMed] [Google Scholar]
- Jiao Y., Zuo Y. (2009). Ultrasonic extraction and HPLC determination of anthraquinones, aloe-emodine, emodine, rheine, chrysophanol and physcione, in roots of polygoni multiflori. Phytochem. Anal. 20, 272–278. 10.1002/pca.1124 [DOI] [PubMed] [Google Scholar]
- Jung K. A., Min H. J., Yoo S. S., Kim H. J., Choi S. N., Ha C. Y., et al. (2011). Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum thunb. Gut Liver 5, 493–499. 10.5009/gnl.2011.5.4.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao K. M., Han D. Q., Chen X. J., Zhao J., Wang T. J., Li S. P. (2013). Simultaneous determination of seven hydrophilic bioactive compounds in water extract of Polygonum multiflorum using pressurized liquid extraction and short-end injection micellar electrokinetic chromatography. Chem. Cent. J. 7:45. 10.1186/1752-153X-7-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. J., Kim H. W., Lee H. Y., Son C. G. (2015). Systematic review on herb-induced liver injury in Korea. Food Chem. Toxicol. 84, 47–54. 10.1016/j.fct.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Lei X., Chen J., Ren J., Li Y., Zhai J., Mu W., et al. (2015). Liver damage associated with Polygonum multiflorum thunb.: a systematic review of case reports and case series. Evid. Based Complement. Alternat. Med. 2015:459749. 10.1155/2015/459749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. L., Ma J., Zheng L., Li H. J., Li P. (2012). Determination of emodin in L–02 cells and cell culture media with liquid chromatography-mass spectrometry: application to a cellular toxicokinetic study. J. Pharm. Biomed. Anal. 71, 71–78. 10.1016/j.jpba.2012.07.031 [DOI] [PubMed] [Google Scholar]
- Li C. Y., Tu C., Gao D., Wang R. L., Zhang H. Z., Niu M., et al. (2016). Metabolomic study on idiosyncratic liver injury induced by different extracts of Polygonum multiflorum in rats integrated with pattern recognition and enriched pathways analysis. Front. Pharmacol. 7:483. 10.3389/fphar.2016.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Niu M., Bai Z., Zhang C., Zhao Y., Li R., et al. (2017). Screening for main components associated with the idiosyncratic hepatotoxicity of a tonic herb, Polygonum multiflorum. Front. Med. 11, 253–265. 10.1007/s11684-017-0508-9 [DOI] [PubMed] [Google Scholar]
- Li D. L., Zhao Y. L., Sun X. L., Hu B. R., Ma M. L. (2014). Rapid screening of natural free radical scavengers in two Polygonum herbs by fast HPLC-DAD-ESI-MSn with precolumn incubation method. Anal. Methods 6, 2299–2305. 10.1039/c3ay41897a [DOI] [Google Scholar]
- Li H., Cao S., Wang X., Zuo Q., Chen P., Liu Y., et al. (2016b). Quality evaluation of heshouwu, a taoist medicine in wudang, China. Exp. Ther. Med. 12, 2317–2323. 10.3892/etm.2016.3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang X., Liu Y., Pan D., Wang Y., Yang N., et al. (2016a). Hepatoprotection and hepatotoxicity of heshouwu, a Chinese medicinal herb: context of the paradoxical effect. Food Chem. Toxicol. 108, 407–418. 10.1016/j.fct.2016.07.035 [DOI] [PubMed] [Google Scholar]
- Li R. Y., Feng W. W., Li X. F., Zhang D. K., Li C. Y., Meng Y. K., et al. (2016). Influence of metal ions on stability of 2,3,5,4'-tetrahydroxystilbene-2-O-β- glucoside contained in polygoni multiflori radix. Acta Pharm. Sin. 51, 116–121. 10.16438/j.0513-4870.2015-0419 [DOI] [PubMed] [Google Scholar]
- Li Y. M., Li R. Y., Niu M., Li C. Y., Bai Z. F., Feng W. W., et al. (2016). Influence of specification on chemical composition of dissolution and hepatocytes toxicity of Polygonum multiflorum. Chin. J. Chin. Mater. Med. 41, 1033–1039. 10.4268/cjcmm20160610 [DOI] [PubMed] [Google Scholar]
