Abstract
Background
Small vessel vasculitis commonly affects the kidney and can progress to end-stage renal disease. The goal of this study is to compare outcomes of patients who received a renal transplant as a result of small vessel vasculitis (group A) with those who received kidney transplants because of other causes (group B).
Methods
This is a retrospective analysis of United Network for Organ Sharing registry data for adult primary kidney transplants from January 2000 to December 2014. Group A patients (N = 2196) were compared with a group B (N = 6588); groups were case matched for age, race, sex, donor type, and year of transplant in a 1:3 ratio.
Results
Renal and patient survivals were better in the group A (P < 0.001). New-onset diabetes after transplant developed in 8.3% of the group A and 11.3% of group B (P < 0.001). Seventeen (0.8%) patients in group A developed recurrent disease. Of these, 7 patients had graft failure, 3 of which were due to disease recurrence. Group A patients had significantly higher risk of developing posttransplant solid organ malignancies (11.3% vs 9.3%, P = 0.006) and lymphoproliferative disorder (1.3% vs 0.8%, P = 0.026). Independent predictors of graft failure and patient mortality were recipients' morbid obesity, diabetes, age, and dialysis duration (hazard ratio of 1.7, 1.4, 1.1/10 years, and 1.1/year for graft failure, and 1.7, 1.7, 1.6/10 years and 1.1/year for patient mortality, respectively).
Conclusions
Renal transplantation in patients with has favorable long-term graft and patient outcomes with a low disease recurrence rate. However, they may have a higher risk of developing posttransplant malignancies.
Vasculitides, an immune reactive inflammation in vessel walls, often presents as serious and sometimes fatal diseases that require prompt diagnosis and therapy.1 In 2012, the International Chapel Hill Consensus Conference revised the nomenclature of vasculitides.2 Noninfectious vasculitides are classified based on size: large-, medium-, and small-sized vessel vasculitis. Small vessel vasculitis (SVV) includes a variety of serious diseases, such as Henoch-Schoenlein purpura (HSP), anti-glomerular basement membrane (GBM) disease, and antineutrophil cytoplasmic antibody (ANCA) associated vasculitis, which includes granulomatosis with polyangiitis (GPA), eosinophilic GPA (Churg-Strauss syndrome), microscopic polyangiitis (MPA), and renal limited ANCA vasculitis.2-4 SVV affects the kidney and can progress to end-stage renal disease (ESRD) in approximately 20% to 40% of cases.5-8 Of these ESRD patients, around 30% have received a renal transplant in the last 2 decades.7
Patients with SVV and anti-GBM have often undergone cytotoxic treatments for their disease and generally have longer exposure to immunosuppressive medications, which may increase their risk of cancer and infection. Subsequently, this can negatively impact graft and patient survival. However, using Organ Procurement and Transplantation Network/United Network of Organ Sharing (UNOS) data, Shen et al5 reported that patient and graft outcomes in kidney transplant recipients with GPA were superior to those who received renal transplants due to other causes. Similarly, Kanaan et al6 reported favorable graft and patient outcomes in patients with HSP after kidney transplantation. These studies, along with others, have shown that the risk of disease recurrence in SVV patients, although relatively low, may still occur and contribute to graft loss.5,6,9 There have been limited data on postrenal transplant nonvasculitic comorbidities, such as diabetes, malignancies, cytomegalovirus (CMV), or other infections, which are known to result from immunosuppression in these patients.9
To date, there is no large study that looks at posttransplant outcomes in patients with SVV and anti-GBM disease other than survival. Moreover, most previous studies focused only on ANCA-associated vasculitis. The goal of this study is to analyze 1-, 5- and 10-year graft and patient survivals using UNOS database in patients with SVV and anti-GBM disease after renal transplantation and comparing their outcomes with case-matched control patients. Furthermore, this study assesses other outcomes, such as disease recurrence, new-onset diabetes after transplantation (NODAT), posttransplant CMV infection, and malignancies.