- Liang L., Zhao Z., Kang T. (2014). Application of microscopy technique and high performance liquid chromatography for quality assessment of Polygonum multiflorum thunb. Pharmacogn. Mag. 10, 415–421. 10.4103/0973-1296.141788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z., Chen H., Yu Z., Zhao Z. Z. (2010). Comparison of raw and processed radix polygoni multiflori (heshouwu) by high performance liquid chromatography and mass spectrometry. Chin. Med. 5:29. 10.1186/1749-8546-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z. T., Shi Y. X., Chen H. B., Zhao Z. Z. (2011). Histochemical analysis of the root tuber of Polygonum multiflorum thunb. (Fam. Polygonaceae), Microsc. Res. Tech. 74, 488–495. 10.1002/jemt.20936 [DOI] [PubMed] [Google Scholar]
- Lin L., Li H., Lin H., Zhang M., Qu C., Yan L., et al. (2017). A new perspective on liver injury by traditional Chinese herbs such as Polygonum multiflorum: the geographical area of harvest as an important contributory factor. Front. Pharmacol. 8:349. 10.3389/fphar.2017.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. F., Lin H. M., Yin X. B., Zhao Y., Xia Z. W., Zhang M., et al. (2015c). Characterization of the constituents in rat plasma after oral administration of radix polygoni multiflori extracts by ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 29, 1541–1547. 10.1002/bmc.3456 [DOI] [PubMed] [Google Scholar]
- Lin L. F., Lin H. M., Zhang M., Dong X. X., Yin X. B., Qu C. H., et al. (2015d). Types, principle, and characteristics of tandem high-resolution mass spectrometry and its applications. RSC Adv. 5, 107623–107636. 10.1039/C5RA22856E [DOI] [Google Scholar]
- Lin L., Lin H., Zhang M., Ni B., Yin X., Qu C., et al. (2015e). A novel method to analyze hepatotoxic components in Polygonum multiflorum using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Hazard. Mater. 299, 249–259. 10.1016/j.jhazmat.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Lin L., Ni B., Lin H., Zhang M., Li X., Yin X., et al. (2015a). Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum thunb: a review. J. Ethnopharmacol. 159, 158–183. 10.1016/j.jep.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Ni B., Lin H., Zhang M., Yan L., Qu C., et al. (2015b). Simultaneous determination of 14 constituents of radix polygoni multiflori from different geographical areas by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 29, 1048–1055. 10.1002/bmc.3391 [DOI] [PubMed] [Google Scholar]
- Lin P., Lu J. M., Zhang G. Y., Li Y. F., Geng C. A., Chen J. J., et al. (2015). Study on absorption and metabolism of active compounds of polygoni multiflori radix in hepatic L-02 cells by liquid chromatography tandem-mass spectrometry. Chin. Pharm. J. 50, 1048–1053. 10.11669/cpj.2015.12.013 [DOI] [Google Scholar]
- Ling S., Xu J. W. (2016). Biological activities of 2,3,5,4'-tetrahydroxystilbene−2-O-β-D-glucoside in antiaging and antiaging-related disease treatments. Oxid. Med. Cell. Longev. 2016:4973239. 10.1155/2016/4973239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Q., Wang L., Yue L. (2013). Influence of high pressure processing on contents of active ingredients from polygoni multiflori radix. Chin. J. Exp. Tradit. Med. Form. 19, 37–40. [Google Scholar]
- Liu X. Y., Liu Y. Q., Qu Y., Cheng M. C., Xiao H. B. (2015). Metabolomic profiling of emodin-induced cytotoxicity in human liver cells and mechanistic study. Toxicol. Res. 4, 948–955. 10.1039/C4TX00246F [DOI] [Google Scholar]