MATERIALS AND METHODS
The current study is a retrospective analysis of 15 years of UNOS registry data for primary kidney-alone transplantation from January 1, 2000, to December 31, 2014. Outcomes of adult patients with SVV and anti-GBM disease are compared with a control group of renal transplant patients. Case controls were matched in a 1:3 ratio for recipients' age (exact age by years), sex, race, year of transplantation (categorized to 3 groups), donors' type (deceased or live). The matching was undertaken to eliminate the confounding effect of significant recipient and donor variables on outcomes. Three matched controls were selected for each study patient yielding a total sample size of 8784 with 2196 patients in the cohort and 6588 case controls. We excluded patients younger than 18 years at the time of kidney transplant and those with simultaneous or more than 1 organ transplantation. Data analysis included the cause of ESRD, presence of pretransplant diabetes, whether the patient received preemptive kidney transplantation, dialysis duration before transplantation, body mass index (BMI), HLA mismatch, cold ischemia time, delayed graft function, acute cellular rejection (ACR) within 6 and 12 months of renal transplantation, induction agents used, and maintenance immunosuppressive regimen.
The outcomes studied included renal allograft and patient survival, vasculitis recurrence, NODAT, the development of posttransplant malignancies, and CMV seroconversion. Univariate comparisons of continuous variables were performed using t test or Mann-Whitney U test, depending on the distribution. Variables were analyzed across categories using analysis of variance or χ2 tests as appropriate. A P value less than 0.05 was considered significant for all tests. A Kaplan-Meier analysis was performed to compare renal allograft and patient survival differences among the groups using log rank tests. Using Cox regression modeling, graft failure and patient death were adjusted to the following covariates: BMI, diabetes, age, dialysis duration, PRA percentage, and HLA mismatch. The proportional hazard’s assumption was tested through insertion of a time and effect interactive term in the Cox models. Sensitivity to competing risk was assessed by analyzing the impact of competing death risk on the allograft survival. We compared graft and patient survival based on etiology of ESRD and diabetic status of the patients as well as according to the type of vasculitis (anti-GBM disease, GPA, MPA, and HSP). Statistical analysis was performed using SPSS version 24 (IBM Inc., Armonk, NY, USA) except for the competing risk modeling which was performed using SAS version 9 (SAS Inc., Cary, NC).
RESULTS
We identified 2197 patients with SVV and anti-GBM disease from the UNOS data set (group A), and successfully matched 2196 of them with 6588 patients in the data set (group B). The mean age of patients was 48.8 ± 15.9 years. The majority were white (80.0%) and male (55.1%). Most of the organs came from young, white, deceased donors (Table 1). In group A, 1167 had GPA, 675 had anti-GBM disease, 174 had HSP vasculitis, 173 had MPA, and 7 patients had Churg-Strauss syndrome. The main reason for kidney failure in group B was glomerular diseases (25.2%), diabetes (21.8%), hypertension (17.4%), and polycystic kidney disease (11.8%).
TABLE 1.
Demographic and transplant-specific characteristics of matched patients

The mean ± SD BMI for the group A was 26.55 ± 5.29 and 27.44 ± 5.54 in group B (P < 0.001). Before renal transplantation, 6.3% of group A patients had diabetes mellitus compared with 27.9% in group B (P < 0.001). More patients in group A had dialysis before renal transplantation with longer mean dialysis duration (P < 0.001 and 0.014, respectively). Group A patients had higher PRA% but better HLA crossmatching (P = 0.008 and P < 0.001, respectively). Thymoglobulin was the most widely used induction agent in both groups. The majority of patients in both groups received maintenance steroids, tacrolimus, and mycophenolate mofetil. There were no statistical significant differences between groups regarding induction and maintenance immunosuppressive regimens. The demographic and transplant-specific characteristics of each group are detailed in Table 1.
The median graft survival time was 13.0 years (95% confidence interval [CI], 11.9-14.2) in group A and 10.6 years (95% CI, 10.5-10.7) in group B patients (P < 0.001). The median patient survival time was 14.3 years (95% CI, 13.4-15.2) in group A and 12.3 years (95% CI, 12.2-12.4) in group B patients (P < 0.001). The 1-, 5-, and 10-year graft survival was 95.5%, 83.1%, and 59.6% in group A, respectively, and 93.9%, 77.0%, and 54.3% in group B patients, respectively (Figure 1). The 1-, 5-, and 10-year patient survival was 98.3%, 90.2%, and 68.9% in group A, respectively, and 97.1%, 85.0%, and 62.8% in group B patients, respectively (Figure 2). In group A, 9.7% of patients were lost to follow-up at a median time of 3.9 years. For the group B patients, 10.0% of patients were lost to follow-up at a median time of 3.5 years.
FIGURE 1.

Graft survival among the 2 groups using Kaplan-Meier curves.
FIGURE 2.

Patient survival among the 2 groups using Kaplan-Meier curves.
Compared with diabetic patients, those who did not have diabetes had better graft and patient survivals in both groups (Figures 3 and 4, P < 0.001 in both). The estimated graft and patient survivals were better in patients with polycystic kidney disease and were worst for patients who had diabetic nephropathy as a reason for ESRD (Figures 5 and 6, P <0.001 in both). There were no statistical significant differences in graft survival between the 4 vasculitis subgroups (Figure 7, P = 0.375). Kidney transplant recipients with GPA had the lowest, whereas HSP patients had the highest patient survival compared with the other 2 subgroups with MPO and anti-GBM disease (Figure 8, P < 0.001). There was no significant difference in CMV seroconversion in both groups (P = 0.187 and 0.841, for IgG and IgM, respectively). NODAT developed in 8.3% of patients among group A and 11.3% in group B (P < 0.001).
FIGURE 3.

Graft survival in patients with and without vasculitis and diabetes using Kaplan-Meier curves.
FIGURE 4.

Patient survival in patients with and without vasculitis and diabetes using Kaplan-Meier curves.
FIGURE 5.

Graft survival stratified to the reason for ESRD.
FIGURE 6.

Patient survival stratified to the reason for ESRD.
FIGURE 7.

Graft survival in group A according to the disease type.
FIGURE 8.

Patient survival in group A according to the disease type.
Disease Recurrence
Seventeen patients in the group A developed disease recurrence (0.8%). The mean time to disease recurrence was 24.4 ± 15.0 months. Seven of these patients had graft failure, 3 due to disease recurrence, with the remaining 4 due to other reasons. Details of the timing and impact of recurrence on renal transplant and patient survival are described in Table 2.
TABLE 2.
Recurrent vasculitis after renal transplantation (17 patients)

Posttransplant Malignancies
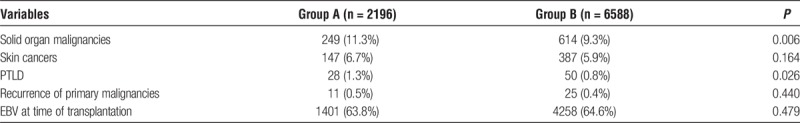
More patients in group A developed solid-organ malignancies and posttransplant lymphoproliferative disorder (PTLD) (P = 0.006 and 0.026, respectively). There was no significant difference between both groups in the occurrence of skin cancers or recurrence of primary malignancies after transplantation. There was no available information about Epstein-Barr virus (EBV) status at the time of cancer diagnosis. However, at the time of transplantation, there was no significant difference in the EBV serology status between the groups (Table 3).
TABLE 3.
Posttransplant malignancies and EBV status
Adjusted Graft and Patient Survival Using Cox Regression Modeling
Multivariable Cox regression analysis showed that independent predictors of graft failure and patient mortality were recipients' morbid obesity, diabetes, age, and dialysis duration (hazard ratio [HR] of 1.7, 1.4, 1.1/10 years, and 1.1/year for graft failure, and 1.7, 1.7, 1.6/10 years and 1.1/year for patient mortality, respectively). After adjustment for these factors, patients with vasculitis or anti-GBM disease maintained favorable graft and patient survival (HR, 0.8 and 0.9 for graft failure and patient death, respectively) (Table 4 and 5). Application of a competing risks (graft failure and death) model had no impact on the vasculitis HRs. Analyses of interactions with a time-dependent covariate were not significant, confirming the proportional hazards assumptions were met.
TABLE 4.
Cox model of graft failure

TABLE 5.
Cox model of patient death

Causes of Kidney Allograft Loss
Chronic allograft nephropathy was the most common cause of graft loss among both groups (65% in group A and 64% in group B). Acute rejection was the second most common cause of graft loss (23% and 29%, in group A and group B, respectively). Death with a functioning graft occurred in 11% of patients in group A and 15% in group B. None of these results were statistically significant between the 2 groups.