- Liu Z., Chao Z., Liu Y., Song Z., Lu A. (2009). Maillard reaction involved in the steaming process of the root of Polygonum multiflorum. Planta Med. 75, 84–88. 10.1055/s-0028-1088349 [DOI] [PubMed] [Google Scholar]
- Liu Z. L., Liu Y. Y., Wang C., Guo N., Song Z. Q., Wang C., et al. (2011). Comparative analyses of chromatographic fingerprints of the roots of Polygonum multiflorum Thunb. and their processed products using RRLC/DAD/ESI-MSn. Planta Med. 77, 1855–1860. 10.1055/s-0030-1271200 [DOI] [PubMed] [Google Scholar]
- Liu Z. L., Song Z. Q., Chao Z. M., Lv S. Y., Wang C., Li L. F. (2008). HPLC determination of chemical constituents produced in radix polygoni multiflori after processing. Chin. J. Chin. Mater. Med. 33, 2326–2329. [PubMed] [Google Scholar]
- Liu Z. L., Song Z. Q., Zhang L., Li S. L. (2005). Influence of process methods on contents of chemical component radix polygoni multiflori. Chin. J. Chin. Mater. Med. 30, 337–340. [PubMed] [Google Scholar]
- Luo Y. Y., Liu J. X., Hou Y., Liu X. H., Lan C. W., Ma Y., et al. (2015f). Study on the differences of inorganic elements between wild and cultivated Polygonum multiflorum based on ICP-MS technique. J. Nanjing Univ. Tradit. Chin. Med. 31, 64–67. 10.14148/j.issn.1672-0482.2015.0064 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Hou Y., Liu X. H., Lan C. W., Ma Y., et al. (2015g). ICP-MS analysis on inorganic elements in polygoni multiflori radix from different habitats and commercial herbs. Chin. Tradit. Herbal Drugs 46, 1056–1064. 10.7501/j.issn.0253-2670.2014.07.022 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu T., Liu X. H., Lan C. W., Wang S. N., et al. (2016a). Simultaneous determination of stilbenes, anthraquinones, flavonoids and phenolic acids in Polygoni multiflori radix by UPLC-MS/MS. Zhipu Xuebao 37, 327–335. 10.7538/zpxb.youxian.2016.0016 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2015a). Dynamic accumulation analysis on bioactive constituents of Polygonum multiflorum in different collection periods. Chin. J. Chin. Mater. Med. 40, 2565–2570. 10.4268/cjcmm.20151315 [DOI] [PubMed] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2015b). Simultaneous determination of seven components in polygoni multiflori radix by MEKC-DAD. Chin. Pharm. J. 50, 802–807. 10.11669/cpj.2015.09.015 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2015c). Dynamic changes of nucleosides and nucleobases in different harvest periods of polygoni multiflori radix by UPLC-QTRAP-MS/MS. J. Chin. Med. Mater. 38, 919–922. 10.13863/j.issn1001-4454.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2015d). Content determination and principal component analysis of nucleosides and nucleobases in different processed products of polygoni multiflori radix. Chin. J. Pharm. Anal. 35, 1474–1482. 10.16155/j.0254-1793.2015.26 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2015e). Simultaneous determination of nucleosides and nucleobases in polygoni multiflori radix from different origins by UPLC-QTRAP-MS/MS. Fenxi Ceshi Xuebao 34, 519–524. 10.3969/j.issn.1004-4957.2015.05.004 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2014). Determination of the dynamic changes of inorganic elements in polygoni multiflori radix by ICP-MS. Chin. Pharm. J. 49, 1978–1982. 10.11669/cpj.2014.22.004 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Hou Y., Ma Y., et al. (2016b). Determination of multiple functional substances in different processed products of polygoni multiflori radix and principal component analysis. Chin. Tradit. Herbal Drugs 47, 318–323. 10.7501/j.issn.0253-2670.2016.02.022 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Lan C. W., Xu L., Hou Y., et al. (2016c). Analysis of nucleosides and nucleobases of Polygonum multiflorum by stepwise regression analysis. Shipin Kexue 37, 104–108. 10.7506/spkx1002-6630-201602018 [DOI] [Google Scholar]