Cause of Death
The most common cause of death in group A was malignancies (21%), followed by infections (16%), then cardiovascular complications (14%). The most common cause of death in group B was cardiovascular events (21%), followed by infections (15%), then malignancies (12%). Unfortunately, the cause of death was frequently missing in the UNOS data set (24% for group A and 28% for group B).
DISCUSSION
Because of the low incidence of SVV and anti-GBM diseases among renal transplant recipients, there is a paucity of large studies evaluating these patients' outcomes. Most of these studies focused only on ANCA-associated vasculitis especially GPA. In this study, using the most recent UNOS data, we compared outcomes of renal transplant patients with anti-GBM disease, ANCA-associated vasculitis (GPA and MPA), and HSP with those who had ESRD secondary to other nonvasculitis causes. Overall, patients with SVV or anti-GBM diseases had relatively better outcome in terms of graft and patient survivals. The SVV and anti-GBM groups were matched to the comparison group by age, race, sex, donor type, and year of transplantation to mitigate the effect of differences in baseline characteristics between both groups. The SVV and anti-GBM cohorts had some favorable characteristics, including fewer HLA mismatches and particularly less diabetes. However, more patients in this group had dialysis before renal transplantation with a longer dialysis duration and overall higher PRA%. Some single-center and limited national studies have reported that the graft and patient survivals for renal transplant recipients with SVV are comparable to other renal transplants.5,6,10-13 However, several other studies have also reported high recurrence rates of vasculitis after renal transplantation.14-20 When we compared survivals after excluding diabetic patients in both the SVV and non-SVV groups, the SVV group still had better graft survival. Yet, when we compared only patients with diabetes in both groups, superior survivals were no longer appreciative in the SVV group. In this study, diabetic renal transplant patients had worse outcomes whether they have SVV, anti-GBM disease, or not. Other studies had reported a similar observation which was mostly attributed to increased cardiovascular risks in diabetic patients.5,21 Lim and colleagues22 investigated in a population cohort study the long-term outcome of kidney transplantation in patients with type 2 diabetes. They found that kidney transplant recipients with type 2 diabetes had significantly poorer patient survival, with 5-year mortality rates exceeding those of nondiabetic patients by over twofold, especially in those younger than 40 years. Furthermore, compared with the general population, there was no evidence of improvement in mortality over time among diabetic patients after kidney transplantation.
There is paucity of information on the nonvasculitic comorbidities, such as infections and malignancies that affect SVV patients after renal transplantation. Also, the frequency of relapses and the influence of disease type on these patients are lacking. Hruskova et al23 suggested that such information will require collaboration among the transplant and vasculitis centers and the establishment of a large registry. In this study, we tried to explore some of these issues at a national level.
Recurrence
Posttransplant recurrence of vasculitis and anti-GBM disease is a major concern for this group of patients. The reported recurrence rate in the literature has been quite variable.6,24-29 Interestingly, in this study, we found a very low recurrent vasculitis rate that was rarely the cause of graft loss in this cohort. We do not have data on disease control or antibody titers at the time of transplantation; however, most of these patients were on dialysis for more than a year, and they were likely in remission at the time of transplantation. Six (0.9%) of 675 patients had recurrence in the anti-GBM subgroup. A study in the Australia and New Zealand registry found that 2.7% of patients developed biopsy-proven recurrent anti-GBM disease, which led to graft failure in less than 0.5% of patients.30 They also reported that the graft and patient survivals were better than other patients transplanted for ESRD from other causes. In an older European study, the frequency of recurrent disease was much higher at 14%,31 which may reflect differences in immunosuppressive use during an earlier era and shorter dialysis duration before renal transplantation in the European study. For example, Briganti et al32 studied the risk of renal allograft loss from recurrent glomerulonephritis in Australia. They did not observe any recurrence in patients with anti-GBM disease up to 10 years after transplantation. Of note, the practice in Australia is to defer transplantation for 12 months after the completion of treatment in cases of anti-GBM disease.
In this study, the recurrence rates of other vasculitis subgroups are as follows: HSP, 5.7%; GPA, 0.1%; and MPA, 0%. The recurrence rate of our patients with HSP was higher compared with other types of vasculitis. In concordance with our results, several studies have reported a high recurrence rate (15% to 53%) after renal transplantation in HSP patients.13,33,34 Gera et al,25 in a single-center study, found the recurrence rate of ANCA-associated vasculitis after renal transplantation to be 8.6%. They were unable to identify clear risk factors for recurrence, and their patients had satisfactory response to treatment with no deleterious effect on renal transplant function. Other studies have reported quite variable relapse rates for ANCA-associated vasculitis. For example, Marco et al35 reported a relapse rate of 0.01 per patient per year, whereas Nachman et al19 in an older study reported rates as high as 17%. In concordance with our study, Westman et al28 reported that the relapse rate may be higher in GPA compared with MPA patients.
Influence of Disease Type on Graft and Patient Survival in Group A Patients (Subgroup Analysis)
We did not find a significant difference in graft survival in group A patients based on the type of the disease. However, patients with GPA had a lower patient survival. Tang et al36 studied the outcomes of 228 MPA and 221 GPA patients in Australia and New Zealand in a multicenter study. They found that graft survival in GPA patients was comparable to non-SVV patients but superior to MPA patients, and MPA patients had worse renal and patient survivals. There were no significant differences in baseline characteristics between MPA and GPA patients except that MPA patients were older. The mean age of our patients with SVV was 48.8 years, whereas the mean age in Tang et al's study was older than 60 years. Suppiah et al37 studied the cardiovascular events in patients with GPA and MPA. They found that those with a PR3-ANCA showed a reduced cardiovascular risk compared with those with a MPO-ANCA. Cardiovascular risk factors were not assessed in our study.
NODAT
The incidence and impact of NODAT on patient and renal survival has not been clearly reported in the literature in patients with vasculitis. In this study, fewer patients in the SVV and anti-GBM disease group developed NODAT after transplantation compared with the nonvasculitis group. This might be explained by the fact that SVV and anti-GBM patients had a significantly lower BMI and less metabolic syndrome compared with the comparison group. The reported incidence of NODAT is variable and must be interpreted in the context of definition used, time from transplant, study population, and immunosuppressive agents used. Studies reported rates ranging from 7% to 46%.38,39 NODAT adversely affects long-term allograft survival. In 1 study, graft survival at 12 years was 22% less in those with NODAT; this was associated with a 3.7 relative risk of graft loss.40 The consequences of NODAT in vasculitis patients require further studies.
Infections
CMV seroconversion was not significantly different between the 2 study groups. In previous studies, the frequency of infections posttransplantation, in general, was not a major concern in patients with ANCA-associated vasculitis23 and HSP patients.33 The details of infections are not reported in the UNOS database; therefore, we were unable to study in depth the rate and outcomes of infections in our cohort.
Malignancies
In this study, more patients in the group A developed solid organ malignancies and PTLD. In agreement with this finding, several studies have reported an increased cancer risk in patients with SVV and anti-GBM diseases. Hoffman et al41 reported an overall increased cancer risk of 2.4 with a 33-fold increased risk for urinary bladder cancer and an 11-fold increased risk for lymphoma in patients with GPA. Similar findings of an increased risk of 1.6 to 3.8 for all sites of cancer were later reported in the literature.42-45 In a recent analysis of patients with SVV and anti-GBM disease, Deegens et al46 noted a significant increase in malignancies (mainly skin cancer) after renal transplantation compared with a matched control group. In contrast, a study from Germany showed no increased risk of cancer at all sites among patients with ANCA-associated vasculitis.47 Also in a single-center study, Marco et al35 did not find differences in the incidence of cancer in patients with ANCA-associated vasculitis after renal transplantation. In a previous UNOS analysis, the reported incidence of cancer in patients with GPA was not different (2.9%) compared with other ESRD transplant recipients (3.1%).5 Our reported cancer rate was relatively higher compared with this previous UNOS study. This could be explained by the younger population age, shorter follow-up period, and difference in immunosuppression regimen in the previous UNOS study.5
Interestingly, in our study malignancies were the most common cause of death in the SVV and anti-GBM group (21% of all deaths). Geetha et al48 in a multicenter study reported that cancer accounted for 27% of deaths in renal transplant patients with GPA and MPA, and it was the most common cause of death among these patients. Similarly, Little et al49 reported in a survey of European transplant centers that the primary cause of death among ANCA-associated vasculitis patients was malignancies (26% of cases), followed by cardiovascular complications in 14% of cases. Geera et al25 also reported in their single-center study that cancer was the leading cause of death (50% of deaths) in patients with GPA and MPA. On the other hand, Shen et al5 reported that cancer accounted for a small proportion of deaths among both GPA (1.2%) and non-GPA (0.95%) transplant recipients. These conflicting results can be explained by the use of less toxic therapy, such as less exposure to cyclophosphamide, or a shorter follow-up period. Several studies have shown that the long-term use of immunosuppressive agents after renal transplantation is associated with an increased risk of malignancies, particularly PTLD and skin cancers.50-52 Given the higher risk of malignancy and consequent mortalities in patients with SVV and anti-GBM diseases, perhaps, we should appropriately risk stratify and advocate the use of less intense immunosuppressive induction and maintenance regimens.