- Luo Y. Y., Liu J. X., Liu X. H., Wang S. N., Hua Y. J. (2016d). Determination of chemical constituents in polygoni multiflori radix and polygoni multifori caulis from the same origin. Tianran Chanwu Yanjiu Yu Kaifa 28, 1035–1044. 10.16333/j.1001-6880.2016.7.009 [DOI] [Google Scholar]
- Lv G. P., Meng L. Z., Han D. Q., Li H. Y., Zhao J., Li S. P. (2015). Effect of sample preparation on components and liver toxicity of Polygonum multiflorum. J. Pharm. Biomed. Anal. 109, 105–111. 10.1016/j.jpba.2015.02.029 [DOI] [PubMed] [Google Scholar]
- Lv L. S., Tang J., Ho C. T. (2008). Identification of oxidation products of 2,3,5,4'-tetrahydroxystilbene-2-O-β-glucopyranosid from Polygonum mutiflorum Thunb with H2O2. J. Food Lipids 15, 231–239. 10.1111/j.1745-4522.2008.00115.x [DOI] [Google Scholar]
- Lv Y., Wang J. B., Ji Y., Zhao Y. L., Ma Z. J., Li Q., et al. (2013). Influence of extracting solvent on hepatocytes toxicity of Polygonum multiflorum. Chin. J. Exp. Tradit. Med. Form. 19, 268–272. 10.11653/syfj2013200268 [DOI] [Google Scholar]
- Ma J., Qi L. W., Li H. J., Li P. (2012). A segmental monitoring strategy based on variable wavelength detection for quality control of three Polygonaceae herbs. J. Pharm. Biomed. Anal. 62, 155–161. 10.1016/j.jpba.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Ma J., Zheng L., Deng T., Li C. L., He Y. S., Li H. J., et al. (2013). Stilbene glucoside inhibits the glucuronidation of emodin in rats through the down-regulation of UDP-glucuronosyltransferases 1A8: application to a drug-drug interaction study in radix polygoni multiflori. J. Ethnopharmacol. 147, 335–340. 10.1016/j.jep.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Ma J., Zheng L., He Y. S., Li H. J. (2015). Hepatotoxic assessment of polygoni multiflori radix extract and toxicokinetic study of stilbene glucoside and anthraquinones in rats. J. Ethnopharmacol. 162, 61–68. 10.1016/j.jep.2014.12.045 [DOI] [PubMed] [Google Scholar]
- Ma Z. J., Li Q., Zhao K. J., Wang J. B., Xiao X. H. (2017). Dynamic serum metabolomics study of liver injury in rats caused by Polygonum multifulorum. Chin. J. Chin. Mater. Med. 42, 152–156. 10.19540/j.cnki.cjcmm.20161222.004 [DOI] [PubMed] [Google Scholar]
- Ma Z. J., Li X. F., Lv Y., Jiang B. Q., Zhao Y. L., Zhang Y. M., et al. (2015). Comparative study on preparation of polygoni multiflori radix based on hepatotoxic bioassay. Chin. J. Chin. Mater. Med. 40, 2325–2329. 10.4268/cjcmm20151212 [DOI] [PubMed] [Google Scholar]
- Mazzanti G., Battinelli L., Daniele C., Mastroianni C. M., Lichtner M., Coletta S., et al. (2004). New case of acute hepatitis following the consumption of Shou Wu Pian, a Chinese herbal product derived from Polygonum multiflorum. Ann. Intern. Med. 140:W30. 10.7326/0003-4819-140-7-200404060-00042-w3 [DOI] [PubMed] [Google Scholar]
- Pang J. Y., Wang J. B., Ma Z. J., Zhu Y., Zou Z. S., Teng G. J., et al. (2014). Quality evaluation and control of polygoni multiflori radix based on chemical fingerprint and toxicity monitoring. Chin. Tradit. Herbal Drugs 45, 3392–3396. 10.7501/j.issn.0253-2670.2014.23.007 [DOI] [Google Scholar]