Study Limitations
Even though this study is the largest in renal transplant patients with SVV and anti-GBM disease, it has some inherent limitations. In particular, it is a retrospective analysis with lack of detailed information on kidney allograft function, complications, and cause of death. Because this study depends on a national database, a relatively small number of unreported or misreported variables could potentially affect the significance of the results. Information regarding vasculitis’ disease activity, treatment, and serology status before and at the time of transplantation are lacking in the UNOS registry. Although we did not adjust for the competing risk of death while a graft was still viable, the adjustment to the reported HR for vasculitis was modest. Finally, the Cox regression models were limited to the available factors in the database.
CONCLUSIONS
Renal transplantation in patients with SVV appears to have a favorable graft and patient survival compared with other ESRD patients. The risk of disease recurrence appears to be minimal. However, the risk of malignancy may be increased and contributes to the cause of death in this population.
Footnotes
Published online 20 February, 2018.
The project described herein was supported by the NIH National Center for Advancing Translational Sciences through grant number UL1TR001998.
The authors declare no conflicts of interest.
A.E.-H. participated in research design, data analysis, performance of the research, and writing of the article. S.S. participated in research design, performance of the research, and writing of the article. O.H. participated in performance of the research and writing of the article. X.M. contributed in analytic tools and data management. A.L.C. participated in research design, performance of the research, and writing of the article. D.L.D. contributed in analytic tools and data management. R.G. participated in research design, performance of the research, and writing of the article. B.P.S. participated in research design, performance of the research, and writing of the article.
REFERENCES
- 1.Silva-Fernández L, Loza E, Martínez-Taboada VM, et al. Biological therapy for systemic vasculitis: a systematic review. Semin Arthritis Rheum. 2014;4:542–557. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 3.Moiseev S, Novikov P, Jayne D, et al. End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant. 2017;32:248–253. [DOI] [PubMed] [Google Scholar]
- 4.Booth AD, Almond MK, Burns A, et al. Outcome of ANCA- associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–784. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Gill J, Shangguan M, et al. Outcomes of renal transplantation in recipients with Wegener’s granulomatosis. Clin Transplant. 2011;25:380–387. [DOI] [PubMed] [Google Scholar]
- 6.Kanaan N, Mourad G, Thervet E, et al. Recurrence and graft loss after kidney transplantation for Henoch-Schönlein purpura nephritis: a multicenter analysis. Clin J Am Soc Nephrol. 2011;6:1768–1772. [DOI] [PubMed] [Google Scholar]
- 7.Buttigieg J, Henderson L, Kidder D. Outcome of kidney transplant in antineutrophil cytoplasmic antibody-associated vasculitis. Exp Clin Transplant. 2016;20:6002. [DOI] [PubMed] [Google Scholar]
- 8.Slot MC, Tervaert JW, Franssen CF, et al. Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int. 2003;63:670–677. [DOI] [PubMed] [Google Scholar]
- 9.Hruskova Z, Geetha D, Tesar V. Renal transplantation in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2015;30:il59–il63. [DOI] [PubMed] [Google Scholar]
- 10.Wrenger E, Pirsch JD, Cangro CB, et al. Single-center experience with renal transplantation in patients with Wegener’s granulomatosis. Transpl Int. 1997;10:152–156. [DOI] [PubMed] [Google Scholar]
- 11.Haubitz M, Kliem V, Koch KM, et al. Renal transplantation for patients with autoimmune diseases: single-center experience with 42 patients. Transplantation. 1997;63:1251–1257. [DOI] [PubMed] [Google Scholar]
- 12.Moroni G, Torri A, Gallelli B, et al. The long-term prognosis of renal transplant in patients with systemic vasculitis. Am J Transplant. 2007;7:2133–2139. [DOI] [PubMed] [Google Scholar]
- 13.Han SS, Sun HK, Lee JP, et al. Outcome of renal allograft in patients with Henoch-Schönlein nephritis: single-center experience and systematic review. Transplantation. 2010;89:721–726. [DOI] [PubMed] [Google Scholar]
- 14.Geetha D, Seo P. Renal transplantation in the ANCA associated vasculitides. Am J Transplant. 2007;7:2657–2662. [DOI] [PubMed] [Google Scholar]
- 15.Rostaing L, Modesto A, Oksman F, et al. Outcome of patients with antineutrophil cytoplasmic autoantibody-associated vasculitis following cadaveric kidney transplantation. Am J Kidney Dis. 1997;29:96–102. [DOI] [PubMed] [Google Scholar]
- 16.Nyberg G, Akesson P, Nordén G, et al. Systemic vasculitis in a kidney transplant population. Transplantation. 1997;63 :1273–1277. [DOI] [PubMed] [Google Scholar]
- 17.Fan SL, Lewis KE, Ball E, et al. Recurrence of Wegener's granulomatosis 13 years after renal transplantation. Am J Kidney Dis. 2001;38:E32. [DOI] [PubMed] [Google Scholar]
- 18.Elmedhem A, Adu D, Savage CO. Relapse rate and outcome of ANCA-associated small vessel vasculitis after transplantation. Nephrol Dial Transplant. 2003;18:1001–1004. [DOI] [PubMed] [Google Scholar]
- 19.Nachman PH, Segelmark M, Westman K, et al. Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int. 1999;56:1544–1550. [DOI] [PubMed] [Google Scholar]
- 20.Samuel JP, Bell CS, Molony DA, et al. Long-term outcome of renal transplantation patients with Henoch-Schonlein purpura. Clin J Am Soc Nephrol. 2011;6:2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosio FG, Hickson LJ, Griffin MD, et al. Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. Am J Transplant. 2008:8:593–599. [DOI] [PubMed] [Google Scholar]
- 22.Lim WH, Wong G, Pilmore HL, et al. Long-term outcomes of kidney transplantation in people with type 2 diabetes: a population cohort study. Lancet Diabetes Endocrinol. 2017;5:26–33. [DOI] [PubMed] [Google Scholar]
- 23.Hruskova Z, Geetha D, Tesar V. Renal transplantation in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2015;30(Suppl 1):i159–i163. [DOI] [PubMed] [Google Scholar]
- 24.Hariharan S, Peddi VR, Savin VJ, et al. Recurrent and de novo renal diseases after renal transplantation: a report from the renal allograft disease registry. Am J Kidney Dis. 1998;31:928–931. [DOI] [PubMed] [Google Scholar]
- 25.Gera M, Griffin MD, Specks U, et al. Recurrence of ANCA-associated vasculitis following renal transplantation in the modern era of immunosupression. Kidney Int. 2007;71:1296–1301. [DOI] [PubMed] [Google Scholar]
- 26.Sauter M, Schmid H, Anders HJ, et al. Loss of a renal graft due to recurrence of anti-GBM disease despite rituximab therapy. Clin Transplant. 2009;23:132–136. [DOI] [PubMed] [Google Scholar]
- 27.Lau D, Summers S, Amos L, et al. Recurrence of anti-neutrophil cytoplasmic antibody vasculitis in the kidney allograft. Nephrology (Carlton). 2012;17(Suppl 1):16–19. [DOI] [PubMed] [Google Scholar]
- 28.Westman K, Flossmann O, Gregorini G. The long-term outcomes of systemic vasculitis. Nephrol Dial Transplant. 2015;30(Suppl 1):i60–i66. [DOI] [PubMed] [Google Scholar]
- 29.Barbouch S, Hajji M, Aoudia R, et al. Outcome of renal transplant in recipients with vasculitis. Exp Clin Transplant. 2017;15(Suppl 1):93–96. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, McDonald SP, Hawley CM, et al. Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney Int. 2013;83:503–510. [DOI] [PubMed] [Google Scholar]
- 31.Briggs JD, Jones E. Renal transplantation for uncommon diseases. Scientific Advisory Board of the ERA-EDTA Registry. European Renal Association-European Dialysis and Transplant Association. Nephrol Dial Transplant. 1999;14:570–575. [DOI] [PubMed] [Google Scholar]