- Panis B., Wong D. R., Hooymans P. M., De Smet P. A., Rosias P. P. (2005). Recurrent toxic hepatitis in a Caucasian girl related to the use of Shou-Wu-Pian, a Chinese herba lpreparation. J. Pediatr. Gastroenterol. Nutr. 41, 256–258. 10.1097/01.MPG.0000164699.41282.67 [DOI] [PubMed] [Google Scholar]
- Park G. J., Mann S. P., Ngu M. C. (2001). Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum. J. Gastroenterol. Hepatol. 16, 115–117. 10.1046/j.1440-1746.2001.02309.x [DOI] [PubMed] [Google Scholar]
- Park S. Y., Jin M. L., Chae S. Y., Ko M. J., Choi Y. H., Park G., et al. (2016). Novel compound from Polygonum multiflorum inhibits inflammatory response in LPS stimulated microglia by upregulating AMPK/Nrf2 pathways. Neurochem. Int. 100, 21–29. 10.1016/j.neuint.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Qin B., Xu Y., Chen J., Huang W., Peng Y., Zheng J. (2016). Chemical reactivity of emodin and its oxidative metabolites to thiols. Chem. Res. Toxicol. 29, 2114–2124. 10.1021/acs.chemrestox.6b00191 [DOI] [PubMed] [Google Scholar]
- Qiu X. H., Sun J. B., Xu D. J., Min J. (2007). Study on content and molecular weight of water-soluble polysaccharides in radix polygoni multiflori by different processing and its correlation to activity. Shanghai Zhongyiyao Zazhi 41, 69–71. 10.16305/j.1007-1334.2007.04.033 [DOI] [Google Scholar]
- Qiu X. H., Zhang J., Huang Z. H., Zhu D. Y., Xu W. (2013). Profiling of phenolic constituents in Polygonum multiflorum thunb. by combination of ultra-high-pressure liquid chromatography with linear ion trap-Orbitrap mass spectrometry. J. Chromatogr. A 1292, 121–131. 10.1016/j.chroma.2012.11.051 [DOI] [PubMed] [Google Scholar]
- Ren X. L., Wang G. F., Wang M., Ouyang H. Z., Qi A. D. (2011). Kinetics and mechanism of 2,3,5,4'-tetrahydroxystilbene-2-O-β-glucoside (THSG) degradation in aqueous solutions. J. Pharm. Biomed. Anal. 55, 211–215. 10.1016/j.jpba.2010.12.026 [DOI] [PubMed] [Google Scholar]
- Severin I., Dumont C., Jondeau-Cabaton A., Graillot V., Chagnon M. C. (2010). Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol. Lett. 192, 189–194. 10.1016/j.toxlet.2009.10.022 [DOI] [PubMed] [Google Scholar]
- Shaw D. (2010). Toxicological risk of Chinese herbs. Planta Med. 76, 2012–2018. 10.1055/s-0030-1250533 [DOI] [PubMed] [Google Scholar]
- Shi K., Xie J., Luo L. J., Yang H. L., Zhang Y. (2011). ASS determination of iron black in radix polygoni multiflori preparata. Chin. J. Pharm. Anal. 31, 583–585. [Google Scholar]
- Stickel F., Shouval D. (2015). Hepatotoxicity of herbal and dietary supplements: an update. Arch. Toxicol. 89, 851–865. 10.1007/s00204-015-1471-3 [DOI] [PubMed] [Google Scholar]
- Sun H., Luo G., Chen D., Xiang Z. (2016). A comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front. Pharmacol. 7:247. 10.3389/fphar.2016.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. L., Huang X. L., Wu H. Q., Huang F. (2009a). HPLC/IT-MS analysis of glycosides in radix polygoni multiflori. Tianran Chanwu Yanjiu Yu Kaifa 21, 806–812. [Google Scholar]
- Sun J. L., Huang X. L., Wu H. Q., Huang F. (2009b). Determination of content and light stability of cis- and trans-2,3,5,4'-tetrahydroxystilbene-2-O-β-glucoside in radix polygoni multiflori by HPLC/DAD/MS. Chin. Pharm. J. 44, 541–544. [Google Scholar]
- Sun L. L., Ren X. L., Zhang H. J., Wang M., Liu Y. N., Deng Y. R., et al. (2017). Research on processing of radix polygoni multiflori based on UPLC fingerprints and chemometrics. Zhonghua Zhongyiyao Zazhi 32, 2194–2197. [Google Scholar]
- Sun Z. X., Zhang L. (2010). Liver damage related to Polygonum multiflorum and its preparations: domestic literature review and analysis. Yaowu Buliang Fanying Zazhi 12, 26–30. [Google Scholar]
- Teschke R., Eickhoff A. (2015a). Herbal hepatotoxicity in traditional and modern medicine: actual key issues and new encouraging steps. Front. Pharmacol. 6:72. 10.3389/fphar.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R., Genthner A., Wolff A., Frenzel C., Schulze J., Eickhoff A. (2014a). Herbal hepatotoxicity: an alysis of cases with initially reported positive re-exposure tests. Dig. Liv. Dis. 46, 264–269. 10.1016/j.dld.2013.10.020 [DOI] [PubMed] [Google Scholar]
- Teschke R., Larrey D., Melchart D., Danan G. (2016). Traditional Chinese Medicine (TCM) and Herbal Hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines 3:18. 10.3390/medicines3030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R., Wolff A., Frenzel C., Schulze J. (2014b). Review article: herbal hepatotoxicity-an update on traditional Chinese medicine preparations. Aliment. Pharmacol. Ther. 40, 32–50. 10.1111/apt.12798 [DOI] [PubMed] [Google Scholar]
- Teschke R., Zhang L., Long H., Schwarzenboeck A., Schmidt-Taenzer W., Genthner A., et al. (2015b). Traditional Chinese medicine and herbal hepatotoxicity: a tabular compilation of reported cases. Ann. Hepatol. 14, 7–19. [PubMed] [Google Scholar]
- Valente G., Sanges M., Campione S., Bellevicine C., De Franchis G., Sollazzo R., et al. (2010). Herbal hepatotoxicity: a case of difficult interpretation. Eur. Rev. Med. Pharmacol. Sci. 14, 865–870. 10.14218/JCTH.2014.00009 [DOI] [PubMed] [Google Scholar]
- Wang G. F., Ren X. L., Ouyang H. Z., Qi A. D., Zhao M. (2011). Stability study of stilbene glucoside by chemical kinetics under four illuminant using. Chin. J. Pharm. Anal. 31, 458–462. 10.16155/j.0254-1793.2011.03.006 [DOI] [Google Scholar]
- Wang G. Q., Deng Y. Q., Hou F. Q. (2014). Overview of drug-induced liver injury in China. Clin. Liver Dis. 4, 26–29. 10.1002/cld.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. B., Li C. Y., Zhu Y., Song H. B., Bai Z. F., Xiao X. H. (2016). Integrated evidence chain-based identification of Chinese herbal medicine-induced hepatotoxicity and rational usage: exemplification by Polygonum multiflorum (he shou wu) (in Chinese). Chin. Sci. Bull. 61, 971–980. 10.1360/N972015-01289 [DOI] [Google Scholar]
- Wang J., Ma Z., Niu M., Zhu Y., Liang Q., Zhao Y., et al. (2015). Evidence chain-based causality identification in herb-induced liver injury: exemplification of a well-known liver-restorative herb Polygonum multiflorum. Front. Med. 9, 457–467. 10.1007/s11684-015-0417-8 [DOI] [PubMed] [Google Scholar]
- Wang L., Sang M., Liu E., Banahene P. O., Zhang Y., Wang T., et al. (2017). Rapid profiling and pharmacokinetic studies of major compounds in crude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC-MS/MS. J. Pharm. Biomed. Anal. 140, 45–61. 10.1016/j.jpba.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Wang T. H., Zhang J., Qiu X. H., Bai J. Q., Gao Y. H., Xu W. (2015). Application of ultra-high-performance liquid chromatography coupled with LTQ-orbitrap mass spectrometry for the qualitative and quantitative analysis of Polygonum multiflorum Thumb. and its processed products. Molecules 21:40. 10.3390/molecules21010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Chen X., Huang Q., Fang D., Li G., Zhang G. (2012). Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia 83, 469–475. 10.1016/j.fitote.2011.12.012 [DOI] [PubMed] [Google Scholar]