- 32.Briganti EM, Russ GR, McNeil JJ, et al. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103–109. [DOI] [PubMed] [Google Scholar]
- 33.Moroni G, Gallelli B, Diana A, et al. Renal transplantation in adults with Henoch-Schonlein purpura: long-term outcome. Nephrol Dial Transplant. 2008;23:3010–3016. [DOI] [PubMed] [Google Scholar]
- 34.Soler MJ, Mir M, Rodriguez E, et al. Recurrence of IgA nephropathy and Henoch-Schönlein purpura after kidney transplantation: risk factors and graft survival. Transplant Proc. 2005;37:3705–3709. [DOI] [PubMed] [Google Scholar]
- 35.Marco H, Mirapeix E, Arcos E, et al. , Catalan Study Group of Glomerular Diseases (GLOMCAT). Long-term outcome of antineutrophil cytoplasmic antibody-associated small vessel vasculitis after renal transplantation. Clin Transplant. 2013;27:338–347. [DOI] [PubMed] [Google Scholar]
- 36.Tang W, Bose B, McDonald SP, et al. The outcomes of patients with ESRD and ANCA-associated vasculitis in Australia and New Zealand. Clin J Am Soc Nephrol. 2013;8:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken). 2011;63:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heisel O, Heisel R, Balshaw R, et al. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4:583–595. [DOI] [PubMed] [Google Scholar]
- 39.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. [DOI] [PubMed] [Google Scholar]
- 40.Miles AM, Sumrani N, Horowitz R, et al. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998;65:380–384. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman GS, Leavitt RY, Kerr GS, et al. The treatment of Wegener's granulomatosis with glucocorticoids and methotrexate. Arthritis Rheum. 1992;35:1322–1329. [DOI] [PubMed] [Google Scholar]
- 42.Knight A, Askling J, Ekbom A. Cancer incidence in a population-based cohort of patients with Wegener's granulomatosis. Int J Cancer. 2002;100:82–85. [DOI] [PubMed] [Google Scholar]
- 43.Faurschou M, Sorensen IJ, Mellemkjaer L, et al. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35:100–105. [PubMed] [Google Scholar]
- 44.Mukhtyar C, Flossmann O, Hellmich B, et al. ; European Vasculitis Study Group (EUVAS). Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League against Rheumatism Systemic Vasculitis Task Force. Ann Rheum Dis. 2008;67:1004–1010. [DOI] [PubMed] [Google Scholar]
- 45.Silva F, Seo P, Schroeder DR, et al. ; Wegener's Granulomatosis Etanercept Trial Research Group. Solid malignancies among etanercept-treated patients with granulomatosis with polyangiitis (Wegener's): long-term followup of a multicenter longitudinal cohort. Arthritis Rheum. 2011;63:2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deegens JK, Artz MA, Hoitsma AJ, et al. Outcome of renal transplantation in patients with pauci-immune small vessel vasculitis or anti-GBM disease. Clin Nephrol. 2003;59:1–9. [DOI] [PubMed] [Google Scholar]
- 47.Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener's granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63:257–266. [DOI] [PubMed] [Google Scholar]
- 48.Geetha D, Eirin A, True K, et al. Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis: a multicenter experience. Transplantation. 2011;91:1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Little MA, Hassan B, Jacques S, et al. Renal transplantation in systemic vasculitis: when is it safe? Nephrol Dial Transplant. 2009;24:3219–3225. [DOI] [PubMed] [Google Scholar]
- 50.Morath C, Mueller M, Goldschmidt H, et al. Malignancy in renal transplantation. J Am Soc Nephrol. 2004;15:1582–1588. [DOI] [PubMed] [Google Scholar]
- 51.Eccher A, Boschiero L, Delahunt B, et al. De novo renal neoplasia after kidney transplantation according to new 2016 WHO Classification of Renal Tumors. Ann Transplant. 2016;21:745–754. [DOI] [PubMed] [Google Scholar]
- 52.Hortlund M, Arroyo Mühr LS, Storm H, et al. Cancer risks after solid organ transplantation and after long-term dialysis. Int J Cancer. 2017;140:1091–1101. [DOI] [PubMed] [Google Scholar]