- Xia X. H., Yuan Y. Y., Liu M. (2017). The assessment of the chronic hepatotoxicity induced by polygoni multiflori radix in rats: a pilot study by using untargeted metabolomics method. J. Ethnopharmacol. 203, 182–190. 10.1016/j.jep.2017.03.046 [DOI] [PubMed] [Google Scholar]
- Xu D. J., Tao Y., Wang S. X., Cheng W. S., Zhong X. F., You B. H. (2011). Effect of high pressure processing on stilbene glycoside and phosphatidylcholine in polygoni multiflori radix. Chin. Tradit. Herbal Drugs 42, 78–80. [Google Scholar]
- Xu L., Luo Y. Y., Liu J. X., Liu X. H., Fang K. H., Lan C. W., et al. (2015). Analysis of nucleosides and nucleobases in polygoni multiflori radix by UPLC-MS/MS. Chin. J. Exp. Tradit. Med. Form. 21, 55–58. 10.13422/j.cnki.syfjx.2015200055 [DOI] [Google Scholar]
- Xu W., Zhang J., Huang Z. H., Qiu X. H. (2012). Identification of new dianthrone glycosides from Polygonum multiflorum thunb. using high-performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry detection: a strategy for the rapid detection of new low abundant metabolites from traditional Chinese medicines. Anal. Methods 4, 1806–1812. 10.1039/c2ay00009a [DOI] [Google Scholar]
- Yan H. J., Fang Z. J., Fu J., Yu S. X. (2010). The correlation between bioactive components of fallopia multiflora root and environmental factors. Am. J. Chin. Med. 38, 473–483. 10.1142/S0192415X10007993 [DOI] [PubMed] [Google Scholar]
- Yan S. L., Su Y. F., Chen L., Que M., Gao X. M., Chang J. B. (2014). Polygonumosides A-D, stilbene derivatives from processed roots of Polygonum multiflorum. J. Nat. Prod. 77, 397–401. 10.1021/np400720y [DOI] [PubMed] [Google Scholar]
- Yang J. B., Li L., Dai Z., Wu Y., Geng X. C., Li B., et al. (2016). Polygonumnolides C1-C4; minor dianthrone glycosides from the roots of Polygonum multiflorum thunb. J. Asian Nat. Prod. Res. 18, 813–822. 10.1080/10286020.2016.1171758 [DOI] [PubMed] [Google Scholar]
- Yang J. B., Tian J. Y., Dai Z., Ye F., Ma S. C., Wang A. G. (2017a). α-Glucosidase inhibitors extracted from the roots of Polygonum multiflorum thunb. Fitoterapia 117, 65–70. 10.1016/j.fitote.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Yang J., Yan Z., Ren J., Dai Z., Ma S., Wang A. G., et al. (2017b). Polygonumnolides A1-B3, minor dianthrone derivatives from the roots of Polygonum multiflorum thunb. Arch. Pharm. Res. [Epub ahead of print]. 10.1007/s12272-016-0816-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Liu T., Feng W. H., Hui L. Q., Li R. R., Liu X. Q., et al. (2016). Exploration research on hepatotoxic constituents from Polygonum multiflorum root. Chin. J. Chin. Mater. Med. 41, 1289–1296. 10.4268/cjcmm20160721 [DOI] [PubMed] [Google Scholar]
- Yang W. Y., Chen X. G., Shi H., Shi Y. D. (2015). Quality changes of root of Polygonum multiflorum during a nine-time repeat of the steaming and sun-drying process. Shizhen Guoyi Guoyao 25, 2414–2418. 10.3969/j.issn.1008-0805.2015.10.040 [DOI] [Google Scholar]
- Yi T., Leung K. S., Lu G. H., Zhang H., Chan K. (2007). Identification and determination of the major constituents in traditional Chinese medicinal plant Polygonum multiflorum thunb by HPLC coupled with PAD and ESI/MS. Phytochem. Anal. 18, 181–187. 10.1002/pca.963 [DOI] [PubMed] [Google Scholar]
- Yu J., Xie J., Mao X. J., Wang M. J., Li N., Wang J., et al. (2011). Hepatoxicity of major constituents and extractions of radix polygoni multiflori and radix polygoni multiflori praeparata. J. Ethnopharmacol. 137, 1291–1299. 10.1016/j.jep.2011.07.055 [DOI] [PubMed] [Google Scholar]
- Zhai X. F., Li K., Lou Y. J., Xiao X. C., Chen D., Jiang B., et al. (2016). Characteristic chemical components in unprocessed and processed Polygonum multiflorum. Zhongnan Yaoxue 14, 704–708. 10.7539/j.issn.1672-2981.2016.07.007 [DOI] [Google Scholar]
- Zhang C. E., Niu M., Li Q., Zhao Y. L., Ma Z. J., Xiong Y., et al. (2016). Urine metabolomics study on the liver injury in rats induced by raw and processed Polygonum multiflorum integrated with pattern recognition and pathways analysis. J. Ethnopharmacol. 194, 299–306. 10.1016/j.jep.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Zhang J. X., Cui Y. M. (2016). Chemical constituents from Polygonum multiflorum. Chin. J. Chin. Mater. Med. 41, 3252–3255. 10.4268/cjcmm20161721 [DOI] [PubMed] [Google Scholar]
- Zhang L., Yang X., Deng Y. (2009). Evaluation and consideration on safety information abroad of Polygonum multiflorum and its preparations. Chin. J. Chin. Mater. Med. 34, 2414–2418. [PubMed] [Google Scholar]
- Zhang P., Ye Y., Yang X., Jiao Y. (2016). Systematic review on Chinese herbal medicine induced liver injury. Evid. Based Complement. Alternat. Med. 2016:3560812. 10.1155/2016/3560812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang N., Zhang M., Diao T., Tang J., Dai M., et al. (2015). Metabonomics study on polygonum multiflorum induced liver toxicity in rats by GC-MS. Int. J. Clin. Exp. Med. 8, 10986–10992. [PMC free article] [PubMed] [Google Scholar]
- Zhao M. J., Gong X. H., Dang J., Yuan A., Li Y., Lou L., et al. (2017). Effect of processing time of polygoni multiflori radix on content changes of 16 componens. Chin. J. Chin. Mater. Med. 42, 1344–1349. 10.19540/j.cnki.cjcmm.20170121.030 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Kao C. P., Chang Y. S., Ho Y. L. (2013). Quality assessment on polygoni multiflori caulis using HPLC/UV/MS combined with principle component analysis. Chem. Cent. J. 7:106. 10.1186/1752-153X-7-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. Q., Su Y. F., Yan S. L., Li T. X., Li J., Gao X. M. (2016). Chromenone derivatives from processed roots of Polygonum multiflorum. Chem. Nat. Compd. 52, 838–840. 10.1007/s10600-016-1791-4 [DOI] [Google Scholar]
- Zhou Y. X., Xia W., Yue W., Peng C., Rahman K., Zhang H. (2015). Rhein: a review of pharmacological activities. Evid. Based Complement. Alternat. Med. 2015:578107. 10.1155/2015/578107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Xue X., Zhang Z. (2016). Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 91, 132–142. 10.1016/j.ijbiomac.2016.05.061 [DOI] [PubMed] [Google Scholar]
- Zhu W. L., Xue X. P., Zhang Z. J. (2017). Structural, physicochemical, antioxidant and antitumor property of anacidic polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 96, 494–500. 10.1016/j.ijbiomac.2016.12.064 [DOI] [PubMed] [Google Scholar]
- Zhu Z. W., Li J., Gao X. M., Amponsem E., Kang L. Y., Hu L. M., et al. (2012). Simultaneous determination of stilbenes, phenolic acids, flavonoids and anthraquinones in radix polygoni multiflori by LC-MS/MS. J. Pharm. Biomed. Anal. 62, 162–166. 10.1016/j.jpba.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Zuo Y., Wang C., Lin Y., Guo J., Deng Y. (2008). Simultaneous determination of anthraquinones in radix polygoni multiflori by capillary gas chromatography coupled with flame ionization and mass spectrometric detection. J. Chromatogr. A 1200, 43–48. 10.1016/j.chroma.2008.01.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.